Abstract
Antibodies directed to the Sa antigen are highly specific for rheumatoid arthritis (RA) and can be detected in approximately 40% of RA sera. The antigen, a doublet of protein bands of about 50 kDa, is present in placenta and in RA synovial tissue. Although it has been stated that the Sa antigen is citrullinated vimentin, experimental proof for this claim has never been published. In this study, we investigated the precise nature of the antigen. Peptide sequences that were obtained from highly purified Sa antigen were unique to vimentin. Recombinant vimentin, however, was not recognized by anti-Sa reference sera. In vivo, vimentin is subjected to various post-translational modifications, including citrullination. Since antibodies to citrullinated proteins are known to be highly specific for RA, we investigated whether Sa is citrullinated and found that Sa indeed is citrullinated vimentin. Anti-Sa antibodies thus belong to the family of anticitrullinated protein/peptide antibodies. The presence of the Sa antigen in RA synovial tissue, and the recent observation that vimentin is citrullinated in dying human macrophages, make citrullinated vimentin an interesting candidate autoantigen in RA and may provide new insights into the potential role of citrullinated synovial antigens and the antibodies directed to them in the pathophysiology of RA.
Keywords: anticitrullinated protein/peptide antibodies, anti-Sa antibodies, citrullinated vimentin, rheumatoid arthritis, Sa antigen
Introduction
Many autoantibodies, directed against a variety of autoantigens, can be detected in the serum of rheumatoid arthritis (RA) patients. Most of these autoantibodies (reviewed in [1,2]), are also found in patients with other diseases and are therefore not specific for RA. Even the well known rheumatoid factor (RF) antibodies, directed against the Fc part of IgG (reviewed in [3]), are not very specific for RA [4,5]. Nevertheless, RF still is the most commonly used serological marker for RA. Antibodies directed to the Sa antigen have a much higher specificity for RA. (This autoimmune system was first described using the serum of an RA patient whose name began with 'Sa'.) The antibodies target a doublet of protein bands of approximately 50 kDa on western blots of extracts from normal human placenta, spleen, and rheumatoid synovial tissue [6].
In a recent review [7], the Sa antigen was suggested to be identical to citrullinated vimentin, but data to support that statement were not given at that time. In this report, we provide such data and show that the Sa antigen is indeed citrullinated vimentin. We show that anti-Sa antibodies target citrullinated epitopes and not unmodified vimentin, which makes them a member of the family of antibodies directed to citrullinated proteins (reviewed in [8]). Because citrulline, the antigenic determinant for these autoantibodies, is a nonstandard amino acid, it is not incorporated into proteins during translation. It can, however, be generated post-translationally by enzymatic citrullination (deimination) of arginine residues. This conversion is catalyzed by the enzyme peptidylarginine deiminase (PAD, EC 3.5.3.15; reviewed in [9]). Because antibodies to citrullinated proteins are very specific for RA, are detectable very early in the disease, sometimes even during the preclinical phase of RA [10,11], and are able to predict clinical disease outcome [12-14], it is likely that these antibodies will become progressively more valuable for the clinician.
We discuss the new perspective provided by our observation that Sa is citrullinated vimentin on the potential role of citrullinated antigens and the antibodies directed to them in RA pathophysiology.
Materials and methods
Patient sera and antibodies
In this study, we used 87 serum samples from patients attending the Rheumatic Diseases Unit, Faculté de Medicine, Université de Sherbrooke, Sherbrooke, QC, Canada. Sixty-one sera were from RA patients satisfying the 1987 American Rheumatism Association criteria for the classification of RA [15]: of these, 46 were anti-Sa-positive and 15, anti-Sa-negative. The 26 non-RA sera (8 osteoarthritis patients, 14 systemic lupus erythematosus patients, 2 psoriatic arthritis patients, and 2 healthy individuals) were all anti-SA negative. Experiments were approved by the the ethics committee of the Université de Sherbrooke.
Rabbit antibodies directed against chemically modified citrulline (anti-MC) were described previously [16,17]. Mouse monoclonal antibodies (RV202) [18] and affinity-purified rabbit polyclonal antibodies against vimentin were a kind gift of Dr F Ramaekers (Maastricht, The Netherlands).
Preparation of placental Sa antigen
Semipurified placental extracts were prepared as described previously [6,19]. Briefly, fresh human placenta was homogenized in a low-salt Tris buffer (50 mM Tris–HCl (pH 7.4), 120 mM NaCl, 0.02% NaN3, 1 mM dithiothreitol, 1.5 mM phenylmethylsulfonyl fluoride (PMSF), and 0.5 μg/ml each of chemostatin, leupeptin, antipain, and pepstatin). The soluble fraction was separated by anion-exchange chromatography. Sa proteins were eluted with 300 mM of NaCl and subsequently desalted and lyophilized for storage at -80°C.
Characterization of placental Sa antigen
To prepare Sa for amino acid sequencing, a three-step purification procedure was performed essentially as described by Liang and colleagues [20]. In the first step, 1 mg of the semipurified Sa (prepared as described above) was resolved on a preparative 10% SDS–polyacrylamide gel. Vertical strips were cut from each side of the gel and were blotted onto nitrocellulose membrane. The blotted strips were incubated with anti-Sa reference serum and served to determine the exact position of the Sa bands.
In the second step of the procedure, the Sa-rich horizontal strip was cut out of the preparative gel and washed extensively in a freshly prepared, filtered and prewarmed (37°C) wash solution (2 mM Tris–HCl [pH 8.0], 8 M deionized urea, and 1% Nonidet P40 [NP-40]). The washed gel strip was then loaded on an isoelectric focusing (IEF) gel (6 M urea, 1% NP-40, 6% acrylamide [39:1], 2% carrier ampholytes [BioRad, Hercules, CA, USA] [pH range 3–10] and 0.067% riboflavin). IEF was performed as described by Bouffard and colleagues [21]. IEF standards for pH range 3.6–6.6 (Sigma, St Louis, MO, USA) were used to estimate the pI of the proteins. Immediately after migration, the gel was fixed in 20% trichloroacetic acid (TCA) for 20 min and then colored with Coomassie brilliant blue. Each individual band was cut out and frozen at -20°C.
The third step of the procedure was to load excised bands on a second preparative 10% SDS–polyacrylamide gel. Each band from the IEF gel was divided over two lanes: one was used for western blotting with anti-Sa reference serum to determine the exact position of the Sa antigen, and the other was stained with Coomassie brilliant blue. The bands that matched the Sa antigen were excised from the Coomassie-stained gel and washed twice with 50% acetonitrile before being frozen and sent for microsequencing to the Harvard microchemistry facility (Harvard University, Cambridge, MA, USA) [22]. Peptides, generated by in-gel digestion with trypsin, were separated by liquid chromatography followed by double mass spectrometry (LC/MS/MS) and finally sequenced by Edman degradation. The obtained peptide sequences were used to identify the purified Sa antigen using the US National Center for Biotechnology Information (NCBI) nonredundant protein database.
Detection of autoantibodies
The presence of anti-Sa antibodies was determined by immunoblotting as described previously [6,19]. Briefly, semipurified Sa was loaded on 15% SDS–polyacrylamide slab gels (0.1–0.2 mg total protein per centimeter of gel width). After migration, proteins were blotted onto nitrocellulose membranes. Sera were diluted 1:40 and tested for IgG anti-Sa. Immunoblots were scored independently by two individuals.
Anti-CCP (cyclic citrullinated peptide) autoantibodies were detected using the Rapscan RA mk2 kit (CCP2; Euro-Diagnostica, Arnhem, The Netherlands) in accordance with the instructions of the manufacturer.
Immunoprecipitation
Immunoprecipitations (IPs) were performed essentially as described elsewhere [23]. Briefly, 2.5 mg of semipurified Sa was dissolved by heating to 95°C for 1 min in 250 μl buffer containing 20 mM Tris–HCl (pH 7.4), 20 mM ethylene glycol bis(β-aminoethylether) N,N'-tetraacetic acid (EGTA), 1 mM dithioerythritol, and 2% SDS. Insoluble proteins were removed by centrifugation for 5 min at 13,000 g. The supernatant was diluted with IP buffer (IPB: 50 mM Tris–HCl (pH 7.2), 150 mM NaCl, 0.25% sodium deoxycholate, 1% Triton X-100, and Complete protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany]) to a final concentration of 0.1% SDS. The diluted solution was centrifuged for another 5 min at 13,000 g to remove insoluble proteins. Protein G agarose beads (150 μl 50% slurry) were washed three times with IPB–SDS (IPB containing 0.1% SDS). RV202 monoclonal antivimentin antibodies (150 μl) were incubated with the beads in IPB–SDS for 4 hours at room temperature. After removal of unbound antibody by three washes with IPB–SDS, 500 μg Sa (1 ml of diluted solution) was incubated with the beads overnight at 4°C. After extensive washing (three washes with IPB–SDS, one wash with IPB–SDS supplemented with 100 mM KCl, and a last wash with IPB–SDS), bound proteins were eluted by boiling in SDS–sample buffer (250 mM Tris–HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol). The immunoprecipitate was divided over three 13% SDS–polyacrylamide gels and transferred to Hybond-C Extra membranes (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). The blots were stained with either anti-MC antibodies, anti-Sa reference serum, or polyclonal antivimentin antibodies, as described below. As a negative control, an IP was performed with an isotype-matched, unrelated control antibody (4G3 directed to U2 snRNP B'' protein [24]).
Citrullination of vimentin
Human recombinant vimentin (Research Diagnostics Inc, Flanders, NJ, USA) was citrullinated in vitro by rabbit muscle PAD (Sigma; 40 U of PAD per milligram of vimentin) for 3 hours at 55°C in a buffer containing 0.1 M Tris–HCl (pH 7.6), 10 mM CaCl2, and 5 mM dithioerythritol. The reaction was stopped by adding EGTA (pH 8.0) to a final concentration of 50 mM. The extent of the citrullination was estimated by immunoblotting with anti-MC antibodies (described below).
Western blotting
Blots were incubated in blocking buffer (PBS containing 5% nonfat dried milk and 0.1% NP-40) for 1 hour at room temperature and 1–3 hours with the antibody of interest diluted in blocking buffer. After washing the blot with blocking buffer, bound antibodies were detected by incubation with horseradish-peroxidase-conjugated secondary antibodies, followed by chemiluminescence. For the detection of citrullinated proteins, blots were chemically treated before immunostaining with anti-MC antibodies, as described previously [16].
Results
Characterization of the Sa antigen
Antibodies directed to Sa are highly specific for RA. To investigate the identity of the Sa antigen, we performed a multistep purification procedure as described in Materials and methods. The accuracy of each step of the procedure was monitored by immunoblotting using anti-Sa reference serum. The result of final purification is shown in Fig. 1, where the estimated molecular weight of Sa is ~50 kDa and its pI is 5.0. Two peptide sequences were obtained (indicated in Fig. 2; peptide 72–86 was obtained twice) that uniquely matched the sequence of vimentin. The calculated molecular weight (54 kDa) and pI (5.1) of vimentin closely resemble those of Sa.
Figure 1.
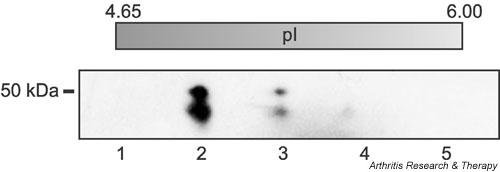
Purification of placental Sa antigen. The antigen was purified by anion-exchange chromatography from extracts of placenta and subsequently purified by two-dimensional gel electrophoresis according to a three-step procedure described by Liang and colleagues [20]. First, proteins were separated by molecular weight, then proteins of appropriate molecular weight were separated by isoelectric focusing (IEF), and finally proteins with appropriate pI were separated once more by molecular weight. Each step of the procedure was monitored by western blotting with an anti-Sa reference serum. Shown here is the final gel, which was stained with Coomassie brilliant blue. The double band in lane 2 is the Sa antigen that was cut out and used for microsequencing. Each lane represents a portion of the IEF gel (approximate pI is listed above each lane).
Figure 2.

Amino acid sequence of vimentin with Sa microsequences and sequence of human vimentin (Swiss-Prot database number P08670). Two distinct peptides that were obtained by microsequencing are indicated in grey. Peptide 72–86 was obtained twice, VD84–85 was ambiguous in one of the peptides, and R78 could not be sequenced in both peptides. All arginines are given in capital and bold, because they can potentially be modified to citrulline by peptidylarginine deiminase.
Discordance between anti-Sa and anti-unmodified vimentin but strong concordance between anti-Sa and anti-CCP status
To confirm that Sa is vimentin, we prepared immunoblots containing human recombinant vimentin. Antivimentin reactivity was observed at lower serum dilutions in only a subset of the patients, irrespective of their anti-Sa or disease status (results not shown). Concordance between reactivity to Sa and reactivity to vimentin was thus not observed. Vimentin is known to undergo several post-translational modifications, including phosphorylation and citrullination [25,26]. Since antibodies directed to citrullinated proteins are very specific for RA [1], we investigated whether anti-Sa sera are reactive with citrullinated epitopes. The vast majority (96%) of anti-Sa-positive RA patients tested positive for anti-CCP2, using the CCP2 test kit, as did a substantial proportion (60%) of the anti-Sa-negative RA patients. Anti-SA-negative RA sera had significantly lower anti-CCP2 titers than anti-Sa-positive sera (Fig. 3). These results suggest that anti-Sa sera contain antibodies that are reactive with citrullinated epitopes.
Figure 3.
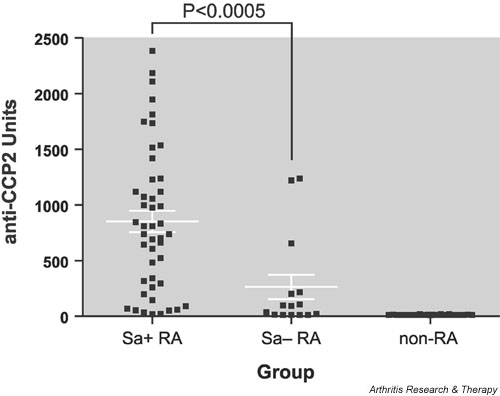
Comparison of anti-CCP titers in Sa+ and Sa- patients. To investigate a possible relationship between anti-Sa and anti-CCP autoantibodies, we compared anti-CCP2 antibody titers in 46 anti-Sa-positive rheumatoid arthritis (RA) sera, 15 anti-Sa-negative RA sera, and 26 control sera, using the CCP2 test kit. Ninety-six percent of anti-Sa-positive RA patients, 60% of Sa-negative RA-patients, and none of the control patients was positive for anti-CCP2. Anti-Sa-positive RA sera had a significantly higher anti-CCP titer (852 ± 96 U; mean ± SEM) than anti-SA-negative sera (263 ± 110 U) (P < 0.0005; Mann–Whitney test). None of the control sera tested positive in either of the two assays (12 ± 0.4 U).
The placental Sa antigen is citrullinated vimentin
To investigate whether Sa is citrullinated vimentin, we used a method to detect citrullinated proteins in cell extracts or fixed cells. In this method, the citrulline sidechain is specifically modified by chemical treatment into complex structures that are so bulky that the influence of flanking amino acids for epitope recognition becomes negligible [16,17,26-28]. Citrullinated proteins are detected (after chemical treatment) by antibodies specifically targeting those modified citrullines (anti-MC). Noncitrullinated proteins cannot be modified by the chemical treatment and are thus not recognized by the specific antibodies.
We prepared three identical immunoblots (Fig. 4) containing semipurified placental Sa antigen, human recombinant vimentin, and human recombinant vimentin that had been citrullinated in vitro. The Sa antigen was recognized by anti-Sa reference serum, by antivimentin antibodies, and by anti-MC antibodies (lanes 1, 4, and 7, respectively), indicating that the antigen is indeed citrullinated vimentin. Furthermore, citrullinated vimentin was recognized by the anti-Sa serum, whereas unmodified vimentin was not (lanes 3 and 2, respectively), indicating that the presence of citrulline residues is essential for the autoantigenicity of vimentin. The differences in the positions of the bands can be attributed to the extent of post-translational processing. Data in the literature show decreased mobilities with increased citrullination of filaggrin and trichohyalin [29]. Furthermore, the increased mobility of the Sa antigen in comparison with the recombinant proteins may be the result of partial proteolytic processing, as this has been described for keratins in the cornified layer of the epidermis [30-32].
Figure 4.

Placental Sa antigen is citrullinated vimentin. Three identical immunoblots containing semipurified placental Sa antigen (100 μg; lanes 1, 4, 7), human recombinant vimentin (Vim) (50 ng; lanes 2, 5, 8), and human recombinant vimentin that had been citrullinated in vitro (cit-Vim) (50 ng; lanes 3, 6, 9) were stained with either anti-Sa reference serum (left panel), anti-modified citrulline (anti-MC) antibodies (middle panel), or antivimentin (anti-Vim) antibodies (right panel). Sa antigen is recognized both by anti-MC antibodies and by anti-Vim antibodies (lanes 4 and 7, respectively), indicating that the antigen is indeed citrullinated vimentin. Citrullinated vimentin was recognized by the anti-Sa serum, whereas unmodified vimentin was not (lanes 3 and 2, respectively), indicating that the presence of citrulline residues is essential for the autoantigenicity of Sa. Molecular weight markers are indicated on the left.
In addition, we performed IP experiments. Vimentin was immunoprecipitated from the semipurified Sa preparation with monoclonal antivimentin antibody. Immunoprecipitated vimentin was stained both by anti-MC antibodies and by anti-Sa reference serum (Fig. 5), confirming that citrullinated vimentin is the antigenic Sa protein.
Figure 5.
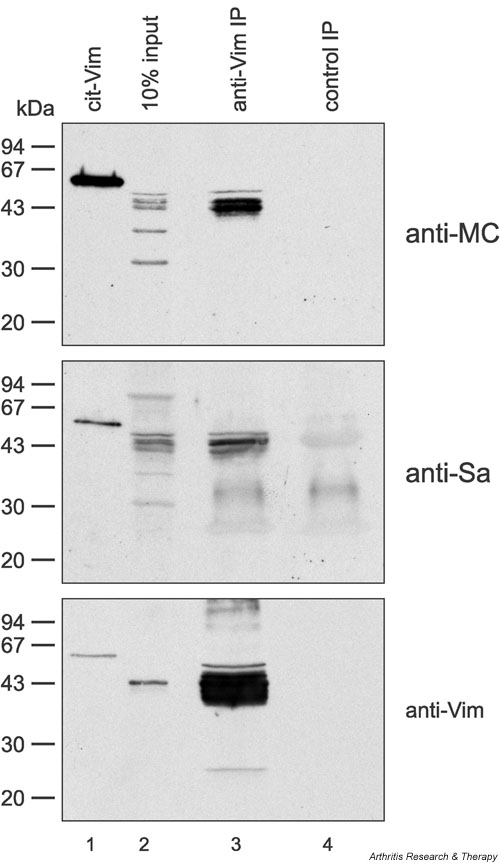
Antivimentin (anti-Vim) can immunoprecipitate Sa antigen from placental extract. Vimentin was immunoprecipitated from semipurified placental extract with monoclonal antibody RV202 (lanes 3). Immunoprecipitated vimentin was stained by anti-modified citrulline (anti-MC) antibodies (upper panel), anti-Sa serum (middle panel), or polyclonal antivimentin (anti-Vim) antibodies (lower panel), indicating that Sa is citrullinated vimentin. An immunoprecipitation (IP) with an isotype-matched monoclonal control antibody 4G3 (lanes 4) served as a negative control. Lanes 1 contain human recombinant vimentin citrullinated in vitro (cit-Vim) (50 ng) as a positive control. Lanes 2 show 10% input. Molecular weight markers are indicated on the left.
Discussion
Autoantibodies against the placental Sa antigen were described for the first time over a decade ago [6]. Since then, this autoimmune system has proven to be highly specific for RA in populations of patients from Europe, America, and Asia [7]. The mean sensitivity of the assay is 37% (range 21–43%) with 98% specificity (range 92–100%) and a positive predictive value between 95 and 99% [7].
Although it was previously suggested that Sa might be identical to citrullinated vimentin [7], evidence for this statement has not been published. This had led to confusion in the literature regarding the exact nature of the antigen. Indeed, it was proposed that Sa could be identical to the glycolytic enzyme α-enolase [33] or to calpastatin [34], the natural inhibitor of calpains. Both types of autoantibody, however, turned out to be independent autoimmune systems, associated with but not specific for RA [35,36]. Other workers have claimed that apolipoprotein-A-1-binding protein could be (part of) the Sa antigen [37]. More recently, autoantibodies not specific for RA and directed to a 68-kDa placental protein were reported [38]. Nevertheless, the authors of that study still chose, inappropriately, to label this antigen Sa. We thus deemed it important to report the experimental evidence relating to the exact nature of the Sa antigen.
We obtained several peptide sequences from highly purified preparations of Sa antigen that were unique to the intermediate filament protein vimentin. Autoantibodies directed to vimentin were described many years ago in RA [39] as well as in other autoimmune diseases [39,40], in infectious diseases [41,42], and even in healthy individuals [43]. Those antivimentin autoantibodies, which are mainly of the IgM class, are not specific for RA. In contrast, anti-Sa antibodies are highly specific for RA and are predominantly IgG [6].
Here, using recombinant human vimentin, we found IgG antivimentin antibodies in only a few sera. The antibodies were only found in low titers, were not related to anti-Sa positivity, and had no disease specificity. Thus we conclude that anti-Sa sera do not target native (unmodified) vimentin.
Many known autoantibodies are directed against proteins that become modified during cell death and in particular during apoptosis (reviewed in [44,45]). These modifications include proteolytic cleavage by caspases or granzyme B [46], transglutamination [47], (de)phosphorylation [48,49], and also citrullination [26,50]. When these modified self proteins are inefficiently cleared, they may be presented to the immune system and might be recognized as 'nonself' [51]. If sufficient 'danger signals' are present (as in an inflammatory environment), this can lead to an immune response against the modified proteins [51,52]. Vimentin can be subjected to various post-translational modifications that could be important for its autoantigenicity. Those modifications are not present in the recombinant vimentin, and this fact could readily explain why we did not observe significant antivimentin reactivity in anti-Sa-positive patients.
Intermediate filaments are a major component of the cytoskeleton of eukaryotic cells. Together with the actin microfilaments and microtubules, they form an integrated network that is responsible for the mechanical integrity of the cell and is critically involved in processes such as cell division, motility, and plasticity. Although there are at least five distinct classes of intermediate filament, cells of mesenchymal origin and most cells in culture contain intermediate filaments composed of vimentin. Vimentin intermediate filaments are dynamic structures [53] and their flexible organization is important for various cellular processes [54]. The filaments are composed of homopolymers of vimentin subunits [55]. The polymerization of the free subunits into filaments is a reversible process, in which phosphorylation is an important regulating factor; free vimentin subunits are more heavily phosphorylated (on their amino terminal head domain) than polymerized vimentin [25,53]. Vimentin can also be citrullinated, which means that some of its arginine residues are deiminated to citrulline residues. This modification of vimentin has been described as occurring in dying macrophages [26,28]. It is known that citrullination of the amino terminal head domain by PAD induces disassembly of the vimentin filaments in vitro [50]. Therefore, citrullination may be involved in the disassembly of the vimentin cytoskeleton during cell death, when the network of vimentin filaments collapses into perinuclear aggregates. The phosphorylation and citrullination of vimentin may account for the small differences between calculated and observed molecular weight/pI values (53.6/5.1 vs ~50/5.0) and also for the differences in gel mobility observed in Fig. 4. Larger variations in molecular weight/pI values of vimentin have been reported elsewhere (66.9/5.6 and 48.6/4.6) [56].
The existence of citrullinated vimentin provided us with new clues to the nature of the Sa antigen. Autoantibodies directed to citrullinated proteins are highly specific for RA (reviewed in [8]). Besides their high specificity, they share more features with the anti-Sa antibodies. They can be detected very early in the disease and can often predict the clinical outcome of the disease [10,57,58]. Furthermore, both types of autoantibody correlate with the presence of HLA-DR shared epitope [58,59]. To investigate whether anti-Sa sera are indeed directed against citrullinated epitopes, we tested 87 sera of known anti-Sa status in the anti-CCP2 assay. Our aim was not to compare the two assays, since we did not test large numbers of randomly selected patients, but to investigate the concordance or lack of it between anti-Sa and anti-CCP antibody status. Of the anti-Sa-positive RA sera, most were also positive for anti-CCP. Of anti-SA-negative RA sera, a considerable proportion was anti-CCP-positive, albeit at a lower titer. The anti-CCP titers of anti-Sa-positive patients were on average more than three times as high as those of anti-Sa-negative RA patients. It thus appears that mainly sera that are strongly positive for anti-CCP will score positive for anti-Sa. This observation is in agreement with the difference in sensitivities of the two assays (70–80% for anti-CCP2, 30–40% for anti-Sa) [7,8]. Other assays that detect anticitrullinated protein antibodies by immunoblotting (using filaggrin as the antigen) have sensitivities (~40%) comparable with the sensitivity of the anti-Sa assay [60-62].
The presence of antibodies to citrullinated proteins is correlated with a more severe disease outcome, especially when high titers of the antibody are present (reviewed in [8]). In an early RA cohort study, the presence of anti-Sa antibodies appeared to be slightly more correlated with erosive disease outcome than the presence of anti-CCP [63], a finding that is in agreement with the idea that the Sa antigen is recognized by patients with high titers of anticitrullinated protein antibodies.
To actually prove that the placental Sa antigen is citrullinated vimentin, we performed western blotting and IP experiments. Sa was recognized by anti-MC antibodies, showing that it does contain citrulline residues. Furthermore, anti-Sa serum was reactive with vimentin citrullinated in vitro but not with unmodified vimentin, showing that citrulline is essential for antigenicity. Finally, antigenic Sa protein could be immunoprecipitated from semipurified placental extracts by antivimentin antibodies, showing that the antigenic citrulline residues are indeed carried by vimentin. Taken together, our results show that the placental Sa antigen is citrullinated vimentin. Therefore, anti-Sa antibodies belong to the expanding family of anticitrullinated protein antibodies (reviewed in [8]), which includes antiperinuclear factor [64], anti-'keratin' antibodies [65], antifilaggrin antibodies [66,67], and anti-CCP antibodies [68,69]. Their common antigenic determinant is the nonstandard amino acid citrulline; the name of the antibody is simply determined by the antigen used to detect them. Not every citrulline residue in a protein will provide a good epitope, however, because the amino acids flanking the citrulline residue are important for the presentation of the antigenic citrulline residue. Therefore, proteins with a high arginine content, such as filaggrin, fibrinogen, vimentin, histones, or myelin basic protein, are more likely to contain reactive epitopes upon citrullination than are proteins with low arginine content, such as albumin [70]. In fact, most of the in vitro-citrullinated arginine-rich proteins mentioned here have been used in diagnostic assays to detect the RA specific anticitrullinated protein antibodies (filaggrin [62,71], fibrinogen [72], myelin basic protein [70]). Because each antigen and each test format (immunofluorescence, immunoblot, or ELISA) will show different values for sensitivity and specificity for RA, care should be taken in using the proper nomenclature and standardization.
The commercially available anti-CCP2 test has a reported sensitivity of almost 80% and a specificity of 98% [73,74]. Interestingly, some (2 of 46) of the anti-Sa-positive RA patients tested negative for anti-CCP. Vimentin contains 43 arginine residues (see Fig. 2). Each of them can potentially be citrullinated by PAD, resulting in a large variety of citrullinated epitopes. In contrast, in the anti-CCP2 test only a few epitopes are presented. It had been previously established that RA sera show a remarkable variety in the reactivity pattern towards different citrulline-containing peptides, indicating, as previously mentioned, that the amino acids flanking the citrulline residue are important for the antigenicity of the epitope and that anticitrullinated protein reactivity is a strongly polyclonal response [68]. It follows that anti-Sa-positive/anti-CCP-negative sera are most likely directed to citrullinated epitopes that are present only on the Sa antigen.
Conclusion
The placental Sa antigen specifically recognized by antibodies in serum from RA patients has been identified as citrullinated vimentin. Anti-Sa antibodies, therefore, belong to the family of anticitrullinated protein antibodies. The Sa antigen is present in the rheumatoid pannus [6]. We recently observed that vimentin is citrullinated in dying human macrophages [28]. Furthermore, it has been reported that vimentin-derived citrullinated peptides were able to bind to HLA-DR4 shared epitope much more efficiently than noncitrullinated peptides [75]. These findings, together with our identification of the Sa antigen, make citrullinated vimentin an interesting candidate autoantigen in RA and may provide new insights into the potential role of citrullinated synovial antigens and the antibodies directed to them in the pathophysiology of RA.
Competing interests
None declared.
Abbreviations
CCP = cyclic citrullinated peptide; EGTA = ethylene glycol bis(β-aminoethylether) N,N'-tetraacetic acid; IEF = isoelectric focusing; IP = immunoprecipitation; IPB = immunoprecipitation buffer; IPB–SDS = IPB containing 0.1% SDS; MC = modified citrulline; NP-40 = Nonidet P40; PAD = peptidylarginine deiminase; PBS = phosphate-buffered saline; pI = isoelectric point; RA = rheumatoid arthritis; RF = rheumatoid factor; SEM = standard error of the mean; Tris = tris(hydroxymethyl)aminomethane.
Acknowledgments
Acknowledgements
The authors wish to thank Dr Frans Ramaekers (Maastricht, The Netherlands) for providing antivimentin antibodies and Dr Han Zendman (Nijmegen, The Netherlands) for critical reading of the manuscript. These studies were financially supported in The Netherlands by "Het Nationaal Reumafonds" of the Netherlands (Dutch League against Rheumatism), The Netherlands Foundation for Research (NWO grant 940-35-037), and the Netherlands Research Council for Chemical Sciences (CW) with financial aid from the Netherlands Technology Foundation (STW). In Canada, the Canadian Institutes for Health Research (formerly the Medical Research Council of Canada), The Arthritis Society, and the "Fonds de recherché en Santé du Québec" provided salaries and operational fund support.
References
- van Boekel MA, Vossenaar ER, van den Hoogen FH, van Venrooij WJ. Autoantibody systems in rheumatoid arthritis: specificity, sensitivity and diagnostic value. Arthritis Res. 2002;4:87–93. doi: 10.1186/ar395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002;Suppl 2:S1–S5. doi: 10.1186/ar551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mageed RA. The RF antigen. In: van Venrooij WJ, Maini RN, editor. In Manual of Biological Markers of Disease. Section B1.1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 1–27. [Google Scholar]
- Lisse JR. Does rheumatoid factor always mean arthritis? Postgrad Med. 1993;94:133–134. doi: 10.1080/00325481.1993.11945749. [DOI] [PubMed] [Google Scholar]
- Palosuo T, Tilvis R, Strandberg T, Aho K. Filaggrin related antibodies among the aged. Ann Rheum Dis. 2003;62:261–263. doi: 10.1136/ard.62.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres N, Boire G, Lopez LF, Menard HA. The Sa system: a novel antigen-antibody system specific for rheumatoid arthritis. J Rheumatol. 1994;21:1027–1033. [PubMed] [Google Scholar]
- Menard HA, Lapointe E, Rochdi MD, Zhou ZJ. Insights into rheumatoid arthritis derived from the Sa immune system. Arthritis Res. 2000;2:429–432. doi: 10.1186/ar122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a specific marker for (early) rheumatoid arthritis. Clin Applied Immunol Rev.
- Vossenaar ER, Zendman AJW, van Venrooij WJ, Pruijn G. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- Rantapää-Dahlqvist , de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- Nielen MMJ, Van Schaardenburg D, Reesink HWR, van de Stadt RJ, van der Horst-Bruinsma I, de Koning MHM, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. [DOI] [PubMed]
- Kroot EJ, de Jong BA, van Leeuwen MA, Swinkels H, van den Hoogen FH, van't Hof M, van de Putte LB, van Rijswijk MH, van Venrooij WJ, van Riel PL. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2000;43:1831–1835. doi: 10.1002/1529-0131(200008)43:8<1831::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V, Ruzockova S. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis. 2003;62:427–430. doi: 10.1136/ard.62.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, Nicaise-Roland P, Sibilia J, Combe B. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–126. doi: 10.1136/ard.62.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Senshu T, Sato T, Inoue T, Akiyama K, Asaga H. Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Anal Biochem. 1992;203:94–100. doi: 10.1016/0003-2697(92)90047-b. [DOI] [PubMed] [Google Scholar]
- Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- Ramaekers F, Huysmans A, Schaart G, Moesker O, Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987;170:235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Hayem G, Chazerain P, Combe B, Elias A, Haim T, Nicaise P, Benali K, Eliaou JF, Kahn MF, Sany J, Meyer O. Anti-Sa antibody is an accurate diagnostic and prognostic marker in adult rheumatoid arthritis. J Rheumatol. 1999;26:7–13. [PubMed] [Google Scholar]
- Liang FT, Granstrom DE, Timoney JF, Shi YF. Micropreparative high resolution purification of proteins by a combination of sodium dodecyl sulfate-polyacrylamide gel electrophoresis, isoelectric focusing, and membrane blotting. Anal Biochem. 1997;250:61–65. doi: 10.1006/abio.1997.2196. [DOI] [PubMed] [Google Scholar]
- Bouffard P, Gagnon C, Cloutier D, MacLean SJ, Souleimani A, Nallainathan D, Home WA, Pilon N, Gibson DM. Analysis of T cell receptor beta expression by isoelectric focusing following gene amplification and in vitro translation. J Immunol Methods. 1995;187:9–21. doi: 10.1016/0022-1759(95)00161-3. [DOI] [PubMed] [Google Scholar]
- Brewer E, Henion J. Atmospheric pressure ionization LC/MS/MS techniques for drug disposition studies. J Pharm Sci. 1998;87:395–402. doi: 10.1021/js9701059. [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel SP, Harris CC. Tissue-specific expression of keratin proteins in human esophageal and epidermal epithelium and their cultured keratinocytes. Exp Cell Res. 1983;146:271–280. doi: 10.1016/0014-4827(83)90129-5. [DOI] [PubMed] [Google Scholar]
- Habets WJ, Hoet MH, de Jong BA, Van der KA, van Venrooij WJ. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989;143:2560–2566. [PubMed] [Google Scholar]
- Inagaki M, Nishi Y, Nishizawa K, Matsuyama M, Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328:649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Asaga H, Yamada M, Senshu T. Selective deimination of vimentin in calcium ionophore-induced apoptosis of mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;243:641–646. doi: 10.1006/bbrc.1998.8148. [DOI] [PubMed] [Google Scholar]
- Asaga H, Senshu T. Combined biochemical and immunocytochemical analyses of postmortem protein deimination in the rat spinal cord. Cell Biol Int. 1993;17:525–532. doi: 10.1006/cbir.1993.1094. [DOI] [PubMed] [Google Scholar]
- Vossenaar ER, Radstake TR, van der Heijden A, Van Mansum WAM, Dieteren C, de Rooij DJ, Barrera P, Zendman AJW, van Venrooij WJ. Expression and activity of citrullinating PAD enzymes in monocytes and macrophages. Ann Rheum Dis. [DOI] [PMC free article] [PubMed]
- Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- Senshu T, Kan S, Ogawa H, Manabe M, Asaga H. Preferential deimination of keratin K1 and filaggrin during the terminal differentiation of human epidermis. Biochem Biophys Res Commun. 1996;225:712–719. doi: 10.1006/bbrc.1996.1240. [DOI] [PubMed] [Google Scholar]
- Senshu T, Akiyama K, Nomura K. Identification of citrulline residues in the V subdomains of keratin K1 derived from the cornified layer of newborn mouse epidermis. Exp Dermatol. 1999;8:392–401. doi: 10.1111/j.1600-0625.1999.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Senshu T, Eady RA, Takahashi H, Shimizu H, Akiyama M, Iizuka H. Sequential reorganization of cornified cell keratin filaments involving filaggrin-mediated compaction and keratin 1 deimination. J Invest Dermatol. 2002;118:282–287. doi: 10.1046/j.0022-202x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- Saulot V, Yon G, Vittecoq O, Charlionnet G, Manchour N, Lange C, Marvin L, Gilbert D, Le Loet X. Sa, alpha-enolase and rheumatoid arthritis [abstract] Arthritis Rheum. 2000;43(Suppl):S68. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- Despres N, Talbot G, Plouffe B, Boire G, Menard HA. Detection and expression of a cDNA clone that encodes a polypeptide containing two inhibitory domains of human calpastatin and its recognition by rheumatoid arthritis sera. J Clin Invest. 1995;95:1891–1896. doi: 10.1172/JCI117870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulot V, Vittecoq O, Charlionet R, Fardellone P, Lange C, Marvin L, Machour N, Le LX, Gilbert D, Tron F. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 2002;46:1196–1201. doi: 10.1002/art.10252. [DOI] [PubMed] [Google Scholar]
- Menard HA, El-Amine M. The calpain-calpastatin system in rheumatoid arthritis. Immunol Today. 1996;17:545–547. doi: 10.1016/S0167-5699(96)30064-9. [DOI] [PubMed] [Google Scholar]
- Escalona M, Lopez LF, Rodriguez-Mahou M, Gonzalez C, Monteagudo I, Del Castillo R, Caro N, Gonzalez-Montagut C, Cebrian L, Carreno L. Sa antigen is an apolipoprotein-a-1-binding protein [abstract] Arthritis Rheum. 2001;44(Suppl):S304. [Google Scholar]
- Escalona M, Lopez-Longo FJ, Gonzalez CM, Monteagudo I, Rodriguez-Mahou M, Grau R, Carreno L. Anti-Sa sera from patients with rheumatoid arthritis contain at least 2 different subpopulations of anti-Sa antibodies. J Rheumatol. 2002;29:2053–2060. [PubMed] [Google Scholar]
- Kurki P, Helve T, Virtanen I. Antibodies to cytoplasmic intermediate filaments in rheumatic diseases. J Rheumatol. 1983;10:558–562. [PubMed] [Google Scholar]
- Senecal JL, Rothfield NF, Oliver JM. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982;69:716–721. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Pedersen J, Underwood JR, Gust I, Toh BH. Autoantibodies to intermediate filaments in acute viral hepatitis A, B and non-A, non-B are directed against vimentin. J Clin Lab Immunol. 1986;19:1–4. [PubMed] [Google Scholar]
- Yang Y, Fujita J, Bandoh S, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ishida T. Detection of antivimentin antibody in sera of patients with idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. Clin Exp Immunol. 2002;128:169–174. doi: 10.1046/j.1365-2249.2002.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Nobunaga M. Antibodies to cytoskeletal systems in normal human serum. Fukuoka Igaku Zasshi. 1990;81:323–330. [PubMed] [Google Scholar]
- Doyle HA, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol. 2002;14:244–249. doi: 10.1097/00002281-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Utz PJ, Gensler TJ, Anderson P. Death, autoantigen modifications, and tolerance. Arthritis Res. 2000;2:101–114. doi: 10.1186/ar75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini M, Colizzi V. Tissue transglutaminase: apoptosis versus autoimmunity. Immunol Today. 1999;20:130–134. doi: 10.1016/S0167-5699(98)01416-9. [DOI] [PubMed] [Google Scholar]
- Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri S, Degen W, Ghirardello A, Doria A, van Venrooij WJ. Dephosphorylation of autoantigenic ribosomal P proteins during Fas-L induced apoptosis: a possible trigger for the development of the autoimmune response in patients with systemic lupus erythematosus. Ann Rheum Dis. 2001;60:72–76. doi: 10.1136/ard.60.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. Ca(2+)-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem. 1989;264:18119–18127. [PubMed] [Google Scholar]
- Rodenburg RJ, Raats JM, Pruijn GJ, van Venrooij WJ. Cell death: a trigger of autoimmunity? Bioessays. 2000;22:627–636. doi: 10.1002/1521-1878(200007)22:7<627::AID-BIES5>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Opal P, Goldman RD. Intermediate filament dynamics. Curr Opin Cell Biol. 1992;4:99–104. doi: 10.1016/0955-0674(92)90065-K. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Strelkov SV, Herrmann H, Aebi U. Molecular architecture of intermediate filaments. Bioessays. 2003;25:243–251. doi: 10.1002/bies.10246. [DOI] [PubMed] [Google Scholar]
- Bruneel A, Labas V, Mailloux A, Sharma S, Vinh J, Vaubourdolle M, Baudin B. Proteomic study of human umbilical vein endothelial cells in culture. Proteomics. 2003;3:714–723. doi: 10.1002/pmic.200300409. [DOI] [PubMed] [Google Scholar]
- Visser H, Le Cessie S, Vos K, Breedveld FC, Hazes JM. How to diagnose rheumatoid arthritis early? A prediction model for persistent (erosive) arthritis. Arthritis Rheum. 2002;46:357–365. doi: 10.1002/art.10117. [DOI] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, Steiner G, Rosen A, Zhang C, Menard HA, Zhou ZJ, Palosuo T, van Venrooij WJ, Wilder RL, Klippel JH, Schumacher HRJ, El-Gabalawy HS. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglin E, Padyukov L, Hallmans G, van Venrooij WJ, Klareskog L, Rantapää-Dahlquist S. Presence of the shared epitope genes increase the predictive value of antibodies against cyclic citrullinated peptide (CCP) for rheumatoid arthritis [abstract] Arthritis Rheum. 2003;48(Suppl):S678. [Google Scholar]
- Vincent C, Simon M, Sebbag M, Girbal NE, Durieux JJ, Cantagrel A, Fournie B, Mazieres B, Serre G. Immunoblotting detection of autoantibodies to human epidermis filaggrin: a new diagnostic test for rheumatoid arthritis. J Rheumatol. 1998;25:838–846. [PubMed] [Google Scholar]
- Palosuo T, Lukka M, Alenius H, Kalkkinen N, Aho K, Kurki P, Heikkila R, Nykanen M, von Essen R. Purification of filaggrin from human epidermis and measurement of antifilaggrin autoantibodies in sera from patients with rheumatoid arthritis by an enzyme-linked immunosorbent assay. Int Arch Allergy Immunol. 1998;115:294–302. doi: 10.1159/000069460. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Sebbag M, Vincent C, Arnaud M, Fournie B, Cantagrel A, Jolivet M, Serre G. Performance of two ELISAs for antifilaggrin autoantibodies, using either affinity purified or deiminated recombinant human filaggrin, in the diagnosis of rheumatoid arthritis. Ann Rheum Dis. 2001;60:882–887. [PMC free article] [PubMed] [Google Scholar]
- Boire G, Cossette P, de Brum-Fernandes AJ, Liang P, Nyonsenga T, Gingras M, Daniel C, Beauchemin J, Menard HA. Anti-Sa/citrullinated vimentin antibodies (anti-Sa Abs), anti-cyclic citrullinated peptide (anti-CCP) Abs and IgM rheumatois factor (RF) as prognostic markers of disease severity in early polyarthritis (EPA) patients [abstract] Arthritis Rheum. 2003;48(Suppl):S666. [Google Scholar]
- Nienhuis RLF, Mandema EA. A new serum factor in patients with rheumatoid arthritis. The antiperinuclear factor. Ann Rheum Dis. 1964;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Mallya RK, Leslie RD, Clark CJ, Hamblin TJ. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979;2:97–99. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Girbal E, Sebbag M, Gomes-Daudrix V, Vincent C, Salama , Serre G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called "antikeratin antibodies," autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993;92:1387–1393. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbag M, Simon M, Vincent C, Masson BC, Girbal E, Durieux JJ, Serre G. The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1995;95:2672–2679. doi: 10.1172/JCI117969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–163. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lapointe E, Déry U, Vaillancourt F, Menard HA, Senshu T. Rheumatoid sera recognize all citrullinated proteins [abstract] Arthritis Rheum. 1999;42(Suppl):S86. [Google Scholar]
- Vincent C, Nogueira L, Sebbag M, Chapuy-Regaud S, Arnaud M, Letourneur O, Rolland D, Fournie B, Cantagrel A, Jolivet M, Serre G. Detection of antibodies to deiminated recombinant rat filaggrin by enzyme-linked immunosorbent assay: a highly effective test for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2002;46:2051–2058. doi: 10.1002/art.10436. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Chapuy-Ragaud S, Constantin A, Clavel C, Sebbag M, Cantagrel A, Vincent C, Serre G. Autoantibodies to deiminated fibrinogen are the most efficient serological criterion for early rheumatoid arthritis [abstract] Arthritis Res Ther. 2003;Suppl 1:18. doi: 10.1186/ar648. [DOI] [Google Scholar]
- van Venrooij WJ, Hazes JM, Visser H. Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med. 2002;60:383–388. [PubMed] [Google Scholar]
- Vasishta A. Diagnosing early-onset rheumatoid arthritis: the role of anti-CCP antibodies. Am Clin Lab. 2002;21:34–36. [PubMed] [Google Scholar]
- Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting Edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]


