Abstract
Plerixafor enhances CD34+ cell mobilization, however, its optimal use is unknown. We hypothesized that plerixafor could “rescue” patients in the midst of mobilization when factors indicated a poor CD34+ yield. Of 295 consecutive autologous peripheral blood mobilization attempts at our center, 39 (13%) utilized plerixafor as rescue strategy due to a CD34+ cell concentration <10/µL (median 5.95/µL, n=30), low CD34+ cell yield from prior apheresis day (median 1.06 × 106 CD34+ cells/kg, n=7), or other (n=2). Patients received a median of 1 plerixafor dose (range 1–4). Thirty-four (87%) collected ≥ 2 × 106 CD34+ cells /kg and 26 (67%) collected ≥ 4 × 106 CD34+ cells /kg. Median collections for lymphoma (n=24) and myeloma (n=15) patients were 4.1 × 106 and 8.3 × 106 CD34/kg, respectively. A single dose of plerixafor was associated with an increase in the mean peripheral blood CD34+ concentration of 17.2 cells/µL (p<0.001) and mean increased CD34+ cell yield following a single apheresis of 5.11 × 106/kg (p<0.03). A real-time rescue use of plerixafor is feasible and may allow targeted use of this agent.
Keywords: AMD-3100, peripheral-blood stem cell mobilization, salvage
Introduction
High dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) has been shown to improve outcomes in patients with multiple myeloma (MM), Hodgkin lymphoma (HL), and non Hodgkin lymphoma (NHL) [1–3]. Current strategies primarily center around the use of granulocyte colony-stimulating factor (G-CSF) or granulocyte/macrophage colony-stimulating factor (GM-CSF) with or without chemotherapy to mobilize peripheral blood progenitor cells that are then collected by means of apheresis [4] [5,6]. A goal of 5 × 106 CD34+ cells / kg per transplant represents a commonly used target though yields as low as 2 × 106 CD 34+ cells/kg can be acceptable for reconstitution of autologous hematopoiesis [7]. Unfortunately, patients failing to collect sufficient autologous CD 34+ cells might be denied this potentially life saving procedure. Factors associated with poor CD34+ yields include extensive prior chemotherapy, pelvic or spinal radiation, and specific prior agents including nucleoside analogs, and lenalidomide [8–10]. However, prospectively identifying specific patients destined to fail collection remains challenging.
Plerixafor, (AMD3100) is a potent, reversible CXCR4 antagonist that blocks the adhesion of hematopoietic stem cells to the bone marrow stroma thereby releasing them into the peripheral blood circulation [11,12]. Prospective randomized trials in patients with NHL and MM observed a more than doubling in the product CD34+ cell yields and rates of protocol-defined successful collections with the addition of Plerixafor to G-CSF as compared to G-CSF alone [13,14]. These studies led to the approval of this agent in 2008 for steady-state mobilization with G-CSF for patients with NHL and MM [15] and more recent data have suggested that plerixafor can be used to successfully mobilize patients who have failed a prior mobilization attempt [16,17]. Despite the recent widespread availability of this agent, the majority of patients don’t require plerixafor for adequate stem cell mobilization and its optimal application remains undefined. Furthermore, only limited data were previously available on the utility of this agent in combination with G-CSF after mobilization chemotherapy[18].
We hypothesized that the use of plerixafor as a real time rescue strategy during stem cell mobilization for patients with factors suggesting a poor yield could be a cost and time effective method to employ this agent only in settings where it was required. We present a series from our center using this technique for patients with poorly mobilizing CD34+ cells, with an emphasis on patients failing chemomobilization, and describe the outcomes related to the kinetics of blood CD34+ cell concentration, apheresis product CD34+ cell yields and post transplant engraftment using such an approach.
PATIENTS AND METHODS
Patients
We evaluated consecutive patients between February 2009 and May 2010 undergoing autologous peripheral blood stem cell (PBSC) mobilization attempts at our center. Patients utilizing plerixafor were categorized into two groups: (1) patients where plerixafor was a planned strategy set forth prior to initiation of mobilization regimen, (2) patients where plerixafor was used as a rescue step in the midst of mobilization based on factors suggesting a poor CD34+ cell yield. Patients in group 2 were the focus of this study. The primary endpoint was evaluating the number of CD34+ cells/ kg actual body weight. Secondary endpoints included the impact of plerixafor on peripheral blood CD34+ cell concentration and apheresis CD34+ cell yields. Engraftment kinetics were also evaluated for patients that went onto transplant. The Fred Hutchinson Cancer Research Center and the University of Washington Consortium Institutional Review Board granted approval for review of these data.
Mobilization Regimens
Chemomobilization regimens included cyclophosphamide 2–4 grams / m2 × 1 with dexamethasone 40 mg daily × 4 (CD), cyclophosphamide 2–4 grams / m2 day 1, etoposide 200 mg / m2 days 1,2,3, and dexamethasone 40 mg days 1– 4 (CED), single agent etoposide 500 mg/ m2 daily 1–2 [19], cyclophosphamide 2–4 grams / m2 day 1, etoposide 200 mg / m2 days 1,2,3, and cisplatin 35mg / m2 days 1,2,3 (CEP), ifosphamide, carboplatin, etoposide,+/− rituximab (ICE / RICE)[20], etoposide, etoposide, methyprednisolone, high dose cytarabine, cisplatin, with rituximab (R-ESHAP)[21], rituximab, cyclophosamide, adriamycin, vincristine, prednisone (R-CHOP), and hyper-CVAD[22].
Granulocyte colony-stimulating factor (filgrastim) was initially administered at 10 µg/kg daily, starting at 24 hours after completion of chemotherapy, for patients undergoing chemomobilization. For those mobilized with G-CSF alone, 16 µg/kg twice a day was used. Plerixafor was administered at the standard dose of 0.24 mg/kg actual body weight (maximum of 40 mg) and given at 12 +/− 2 hours prior to apheresis. Patients with creatinine clearance < 50 ml/min had the dose reduced to 0.16 mg/kg up to a maximum of 27 mg/dose. G-CSF was continued concurrently with plerixafor.
Apheresis Procedure and Collection Goals
Mononuclear cell apheresis was performed on all patients using the COBE Spectra instrument (CaridianBCT, Lakewood, CO) with the white blood cell (WBC) kit and manual V4.7 program, as previously described [23]. Such adult patients typically undergo large-volume leukapheresis, with processing of up to 6-times total blood volume or maximum of 36 liters. Daily apheresis routinely commences when the peripheral blood CD34+ cell count reaches 10/µl. or, less commonly, when the blood WBC count reaches at least 1000/µl after chemomobilization. Daily apheresis collections continued until achieving the standard product goals of ≥ 5 × 106 CD34+ cells/kg for a single autologous transplant, ≥ 10 × 106 CD34+ cells/kg for two autologous transplants or at least 2 × 106 CD34+ cells/kg for a single transplant if the goal of 5 × 106 CD34+ cells/kg was not achievable. Aliquots were removed from the fresh PBSC products to assess CD34+ cell content, TNC content, viability and sterility prior to product centrifugation and removal of plasma supernatant in preparation of cryopreservation in a final concentration of 10% DMSO, as previously described [23]. CD34 quantification was performed as previously reported [23]. Briefly, CD34 emumeration was determined by flow cytometric analysis after RBC lysis and washing with phosphate buffered saline with human serum albumin. A sample of 1 ×106 nucleated cells was stained with phycoerythrin (PE)-conjugated anti-CD34 and fluorescein-isothiocyanate-conjugated anti-CD14. Additional cells were stained with the anti-CD14-conjugate and PE-conjugated-IgG1 for use an isotype-matched control. After incubation, washing, and resuspension, analysis was performed on a fluorescence activated cell sorter.
Statistical Analysis
Baseline characteristics and outcomes were summarized descriptively. Non-normally distributed continuous linear variables were compared using the median test.
RESULTS
Patient Characteristics
Between February 2009 and May 2010, 522 apheresis procedures were performed as a part of 295 autologous PBSC transplantations at our center. Of these mobilization attempts 4 (1%) utilized plerixafor as a preplanned approach. By comparison, 39 (13%) employed plerixafor as a rescue strategy in the midst of mobilization based on the treating physician’s interpretation of clinical factors predicting poor CD34+ cell yield. Baseline characteristics of the 38 patients undergoing 39 plerixafor rescue attempts during mobilization are noted in table 1. Reasons for salvage use of plerixafor included: pre-collection blood CD34+ cell concentration < 10/µl (n = 30, 77%, median CD34 + cell count = 5.95/µl (range 1.1 2 – 8.64), low CD34+ cell yield from first apheresis day (n=7, 18%, median prior apheresis yield = 1.06 × 106 CD34+ cells/kg range 0.54 – 1.7 × 106/kg), waning CD34 + cell yield (n=1), and clinical delay of apheresis (n=1). Twenty-six (67%) patients were mobilized following chemotherapy including ICE /RICE (n=9), CD (n=8), CED (n=3), VP-16 (n=2), CEP (n=1), R-CHOP (n=1), hyper-CVAD (n=1), R-ESHAP (n=1) (Table 2). In addition four patients received oral vorinostat with ICE/RICE. Lymphoma and MM/amyloidosis (MM/AL) patients had received a median of 2 (range 1–4), and 1 (range 1–8) prior regimens, respectively. Thirteen of 15 (87%) MM/AL patients had received prior lenalidomide, while only 2 of 23 (9%) of NHL patients received prior fludarabine.
Table 1.
Baseline characteristics of patients undergoing real-time rescue use of plerixafor
| G-CSF Alone (n=13) |
Chemo + G-CSF (n=26) |
Total (n=39) |
|
|---|---|---|---|
| Median age (range) | 64y (55–73y) | 57y (42–68y) | 62y (26–73y) |
| Diagnosis | |||
| Non-Hodgkin lymphoma | 7 | 16 | 23 |
| Hodgkin lymphoma | 0 | 1 | 1 |
| Multiple Myeloma | 5 | 9 | 14 |
| Amyloidosis | 1 | 0 | 1 |
| Median # of prior regimens (range) | 2 (1–4) | 2 (1–8) | 2 (1–8) |
| Prior lenalidomide (%) | 5 | 9 | 14 (36%) |
| Prior fludarabine (%) | 0 | 2 | 2 (5%) |
| Prior pelvic or spinal radiation | Spinal 2 Pelvic 1 |
Spinal 1 Pelvic 2 |
6 (15%) |
| Prior failed mobilization (%) | 3 | 5 | 8 (21%) |
| Prior ASCT (%) | 1 | 0 | 1 (3%) |
| Reason for rescue use of plerixafor (%) | |||
| Low blood CD34+ cell concentration | 8 | 22 | 30 (77%) |
| Low initial apheresis CD34+ cell yield | 4 | 3 | 7 (18%) |
| Waning apheresis CD34+ cell yield | 0 | 1 | 1 (3%) |
| Clinical delay in apheresis | 1 | 0 | 1 (3%) |
Note: To provide complete data for each rescue use of plerixafor the one patient receiving 2 rescue attempts is listed twice.
Table 2.
Chemotherapy mobilization regimens
| Regimen | n |
|---|---|
| Ifosfamide, carboplatin, etoposide, +/−rituximab | 9 |
| Cyclophosphamide, dexamethasone | 8 |
| Cyclophosphamide, etoposide, dexamethasone | 3 |
| Etoposide | 2 |
| Cyclophosphamide, etoposide, cisplatin | 1 |
| Cyclophosphamide, hydroxydaunomycin, vincristine, prednisone, rituximab | 1 |
| Cyclophosphamide, hydroxydaunomycin, vincristine, dexamethasone | 1 |
| Etoposide, methylprednisolone, cytarabine, cisplatin, rituximab | 1 |
Mobilization Yields
The median CD34+ cells collected for the entire group was 5 × 106/kg (range 0.5 – 14.54 × 106 CD34+ cells/kg). Thirty-four (87%) collected ≥ 2 × 106 CD34+ cells /kg and 26 (67%) collected ≥ 4 × 106 CD34+ cells /kg. The median CD34+ cell yield for patients collected following chemotherapy G-CSF and rescue plerixafor was 5 × 106 CD34+ cells /kg (range 0.05 – 12.02 × 106 CD34+ cells /kg). For patients mobilized after G-CSF alone followed by rescue plerixafor the median CD34+ cell yield was 4.84 × 106 /kg (range 1.1 – 14.54 × 106 CD34+ cells/kg). The median (range) collections for those receiving plerixafor for low CD34 concentrations (n=30) and low first day apheresis (n=7) were 4.8 ×106 CD34/kg (0.5–12.02) and 4.87 ×106 CD34/kg (1.53–12.84). The median CD34/kg collected in patients who received prior lenalidomide was 8.4×106 (range 2.9–14.5). Full details of the CD34+ cell yields grouped by disease are summarized in table 3.
Table 3.
Mobilization data for patients undergoing real-time rescue
| Disease group | Myeloma/Amyloid (n=15) |
Lymphoma (n=24) |
Total (n=39) |
|---|---|---|---|
| Median # plerixafor doses* (range) | 1 (1–4) | 1 (1–4) | 1 (1–4) |
| Median CD34+ cells/kg collected (range) | 8.3 × 106 (2.9 – 15) |
4.1 × 106 (0.05–6.8) |
5.0 ×106 (0.5–15) |
| ≥2 × 106 CD34+ cells/kg | 15 (100%) | 19 (79%) | 34 (87%) |
| ≥ 4 × 106 CD34+ cells /kg | 13 (87%) | 13 (54%) | 26 (67%) |
| ≥ 8 × 106 CD34+ cells /kg. | 8 (53%) | 0 | 8 (24%) |
CD34+ Cell Mobilization Kinetics
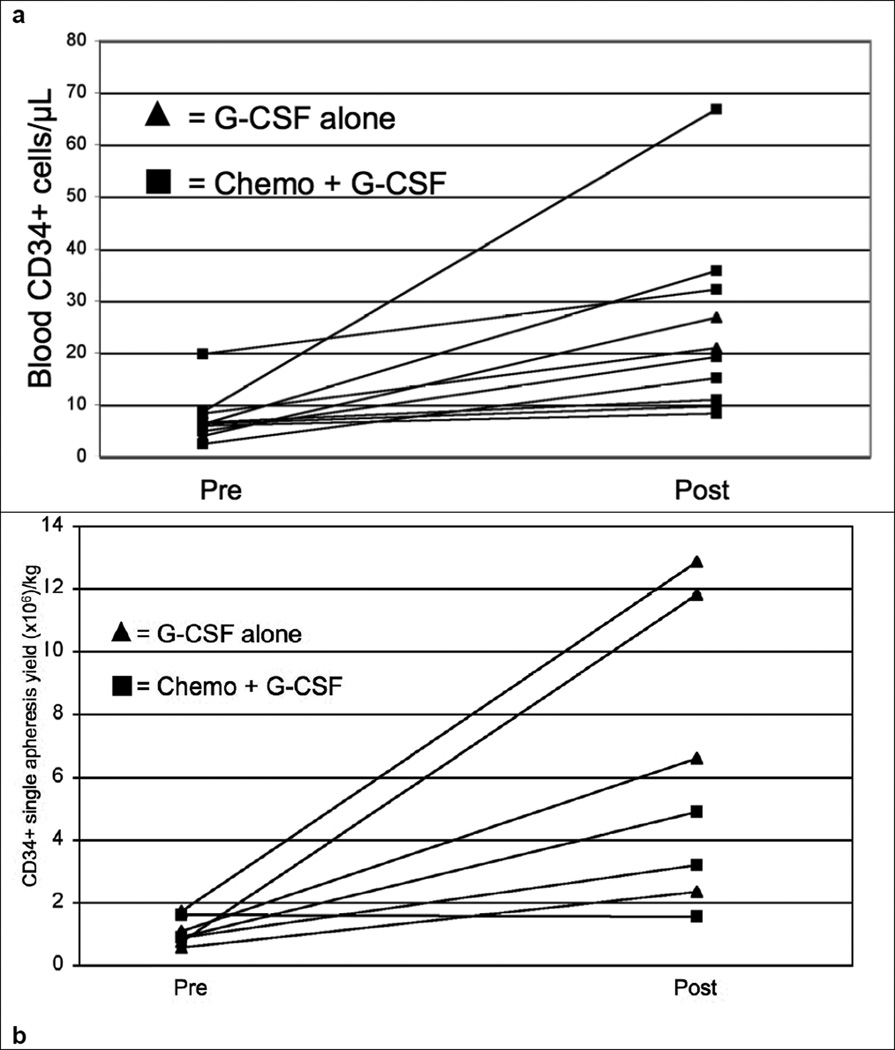
In patients (n=10) with available pre and post plerixafor peripheral blood CD34+ cell concentrations a single dose of plerixafor was associated with an increase of the mean peripheral blood CD34+ cell concentration of 17.2 cells/ µl (p<0.001) Similarly for patients (n=7) with large-volume apheresis attempts pre- and post-plerixafor the mean CD34+ cells/kg increased by 5.11 × 106/kg (p<0.03). Specific details of pre and post plerixafor CD34+ cell concentrations and yields are described in table 4. Even though the mean and median CD34+ cell concentration increased over three-fold after plerixafor administration the effect on individual patients was less predictable. A similar observation was noted concerning pre- and post-plerixafor CD34+ cell yields/kg. Individual patient data is displayed in figures 1a and 1b illustrating the trajectory of each individual patient in CD34+ cell concentration and yields. The median number of collection days for patients that received plerixafor for low CD34 was 2 (range 1–4).
Table 4.
Pre and post plerixafor single-collection blood CD34+ cell concentrations and apheresis product yields in patients treated with a real-time rescue strategy (evaluable patients with both values). P values represent comparisons by the median test.
| n | Pre-Plerixafor (standard deviation) |
Post-Plerixafor (standard deviation) |
P value | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |||
| Blood CD34+ cell concentration (cells/µL) | 10 | 7.30 (4.7) |
6.26 | 2.46–19.74 | 24.6 (18) |
20.02 | 8.3–66.9 | p<0.001 |
| Product CD34+ cell yield (× 106/kg) | 7 | 1.05 (0.43) |
0.87 | 0.54–1.7 | 6.16 (4.5) |
4.87 | 1.53–12.84 | p<0.03 |
Figure 1.
Individual patient pre and post plerixafor CD34 concentrations (a) and apheresis yields (b).
Engraftment and Patient Outcomes
Twenty-four of 38 (63%) patients proceeded to transplant and 23 were evaluable for engraftment. One patient, who died at day 10, was not evaluable for engraftment. Reasons for not proceeding to transplant included inadequate disease control (n=11), insufficient CD34+ cell collection in (n=2), and one who was deemed to not be a transplant candidate due to low cardiac ejection fraction. For the 23 engraftment-evaluable patients the median time to absolute neutrophil counts (ANC) >500µ/L was 16 days (range 14 – 38) and the median time to platelet count over 50,000/µl was 19 days (range 14–33). The median time to ANC >500/µL for those patient who received >5×106 CD34/kg was also 16 days.
DISCUSSION
The optimal strategy for mobilization of autologous PBSC remains controversial. Data to-date indicate that myelosuppressive chemotherapy with hematopoietic growth factor support or the addition of plerixafor to growth factor without prior chemotherapy result in superior CD34+ cell yields compared to mobilization with growth factor alone [13,14]. The decision to use chemotherapy is often based, in part, on the added need for further cytoreduction prior to transplant, whereas the optimal application of plerixafor, particularly after chemomobilization, remains undefined. We present a large series of patients wherein plerixafor was solely used as a rescue option in the midst of prior failed mobilization or for those with clinical risk factors suggesting a poor CD34+ cell yield. Our observations provide useful insight into strategies to optimally apply this important but expensive agent.
Most of the patients in our study utilized plerixafor for a low peripheral blood CD34+ cell concentration at < 10 CD34+ cells/µl. A blood concentration of at least 10 CD34+ cells/µl serves as a strong predictor for optimal timing of apheresis and PBSC product yield [23]. In our study population, plerixafor resulted in a mean increase of peripheral blood CD34+ cell concentration of 17.2 cells/µl. Notably, other groups have utilized a more aggressive threshold of 25 peripheral blood CD34+ cells/µl for the trigger to start apheresis for patients with a target of 5 × 106 CD34+ cells/kg [24]. Among our poorly mobilizing cohort, less than half of patients would have achieved this high threshold to initiate PBSC collection even after using plerixafor as a salvage strategy. Despite using the lower blood CD34+ cell threshold, 87 % of the patients in our study collected at least 2 × 106 CD34+ cells/kg. One could, thus, hypothesize that those with pre-plerixafor CD34+ concentrations > 10 cells/µl may not require the use of this agent, as is the practice at our center, unless apheresis yields are suboptimal.
A key observation of our study was that the mean post-plerixafor increase in blood CD34+ cell concentration and PBSC product yield were more than three-fold and five-fold, respectively, though the kinetics for individual patients was difficult to predict. For example, relative increases in blood CD34+ cell concentrations in individual patients ranged from less than two-fold to more than seven-fold, similar to other reports[25]. This suggests that other factors may be involved in determining the final blood CD 34+ cell concentration and ultimate yield of product CD34+ cells rather than simply the effects of plerixafor and the pre-treatment CD34+ cell quantification. It is possible that plerixafor variably enhances the active trafficking of CD34+ cells into the peripheral blood during the apheresis procedure. Previous data from our center and others have shown that the blood CD34+ cell concentration rapidly declines to an equilibrium point during apheresis in both healthy donors and patients but the apheresis product CD34+ cell yield is greater than predicted by the blood concentration, suggesting an active egress of CD34+ cells into the blood during stem cell collection[26] [27]. Further studies will need to be done to determine whether plerixafor affects this trafficking phenomenon and if this might be related to schedule of administration, timing and volume of blood processed during apheresis.
Unfortunately, despite adequate CD34+ cell collections in 87% of the attempts, only 24 patients proceeded to transplant, primarily due to inadequate control of the underlying disease. These data suggest that patients requiring rescue use of plerixafor may also have a higher likelihood of not being transplant candidates for other reasons. Thus, close scrutiny of the overall clinical scenario may need to be applied before attempting mobilization or utilizing this agent. Nevertheless, those that did go on to transplant engrafted, though with somewhat delayed neutrophil and platelet recovery at sixteen and nineteen days, respectively. Interestingly. patients that went to transplant and received >5×106 CD34/kg had no sooner neutrophil engraftment, suggesting the “quality” of the stem cell product may also be impaired in poor mobilizers.
Despite the utility of our findings we were not able to address whether or not these patients would have experienced an increase in their CD34+ cell concentrations and successful PBSC collections in the absence of plerixafor. Unfortunately, no readily identifiable comparison group that could be matched at multiple relevant baseline factors was available at our center. One recent study did attempt to address this question by comparing outcomes in patients collected before and after the availability of plerixafor, though a higher proportion of patients (36%) received this agent than in our trial (13%) [28]. Nevertheless, our data do parallel results from other similar studies suggesting that the rescue use of plerixafor can be an effective option for poorly mobilizing patients[29], though our data is unique in that we included patients who had failed twice daily G-CSF at16µg/kg. Future directions to evaluate this strategy could randomly assign patients with baseline factors suggesting a high risk of stem cell collection failure to a scheduled use of a plerixafor-based rescue strategy versus placebo or some other alternative strategy such escalated G-CSF doses. In addition, future pharmacoeconomic studies should be performed to identify the cost-effective thresholds for rescue or standard use of plerixafor after G-CSF and chemomobilization. Until such data are available, our study supports the feasibility of a real-time rescue use of plerixafor to judiciously incorporate this agent into mobilization regimens resulting in successful CD34+ cell yields for most candidates while obviating its use in the majority of patients.
Acknowledgments
Special thanks to administrative and research support from Tina Bogne, Jennifer Roden, and Caroline Stamato.
Financial support: Lymphoma Research Foundation Mantle Cell Lymphoma Research Initiative, SCOR Grant 7040 from the Leukemia and Lymphoma Society, the Mary A. Wright Memorial Research Fund, and a donation from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Footnotes
Presented in part at the Presented in part at the 52nd Annual Meeting of The American Society of Hematology, Sunday, December 5, 2010: 5:00 PM, Orlando, FL. Abstract # 2251
Disclosures:
AKG: Research support: GSK, Biogen-Idec, Lilly, Seattle Genetics, Merck, Cephalon, Piramal, NCI, LRF, LLS, LSDF, CLL topics; Speakers Bureau: Millenium, Genzyme; Honoraria: Millenium, Amgen, Seattle-Genetics, CTI.
References
- 1.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F, Zschaber R, Muller P, Kirchner H, Lohri A, Decker S, Koch B, Hasenclever D, Goldstone AH, Diehl V. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 3.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 4.Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, Munshi N, Singhal S, Mehta J, Tindle S, Nelson J, Bracy D, Mattox S, Tricot G. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547–1553. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 5.Gianni AM, Siena S, Bregni M, Tarella C, Stern AC, Pileri A, Bonadonna G. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet. 1989;2(8663):580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, Demuynck HM, Link H, Zander A, Barge A. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347(8998):353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 7.Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, Cheson B, Crowley J, Barlogie B. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85(2):588–596. [PubMed] [Google Scholar]
- 8.Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Lentzsch S, Munshi N, Niesvizky R, San Miguel J, Ludwig H, Bergsagel L, Blade J, Lonial S, Anderson KC, Tosi P, Sonneveld P, Sezer O, Vesole D, Cavo M, Einsele H, Richardson PG, Durie BG, Rajkumar SV. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009;114(9):1729–1735. doi: 10.1182/blood-2009-04-205013. [DOI] [PubMed] [Google Scholar]
- 9.Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. Prediction of mobilisation failure in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33(9):907–912. doi: 10.1038/sj.bmt.1704466. [DOI] [PubMed] [Google Scholar]
- 10.Tournilhac O, Cazin B, Lepretre S, Divine M, Maloum K, Delmer A, Grosbois B, Feugier P, Maloisel F, Villard F, Villemagne B, Bastit D, Belhadj K, Azar N, Michallet M, Manhes G, Travade P. Impact of frontline fludarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemia. Blood. 2004;103(1):363–365. doi: 10.1182/blood-2003-05-1449. [DOI] [PubMed] [Google Scholar]
- 11.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, Calandra G, DiPersio JF. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22(6):1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 12.Grignani G, Perissinotto E, Cavalloni G, Carnevale Schianca F, Aglietta M. Clinical use of AMD3100 to mobilize CD34+ cells in patients affected by non-Hodgkin's lymphoma or multiple myeloma. J Clin Oncol. 2005;23(16):3871–3872. doi: 10.1200/JCO.2005.55.250. author reply 3872–3873. [DOI] [PubMed] [Google Scholar]
- 13.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 14.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 15.Brave M, Farrell A, Ching Lin S, Ocheltree T, Pope Miksinski S, Lee SL, Saber H, Fourie J, Tornoe C, Booth B, Yuan W, He K, Justice R, Pazdur R. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 78(3–4):282–288. doi: 10.1159/000315736. [DOI] [PubMed] [Google Scholar]
- 16.Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K, Nelson V, Kovacsovics T, Leis J, Calandra G, Maziarz RT. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone marrow transplantation. 2009;43(12):909–917. doi: 10.1038/bmt.2008.409. [DOI] [PubMed] [Google Scholar]
- 17.Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, Calandra G. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1578–1586. doi: 10.1016/j.bbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Hubel K, Fresen MM, Salwender H, Basara N, Beier R, Theurich S, Christopeit M, Bogner C, Galm O, Hartwig R, Heits F, Lordick F, Rosler W, Wehler D, Zander AR, Albert MH, Dressler S, Ebinger M, Frickhofen N, Hertenstein B, Kiehl M, Liebler S, von Lilienfeld-Toal M, Weidmann E, Weigelt C, Lange F, Kroger N. Plerixafor with and without chemotherapy in poor mobilizers: results from the German compassionate use program. Bone marrow transplantation. 2011;46(8):1045–1052. doi: 10.1038/bmt.2010.249. [DOI] [PubMed] [Google Scholar]
- 19.Reiser M, Josting A, Draube A, Mapara MY, Scheid C, Chemnitz J, Tesch H, Wolf J, Diehl V, Sohngen D, Engert A. Successful peripheral blood stem cell mobilization with etoposide (VP-16) in patients with relapsed or resistant lymphoma who failed cyclophosphamide mobilization. Bone Marrow Transplant. 1999;23(12):1223–1228. doi: 10.1038/sj.bmt.1701791. [DOI] [PubMed] [Google Scholar]
- 20.Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lyons N, Agus DB, Goy A, Jurcic J, Noy A, O'Brien J, Portlock CS, Straus DS, Childs B, Frank R, Yahalom J, Filippa D, Louie D, Nimer SD, Zelenetz AD. Ifosfamide, carboplatin, and etoposide: a highly effective cytoreduction and peripheral-blood progenitor-cell mobilization regimen for transplant-eligible patients with non-Hodgkin's lymphoma. J Clin Oncol. 1999;17(12):3776–3785. doi: 10.1200/JCO.1999.17.12.3776. [DOI] [PubMed] [Google Scholar]
- 21.Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, Romaguera J, Rubenstein E, Cabanillas F. ESHAP--an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12(6):1169–1176. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 22.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Sarris AH, Dang NH, Wang M, Beasley V, Medeiros LJ, Katz RL, Gagneja H, Samuels BI, Smith TL, Cabanillas FF. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Leisenring W, Bensinger WI, Holmberg LA, Rowley SD. The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 1999;39(5):442–450. doi: 10.1046/j.1537-2995.1999.39050442.x. [DOI] [PubMed] [Google Scholar]
- 24.Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C, Stuart RK. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant. 46(4):523–528. doi: 10.1038/bmt.2010.170. [DOI] [PubMed] [Google Scholar]
- 25.D'Addio A, Curti A, Worel N, Douglas K, Motta MR, Rizzi S, Dan E, Taioli S, Giudice V, Agis H, Kopetzky G, Soutar R, Casadei B, Baccarani M, Lemoli RM. The addition of plerixafor is safe and allows adequate PBSC collection in multiple myeloma and lymphoma patients poor mobilizers after chemotherapy and G-CSF. Bone Marrow Transplant. 46(3):356–363. doi: 10.1038/bmt.2010.128. [DOI] [PubMed] [Google Scholar]
- 26.Ford CD, Greenwood J, Strupp A, Lehman CM. Change in CD34+ cell concentration during peripheral blood progenitor cell collection: effects on collection efficiency and efficacy. Transfusion. 2002;42(7):904–911. doi: 10.1046/j.1537-2995.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- 27.Rowley SD, Yu J, Gooley T, Heimfeld S, Holmberg L, Maloney D, Bensinger WI. Trafficking of CD34+ cells into the peripheral circulation during collection of peripheral blood stem cells by apheresis. Bone Marrow Transplant. 2001;28(7):649–656. doi: 10.1038/sj.bmt.1703217. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, Langston A, Prichard JM, Anderson D, Gleason C, Lonial S, Flowers CR, Kaufman JL, Waller EK. Effectiveness and cost analysis of "just-in-time" salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51(10):2175–2182. doi: 10.1111/j.1537-2995.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 29.Jantunen E, Kuittinen T, Mahlamaki E, Pyorala M, Mantymaa P, Nousiainen T. Efficacy of pre-emptively used plerixafor in patients mobilizing poorly after chemomobilization: a single centre experience. Eur J Haematol. 2011;86(4):299–304. doi: 10.1111/j.1600-0609.2010.01573.x. [DOI] [PubMed] [Google Scholar]