Abstract
The plasma contact activation (CAS) and kallikrein/kinin (KKS) systems consist of 4 proteins: factor XII, prekallikrein, high molecular weight kininogen, and the bradykinin B2 receptor. Murine genetic deletion of factor XII (F12−/−), prekallikrein (Klkb1−/−), high molecular weight kininogen (Kgn1−/−) and the bradykinin B2 receptor (Bdkrb2−/−) yield animals protected from thrombosis. With possible exception of F12−/− and Kgn1−/− mice, the mechanism(s) for thrombosis protection is not reduced contact activation. Bdkrb2−/− mice are best characterized and they are protected from thrombosis through over expression of components of the renin angiotensin system (RAS) leading to elevated prostacyclin with vascular and platelet inhibition. Alternatively, prolylcarboxypeptidase, a PK activator and degrader of angiotensin II, when deficient in the mouse leads to a prothrombotic state. Its mechanism for increased thrombosis also is mediated in part by components of the RAS. These observations suggest that thrombosis in mice of the CAS and KKS are mediated in part through the RAS and independent of reduced contact activation.
The plasma contact activation system (CAS) is a group of proteins [factor XII (XII), prekallikrein (PK), high molecular weight kininogen (HK)] that influence surface-activated blood coagulation tests [the activated partial thromboplastin time, activated clotting time (ACT)] but not hemostasis. XII and to a lesser extent PK influence these assays because they autoactivate into enzymes when incubated with biologic or artificial surfaces. In plasma, XII autoactivates on surfaces and activates PK to plasma kallikrein; plasma kallikrein activates XII and they then reciprocally amplify each other’s activation. HK accelerates this process (Fig 1). Contact activation occurs at the interaction of blood with artificial surfaces such as thrombus on catheter tips, blood and platelet activation in cardiopulmonary bypass, or after adulteration of intravenous preparations (e.g. albumin, immunoglobulin or heparin) with the plasma kallikrein, activated forms of XII (Hageman factor fragment, βFXIIa), or a glysoaminoglycans like chondroitin sulfate. Additionally, several medical disorders such as sepsis, acute attacks of hereditary angioedema due to C1 inhibitor deficiency or mutated XII, adult respiratory distress syndrome (ARDS), and allergic reactions have contact activation with plasma kallikrein formation and bradykinin (BK) liberation.
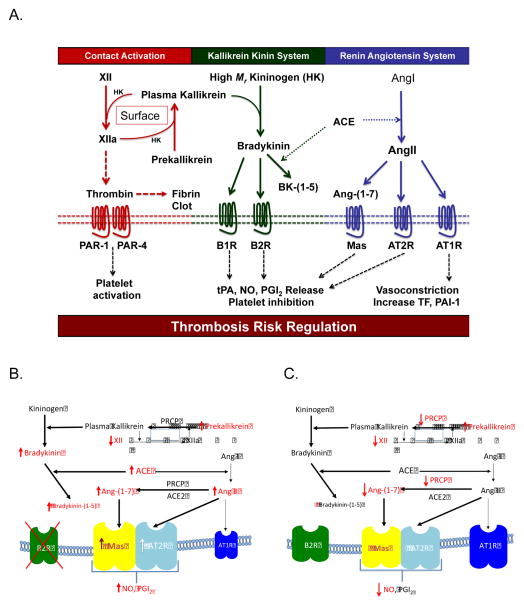
Figure 1.
Panel A Juxtaposition of the plasma contact activation system with the plasma kallikrein kinin and renin angiotensin systems. XII=factor XII; HK or High Mr Kininogen=high molecular weight kininogen; XIIa=activated factor XII; PAR-1=protease activated receptor 1; PAR-4=protease activated receptor 4; BK-(1-5)=bradykinin-(1-5); B1R=bradykinin B1 receptor; B2R=bradykinin B2 receptor; tPA=tissue plasminogen activator; NO=nitrous oxide; PGI2=prostacyclin; ACE=angiotensin converting enzyme; Ang1=angiotensin I; AngII=angiotensin II; Ang-(1–7)=angiotensin-(1–7); Mas: a receptor for Ang-(1–7); AT2R=angiotensin receptor 2; AT1R=angiotensin receptor 1; TF=tissue factor; PAI-1=plasminogen activator inhibitor-1. See text for explanation of the diagram. Panel B: Changes in the plasma KKS and RAS in bradykinin B2 receptor deleted (Bdkrb2−/−) mice that influence thrombosis risk. In Bdkrb2−/− mice there is increased plasma PK and decreased XII. Further there is elevation of BK, bradykinin-(1-5), ACE activity, AngII, and Ang-(1–7). The elevated AngII and Ang-(1–7) stimulate elevated receptors AT2R and Mas to produced increase NO and PGI2. These latter entities inhibit platelet activation and influence vasculature to create an animal that is protected from arterial thrombosis. All abbreviations in this figure are identical to those in Fig. 1A. Panel C: Changes in the plasma KKS and RAS in prolylcarboxypeptidase (PRCP) gene trap mice (PRCPgt/gt) mice that influence thrombosis risk. In PRCPgt/gt, there is reduced XII and increased PK. Additionally there is reduced PK activation and reduced Ang-(1–7) formation. Vascular and cultured endothelial studies indicate that there is reduced and uncoupled eNOS (59). These animals have hypertension and arterial thrombosis. Their vasculature shows inflammation with increased vascular reactive oxygen species associated with reduced and uncoupled eNOS, reduced and dysfunctional thrombomodulin, increased tissue factor, and increased plasminogen activator inhibitor.
A “physiologic process” is one consistent with normal functioning of an organism. Contact activation in vivo always arises under pathophysiologic (disease) circumstances. Contact activation in catheter thrombosis, cardiopulmonary bypass, sepsis, and ARDS leads to thrombin formation (Fig 1). Alternatively, contact activation after infusion of adulterated intravenous preparations, acute attacks of hereditary angioedema, or allergic reactions leads to tissue swelling and hypotension without thrombin formation. Activation of plasma PK by activated XII or prolylcarboxypeptidase (PRCP) (see below) results in plasma kallikrein cleaving HK to liberate BK (1,2). This pathway is the plasma kallikrein/kinin system (KKS) and it generates BK both physiologically and in disease states (Fig 1A).
We have shown that XII binds to endothelial cell uPAR, gC1qR, and cytokeratin 1, and, through uPAR, signals through β1 integrins and EGFR to phosphorylate ERK1/2 and AktS473 (3,4). XII binding results in cell growth and angiogenesis. F12−/− mice have reduced angiogenesis in wound repair and ischemia-reperfusion (unpublished) (4). HK also binds platelets, neutrophils, endothelial cells via uPAR, gC1qR, and cytokeratin 1, is contained in platelet alpha granules and endothelial cells (EC), and is a receptor for PK and factor XI (XI) on EC (5–14). PK and XI bind EC with and without HK (13,14). HK is anti-proliferative, anti-angiogenic, and pro-apoptotic. Deficiencies in HK, XII, and PK (unpublished) are associated with reduced plasma BK, although XII deficiency is associated with half normal plasma BK levels whereas HK and PK deletions are associated with virtually no BK (15,16). BK mediates its activities through constitutive bradykinin B2 receptor (B2R, Bdkrb2) and the B1 receptor (B1R, Bdkrb1) that arises in inflammatory states. BK stimulation of its receptors results in tPA liberation, NO formation, and PGI2 production (Fig 1A).
The CAS and KKS have an intimate relationship with the renin-angiotensin system (RAS) (Fig 1). The crosstalk between KKS and RAS is profound (17). Kininase II, the major enzyme that degrades BK, is angiotensin converting enzyme (ACE) (Fig 1A). ACE degrades BK to make bradykinin-(1–5) [BK-(1–5), (RPPGF)] that is a low affinity direct thrombin inhibitor (18). ACE also creates angiotensin II (AngII). AngII binds to the angiotensin receptor 1 (AT1R) to stimulate vasoconstriction, blood pressure elevation, and increased tissue factor (TF) and PAI-1 release from endothelium (Fig 1A). AngII also binds the angiotensin receptor 2 (AT2R) to produce NO and PGI2. The binding affinity of AngII to the AT1R vs AT2R is the same so the receptor that has higher expression has the dominant effect. The B2R regulates expression of the AT1R and AT2R by formation of heterodimeric complexes (19). Angiotensin-1–7) [Ang-1–7)] which is the angiotensin converting enzyme 2 (ACE2) or PRCP breakdown product of AngII, binds to the receptor Mas (20). Mas activation by Ang-1–7) also elevates tPA, NO and PGI2 (Fig 1A).
Recently several laboratories have recognized a variety of biologic substances, such as exposed vessel collagen, DNA and RNA, aggregated proteins, and long chain polyphosphates, that serve as platforms for XII autoactivation. Further, XII deficient mice (F12−/−) were recognized to have reduced surface activation-induced pulmonary embolism (collagen/epinephrine and long chain polyphosphate) (21). In an inferior vena cava venous stasis model, F12−/− mice also have reduced thrombosis (22). These findings suggest a novel notion that the CAS is involved in thrombosis.
Our laboratory has focused on the contributions of the CAS and KKS on arterial thrombosis. Four animals models of the CAS-KKS with reduced BK delivery to tissues have been examined: the XII KO (F12−/−), the kininogen I KO (Kgn−/−), BK B2 receptor KO (Bdkrb2−/−), and PK (Klkb1−/−) mice (15,21–25). In each case, gene deletion is associated with arterial thrombosis protection. With exception of the Bdkrb2−/− mice, the mechanism(s) for thrombosis protection has not been elucidated in detail.
The finding that Bdkrb2−/− mice are protected from thrombosis was not intuitive since BK has been known to stimulate endothelial cell NO, prostacyclin (PGI2) formation and tissue plasminogen activator (tPA) liberation (23,24). Bdkrb2−/− mice have elevated BK and its ACE metabolite, BK-(1-5), due to less uptake and metabolism by the absent B2R (Fig 1B). BK-(1-5) is elevated because of increased ACE activity that also yields elevated AngII. Usually, AngII stimulates the angiotensin receptor 1 (AT1R) to elevate blood pressure and increase thrombosis risk. However, if the angiotensin receptor 2 (AT2R) is elevated, AngII would bind to the AT2R to produce NO and PGI2. When examined, we found increased AT2R, NO, and PGI2 in Bdkrb2−/− mice (23). Treatment of Bdkrb2−/− with PD123314, an AT2R antagonist, L-NAME, an eNOS antagonist, or nimesulide, a COX2 inhibitor, corrected the thrombosis protection in these animals.
In addition to elevated AngII, we also found elevated angiotensin-(1–7) [Ang-(1–7)] in Bdkrb2−/− mice (Fig 1B). Ang-(1–7) is the prolylcarboxypeptidase (PRCP) or ACE2 breakdown product of AngII (20). Further, the Ang-(1–7) receptor, Mas, was increased as well. Stimulation of the Mas receptor also increases NO and PGI2. In Bdkrb2−/− mice, the Mas antagonist A-779 corrected the prolonged thrombosis time to normal. Thus, in Bdkrb2−/− mice, thrombosis protection is produced by a double increase in AngII and Ang-(1–7) working on increased receptors AT2R and Mas (24).
How do the elevated NO and PGI2 precisely influence thrombosis risk? Bdkrb2 mice have long bleeding times. PGI2, not NO, was mostly responsible for the observed platelet defect (24). Bdkrb2−/− mice have 3-fold PGI2 elevation, elevated cGMP and cAMP, but normal thrombin- and ADP-induced α2bβ3 integrin complex formation and P-selectin expression and fibrinogen binding, respectively. Bdkrb2−/− mice also have an integrin-mediated spreading defect on fibrinogen and collagen and a GPVI activation defect to convulxin, CRP, and collagen (24). The platelet defect is host dependent, not intrinsic to their platelets because on bone marrow transplantation of normal bone marrow into Bdkrb2−/− mice, the normal platelets acquire the same platelet function defect (24). To date, we have not examined the influence of NO and PGI2 on vascular function in Bdkrb2−/− mice, but postulate that it has additional mechanisms for arterial thrombosis protection. Investigations with the PK KO (Klkb1−/−) mice indicate a related mechanism conferring thrombosis protection by influence on vascular function (unpublished). Kgn1−/− mice are deficient in BK because kininogen is the parent protein for BK (15) but as yet, there are no mechanistic data on these animals.
Alterations between the RAS and KKS also lead to higher thrombosis risk. Prolylcarboxypeptidase (PRCP) is a membrane serine protease that degrades biologically active peptides with C-terminus Pro-X bonds and activates PK (1,26,27). Its substrates include αMSH1-13 metabolism regulating central anorexia and AngII metabolism leading to the production of Ang-(1–7) (20,28). In kidney, PRCP is as an important producer of Ang-(1–7) as ACE2 (20,24). PRCP gene trap (PRCPgt/gt) mice are lean, hypertensive, and prothrombotic (29). In PRCP deficiency, there is increased plasma PK and reduced XII (29). Further, there is reduced renal Ang-(1–7) production (Fig 1C) (44). PRCPgt/gt mice have increased vessel ROS with reduced eNOS, uncoupled eNOS, reduced protein C activation due to reduced thrombomodulin expression, increased vascular tissue factor and plasminogen activator inhibitor (59). These animals also have reduced cell growth, angiogenesis, wound injury repair, ischemia/reperfusion repair, and increased arterial neointima/media growth after endothelial cell denudation (30). PRCP’s influence on vascular well-being has nothing to do with contact activation.
In sum, we propose that the proteins of the plasma CAS and KKS have profound effects on thrombosis. With possible exception of F12−/− and Kgn1−/− mice, the regulation of thrombosis risk is not through reduced contact activation leading to thrombin formation, but rather through interactions with the RAS. As we become more knowledgeable in the mechanism(s) by which each of the proteins of the plasma CAS and KKS influence thrombosis risk in vivo, the contributions of RAS are becoming more evident. Finally, the hypothesis for contact activation induced thrombosis is 50 years old this year. Although we are learning new things about contact activation, it is not the only way by which the proteins of the CAS influence thrombosis risk. It is time to think outside the box for new mechanistic pathways.
Acknowledgments
I would like to acknowledge the contributions of Mr. Chao Fang and Dr. Evi Stavrou to the ideas expressed in the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Qui Q, Mahdi F, Shariat-Madar Z, Rojkjaer R, Schmaier AH. Assembly and activation of the HK.PK complex on endothelial cells results in bradykinin liberation and NO formation. Amer J Physiol Heart Circ Physiol. 2001;280:H1821–H1829. doi: 10.1152/ajpheart.2001.280.4.H1821. [DOI] [PubMed] [Google Scholar]
- 3.Mahdi F, Shariat-Madar Z, Figueroa CD, Schmaier AH. Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood. 2002;99:3585–3596. doi: 10.1182/blood.v99.10.3585. [DOI] [PubMed] [Google Scholar]
- 4.LaRusch GA, Madhi F, Shariat-Madar Z, Adams G, Sitrin RG, Zhang WM, McCrae KR, Schmaier AH. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood. 2010;115:5111–5120. doi: 10.1182/blood-2009-08-236430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafson EJ, Schutsky D, Knight L, Schmaier AH. High molecular weight kininogen binds to unstimulated platelets. J Clin Invest. 1986;78:310–318. doi: 10.1172/JCI112567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson EJ, Schmaier AH, Wachtfogel YT, Kaufman N, Kucich U, Colman RW. Human neutrophils contain and bind high moleculae weight kininogen. J Clin Invest. 1989;84:28–35. doi: 10.1172/JCI114151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmaier AH, Kuo A, Lundberg D, Murray S, Cines DB. Expression of high molecular weight kininogen on human umbilical vein endothelial cells. J Biol Chem. 1988;263:16327–16333. [PubMed] [Google Scholar]
- 8.Hasan AAK, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Nat’l Acad Sci. 1998;95:3615–3620. doi: 10.1073/pnas.95.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahdi F, Shariat-Madar Z, Todd RF, III, Figueroa CD, Schmaier AH. Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood. 2001;97:2342–2350. doi: 10.1182/blood.v97.8.2342. [DOI] [PubMed] [Google Scholar]
- 10.Madhi F, Shariat-Madar Z, Schmaier AH. The relative priority of prekallikrein and factors XI/Xia assembly on cultured endothelial cells. J Biol Chem. 2003;278:43983–43990. doi: 10.1074/jbc.M304239200. [DOI] [PubMed] [Google Scholar]
- 11.Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH. Mapping the interaction between high molecular weight kininogen and the urokinase plasminogen activator receptor. J Biol Chem. 2004;279:16621–16628. doi: 10.1074/jbc.M313850200. [DOI] [PubMed] [Google Scholar]
- 12.Schmaier AH, Zuckerberg A, Silverman C, Kuchibhotla J, Tuszynski GP, Colman RW. High molecular weight kininogen: a secreted platelet protein. J Clin Invest. 1983;71:1477–1489. doi: 10.1172/JCI110901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motta G, Rojkjaer R, Hasan AAK, Cines DB, Schmaier AH. High molecular weight kininogen regulates prekallikrein assembly and activation on endothelial cells: a novel mechanism for contact activation. Blood. 1998;91:516–528. [PubMed] [Google Scholar]
- 14.Shariat-Madar Z, Mahdi F, Schmaier AH. Factor XI assembly and activation on human umbilical vein endothelial cells in culture. Thromb Haemost. 2001;85:544–551. [PubMed] [Google Scholar]
- 15.Merkulov S, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Lou G, McCrae KR. Deletion of murine kininogen gene (mKgn1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwaki T, Castellino FJ. Plasma levels of bradykinin are suppressed in factor XII-deficient mice. Thromb Haemost. 2006;95:1003–1010. doi: 10.1160/TH06-03-0128. [DOI] [PubMed] [Google Scholar]
- 17.Schmaier AH. The kallikrein/kinin and the renin angiotensin systems have a multi-layered interaction. Amer J Physiol: Regulatory, Integrative and Comparative Physiology. 2003;285:R1–R13. doi: 10.1152/ajpregu.00535.2002. [DOI] [PubMed] [Google Scholar]
- 18.Hasan AAK, Warnock M, Nieman MT, Srikanth S, Mahdi F, Krishnan R, Tulinsky A, Schmaier AH. The mechanisms of Arg-Pro-Pro-Gly-Phe inhibition of thrombin. Amer J Physiol Heart and Circ Physiol. 2003;285:H183–H193. doi: 10.1152/ajpheart.00490.2002. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PC, Lee M-H, Appleton KM, El-Shewy HM, Morinelli TA, Peterson YK, Luttrell LM, Jaffa AA. The arrestin-selective angiotensin AT1 receptor agonist [Sar1, Ile4,Ile8]-AngII negatively regulates bradykinin B2 receptor signaling via AT1-B2 receptor heterodimers. J Biol Chem. 2013;288:18872–18884. doi: 10.1074/jbc.M113.472381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grobe N, Weir NW, Leiva O, Ong FS, Bernstein KE, Schmaier AH, Morris M, Elased KM. Novel proteomic approaches identify prolyl carboxypeptidase as alternative enzyme for renal angiotensin II processing. Amer J Physiol Cell Physiol. 2013;304:C945–C953. doi: 10.1152/ajpcell.00346.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Bruhl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shariat-Madar Z, Mahdi F, Warnock M, Homeister JW, Srikanth S, Krijanovski Y, Murphey LJ, Jaffa AA, Schmaier AH. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108:192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau MT, Mahdi F, Warnock M, Schmaier AH. Angiotensin-(1-7) and Mas decrease thrombosis in Bdkrb2−/− mice by increasing NO and prostacyclin to reduce platelet spreading and GPVI activation. Blood. 2013;121:3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird JE, Smith PL, Wang X, Schmuacher WA, Barbera F, Revelli J-P, Seiffert D. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher Trait. Thromb Haemost. 2012;107:1141–1150. doi: 10.1160/th-11-10-0682. [DOI] [PubMed] [Google Scholar]
- 26.Odya CE, Marinkovic DV, Hammon KJ, Stewart TA, Erdos EG. Purification and properties of prolylcarboxypeptidase (angiotensinase C) fro human kidney. J Biol Chem. 1978;253:5927–5931. [PubMed] [Google Scholar]
- 27.Shariat-Madar Z, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 204(103):4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 28.Wallingford N, Perroud B, Gao Q, Coppola A, Gao X-B, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase influences food intake by promoting breakdown of α-MSH. J Clin Invest. 2009;119:2291–2302. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams GN, LaRusch GA, Stavrou E, Zhou Y, Nieman M, Jacobs G, Cui Y, Lu Y, Jain MK, Mahdi F, Shariat-Madar Z, Okada Y, D’Alecy LG, Schmaier AH. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. 2011;117:3929–3937. doi: 10.1182/blood-2010-11-318527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams GN, Stavrou EX, Fang C, Merkulova A, Alaiti MA, Nakajima K, Morooka T, Merkulov S, LaRusch GA, Simon DI, Jain MK, Schmaier AH. Blood. 2013;122:1522–1531. doi: 10.1182/blood-2012-10-460360. [DOI] [PMC free article] [PubMed] [Google Scholar]