Abstract
Short sleep duration is associated with increased obesity, however, the role of sleep timing is understudied, particularly in young children.
Objective
To test the independent main and moderating effects of sleep timing on body mass index (BMI) in low-income preschool-aged children (M=4.11 years, SD=0.54).
Methods
Parents reported demographics and children’s sleep concurrently, and a subset of children was followed longitudinally. Child height and weight were measured and BMI z-score (BMIz) calculated. Regression analysis evaluated main effects of sleep timing (bedtime, weekday-to-weekend schedule shifting, napping) on concurrent BMIz and future rate of change, and their moderating effects on the sleep duration-BMIz association.
Results
Of 366 children (longitudinal subsample=273), 50% were male, 57% white, and 37% overweight or obese. Nocturnal sleep duration predicted concurrent BMIz, but not rate of change in BMIz over time. Bedtime was a moderator; the sleep duration-BMIz association was present only among children with bedtimes after 9pm (−0.44; 95% CI −0.69, −0.18). Schedule shifting was a moderator; the association between greater nocturnal sleep duration and lesser rate of future BMIz increase was present only among children with the most consistent sleep schedules (<45 minute delay in weekend bedtime: β = −0.12; 95% CI −0.23, −0.01). Daytime napping did not moderate the nocturnal sleep duration-BMIz association. Covariates (sleep-disordered breathing; soda consumption; home chaos) did not explain these associations.
Conclusions
Among low-income preschoolers, sleep timing moderated the nocturnal sleep duration-BMIz association. Understanding how sleep timing, as well as sleep duration, relates to childhood obesity is important for prevention efforts.
Keywords: sleep timing, sleep duration, obesity, low-income preschool-age children
INTRODUCTION
One in five preschool-aged children in the U.S. is obese.1 Identifying early correlates of and risk factors for obesity is critical for prevention, because once established, obesity typically tracks throughout the lifespan.2 Evidence suggests short sleep duration in early childhood is a risk factor for obesity, 3-5 and low-income children are at high risk for both insufficient sleep6 and obesity.7 Despite such findings, sleep-obesity associations are understudied among young, low-income children. Preschool-age (between 3-5 years) is a sensitive period for the establishment of obesity and sleep-obesity associations,8 yet most studies including this age group are cross-sectional or include only one preschool-age datapoint.5 Children learn to establish independent sleep habits over the preschool period, while parents still retain significant influence on sleep schedules. Preschool-age is thus a time of developmental change when sleep patterns are relatively malleable, and children’s sleep duration has emerged as a target for obesity prevention and intervention.9 The potential additional role of sleep timing in shaping obesity risk during the early childhood years has received little attention, however. Features of sleep timing that could influence obesity risk at this age include bedtime, weekday-to-weekend schedule shifting, and daytime napping.
Five studies have found associations of obesity with late bedtimes.8,10-13 Three of those found that late bedtimes were associated with obesity independent of sleep duration,11-13 but their samples did not include preschoolers. Children as young as preschool-age have been shown to “shift” their bedtimes later on the weekend.14 Maintaining a regular bedtime schedule across weekdays and weekends may be important for reducing obesity risk due to metabolic changes associated with shifted sleep timing.15 Five studies have examined this issue in school-age children or adolescents, with conflicting results: four found that shifted weekday-weekend schedules were associated with obesity, independent of sleep duration.11,16-18 Another found that sleeping more on weekends reduced the increased obesity risk conferred by short weekday sleep, although more variable sleep schedules were associated with poorer metabolic indicators.19 No studies have specifically addressed this issue among preschool-age children, however. Finally, although preschool-age children meet their sleep needs in part via daytime naps, only three studies have examined napping with respect to obesity. One study found that daytime napping was associated with reduced obesity risk,3 whereas two did not find this association.4,20 Only one study20 assessed concurrent napping-obesity associations during the preschool years; however, this was in a Chinese sample with a relatively low obesity rate and high occurrence of napping. To our knowledge, no study has examined the potential main effects of sleep timing, or the moderating effects of sleep timing on the sleep duration-obesity association, in a low-income sample during the early childhood years.
It is also important to consider whether potential sleep duration-obesity associations may be accounted for by other factors that are associated with both sleep and obesity. For example, sleep-disordered breathing (SDB) is an indicator of poor sleep quality, and is associated with obesity in children, so this may be a pathway of association.21 Caffeine consumption (e.g., in soft drinks) may also be another shared pathway.22 Finally, the presence of routines (mealtime; bedtime) in the home environment is also associated with decreased risk for obesity,23 and represents another potential pathway driving the sleep-obesity association, for example by highly-organized families having both consistent bedtimes and healthy eating habits. It is not known whether the association of sleep duration and obesity among low-income preschoolers can be explained by such covariates.
The current study tested whether the association between short sleep duration and increased body mass index (BMI) was present concurrently in a low-income, preschool-age sample. The study also examined whether short sleep duration predicted a greater rate of future increase in BMI longitudinally, in a subsample of the children. The main effect of sleep timing (bedtime, weekday-to-weekend shifting, and napping) on current and future BMI was also tested. This study tested whether the association between nocturnal sleep duration and BMIz was moderated by the same three aspects of sleep timing: bedtime; weekday-to-weekend shifting; and napping. Finally, this study tested whether sleep duration-BMI associations were accounted for by SDB, soda consumption, or home chaos.
METHODS
Participants
Participants were 380 low-income preschoolers from the Midwest region of the United States enrolled in a study of stress, eating behavior, and obesity. Families were recruited from Head Start, a preschool program for low-income families. The study was approved by the University of Michigan Institutional Review Board; parents/legal guardians (typically mothers) provided written informed consent. Families were compensated $90 for participating. Inclusion criteria were that the child was enrolled in Head Start, not in foster care, born at ≥ 35 weeks gestation without serious perinatal/neonatal complications, food allergies, or serious medical problems; parent and child spoke English; and neither parent had ≥ a 4-year college degree. All questionnaires were interviewer-administered. Parents reported child sex and race/ethnicity and mothers were weighed and measured to calculate maternal BMI.
Primary Predictors: Sleep Duration and Timing
Parents were asked to report their child’s “usual bedtime on weekday nights”, “usual waketime on weekday mornings”, “usual bedtime on weekend nights”, and “usual waketime on weekend mornings”, the number of days per week their child napped, and the child’s “typical nap duration”. From these data, average nightly sleep duration across 7 days (“nocturnal sleep duration”) was calculated. For sleep timing, parent-reported weekday bedtime was used to assess “bedtime”. Weekday-to-weekend “shifting” was calculated as the difference between weekday and weekend bedtime (positive values indicated later bedtimes on weekend vs. weekday nights). Average daily nap duration (“napping”) was calculated by multiplying the number of days per week napping by the typical nap duration, and dividing that by 7 (children who did not nap were given a value of zero).
Primary Outcomes: Child Body Mass Index z-score and Rate of Change
Children’s weights and heights were measured without shoes or heavy clothing in a private room at Head Start, by research staff who were trained to reliably weigh and measure children according to standard protocols by a pediatrician, using a +/− 0.1kg calibrated scale (Detecto Physician’s Scale Model DR550) and a +/− 0.1 cm calibrated stadiometer (Seca 217/213). Weight and height measurement was conducted twice, and a third and fourth time if measurements were off by 0.1 kg for weight and 0.5 cm for height. The mean of the two measurements was used. BMI and derived BMI-z score (BMIz) were calculated using sex- and age-specific norms from the US Centers for Disease Control growth charts.24 Overweight or obese was defined as BMI ≥ 85th percentile for age and sex.25
All children in the original cross-sectional study were contacted to participate in the follow-up study, which was focused on stress and self-regulation. In the follow-up study, children were weighed and measured in an identical manner. BMIz rate of change was calculated as the difference between follow-up and original BMIz measures, divided by months in between measurements (M=17.77 months; SD=5.62 months). We multiplied this number by 12 to calculate the rate of BMIz change per year.
Covariates
Sleep-disordered breathing was assessed by parent report using the 22-item SDB subscale of the Pediatric Sleep Questionnaire.26 Items were scored as present/absent and averaged to create a Total SDB Score (α=.70; possible range 0 to 1). Number of servings of soda (“soda, soft drink, pop (not sugar free)”) per day was assessed via parent report using a 90-item food frequency questionnaire validated among parents of low-income children.27 Home chaos was measured as the sum of 15 true/false items on the Confusion, Hubbub, and Order Scale (CHAOS28; α=.79; possible range 0 to 15), with higher scores indicating more chaotic home environments.
Statistical Analyses
Analyses were conducted using SAS version 9.3 (Cary, NC). The sample for cross-sectional analyses was restricted to children in the original study with complete data for nocturnal sleep duration and concurrent BMIz. The sample with complete data included in cross-sectional analyses (n=366) did not differ from the sample without complete data for child sex, age, or sleep duration. Children excluded from analyses (n=13) had higher BMIz’s (M=1.60, SD=0.95) than children included (M=0.74, SD=1.09; p<.01). The sample for longitudinal analysis was restricted to children with complete data for sleep measures and BMIz rate of change, with weight and height measurements at least 6 months apart between the original and follow-up study assessments (n=273). Children with longitudinal BMIz data did not differ from children without longitudinal BMIz data (n=50) in child’s initial BMIz, age, sex, or race/ethnicity; or in mother education, race/ethnicity, or BMI.
The same series of general linear regression models was tested in the cross-sectional and the longitudinal samples. First, the main effect of nocturnal sleep duration on concurrent BMIz was examined in the cross-sectional sample. Next, each of the 3 sleep timing measures (bedtime, shifting, napping) was independently added to this model, to test the main effect of each sleep timing measure on BMIz, after accounting for nocturnal sleep duration. In order to test whether the nocturnal sleep duration-concurrent BMIz association was moderated by any of the 3 sleep timing measures, models were run to test the interaction with nocturnal sleep duration for each measure of sleep timing: bedtime, weekday-to-weekend shifting, and napping. Finally, the above models were repeated including covariates to test whether sleep duration-BMI associations could be explained by SDB, soda consumption, or home chaos. Bivariate associations of BMIz and nocturnal sleep duration with these covariates were also examined.
Parallel models were run in the longitudinal sample to examine the main effect of nocturnal sleep duration; independent effects of each sleep timing variable, the interaction of nocturnal sleep duration with each sleep timing variable, and the role of covariates (SDB, soda consumption, home chaos). In all longitudinal analyses, the outcome was rate of change in BMIz-score per year, controlling for initial BMIz at baseline.
Bivariate analyses showed that child sex and age were unrelated to BMIz or sleep duration, and maternal BMI was related only to child BMIz (r=.22, p<.05). Models with and without maternal BMI produced similar results. As maternal BMI reduced the sample size, unadjusted estimates for all models are presented.
In all models, p<.05 was considered statistically significant. Interactions with p-values < .10 were investigated, and 95% confidence intervals were calculated for parameter estimates.
RESULTS
Table 1 shows sample characteristics. Children’s average weekday bedtime was 8:35pm. Bedtimes averaged over 1 hour later on weekends than weekdays (9:40pm), with greater variability across children on weekends than weekdays. Children who napped during the day (78% of sample) had shorter nocturnal sleep duration than those who did not (p<.01; nappers’ M=10.42 hours/night; SD=0.75; non-nappers’ M=10.68 hours/night; SD=0.79).
Table 1.
Characteristics of the sample (n=366)
| Variable | M (SD) or N (%) |
|---|---|
| Child BMI z-score | 0.75 (1.07) |
| Child rate of BMIz change (per year) (n=273) | 0..048 (0.432) |
| Child Overweight or Obese | 135 (37%) |
| Child age (years) | 4.11 (0.54) |
| Child sex | |
| Male | 184 (50%) |
| Female | 182 (50%) |
| Child race/ethnicity | |
| White, non-Hispanic/Latino | 208 (57%) |
| Black | 56 (15%) |
| Biracial | 59 (16%) |
| Hispanic/Latino | 40 (11%) |
| Other | 3 (1%) |
| Maternal education | |
| < High school | 51 (15%) |
| High school diploma or
Generalized Equivalency Diploma (GED) |
104 (31%) |
| Some college | 177 (53%) |
| Maternal BMI | 32.24 (8.87) |
| Family Income-to-Needs Ratio (1.0=poverty) | 0.88 (0.78) |
| Sleep Variables | |
| Total nocturnal sleep duration (hours per night) | 10.48 (0.76) |
| Child’s usual bedtime (weeknights) | 8:35pm (45 min) |
| Child’s usual bedtime (weekend nights) | 9:40pm (65 min) |
| Weekday to weekend bedtime delay (minutes) | 64.68 (50.76) |
| Number of days napping per week | 3.38 (2.50) |
| Daily nap duration (minutes) | 75.24 (53.70) |
Concurrent Association of Sleep with BMIz
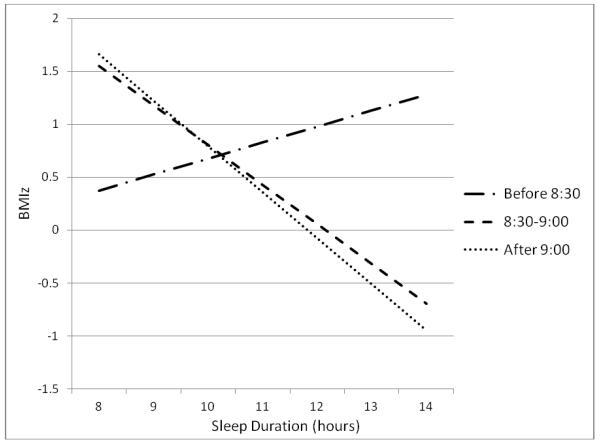
Significant interactions are presented first for both concurrent and longitudinal models. Bedtime moderated the nocturnal sleep duration-concurrent BMIz association (p<.02). To examine this interaction, the sample was divided into tertiles based on bedtime: before 8:30pm (36%), between 8:30 and 9pm (22%), and 9pm or later (40%). There was no association between nocturnal sleep duration and BMIz among children with bedtimes before 8:30pm (β = 0.15; 95% CI −0.14, 0.44), a trend for children with bedtimes between 8:30 and 9pm (β = −0.37; 95% CI −0.75, −0.001), and a significant association for children with bedtimes at 9pm or later (β = −0.44; 95% CI −0.69, −0.18). These associations are shown in Figure 1; slopes for children with bedtimes between 8:30 and 9pm and for those with bedtimes after 9pm did not differ from each other (p<.78). Neither shifting nor napping moderated the nocturnal sleep duration-BMIz association (p<.23 and p<.88 for interaction terms, respectively).
FIGURE 1.
Association between nocturnal sleep duration and concurrent BMIz by tertile of bedtime (cross-sectional sample)
Table 2 presents results for regression models evaluating the main effect of nocturnal sleep duration on concurrent BMIz, as well as the main effects and significant interactions between nocturnal sleep duration and each of the 3 sleep timing measures. Longer nocturnal sleep duration was associated with lower concurrent BMIz (Model A), but there was no independent main effect of shifting or napping on BMIz.
Table 2.
Unadjusted parameter estimates and 95% confidence intervals from final cross-sectional models predicting child concurrent BMI z-score (n = 366)
| B (SE) | ||||
|---|---|---|---|---|
|
| ||||
| Model A: Nocturnal Sleep Duration |
Model B: Nocturnal Sleep + Bedtime |
Model C: Nocturnal Sleep + Shifting |
Model D: Nocturnal Sleep + Napping |
|
| Nocturnal Sleep Duration | −0.15 (0.07)* | −0.23 (0.10)* | −0.15 (0.08)* | −0.15 (0.08)† |
| Bedtime | −0.18 (0.10)* | |||
| Shifting | −0.02 (0.07) | |||
| Napping | 0.05 (0.08) | |||
| Nocturnal Sleep Duration
× Bedtime |
−0.18 (0.08)* | |||
| SDB | 0.43 (0.40) | 0.37 (0.40) | 0.45 (0.41) | 0.43 (0.40) |
| Caffeine | 0.10 (0.10) | 0.08 (0.10) | 0.10 (0.10) | 0.10 (0.10) |
| CHAOS | 0.01 (0.02) | −0.002 (0.02) | 0.01 (0.02) | 0.01 (0.02) |
p<.10.
p<.05
Neither SDB, soda intake, nor home chaos were associated with nocturnal sleep duration or BMIz, nor they did not account for the association between BMIz and sleep duration (all p’s > .05).
Association of Sleep with Future Rate of BMIz Change
In the longitudinal analyses, there was a marginal moderating effect of shifting on the nocturnal sleep duration-rate of BMIz change association (p<.06 for the interaction). To examine this interaction, the sample was divided into tertiles based on bedtime delay: under 45 minutes (32%), between 45 and 75 minutes (33%), and greater than 75 minutes (35%). There was an association between nocturnal sleep duration and rate of BMIz change only for children with the most consistent bedtimes (< 45 minute shift from weekday to weekend: β = −0.12; 95% CI −0.23, −0.01), such that longer sleep duration was associated with lesser increases in BMIz over time. There was no association between sleep duration and BMIz rate of change for children with 45 minute or greater delays in bedtime on the weekend compared to weekdays (45-75 minute delay: β = −0.06; 95% CI −0.18, 0.01, or >75 minute delay: β = −0.02; 95% CI −0.16, 0.10). These associations are shown in Figure 2. Neither bedtime nor napping moderated the nocturnal sleep duration-rate of BMIz change association (p<.59 and p<.84 for the interactions, respectively).
FIGURE 2.
Association between nocturnal sleep duration and rate of BMIz change by tertile of weekday to weekend bedtime shift (longitudinal sample)
The parallel regression models predicting change in BMIz from nocturnal sleep duration and the three sleep timing variables, controlling for initial BMIz, are shown in Table 3. Longer nocturnal sleep duration was marginally associated with a lower rate of change in BMIz (Model A; p<.06). There was no independent main effect of bedtime on rate of BMIz change. Napping was marginally associated with a higher rate of BMIz change (Model D; p<.08).
Table 3.
Unadjusted parameter estimates and 95% confidence intervals from final longitudinal models predicting child rate of change in BMI z-score per year, controlling for baseline BMIz (n = 273)
| B (SE) | ||||
|---|---|---|---|---|
| Model A: Nocturnal Sleep Duration |
Model B: Nocturnal Sleep + Bedtime |
Model C: Nocturnal Sleep + Shifting |
Model D: Nocturnal Sleep + Napping |
|
| Nocturnal Sleep Duration | −0.06 (0.04)† | −0.10 (0.05)* | −0.13 (0.05) | −0.06 (0.04) |
| Bedtime | −0.04 (0.05) | |||
| Shifting | −0.68 (0.40)† | |||
| Napping | 0.06 (0.04) | |||
| Nocturnal Sleep Duration
× Shifting |
0.07 (0.04)† | |||
| Baseline BMIz | −0.11 (0.02)* | −0.11 (0.02)* | −0.11 (0.02)* | −0.11 (0.02)* |
| SDB | 0.08 (0.19) | 0.10 (0.19) | 0.06 (0.19) | 0.11 (0.19) |
| Caffeine | 0.05 (0.05) | 0.05 (0.05) | 0.04 (0.05) | 0.05 (0.05) |
| CHAOS | −0.01 (0.01) | −0.01 (0.01) | −0.01 (0.01) | −0.01 (0.01) |
p<.10.
p<.05
As with the cross-sectional models, neither SDB, soda intake, nor home chaos were associated with change in BMIz, nor they did not account for the association between rate of change in BMIz and nocturnal sleep duration (all p’s > .05).
DISCUSSION
There were three main findings of this study. First, shorter nocturnal sleep duration was associated with higher BMIz, but only among children with bedtimes later than 9pm. Second, longer nocturnal sleep duration was associated with lesser future rate of increase in BMIz, but only among children whose weekend bedtimes shifted less than 45 minutes later than their weekday bedtime. Finally, the association of sleep duration with BMIz was not accounted for by SDB symptoms, soda intake, or home chaos.
Our findings support and extend prior literature in several ways. First, like others,3,4,29 we found an association of short sleep duration with higher BMIz. That we found this association in this population of low-income preschoolers is concerning because poor sleep is more common in groups of lower socioeconomic status.6 Insufficient nighttime sleep early in development could alter biological mechanisms affecting appetite and metabolism, placing sleep-deprived low-income children at risk for obesity through this pathway.30 Short sleep duration may also affect obesity through behavioral pathways, such as sedentary activity or dietary intake. Alternatively, the short sleep-obesity association may be explained by “third variable” causes that relate both to short sleep duration and obesity (e.g., home chaos), some of which we assessed in the current study and found to be unrelated, but should be further investigated in future research.
Our results regarding sleep timing differ from and expand on prior work in several ways. We did not find a main effect of late bedtimes on higher BMIz, unlike several prior studies.8,10-13. We did find that bedtime moderated the association of short sleep duration with high BMIz, however, such that the association was only present among children with bedtimes after 9pm (there was a marginal association present for children with bedtimes after 8:30pm). Time use may in part explain this finding: activities available to children later in the evening tend to be more sedentary (e.g., TV watching), and may involve late-night snacking, which may promote weight gain.
Studies of older children11,16-18 have shown an association between a shifted sleep schedule (i.e., later bedtimes on weekends than weekdays) and obesity. To our knowledge, our study is the first to examine the effect of such shifting among preschool-aged children on BMIz. We found no main effect of shifting to a later bedtime on the weekend on concurrent BMIz; however, we found that longer sleep durations were only associated with lesser future increases in BMIz among children with the most consistent bedtimes between weekdays and weekends (i.e., a bedtime that was no more than 45 minutes later on weekends as compared to weekdays). Thus, children with inconsistent bedtimes were perhaps less able than children with more consistent bedtimes to absorb the potential beneficial effects of longer sleep duration, yet were also somewhat less-sensitive to the ill effects of getting less sleep, at least with regard to obesity. Children with less consistent sleep schedules may also have other home environment factors that could increase risk for obesity, and thus short sleep duration may represent just one of many risks. In the current study, neither SDB nor home chaos explained associations between shifting and BMI. There may be other processes operating in the home environment or in the child that we did not measure in the current study, but that may drive how the consistency of sleep schedules and duration of sleep interact and relate to BMI. As sleep schedules shift increasingly with development, it is important to understand in more detail how implications of sleep timing for obesity emerge during early childhood.8
We did not find an independent effect of napping on BMIz, which is consistent with some prior studies4,20 but not with other work identifying an inverse association between napping and BMIz.3 We found a marginal positive association between napping and rate of BMIz change, which was not expected and could be investigated in future longitudinal studies. At age 4, napping may not relate to concurrent BMIz because children who nap are still meeting part of their overall physiological need for sleep with daytime sleep, and thus cannot make up for the effects of lost nocturnal sleep through napping.31 Napping is clearly important for child functioning in other domains, but there may not be protective effects of “extra” napping for obesity risk at this age. Nocturnal sleep may engage different physiological processes than daytime napping.32 Studies of adults suggest that certain features of sleep architecture, specifically slow-wave sleep and fragmented sleep, are most closely associated with obesity-relevant metabolic functions (e.g., glucose regulation/reduced insulin sensitivity;30 leptin/ghrelin regulation33). Thus, investigating how nocturnal sleep architecture (perhaps versus that of daytime napping) relates to obesity in young children is a complex, yet important area for future research.
Overall, findings suggest that connections between sleep timing and sleep duration are important in understanding obesity during the preschool period: the later children go to bed, the greater adverse effects when they do not obtain sufficient sleep, and the more consistent their weekday-to-weekend sleep schedule is, the more they may benefit from nocturnal sleep. Sleep timing may be an important influence on physiological processes relevant for weight gain. The circadian cycle regulates hormones related to metabolism,15 so when children are sleeping, in addition to how much sleep they obtain, may affect metabolic function. Shorter sleep duration is associated with decreased leptin secretion (which reduces appetite), and increased ghrelin secretion (which increases appetite), both of which are related to circadian rhythms.30,34 Understanding connections between sleep timing and duration, in addition to sleep duration alone, may therefore elucidate how such physiological mechanisms contribute to obesity. Further mechanistic work is needed to understand the role of sleep timing in childhood risk for obesity.
Our study has certain limitations. Most importantly, although we examined SDB, soda consumption, and home chaos as potential confounders of the sleep duration-BMI association, we did not measure other variables that may be important mechanisms of association, such as nighttime snacking, TV or other screen time, or timing of meals. Further, our sleep measures were all parent-reported, although mother-reported sleep correlates well with actigraphy at this age.35 As with all correlational designs, we cannot infer causation. Results may also not generalize to other populations. Study strengths include the relatively large sample of young, low-income children who are at risk for poor sleep and obesity, but who have heretofore not been studied with regard to sleep timing.
Conclusion
Our findings suggest that sleep timing represents an important aspect of the process through which short sleep duration may contribute to obesity. Specifically, the combination of regular sleep schedules and earlier bedtimes may allow preschool-age children to benefit most from nighttime sleep. Pediatric providers should consider emphasizing not only adequate sleep duration, but also earlier bedtimes to prevent obesity. Sleep may be a mechanism through which socioeconomic stress results in poor health. Thus, working with parents of low-income preschoolers to establish more effective sleep habits with respect to both the duration and the timing of sleep may help interrupt the poor sleep-obesity risk cascade for these children early in development.
What’s New.
The combination of regular weekday-to-weekend sleep schedules and a bedtime before 9pm may allow preschool-aged children to most benefit from longer nocturnal sleep duration in preventing obesity. Napping does not compensate for short nocturnal sleep duration in obesity risk.
Acknowledgments
Funding Source: All phases of this study were supported by NIH grant #RC1DK086376, NIH grant #R21DK090718, and American Heart Association grant #10GRNT4460043.
Abbreviations
- BMIz
body mass index z-score
- BMI
body mass index
- SDB
sleep-disordered breathing
Footnotes
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson SE, Whitaker RC. Prevalence of Obesity Among US Preschool Children in Different Racial and Ethnic Groups. Arch Pediatr Adolesc Med. 2009 Apr 1;163(4):344–348. doi: 10.1001/archpediatrics.2009.18. 2009. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. International Journal of Obesity. 2003;27(6):722–727. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- 3.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004 Jul;145(1):20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Bell JF, Zimmerman FJ. Shortened Nighttime Sleep Duration in Early Life and Subsequent Childhood Obesity. Arch Pediatr Adolesc Med. 2010 Sep 1;164(9):840–845. doi: 10.1001/archpediatrics.2010.143. 2010. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Beydoun M, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 6.El-Sheikh M, Bagley EJ, Keiley M, Elmore-Staton L, Chen E, Buckhalt JA. Economic adversity and children’s sleep problems: Multiple indicators and moderation of effects. 2012. [DOI] [PMC free article] [PubMed]
- 7.Feese M, Franklin F, Murdock M, et al. Prevalence of obesity in children in Alabama and Texas participating in social programs. JAMA: The Journal of the American Medical Association. 2003;289(14):1780–1781. doi: 10.1001/jama.289.14.1780-b. [DOI] [PubMed] [Google Scholar]
- 8.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Development. 2007 Jan-Feb;78(1):309–323. doi: 10.1111/j.1467-8624.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 9.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Archives of Disease in Childhood. 2006 Nov;91(11):881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child: Care, Health & Development. 2002;28(2):163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 11.Jarrin D, McGrath J, Drake C. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. International Journal of Obesity. 2013;37:552–558. doi: 10.1038/ijo.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golley R, Maher C, Matricciani L, Olds T. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. International Journal of Obesity. 2013;(37):546–551. doi: 10.1038/ijo.2012.212. [DOI] [PubMed] [Google Scholar]
- 13.Olds TS, Maher CA, Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34(10):1299. doi: 10.5665/SLEEP.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touchette E, Mongrain V, Petit D, Tremblay RE, Montplaisir JY. Development of sleep-wake schedules during childhood and relationship with sleep duration. Arch Pediatr Adolesc Med. 2008 Apr 1;162(4):343–349. doi: 10.1001/archpedi.162.4.343. 2008. [DOI] [PubMed] [Google Scholar]
- 15.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences. 2009 Mar 17;106(11):4453–4458. doi: 10.1073/pnas.0808180106. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olds T, Blunden S, Dollman J, Maher CA. Day type and the relationship between weight status and sleep duration in children and adolescents. Australian and New Zealand Journal of Public Health. 2010;34(2):165–171. doi: 10.1111/j.1753-6405.2010.00502.x. [DOI] [PubMed] [Google Scholar]
- 17.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011 Feb 1;127(2):e345–e352. doi: 10.1542/peds.2010-0497. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung K-F, Kan KK-K, Yeung W-F. Sleep duration, sleep–wake schedule regularity, and body weight in Hong Kong Chinese adolescents. Biological Rhythm Research. 2012 Apr 01;44(2):169–179. 2013/ [Google Scholar]
- 19.Wing YK, Li SX, Li AM, Zhang J, Kong APS. The effect of weekend and holiday sleep compensation on childhood overweight and obesity. Pediatrics. 2009 Nov;124(5):e994–e1000. doi: 10.1542/peds.2008-3602. 2009. [DOI] [PubMed] [Google Scholar]
- 20.Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X. Sleep and obesity in preschool children. The Journal of Pediatrics. 2009;154(6):814–818. doi: 10.1016/j.jpeds.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Medicine Reviews. 2008;12(5):339–346. doi: 10.1016/j.smrv.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Drescher AA, Goodwin JL, Silva GE, Quan SF. Caffeine and screen time in adolescence: associations with short sleep and obesity. Journal of Clinical Sleep Medicine. 2011;7:337–342. doi: 10.5664/JCSM.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SE, Whitaker RC. Household Routines and Obesity in US Preschool-Aged Children. Pediatrics. 2010 Mar 1;125(3):420–428. doi: 10.1542/peds.2009-0417. 2010. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000 Jun 8;314:1–27. [PubMed] [Google Scholar]
- 25.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(Suppl 4):S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 26.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 27.Blum R, Wei E, H R. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Maternal Child Health Journal. 1999;3(3):167–172. doi: 10.1023/a:1022350023163. [DOI] [PubMed] [Google Scholar]
- 28.Matheny JAP, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology. 1995 Sep;16(3):429–444. 1995/ [Google Scholar]
- 29.Carter PJ, Taylor BJ, Williams SM, Taylor RW. Longitudinal analysis of sleep in relation to BMI and body fat in children: the FLAME study. BMJ: British Medical Journal. 2011:342. doi: 10.1136/bmj.d2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Medicine Reviews. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenni O, LeBourgeois M. Understanding sleep-wake behavior and sleep disorders in children: The value of a model. Current Opinion in Psychiatry. 2006;19(3):282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knowles JB, MacLean AW, Salem L, Vetere C, Coulter M. Slow-Wave Sleep in Daytime and Nocturnal Sleep: An Estimate of the Time Course of “Process S”. Journal of Biological Rhythms. 1986 Dec 1;1(4):303–308. doi: 10.1177/074873048600100404. 1986. [DOI] [PubMed] [Google Scholar]
- 33.Weikel JC. Ghrelin promotes slow-wave sleep in humans. American journal of physiology: endocrinology and metabolism. 2003;284(2):E407. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004 Dec 7;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 35.Acebo C. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1-to 5-year-old children. Sleep (New York, N.Y.) 2005;28(12):1568. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]