Abstract
Pediatric primary care is an important setting in which to address obesity prevention, yet relatively few interventions have been evaluated and even fewer have been shown to be effective. The development and evaluation of cost-effective approaches to obesity prevention that leverage opportunities of direct access to families in the pediatric primary care setting, overcome barriers to implementation in busy practice settings, and facilitate sustained involvement of parents is an important public health priority. The goal of the Healthy Homes/Healthy Kids (HHHK 5-10) randomized controlled trial is to evaluate the efficacy of a relatively low-cost primary care-based obesity prevention intervention aimed at 5 to 10 year old children who are at risk for obesity. Four hundred twenty one parent/child dyads were recruited and randomized to either the obesity prevention arm or a contact control condition that focuses on safety and injury prevention. The HHHK 5-10 obesity prevention intervention combines brief counseling with a pediatric primary care provider during routine well-child visits and follow-up telephone coaching that supports parents in making home environmental changes to support healthful eating, activity patterns, and body weight. The contact control condition combines the same provider counseling with telephone coaching focused on safety and injury prevention messages. This manuscript describes the study design and baseline characteristics of participants enrolled in the HHHK 5-10 trial.
Keywords: pediatric obesity, primary care, overweight, motivational interviewing, obesity prevention
Introduction
Given recent obesity trends, the development of effective interventions to prevent unhealthy weight gain in children is a public health priority.[1] Health care providers have the potential for taking a leadership role in this regard with the credibility they are perceived to have on information about health risks and preventive behaviors.[2] A large majority of children have regular contact with primary care providers in the early elementary school years, providing access to families and an opportunity for efficiently identifying and initiating weight gain prevention interventions, if needed. Although health care providers are concerned about pediatric obesity, several barriers may reduce provider's ability to provide effective obesity prevention advice. Some of these barriers include: 1) time limitations in medical care visits, 2) limited experience of health care providers delivering prescriptions for lifestyle behavior change, 4) resistance of some parents, children, and even providers to discuss weight issues, and 5) provider doubts about their behavioral advice being followed. Taken together, these barriers make medical care settings a uniquely challenging environment in which to attempt obesity prevention interventions.
Despite these barriers, there has been increased attention to developing and evaluating interventions that leverage the influential role of the pediatric primary care provider and are integrated into the health care setting, including multiple non-randomized trials and pilot studies[3-19] and a smaller number of completed larger-scale randomized trials[20-24] and randomized trials that are currently in progress.[25-29] These studies have taken steps towards testing primary-care based interventions that take minimal office visit time and supplement physician counseling with a supportive, often clinic-based or home-based intervention component, yet the quality of the studies, role of the pediatric primary care provider, and strength of the supportive intervention components have varied, as have other aspects of the research design, such as participation and follow-up rates, and length of follow-up.
An important next step is to build upon the existing knowledge-base by developing cost-effective approaches to obesity prevention that leverage opportunities of direct access to families in the pediatric primary care setting, overcome barriers to implementation in busy practice settings, and facilitate sustained parent involvement. This paper describes the study design and baseline characteristics of participants in Healthy Homes/Healthy Kids (HHHK 5-10). The study goal is to evaluate the efficacy of a relatively low-cost primary care-based obesity prevention intervention aimed at 5 to 10 year old children who are at risk for obesity that combined a brief counseling session with a pediatric primary care provider with follow up telephone coaching with parents.
Study Design
Four hundred twenty one parent child dyads were randomized to one of two study conditions, 1) a primary care based obesity prevention intervention and 2) a primary care based attention control condition focused on general health, safety, and injury prevention. Thus, families in both conditions receive information valuable in promoting child well-being and behavior change support. Both interventions include a provider component in which primary care providers offer concise messages to parents about the parental practices to reduce the risk of unhealthy outcomes, including obesity and injury. Providers also give parents specific recommendations for promoting healthy eating and activity patterns as well as injury prevention. Both interventions also include follow-up by phone coaches to reinforce the provider message and provide family-specific, tailored guidance. The two conditions differ in the topics covered by coaching sessions. Those in the Obesity Prevention arm focus on healthy eating and activity advice while the Contact Control arm focuses on injury prevention and safety. The primary hypothesis is that at 12- and 24-month follow-up, Obesity Prevention arm children will have a significantly lower body mass index (BMI) percentile than Contact Control arm children. Secondary outcomes include changes in child physical activity level and dietary intake, parenting practices related to child diet, physical activity, and injury prevention/safety, psychosocial variables, treatment adherence, and parent BMI.
Recruitment and enrollment
The recruitment goal was to obtain baseline data from 400 parent-child dyads. Families were recruited from those making a well child visit with a pediatric primary care provider at one of 20 clinics in the greater Minneapolis-St. Paul area. Eligibility criteria for the study were as follows: 1) 5-10 year old child attending a well child visit conducted by a pediatric or family practice care provider; 2) child BMI was between the 70th and 95th percentile according to 2000 CDC age and sex reference standards; http://www.cdc.gov/nchs/data/nhanes/growthcharts/bmiage.txt; 3) the child's parent/guardian and child agreed to participation in the study and were not planning to move out of the state in the next 24 months; 4) English speaking parent and child; 5) no medical problems that would preclude study participation as determined by the physician conducting the well child visit (e.g. a chromosomal abnormality, chronic condition such as kidney disease, Type I diabetes, lupus, or cancer); 6) child was not using a steroid medication for more than one month; and 7) child was not participating in another health-related research study.
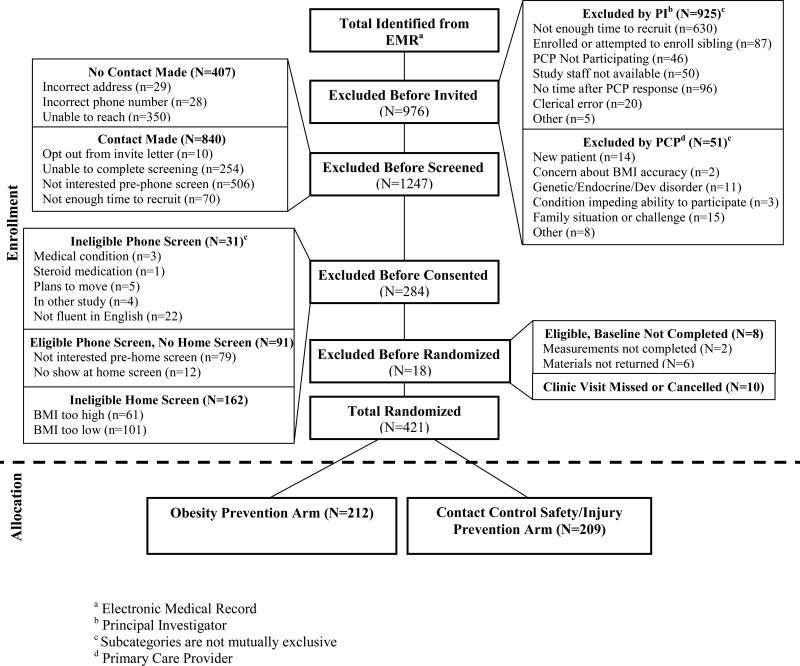
Families were recruited and randomized through a multi-step process coordinated between the research study staff and the participating clinics. Figure 1 depicts a modified CONSORT diagram describing study flow. First, age-eligible children scheduled for a well child visit who, based on prior heights and weights recorded in the Electronic Medical Record (EMR), would likely meet the study eligibility criteria were identified. The child's primary care provider was next consulted via secure messaging through the EMR prior to the visit to confirm preliminary eligibility. This was followed by a study invitation letter from the child's primary care provider and the study principal investigator (PI) to parents. A recruitment phone call followed at which potential interest and eligibility were assessed and a home visit was scheduled. At this visit, eligibility was first confirmed by measuring the child's height and weight and computing BMI percentile. If the child's BMI percentile was outside of the eligible range, the family was given a $10.00 gift card to thank them for their time and information regarding nutrition, physical activity, safety and injury prevention. If BMI eligibility was confirmed, consent and assent forms were reviewed with the parent and child and signed. The HHHK data collection staff scheduled to meet the family at the clinic prior to their well child visit. Detailed information about baseline data collected following informed consent and assent is provided in the Measures section below.
Figure 1.
Healthy Homes/Healthy Kids 5-10 Modified CONSORT Diagram
As shown in Figure 1, 2946 age-eligible children were identified using data from the EMR. Approximately one-third of these children were excluded by either the study PI or the child's pediatric primary care provider. The most common reason for exclusion at this point in the recruitment process was a lack of time to screen, consent, and complete baseline measures prior to the child's well child visit. 1970 children proceeded to the next recruitment step, which involved sending an invitation letter to the parent of the child. We were unable to make phone contact with about 20% of these families. Of the remaining 80% of the families who received a letter, about 40% declined to complete the screening, primarily because they were not interested in participating in the study. Study staff completed a phone screen with 723 parents. Of these, 31 families were considered ineligible based on the phone screening, 91 families were phone screen eligible, but did not complete the baseline home visit, and 162 were phone screen eligible, but ineligible at the baseline home visit based on the child's BMI percentile. Of the 439 families that completed the baseline home visit, 18 were not randomized because they did not complete all of the baseline measures or did not complete the scheduled well child visit. Thus, approximately 20% of parents who were sent a study invitation letter enrolled in the trial.
Randomization
Each participant was randomized to the Obesity Prevention group or to the Contact Control group after completing the baseline measures and the initial well child visit. The study statistician created a 1:1 blocked randomization schedule prior to study enrollment using blocks of 10 to ensure equivalent study group size, and the study programmer embedded it in the “back end” of the study database such that it would be unobservable to the study coordinator. As each participant completed baseline measures, the study coordinator randomly assigned the participant to the condition shown in the next available slot in the pre-defined randomization schedule. The coordinator then sent a letter to the participant to inform them of their randomization assignment, accompanied by the corresponding parent workbook for their assigned study condition.
Measures
Data for this study are collected at baseline, 6, 12, 18, and 24 months. The baseline, 12-, and 24-month visits are conducted in participants’ homes. Six- and 18-month data are collected via a web survey. Participants are compensated for their time at the completion of each measurement time point, ($50 gift card for baseline and 12-month visits and measures, $25 gift card for 6- and 18-month surveys, and $75 gift card for 24-month visit and measures). In order to support the importance of the substantive contact control condition and evaluate safety and injury prevention outcomes, the parental survey includes an approximately equal number of measures of general health, safety, and injury prevention constructs.
Primary Outcome: Index Child Body Mass Index (BMI) Percentile
Child weight and height are measured by study staff in the family home using a Seca 876 flat scale and Seca 217 stadiometer (Seca Corp., Hanover, MD). Weight and height are measured twice. If the first two measurements differ by more than 0.2 kg for weight or more than 1.0 cm for height, the process is repeated a third time. Data for the multiple assessments are averaged. To assess validity, a second trained staff member measures the height and weight of 10% of participants. Primary and secondary rater weight and height measurements are highly correlated (ICC=0.99 and ICC=0.99, respectively). BMI percentile is then calculated using the CDC 2000 Growth Charts.
Secondary Outcomes: Dietary Intake, Physical Activity, and Sedentary Behavior
Diet Recall
To assess child dietary intake, a multi-pass 24-hour recall is administered with parent/child dyads by staff trained and certified to use the Nutrition Data System for Research software versions 2009, 2010, and 2011 (NDSR, Nutrition Coordinating Center, NCC, University of Minnesota, Minneapolis, MN).[30] Children seven years old or older serves as the primary respondent. The parent serves as the respondent for children younger than seven. Before the recall, both parent and child are trained to use a two-dimensional food amounts booklet (NCC, adapted from van Horn et al., 1993)[31] and three-dimensional glasses and bowls to estimate portion sizes. Recalls are analyzed using NDSR version 2011 software to estimate multiple intake dimensions. Total energy intake, percent calories from fat, and servings of fruits and vegetables are reported. The fruit category included the following NDSR food codes: citrus fruit, fruit excluding citrus fruit, avocado and similar. The vegetable category included: dark green vegetables, deep yellow vegetables, tomato, other starchy vegetables, and other vegetables.
Accelerometry
We assess child physical activity using ActiGraph GT1M accelerometers (ActiGraph LLC, Pensacola, FL). Children are asked to wear the accelerometers for seven full days during waking hours, except during water activities. The devices are placed on elastic belts, fitted on the right hip, and initialized to record data in 15-second epochs. Children are included in analyses of the physical activity data if they record at least 4 valid monitoring days, defined as 10 or more hours of wear time. Non-wear time is defined as a string of 60 minutes or more of zero-counts, allowing for a two-minute interruption interval of 100 counts or less. To estimate minutes spent in moderate-to-vigorous physical activity (MVPA), data are aggregated into one-minute epochs; cut points are defined using the Evenson et al. (2008) equations.[32]
Television and Media Use
Time spent viewing television and media is assessed with 4 items.[45] Parents report the amount of time their child uses TV (2-items) and other media (2-items) on an average weekday and weekend day. Response options for the 4-items are: “< 1 hour per day,” “1 hour per day,” “2 hours per day,” “3 hours per day,” “4 hours per day,” and “5+ hours per day.” TV viewing items are dichotomized to classify children as meeting (≤ 2 hours of TV per day) or exceeding (>2 hours per day). The 2 dichotomies for weekday and weekend day viewing are combined to create a third variable that describes meeting American Academy of Pediatric[33] guidelines on both average week and weekend days. The same recoding process is followed for the 2 media use items.
Demographic Characteristics
Demographic characteristics are collected with a 19-item parent-reported questionnaire including child characteristics (age, gender, ethnicity, race), their own characteristics (age, gender, ethnicity, race, marital status, employment status, educational achievement), and household characteristics (free or reduced price school lunch eligibility, household composition, and home ownership).
Parent/Guardian BMI
Primary study parent/guardian (and secondary study parent/guardian, when available) weight and height are measured by study staff in the family home using a Seca 876 flat scale and Seca 217 stadiometer (Seca Corp., Hanover, MD) following the procedures described above. BMI is then calculated as kg/m2.
Diet-Related Factors
Breakfast Behavior
Parents report the number of times the child ate breakfast during the past week.[34] Response options (“0,” “1 or 2,” “3 or 4,” “5 or 6,” and “7+” times) are dichotomized to “eating breakfast daily” vs. “eating breakfast ≤ 6 days.”
TV Use and Eating Behavior
Eating behavior while watching TV is measured with 3 items adapted from Dennison et al. (2004).[35] Parents report the number of days during the past week that the child had snacked, eaten breakfast, or eaten dinner with the TV turned on. Response options (0-7 days) are dichotomized to “0 days” and “1+ day.”
Food Availability
Household food availability is assessed across 5 categories (fruits, vegetables, salty snacks, beverages, and sweet snacks). Items were developed using the food categories included in the Food Frequency Questionnaire.[36, 37] Parents are presented with a list of items for each food and beverage category and asked to select the items available in their home within the last week. Vegetables and fruit categories include fresh, frozen, or canned items. A count variable is created for each food and beverage category, which indicates the number of items from that category available in the home.
Restaurant Behavior
Restaurant Behavior is assessed with 3 items, modified from Boutelle (2007).[38] Parents report the number of times during the past week their child ate something from each of the following restaurant types: fast food (e.g., McDonald's, Burger King, etc), fast casual (e.g., Panera Bread, Chipotle), and casual, full table service restaurant (e.g., Applebee's, Olive Garden). Response options (“Never,” “1-2 times,” “3-4 times,” “5-6 times,” and “7+ times”) are dichotomized for each type of restaurant to “never ate out” and “ate out 1+ times during the past week.”
Caregiver's Feeding Styles Questionnaire (CFSQ)
The Caregiver's Feeding Styles Questionnaire (CFSQ) is a 38-item questionnaire developed to assess parent- and child-centered feeding styles of parents of preschool age children.[39] This study includes 3 CFSQ items from the parent-centered dimension; these questions assess parental use of reward contingencies to encourage the child to eat. Parents report the frequency with which they use each feeding behavior. Items are rated on a 5-point (“never,” to “always”) scale, and scores for each item are reported separately. Questionnaire developers reported a Cronbach's coefficient alpha of 0.86 for the 12 original parent-centered feeding items.[39]
Child Feeding Questionnaire (CFQ)
The Child Feeding Questionnaire (CFQ) is a 31-item measure of parent feeding.[40] Seven factor-analytically-derived subscales assess the following constructs: perceived child weight, perceived parent weight, concern about child weight, feeding responsibility, monitoring, restriction, and pressure to eat. The developers reported internal consistency values ranging from 0.70 to 0.92 based on a sample of 394 parents of 5-9 year-old girls.[40] Cronbach's alpha coefficients in the present sample range from 0.49-0.93.
Children's Eating Behavior Questionnaire (CEBQ)
The Children's Eating Behavior Questionnaire measures child eating style.[41] Five of the 8 subscales are included in the present study: food responsiveness (5-items), enjoyment of food (4-items), emotional overeating (4-items), satiety responsiveness (5-items), and slowness in eating (4-items). The developers reported Cronbach's coefficient alphas ranging from 0.72 to 0.91 in two samples of parents (N=177 and N=222), and 2-week test-retest reliability values between 0.52 and 0.87 based on a sample of 166 parents.[41] In our sample, Cronbach's alpha coefficient is 0.76 for food responsiveness, 0.84 for enjoyment of food, 0.78 for emotional overeating, 0.75 for slowness of eating, and 0.75 for satiety responsiveness, and 0.80 for satiety responsiveness/slowness of eating (combined).
Parental Healthy Eating/PA Self Efficacy
Thirteen questions adapted from Taveras et al. (2009)[42] assess parental confidence in maintaining or making changes to health behaviors in 3 domains: eating, physical activity, and media. The eating self-efficacy subscale (9-items) questions evaluate a range of child and family eating behaviors such as breakfast and family meal occurrence, dietary intake, and home food availability. The physical activity self-efficacy subscale (2-items) assesses parents’ perceived ability to provide physical activity equipment to their child and encourage their child to be physical active for at least one hour per day. The media self-efficacy subscale (2-items) measures parents’ ability to limit their child's media viewing and remove television from their child's bedroom. Cronbach's alpha coefficients in this sample for the eating, physical activity, and media self-efficacy subscales are 0.80, 0.73, and 0.38, respectively. Scores for each item in the media self-efficacy subscale are reported separately, due to low internal consistency.
Parent Physical Activity
Primary study parent physical activity level is self-reported via the Active Australia questionnaire, modified as a paper survey by Brown et al (2008).[43] The questionnaire consists of 8 fill-in-the blank questions about frequency and time spent (minutes and/or hours) in specific moderate and vigorous activities in the past week. The questionnaire distinguishes between walking, leisure-time physical activity and physical activity relating to household chores. Reliability coefficients for frequency/time in each domain of physical activity range from 0.56-0.64 and the percent agreement scores range from 40-65% for the physical activity categories.[43] Total past-week minutes spent in moderate-to-vigorous physical activity (MVPA) are calculated and converted to average minutes/day.
Parent Physical Activity with Child
This is a 2-item questionnaire developed for the present study to assess the amount and type of physical activity a parent participates in with their child. Parents report the number of times they were physically active with their child over the past week. Parents then select the type of physical activity they participated in with their child from a list of 22 types of activity.
Play Equipment Availability
Parents report household availability of play and media equipment for their child using a 47-item inventory, modified from Sherwood et al.[34] Play equipment is categorized into 2 categories: active play equipment (e.g., soccer ball) and media equipment (e.g., active video games). A count variable is created for each category of play equipment to indicate the household availability of items from each category.
Parental Support for Physical Activity
Parental support for child physical activity is assessed with 8 items adapted from Trost et al. (2003).[44] Parents rate the frequency during the past week that they had engaged in the following behaviors: encouraged their child to do PA or play sports, engaged in PA or played sports with their child, provided transportation so their child could engage in PA or play sports, and watched their child engage in PA or play sports. Items are rated twice, once with respect to the parent in the study and once with respect to other adult members of the household and items are averaged across both family members. Questionnaire developers reported a Cronbach's alpha coefficient of 0.78 and a 1-week test-retest reliability of 0.81 for the original 5-item scale.[44] Cronbach's alpha coefficient is 0.87 for the 4-item scale in the present sample.
Parental Importance and Enjoyment of Physical Activity
Parental importance and enjoyment of physical activity are assessed with 4 items adapted from Trost et al. (2003).[44] Parents are asked, “How important is it to you and/or other adults in your household that this child is physically active or plays sports?”, and “How much do you and/or other adults in your family enjoy physical activity or exercise?”. These items are rated twice, once from the perspective of the parent in the study and once from the perspective of other adult household members and ratings are averaged across both adult household members.
Media Availability and Household Media Rules
Three items modified from Dennison et al.[35] and Rideout et al.[45] assess the presence of media items (TV, video game console, and computer) in the child's bedroom. Six additional items assess household rules regarding media.[45] Questions such as, “Do you have any rules for the child in this study about what he/she is allowed to do on a computer,” assess rules about media content, time, and behavior. Response options for these items are “yes” and “no.”
Parent Weight Loss
Parents indicate their current weight loss activity using the following 4 response options: “lose weight,” “stay the same,” “gain weight,” and “not trying anything.” Parents also complete a 12-item inventory of healthy and unhealthy weight-loss methods used during the past year modified from Neumark-Sztainer et al.[46]
Three-Factor Eating Questionnaire (TFEQ)
The Three-Factor Eating Questionnaire (TFEQ) is a 51-item questionnaire includes the following factor analytically-derived subscales: cognitive restraint of eating, disinhibition, and hunger.[47] The 16-item disinhibition subscale (range 0-16) assesses parental eating behavior in the current study. Cronbach's alpha coefficient for the disinhibition subscale in this sample is 0.83. Questionnaire developers reported Cronbach's alpha of 0.91 for the revised 20-item version of this subscale, which included 4 weight fluctuation items that were removed in the final 16-item version.[47]
Parenting Styles and Dimensions Questionnaire-Short Form (PSDQ-32)
The Parenting Styles and Dimensions Questionnaire-Short Form (PSDQ-32)[48] measures multiple subscales and three higher-order factors: authoritative parenting (5-item connection, 5-item regulation, and 5-item autonomy granting subscales); authoritarian parenting (4-item physical coercion, 4-item verbal hostility, and 4-item non-reasoning/ punitive subscales); and permissive parenting (5-item indulgent subscale). The developers reported internal consistency values based on a sample of 1377 as follows: 0.86 for authoritative, 0.82 for authoritarian, and 0.64 for permissive.[48] Values based on the present dataset are 0.86 for authoritative, 0.72 for authoritarian, and 0.69 for permissive parenting.
Children's Sleep Habits Questionnaire (CSHQ)
The Children's Sleep Habits Questionnaire (CSHQ) is 33-item questionnaire assessing child sleep behavior.[49] The present study includes the sleep duration (3-items) and night wakings (3-items) subscales. Cronbach's alphas are acceptable in the present sample for the sleep duration (α = 0.76) and night wakings (α = 0.72) subscales and comparable to the original sample (sleep duration, α = 0.69; night waking, α = 0.54).
Pediatric Quality of Life Inventory, Parent-Report for Young Children (ages 5-7)-Version 4.0 Short Form (PedsQL™ 4.0 SF15)
Items from the parent-proxy report of the short-form version of the Pediatric Quality of Life Inventory (PedsQL™) are used to assess parent perceptions of child HRQOL across the core dimensions of physical, mental, social, and school domains.[50] Internal consistency reliability for the parent-report, short-form generic core module is 0.82 in a sample of 451 healthy children.[51] Cronbach's alpha in our sample is 0.87.
Questionnaire on Pediatric Gastrointestinal Symptoms (QPGS)
Child gastrointestinal (GI) health is assessed with 8-items from the Questionnaire on Pediatric Gastrointestinal Symptoms (QPGS).[52, 53]
Parental Safety Self Efficacy
Parental confidence to make safety-related behavior changes is measured by a 17-item safety self-efficacy scale developed for this study using an approach similar to Taveras et al.[42] Parents rate their ability to make changes to their behavior, their family's behavior, and their child's behavior in the areas of vehicle safety, fire safety, household poison prevention, secondhand smoke, sun safety, internet safety, and gun safety (e.g. gun storage). Item scores are averaged to compute a summary scale score with higher scores denoting greater safety self-efficacy. Cronbach's alpha coefficient for the 17-item scale is 0.87.
Parental Support for Safety
A 3-item measure assessing parental support for child safety behaviors was developed for the current study based on a questionnaire assessing parental support for physical activity.[44] Parents indicate the number of days over the past week that they encouraged their child to wear a bicycle helmet, use sunscreen, or wear a seatbelt in the car. Scores across the three items are averaged to produce a scale score (range 0-4; Cronbach's alpha coefficient = 0.64 ). .
Vehicle Safety
Parent and child vehicle restraint use is measured with 5 items adapted from the Child Car Seat and Air Bags Questionnaire.[54]
Distracted Driving
Seven questions assess parental distracted driving (e.g., putting on make-up, cell phone use, texting while driving), including two questions modified from Laberge-Nadeau.[55]
Safety Equipment Ownership and Use
Fourteen questions, adapted from the 2009 YRBS Standard High School Survey[56] and a home safety practice questionnaire developed by Robertson et al.[57], measure parent and child safety equipment ownership and use, including helmets, knee pads, elbow pads, wrist guards, mouth guards, and shin guards.
Injury Prevention
Three items adapted from a home safety questionnaire are used to assess burn and fall prevention.[58]
Fire Prevention
Parents report several aspects of household fire safety, including smoke detector use and maintenance, space heater use, and fireplace screen use. This 6-item questionnaire was adapted from several fire safety measures, which have adequate reliability and validity.[25, 56, 57, 59]
Carbon Monoxide Safety
A 3-item questionnaire assesses household carbon monoxide (CO) safety, including CO detector use, maintenance, and location adapted from the 2009 Behavioral Risk Factor Surveillance System (BRFSS) Questionnaire.[56]
Water/Ice Safety
Two-items adapted from a water safety survey[60] assess parent practices to promote child safety near water.
Sun Safety
Seven questions modified from the Sun Protection Survey[56] assess child and parent sun safety, including types of sun protection use and child sunburn history.
Secondhand Smoke
Household rules about secondhand smoke are assessed with 2 items. The first item, taken from the 2009 BRFSS Questionnaire, assesses smoking rules inside the home.[56] A second-item, using the same response options, was developed for this study to assess smoking rules inside the family's vehicle.
Family Disaster Plan
A 4-item questionnaire assesses family's natural disaster preparedness, including having a disaster emergency kit and a fire escape plan.[58]
Firearm/Gun Safety
A 7-item questionnaire adapted from 1994 National Health Interview Survey[61] assesses families’ firearm ownership, storage practices, and gun safety discussions.
Internet Safety
7 items assessing child internet safety were modified from an online survey, as there were no published, validated measures assessing the safety of children on the internet at the time of study development.[62]
Intervention Cost
To evaluate program scalability, intervention cost data including program development, materials/supplies, and program implementation will be collected. Development cost will be based on staff time and material for intervention development. Implementation cost will be derived from time spent in interactions between the participants and the phone coaches, using the phone coach salaries (including fringe benefits). Phone coach time will include actual time conducting phone coaching calls, time for documentation and scheduling, and time spent in weekly intervention supervision meetings
Healthy Homes Healthy Kids Intervention Overview
The theoretical and clinical underpinnings of the intervention use a combination of Social Cognitive Theory (SCT), which posits that behavior is determined jointly by knowledge, attitudes, behavioral skills, and environmental factors that facilitate implementing those skills,[63, 64] motivational interviewing,[65-68] which emphasizes the importance of participant self-determination and direction-setting in the change process, and strategies designed to maximize the likelihood of participant adherence to targeted goals.[69-71] The recognition of the complexity of obesity and multiple levels of influence and environmental contexts (e.g., community-level, built environment) that influence the development and maintenance of obesity were also incorporated into our intervention.[72, 73] We specifically target the intersection between the family environment and pediatric primary care as a community-level setting. Furthermore, our approach incorporates the need for all involved to recognize that continuous change in children's lives, in environments and other realities (such as those brought on by the child's maturing) demands flexibility and adaptation in all strategies.
Both HHHK treatment arms include two components: (1) a brief pediatric primary care provider counseling during a scheduled annual well child visit followed by (2) phone coaching to support parents in making changes in the home environment to promote the targets of the treatment arm. Table 1 describes the specific target areas and goals for each treatment arm.
Table 1.
Healthy Homes/Healthy Kids Target Areas and Goals by Treatment Arm
| Obesity Prevention Arm | Safety/Injury Prevention Contact Control Arma | ||
|---|---|---|---|
| Target Area | Specific Goal | Target Area | Specific Goal |
| Keep healthy fruit and vegetable options around the house | Increase fruit and vegetable availability so your child eats at least five servings of fruits and vegetables each day. | Keep your family safe in the car | Make sure everyone is buckled up and children are in child safety seats. Pay attention while driving; don't use cell phones or text. |
| Keep active play equipment around the house | Increase active play equipment to help your child get at least 60 minutes of physical activity each day. | Keep your family safe from injuries and accidents | Make sure your family uses safety equipment (e.g., helmets, knee pads, wrist guards) and put non-slip mats in the bathtub or shower. |
| Limit salty/high fat snacks, sweets, and sugared drinks | Limit salty/high fat snacks, sweets, and sugared drinks to one serving per day or less. | Prevent injury from fires | Have smoke detectors that work and practice fire drills with your family. |
| Limit media use | Limit media use including TV, computer, and video games to two hours per day or less. | Protect your family from household poisons | Have working CO detectors and keep household chemicals in locked cabinets. |
| Eat family meals together | Eat family meals together as often as possible. | Protect your family from second-hand smoke | Smoke outside your home and car or truck and have others do the same |
| Be physically active together as a family and support your child's activity level | Be physically active together as a family and support your child's activity level so he/she gets at least 60 minutes each day. | Keep your family safe by water | Have grownups take turns watching children in and around pools or lakes and have children wear life jackets when needed. |
| Eat a healthy breakfast | Eat a healthy breakfast every day. | Be safe in the sun | Always use sunscreen outside to protect your family from sun damage. |
| Limit eating out at restaurants | Limit eating out at restaurants to once each week or less. | Be ready for a disaster | Make a family disaster plan and put together emergency kits. |
| Portion control and food intake awareness | Be aware of what, when, and how much your child eats to help your child get closer to his/her daily needs. | Be safe with guns | Store guns unloaded in a locked place and keep bullets in a different locked place. |
| Help your child make everyday an active day | At least once a week, help your child replace an inactive routine with something active. | Use the internet safely | Be aware of children's internet activity and move computers into a family area. |
| Be balanced in your parenting | Think about changes you might want to make to be more balanced in your parenting. | Help protect your family from bed bugs | Learn how to protect your family from bedbugs. |
| Help keep your family safe from choking hazards | Help keep your family safe from choking hazards. | ||
| Help your family have clean and safe drinking water | Learn about the water quality in your home and know what to do in an emergency. | ||
| Help protect your family's home and auto from crime | Learn more about home and auto crime prevention. | ||
| Be prepared for winter driving hazards | Learn more about winter car safety and keep a safety kit in your car. | ||
| Protect your family from radon exposure | Learn more about home radon levels and make a change to reduce your risk. | ||
| Help keep your family protected from head lice | Learn to protect your family from exposure to lice. | ||
There are six more target areas in the Safety/Injury Prevention Contact Control Arm. Given that many of the target areas include goals and behaviors that are less complex and/or do not necessarily require daily attention (i.e., changing smoke alarm batteries is recommended twice a year), we increased the number of target areas to ensure that the intervention content would be substantive enough to engage families for 14 coaching calls.
Pediatric Primary Care Provider Well Child Visit Intervention Component
As a major goal of the Healthy Homes/Healthy Kids (HHHK 5-10) trial is to test a realistic intervention that could be implemented in routine pediatric practice, the pediatric primary care provider intervention component is integrated into the regular annual well child visit. This component of the HHHK 5-10 intervention starts the conversation about the study with parents, reinforces obesity prevention and safety issues relevant to the family's unique situation, and offers an opportunity for the provider to answer initial questions about healthy behaviors. This conversation provides an important and necessary link between the well child visit and the subsequent phone coaching component of the intervention. We developed this intervention component in partnership with several of the pediatric primary care providers in the clinic system to ensure that this brief counseling could be easily integrated into the well-child visit and would be accepted by providers. The procedures for this intervention component are as follows: An HHHK staff member meets the family at the clinic at their appointment time, accompanies the family into the well child visit and records the clinic staff-measured height and weight, and computes the child's BMI percentile and records it on an HHHK designed brochure. The brochure and an HHHK study “flipchart” were designed in collaboration with the pediatric clinic leadership to support HHHK message delivery. These are given to the child's primary care provider as a cue to provide brief counseling. The HHHK study “flipchart” includes brief messaging regarding child BMI percentile and the target areas for both the Obesity Prevention and the Contact Control arms.
Phone coaching Intervention Component
As stated previously, participants are randomized after the well child visit to either the Obesity Prevention condition or the Contact Control condition. Parents receive a package in the mail, which includes a letter describing their treatment group assignment and the HHHK workbook for their condition. Families in both conditions receive six bi-weekly phone coaching calls from a trained phone coach over the first three months. Families then receive eight monthly phone coaching calls during the remainder of their first year of the study. Each family is assigned a phone coach who will work with them for the entire intervention year. The purpose of the first call is to establish rapport, briefly review each target area, and discuss where the family is now and where they would like to go within each area. During the second and subsequent calls, the phone coach covers problems/adherence to goals, operationalizes the remainder of the recommendations, and works with the parent to problem solve in identified priority areas and where adherence is a problem. In our experience, families differ with respect to the number of relevant problem areas, the extent to which parents identify a particular behavior or lifestyle factor as a problem, and willingness to work on a goal in a particular area. To accommodate these variations and enhance parent motivation to make changes, we work with parents to identify the problem areas and goals most relevant to them, while also ensuring we address the core household recommendations over the course of the phone coaching calls. During these calls we prioritize problem areas for parents and problem solve specific ways the suggestions can be tailored and implemented for each family, work with the family to resolve adherence obstacles, and reinforce goal plan adherence.
Obesity Prevention Arm
For the Obesity Prevention arm, target areas and specific goals, as recommended by the most recent pediatric obesity guidelines[74], include: limiting consumption of sugar-sweetened beverages, encouraging consumption of fruits and vegetables, limiting television and other screen time, eating breakfast daily, limiting eating out restaurants (fast food in particular), encouraging family meals, and limiting portion size.
Safety/Injury Prevention Arm
The Safety/Injury Prevention Contact Control arm topics and specific goals include home safety and injury prevention, such as information on fire safety, bicycle safety, and skin protection. The Safety/Injury Prevention contact control condition content was developed with consultation from the Home Safety Council, Washington DC.
Treatment Fidelity
Phone coaches participate in standardized training and weekly group supervision meetings run by the lead interventionist and the study PI's. All phone coaching calls are recorded, and session recordings are routinely reviewed and discussed in supervision meetings. After each phone coaching call, the length of time of the call, topics discussed, and specific goals set are recorded. Phone coaches also rate themselves regarding adherence to motivational interviewing principles and adherence to goal setting and problem solving strategies. In addition, we are in the process of randomly selecting twenty percent of audio recordings and will use trained observers to rate treatment fidelity.
Analysis Plan
The primary analysis will evaluate the efficacy of the Obesity Prevention intervention in preventing unhealthy weight gain among children at risk for developing obesity relative to the Contact Control group, as evidenced by lower BMI percentiles at 12 and 24 months post-baseline among children randomized to the Obesity Prevention group.
The primary analysis will predict BMI percentile in all randomized children (intent-to-treat approach) from randomized treatment group (Tx), the time at which BMI% was observed (Time) and the Tx by Time interaction using a general linear mixed model approach. Tx (Contact Control, Obesity Prevention) will be treated as a fixed between subjects effect, and Time (Baseline, 12 months, 24 months) as a fixed within subjects fixed effect. Repeated BMI percentile observations will be nested within the children in which they are measured, and children will be nested within the clinic from which they were recruited. A random clinic intercept will be estimated to account for the possibility that BMI percentile varies systematically by clinic, thus rendering the BMI percentile observations from children within a given clinic dependent on each other (i.e., ICC > 0).
A 2 degree of freedom planned contrast will test whether 12 and 24 month BMI percentile observations among Obesity Prevention (OP) children are significantly different from the 12 and 24 month BMI percentile observations among Contact Control (CC) children, relative to the comparison of OP versus CC BMI% observations at baseline (H0: [(OP12, OP24) – (CC12, CC24)] – [OPB – CCB] = 0). The OP intervention will be considered efficacious if this planned contrast shows that BMI percentile is significantly lower at 12 and 24 months among OP participants relative to Baseline differences, and simple effect tests will test whether BMI percentile is significantly lower at each of these time points.
Secondary outcomes will be analyzed using the same model as the primary outcome, with a 2 degree of freedom planned contrast assessing whether OP children had more favorable outcomes at follow-up relative to CC children, and 2 single degree of freedom simple effect tests depicting the specific time points at which outcomes were different.
Sample Size Justification
A preliminary power analysis relied on pilot data for this trial (N=88) to evaluate whether our plan to randomize N=400 children equally to the two study groups would be sufficient for attaining 80% power to detect a practically meaningful difference in BMI percentile (3-5%) at 12 and 24 months post-baseline. The assumptions that would impact the magnitude of the design effects (children within clinics, BMI percentile observations within children), and hence the effective size of the analytic dataset, were as follows. The intraclass correlation (ICC) of BMI percentile within clinics was assumed to be ICCclin = .00-.01. With N=400 children recruited from 18 clinics, the resulting design effect (DEFF = (1 + (nclus – 1)*ICC) was assumed to be 1.0-1.2. The ICC of BMI percentile within children was assumed to be ICCchild = .60-.70, and with a minimum of 80% retention at 12 and 24 months, the implied DEFF = 1.96-2.12. Effective sample sizes for the data available at baseline, 12 and 24 months were calculated as Neff = N / DEFF so that the minimum detectable standardized effect (MDSE, Cohen's d) could be calculated for the planned contrast and simple effects tests.
Table 2 presents the MDSEs for the planned contrast, d=.56-.64, and for the simple effects tests, d=.44-.50, across the range of design effect assumptions. In our pilot data, baseline BMI% M = 89.3, SD = 8.3. The pilot SD was used as a starting point to estimate the likely variability in BMI% that would be observed in a larger sample following an efficacious intervention. The right three columns of Table 2 express the minimum difference in BMI% at 12 and 24 months, over and above any baseline differences, that the primary analysis would be powered to detect. The primary analysis will be adequately powered to detect practically meaningful differences in BMI% at 12 and 24 months should the observed SD ≈ 6-8 but not if it is as high as 10.
Table 2.
Minimum detectable standardized effects (Cohen's d) and corresponding between groups differences in BMI% at 12 and 24 months for a range of sample size assumptions.
| Cohen's d for... | BMI% for planned contrast when SD = | |||||
|---|---|---|---|---|---|---|
| DEFF clinic | ICC / DEFF child | planned contrast | simple effects | 6 | 8 | 10 |
| 1.2 | .70 / 2.12 | .64 | .50 | 3.81 | 5.08 | 6.35 |
| 1.2 | .65 / 2.01 | .62 | .50 | 3.74 | 4.98 | 6.23 |
| 1.2 | .60 / 1.96 | .61 | .48 | 3.66 | 4.88 | 6.10 |
| 1.1 | .70 / 2.12 | .61 | .48 | 3.65 | 4.86 | 6.08 |
| 1.1 | .65 / 2.01 | .60 | .47 | 3.56 | 4.77 | 5.96 |
| 1.1 | .60 / 1.96 | .58 | .46 | 3.50 | 4.66 | 5.83 |
| 1.0 | .70 / 2.12 | .58 | .46 | 3.47 | 4.62 | 5.78 |
| 1.0 | .65 / 2.01 | .57 | .45 | 3.40 | 4.54 | 5.67 |
| 1.0 | .60 / 1.96 | .56 | .44 | 3.34 | 4.45 | 5.56 |
The HHHK baseline data presented in Table 3 were referenced to assess the viability of the assumptions used in the preliminary power analysis. The N=421 enrollees were recruited from 18 clinics, with the median number of children per clinic being n=17. Descriptive statistics calculated on baseline BMI% were consistent with our assumptions, in that the ICCclin solved to 0, M=84.8 and SD=6.9. It is expected, then, that the primary analyses will be sufficiently powered to assess the efficacy of the OP intervention.
Table 3.
Baseline demographic characteristics, overall and by treatment arm.
| Total | Obesity Prevention | Safety/Injury Prevention | p-value | |
|---|---|---|---|---|
| N | 421 | 212 | 209 | |
| Child demographic characteristics | ||||
| Female, n(%) | 208 (49.4) | 101 (47.6) | 107 (51.2) | 0.47 |
| Age in years, M(SD) | 6.6 (1.7) | 6.6 (1.6) | 6.6 (1.7) | 0.84 |
| Non-Hispanic White, n(%) | 289 (69.1) | 154 (73.0) | 135 (65.2) | 0.09 |
| White, n(%) | 301 (71.5) | 159 (75.0) | 142 (67.9) | 0.06 |
| Multiple racial categories, n(%) | 49 (11.6) | 25 (11.8) | 24 (11.5) | |
| Black or African American, n(%) | 44 (10.5) | 21 (9.9) | 23 (11.0) | |
| Asian, n(%) | 14 (3.3) | 6 (2.8) | 8 (3.8) | |
| American Indian/Alaska Native, n(%) | 2 (0.5) | 0 | 2 (1.0) | |
| Unknown race, n(%) | 11 (2.6) | 1 (0.5) | 10 (4.8) | |
| Hispanic, n(%) | 29 (6.9) | 8 (3.8) | 21 (10.1) | 0.01 |
| BMI a in kg/m2, M(SD) | 17.8 (1.3) | 17.8 (1.3) | 17.9 (1.4) | 0.40 |
| BMI a percentile, M(SD) | 84.9 (6.9) | 84.7 (6.9) | 85.0 (7.0) | 0.67 |
| BMI a 70—84th percentile, n(%) | 206 (48.9) | 105 (49.5) | 101 (48.3) | 0.81 |
| BMI a 85—95th percentile, n(%) | 215 (51.1) | 107 (50.5) | 108 (51.7) | |
| Parent demographic characteristics | ||||
| Female, n(%) | 391 (92.9) | 198 (93.4) | 193 (92.3) | 0.67 |
| Age in yrs, M(SD) | 37.5 (6.5) | 37.7 (6.3) | 37.3 (6.8) | 0.52 |
| Non-Hispanic White, n(%) | 330 (79.0) | 173 (82.0) | 157 (75.9) | 0.12 |
| White, n(%) | 341 (81.0) | 176 (83.0) | 165 (79.0) | 0.18c |
| Multiple racial categories, n(%) | 14 (3.3) | 8 (3.8) | 6 (2.9) | |
| Black or African American, n(%) | 41 (9.7) | 19 (9.0) | 22 (10.5) | |
| Asian, n(%) | 16 (3.8) | 5 (2.4) | 11 (5.3) | |
| American Indian/Alaska Native, n(%) | 1 (0.2) | 1 (0.5) | 0 | |
| Unknown race, n(%) | 6 (1.4) | 1 (0.5) | 5 (2.4) | |
| Other race, n(%) | 2 (0.5) | 2 (1.0) | 0 | |
| Hispanic, n(%) | 15 (3.6) | 3 (1.4) | 12 (5.8) | 0.02 |
| College or graduate degree, n(%) | 299 (71.5) | 155 (73.5) | 144 (70.0) | 0.38 |
| Married/Living as married, n(%) | 338 (80.9) | 179 (84.8) | 159 (76.8) | 0.04 |
| Full or part-time employment, n(%) | 350 (83.7) | 172 (81.5) | 178 (86.0) | 0.22 |
| Eligible for FRPLb, n(%) | 82 (19.7) | 41 (19.5) | 41 (19.9) | 0.96 |
| Owns home, n(%) | 333 (79.5) | 172 (81.1) | 161 (77.8) | 0.40 |
| BMI a in kg/m2, M(SD)* | 28.6 (6.3) | 28.8 (6.4) | 28.4 (6.1) | 0.48 |
| BMI a < 25 kg/m2, n(%) | 137 (34.4) | 61 (31.1) | 76 (37.4) | 0.36 |
| BMI a 25—29 kg/m2, n(%) | 126 (31.6) | 67 (34.2) | 59 (29.1) | |
| BMI a ≥ 30 kg/m2, n(%) | 136 (34.1) | 68 (34.7) | 68 (33.5) | |
Body Mass Index
Free/Reduced Price Lunch
P-value derived from Fisher's Exact Test
Reported for the surveyed-parent or guardian
Missing Data
In spite of efforts to minimize missing follow-up data, some children will nonetheless have missing BMI% at 12 or 24 months. Missing BMI% may result from random (e.g., busy schedule, moving out of the area) or non-random (e.g., perceptions of not responding well to study protocol) processes, and it will not be possible to determine whether missing BMI% values should be considered missing at random(MAR) or not missing at random MNAR).[75] The maximum likelihood estimation used in the primary efficacy analyses will result in unbiased parameter estimates and accurate standard errors provided the missing BMI% values are, or even depart from, MAR.[75-78] The efficacy analyses will be supplemented by re-estimating the efficacy model using a set of multiply imputed datasets, which will also assumes that missing BMI% are MAR.[79] These datasets will be created using fully conditional specification, and the imputation models will include all variables included in the primary analytic model as well as interactions among them to improve precision.
A sensitivity analysis will then be carried out in which a delta-adjusted pattern imputation strategy will be employed to accommodate the possibility that missing BMI% are MNAR.[80] Multiply imputed datasets will be created at 12 and 24 months, separately for OP and CC children, by disturbing a MAR imputation process with increasingly extreme assumptions about the true values of missing BMI%. This will enable a consideration of whether there is a point at which reasonable assumptions about missing BMI% would prompt a reinterpretation of the efficacy analysis. Disturbance values (δ) will range from 0 to 1 SD of observed BMI% at 12 and 24 months, with increments of δ=0.1 SD added (OP) or subtracted (CC) to a MAR imputation process. This will reflect a conservative assumption that there was improvement in BMI% among CC children, and worsening among OP children, with missing values.
Results
Table 3 shows descriptive characteristics of the study sample, overall and by treatment group. The sample includes equal representation of boy and girl participants. The average age of study participants was 6.6 years (SD=1.7) and the majority of participants are non-Hispanic White with approximately 30% other race/ethnicities including Black/African American and Hispanic. The average BMI percentile of the children is 84.9 (SD=6.9); approximately 51% are classified as overweight with the remaining 49% between the 70th and 85th percentile. The majority of participating parents are females, married or living as married, and employed full or part-time. The average BMI of the parents is 28.5 (SD=6.2). Thirty-four percent of parents are normal weight, 32% overweight, and 34% obese. Table 4 presents baseline data for the study population related to the Obesity Prevention arm of the trial including dietary recall data, accelerometry, and parent-reported survey items assessing a wide range of constructs relevant to obesity prevention. The following criteria were used to calculate subscale scores for study measures: 1) Participants were required to complete 80% of subscale items to receive a score on subscales comprised of 5+ items; 2) A response was required for 75% of items in 4-item subscales, 67% of items in 3-item subscales, and 50% of items in 2-item subscales to calculate a subscale score; and 3) Published guidelines for item completion thresholds were followed if available. Table 5 presents baseline data for the study population related to the Contact Control arm of the trial focused on safety and injury prevention. Across all of the constructs assessed, minimal treatment group differences were observed at baseline, indicating the success of the randomization scheme.
Table 4.
Baseline obesogenic and other family characteristics, overall and by treatment arm.
| Total | Healthy Eating/Physical Activity | Safety | p-value | |
|---|---|---|---|---|
| N | 421 | 212 | 209 | |
| Diet-Related Factors | ||||
| NDSRa- Dietary Intake | ||||
| Energy intake in kcal, M(SD) | 1767 (564) | 1784 (536) | 1750 (593) | 0.54 |
| Percent energy from fat, M(Sd) | 29.9 (6.9) | 30.5 (6.7) | 29.3 (7.1) | 0.06 |
| Servings of fruit, M(SD) | 1.5 (1.8) | 1.5 (1.6) | 1.6 (2.0) | 0.60 |
| Servings of vegetables, M(SD) | 1.2 (1.5) | 1.3 (1.5) | 1.2 (1.4) | 0.25 |
| Ate breakfast every day/week, n(%) | 359 (85.3) | 185 (87.3) | 174 (83.3) | 0.25 |
| Media use and eating behaviors | ||||
| Breakfast while watching TV 1+day/week, n(%) | 218 (52.0) | 109 (51.4) | 109 (52.7) | 0.80 |
| Dinner while watching TV 1+day/week, n(%) | 205 (48.9) | 104 (49.1) | 101 (48.8) | 0.96 |
| Snack while watching TV 1+day/week, n(%) | 308 (73.5) | 156 (73.6) | 152 (73.4) | 0.97 |
| Household food availability | ||||
| Count of fruits, M(SD) | 5.6 (1.9) | 5.8 (1.8) | 5.5 (2.0) | 0.21 |
| Count of vegetables, M(SD) | 8.0 (2.6) | 8.0 (2.5) | 8.0 (2.8) | 0.85 |
| Count of salty snacks, M(SD) | 2.5 (1.4) | 2.5 (1.4) | 2.6 (1.4) | 0.43 |
| Count of sweets, M(SD) | 5.4 (2.6) | 5.4 (2.7) | 5.4 (2.5) | 0.93 |
| Count of sweetened beverages, M(SD) | 1.5 (1.2) | 1.4 (1.1) | 1.5 (1.2) | 0.10 |
| Restaurant behavior | ||||
| Eat at fast-food restaurant 1+times/week, n(%) | 278 (66.4) | 141 (66.5) | 137 (66.2) | 0.94 |
| Eat at fast casual food restaurant 1+times/week, n(%) | 91 (22.0) | 48 (22.6) | 43 (21.3) | 0.74 |
| Eat at sit-down restaurant 1+times/week, n(%) | 168 (40.1) | 85 (40.1) | 83 (40.1) | 0.99 |
| Parent self-efficacy for child healthy eating, M(SD) | 3.3 (0.5) | 3.3 (0.5) | 3.3 (0.5) | 0.64 |
| CFSQ b -Parent feeding styles | ||||
| Use food as reward, n(%) | 332 (79.2) | 161 (76.3) | 171 (82.2) | 0.14 |
| Promise child non-food item, n(%) | 274 (65.4) | 132 (62.6) | 142 (68.3) | 0.22 |
| Warn take away non-food item, n(%) | 246 (58.7) | 124 (58.8) | 122 (58.7) | 0.98 |
| CFQ c-Child feeding practices | ||||
| Perceived feeding responsibility, M(SD) | 4.1 (0.7) | 4.1 (0.6) | 4.1 (0.7) | 0.68 |
| Monitoring, M(SD) | 3.7 (1.0) | 3.7 (1.0) | 3.7 (1.0) | 0.99 |
| Restriction, M(SD) | 3.2 (0.8) | 3.2 (0.8) | 3.2 (0.8) | 0.78 |
| Pressure to eat, M(SD) | 2.2 (0.9) | 2.2 (0.9) | 2.2 (0.9) | 0.72 |
| CEBQ d-Child eating behavior | ||||
| Satiety responsiveness/slowness in eating, M(SD) | 2.9 (0.5) | 2.9 (0.5) | 2.9 (0.5) | 0.85 |
| Satiety responsiveness, M(SD) | 2.9 (0.6) | 2.9 (0.6) | 2.9 (0.6) | 0.98 |
| Slowness in eating, M(SD) | 2.8 (0.7) | 2.8 (0.7) | 2.8 (0.7) | 0.67 |
| Food responsiveness, M(SD) | 2.3 (0.7) | 2.3 (0.7) | 2.3 (0.7) | 0.89 |
| Enjoyment of food, M(SD) | 3.6 (0.6) | 3.6 (0.6) | 3.6 (0.7) | 0.61 |
| Emotional overeating, M(SD) | 1.6 (0.6) | 1.5 (0.6) | 1.6 (0.6) | 0.49 |
| Physical Activity-Related Factors | ||||
| Accelerometry-Child MVPAe in minutes/day, M(SD) | 53.6 (30.9) | 53.9 (30.8) | 53.3 (31.2) | 0.85 |
| AAf-Parent MVPAe in minutes/day, M(SD) | 44.5 (35.5) | 47.9 (38.7) | 40.9 (31.4) | 0.07 |
| Parent physically active with child 3+/week, n(%) | 231 (55.5) | 121 (57.6) | 110 (53.4) | 0.39 |
| Count of active play equipment available, M(SD) | 19.3 (6.0) | 19.4 (5.9) | 19.1 (6.1) | 0.53 |
| Parental support for physical activity, M(SD) | 2.5 (0.8) | 2.5 (0.8) | 2.5 (0.9) | 0.85 |
| Parental importance of physical activity, M(SD) | 4.8 (0.9) | 4.8 (0.8) | 4.8 (0.9) | 0.59 |
| Parental enjoyment of physical activity, M(SD) | 4.4 (0.8) | 4.4 (0.8) | 4.4 (0.9) | 0.58 |
| Parent self-efficacy for child physical activity, M(SD) | 3.5 (0.6) | 3.5 (0.6) | 3.5 (0.6) | 0.41 |
| Media-Related Factors | ||||
| Media use | ||||
| TV ≤ 2 hrs, n(%) | 243 (58.1) | 126 (59.4) | 117 (56.8) | 0.58 |
| Other media ≤ 2 hrs, n(%) | 375 (89.7) | 188 (88.7) | 187 (90.8) | 0.48 |
| Media availability | ||||
| TV in child's bedroom, n(%) | 90 (21.5) | 44 (20.8) | 46 (22.3) | 0.70 |
| Computer in child's bedroom, n(%) | 19 (4.6) | 6 (2.8) | 13 (6.3) | 0.09 |
| Video game console in child's bedroom, n(%) | 31 (7.4) | 14 (6.6) | 17 (8.3) | 0.52 |
| Parent self-efficacy in limiting child TV time, M(SD) | 3.1 (0.9) | 3.1 (0.9) | 3.1 (0.9) | 0.99 |
| Parent self-efficacy in removing TV from child's bedroom, M(SD) | 3.5 (1.0) | 3.5 (1.0) | 3.5 (1.0) | 0.50 |
| Family rules about TV time, n(%) | 330 (79.1) | 171 (81.0) | 159 (77.2) | 0.33 |
| Family rules about other media time, n(%) | 336 (82.0) | 173 (83.6) | 163 (80.3) | 0.39 |
| Other Family Factors | ||||
| CFQ c-Parent perceptions of weight | ||||
| Parent perceived own weight, M(SD) | 3.2 (0.4) | 3.2 (0.4) | 3.2 (0.4) | 0.07 |
| Parent perceived child weight, M(SD) | 3.0 (0.3) | 3.0 (0.2) | 3.0 (0.3) | 0.25 |
| Parent concerns re child weight, M(SD) | 2.4 (1.2) | 2.4 (1.2) | 2.4 (1.2) | 0.76 |
| Parent currently trying to lose weight, n(%) | 251 (60.5) | 137 (65.2) | 114 (55.6) | 0.04 |
| EI g-Parent emotional eating | ||||
| Disinhibition, M(SD) | 6.1 (3.8) | 6.2 (3.8) | 6.0 (3.7) | 0.53 |
| PSDQ32 h-General parenting style | ||||
| Authoritative, M(SD) | 4.0 (0.5) | 3.9 (0.5) | 4.0 (0.4) | 0.64 |
| Authoritarian, M(SD) | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.3) | 0.71 |
| Permissive, M(SD) | 1.9 (0.5) | 1.9 (0.5) | 1.9 (0.5) | 0.73 |
| CSHQ i-Child sleep behavior | ||||
| Sleep duration, M(SD) | 1.2 (0.4) | 1.2 (0.4) | 1.3 (0.4) | 0.50 |
| Night wakings, M(SD) | 1.3 (0.5) | 1.3 (0.5) | 1.3 (0.4) | 0.68 |
| PedsQLTM4.0 SF15 j -Child quality of life | ||||
| Physical health, M(SD) | 92.3 (15.3) | 92.1 (16.2) | 92.6 (14.2) | 0.75 |
| Psychosocial health, M(SD) | 78.7 (14.4) | 77.5 (14.3) | 79.8 (14.5) | 0.11 |
| Total score, M(SD) | 83.2 (12.6) | 82.4 (12.9) | 84.1 (12.4) | 0.18 |
| PHQ9 k-Parent depression status, M(SD) | 2.8 (3.2) | 2.9 (3.4) | 2.6 (3.0) | 0.42 |
| Child abdominal pain 2+/month, n(%) | 103 (24.5) | 56 (26.4) | 47 (22.5) | 0.35 |
Nutrition Diet System for Research
Caregiver Feeding Styles Questionnaire
Child Feeding Questionnaire
Child Eating Behavior Questionnaire
Moderate-to-vigorous physical activity
Active Australia
Three-Factor Eating Inventory
Parenting Styles and Dimensions Questionnaire- Short Form
Children's Sleep Habits Questionnaire
Pediatric Quality of Life InventoryTM Version 4.0 Short Form, Parent Report for Young Children (ages 5-7)
Patient Health Questionnaire
Table 5.
Baseline safety characteristics, overall and by treatment arm.
| Total | Healthy Eating/Physical Activity | Safety | p-value | |
|---|---|---|---|---|
| N | 421 | 212 | 209 | |
| Parent-Related Safety Factors | ||||
| Parent safety self-efficacy, M(SD) | 3.5 (0.4) | 3.5 (0.4) | 3.5 (0.4) | 0.39 |
| Parental support for safety a | ||||
| Encouraged child to wear helmet or safety equipment daily/past week, n(%) | 80 (29.3) | 50 (35.0) | 30 (23.0) | 0.03 |
| Encouraged child to wear sunscreen daily/past week, n(%) | 73 (24.6) | 39 (25.7) | 34 (23.5) | 0.66 |
| Reminded child to wear seatbelt or car restraint daily/past week, n(%) | 324 (80.6) | 164 (80.0) | 160 (81.2) | 0.76 |
| Seatbelt and car seat use a | ||||
| Child has car seat or booster seat, n(%) | 325 (85.5) | 172 (86.4) | 153 (84.5) | 0.60 |
| Child always uses booster seat, n(%) | 291 (76.4) | 151 (75.1) | 140 (77.8) | 0.54 |
| Child always uses seatbelt, n(%) | 206 (51.0) | 96 (46.4) | 110 (55.8) | 0.06 |
| Parent always uses seatbelt when driver, n(%) | 392 (94.2) | 202 (95.3) | 190 (93.1) | 0.35 |
| Parent always uses seatbelt as a passenger, n(%) | 379 (90.7) | 194 (91.5) | 185 (89.8) | 0.55 |
| Parent distracted driving | ||||
| Never put on makeup while driving/past week, n(%) | 390 (93.3) | 197 (92.9) | 193 (93.7) | 0.75 |
| Never took hands off wheel to care for child while driving/past week, n(%) | 249 (59.7) | 124 (58.8) | 125 (60.7) | 0.69 |
| Never turned on/changed GPS while driving/past week, n(%) | 343 (82.3) | 175 (82.6) | 168 (82.0) | 0.87 |
| Never talked on cell phone while driving/past week, n(%) | 80 (19.2) | 27 (12.8) | 53 (25.7) | <0.01 |
| Never dialed or texted on cell phone while driving/past week, n(%) | 208 (49.8) | 102 (48.1) | 106 (51.5) | 0.49 |
| Does not make calls while driving, n(%) | 97 (23.1) | 46 (21.7) | 51 (24.5) | 0.49 |
| Waits for red light before making calls, n(%) | 246 (58.6) | 129 (60.9) | 117 (56.3) | 0.34 |
| Slow down before making calls, n(%) | 91 (21.7) | 52 (24.5) | 39 (18.8) | 0.15 |
| Park by side of road before making calls, n(%) | 91 (21.7) | 47 (22.2) | 44 (21.2) | 0.80 |
| Child safety equipment availability | ||||
| Owns a helmet, n(%)a | 378 (91.3) | 195 (92.4) | 183 (90.2) | 0.41 |
| Owns knee pads, n(%) | 172 (41.0) | 95 (44.8) | 77 (37.0) | 0.10 |
| Owns elbow pads, n(%) | 147 (35.0) | 82 (38.7) | 65 (31.3) | 0.11 |
| Owns shin guards, n(%) | 93 (22.1) | 48 (22.6) | 45 (21.6) | 0.80 |
| Owns wrist guards, n(%) | 60 (14.3) | 33 (15.6) | 27 (13.0) | 0.45 |
| Owns a mouth guard, n(%) | 42 (10.0) | 23 (10.9) | 19 (9.1) | 0.56 |
| Child helmet use a | ||||
| Always while riding bike, n(%) | 240 (59.4) | 133 (63.6) | 107 (54.9) | 0.07 |
| Always while riding scooter, n(%) | 89 (32.1) | 44 (31.4) | 45 (32.9) | 0.80 |
| Always while playing hockey/contact sports, n(%) | 60 (60.0) | 31 (59.6) | 29 (60.4) | 0.93 |
| Always while rollerblading, n(%) | 47 (42.3) | 26 (47.3) | 21 (37.5) | 0.30 |
| Always while skiing/snowboarding, n(%) | 35 (48.0) | 16 (50.0) | 19 (46.3) | 0.76 |
| Always while skateboarding, n(%) | 33 (35.9) | 19 (41.3) | 14 (30.4) | 0.28 |
| Parent checks helmet fit regularly, n(%) | 295 (78.5) | 159 (80.7) | 136 (76.0) | 0.26 |
| Parent helmet availability and use a | ||||
| Owns a helmet, n(%) | 185 (48.6) | 96 (49.7) | 89 (47.3) | 0.64 |
| Always wears while riding bike, n(%) | 98 (30.5) | 53 (31.7) | 45 (29.2) | 0.62 |
| Always wears while rollerblading, n(%) | 14 (9.9) | 6 (8.2) | 8 (11.8) | 0.48 |
| Always wears while skiing or snowboarding, n(%) | 11 (9.8) | 4 (7.7) | 7 (11.7) | 0.48 |
| Always wears while playing hockey or contact sports, n(%) | 6 (9.4) | 2 (6.7) | 4 (11.8) | 0.68b |
| Injury prevention a | ||||
| Use non-slip mat in bathtub or shower, n(%)c | 170 (40.5) | 86 (40.6) | 84 (40.4) | 0.97 |
| When cooking, usually use back burners on oven, n(%)c | 126 (30.2) | 65 (30.8) | 61 (29.6) | 0.79 |
| When cooking, usually face pot handles to side or back of oven, n(%)c | 403 (97.6) | 207 (99.0) | 196 (96.1) | 0.06b |
| Have working smoke detectors in home, n(%)c | 409 (97.6) | 209 (98.6) | 200 (96.6) | 0.22b |
| Have smoke detectors on each floor of home, n(%)c | 390 (93.5) | 200 (94.8) | 190 (92.2) | 0.29 |
| Changed smoke detector batteries/past year, n(%)c | 264 (63.0) | 137 (64.9) | 127 (61.1) | 0.41 |
| Carbon monoxide detector in home, n(%)c | 348 (83.1) | 182 (85.9) | 166 (80.2) | 0.12 |
| Changed carbon monoxide detector batteries/past year, n(%)c | 162 (40.8) | 96 (47.8) | 66 (33.7) | <0.01 |
| Use space heaters without safety mechanism in home, n(%)d | 19 (4.5) | 9 (4.3) | 10 (4.8) | 0.79 |
| Have screens on fireplaces/wood stoves in home, n(%)c | 164 (87.7) | 83 (89.3) | 81 (86.2) | 0.52 |
| Parent smokes in bed, n(%)d | 3 (5.6) | 2 (7.7) | 1 (3.6) | 0.60b |
| Adult always present when child near water, n(%) | 408 (97.1) | 204 (96.2) | 204 (98.1) | 0.26 |
| Sun protection use | ||||
| Child had 1+ sunburns/past year, n(%) | 170 (40.5) | 86 (40.6) | 84 (40.4) | 0.97 |
| Child always or usually wears sunscreen when outside, n(%) | 293 (69.8) | 141 (66.5) | 152 (73.1) | 0.14 |
| Child uses > SPF30 sunscreen, n(%) | 267 (64.5) | 137 (65.2) | 130 (63.7) | 0.75 |
| Child uses sunscreen on cloudy days, n(%) | 146 (35.0) | 77 (36.7) | 69 (33.3) | 0.48 |
| Child uses sunglasses, n(%) | 285 (67.9) | 139 (65.6) | 146 (70.2) | 0.31 |
| Child uses hat, n(%) | 258 (61.4) | 121 (57.1) | 137 (65.9) | 0.06 |
| Child stays in shade, n(%) | 254 (60.5) | 125 (59.0) | 129 (62.0) | 0.52 |
| Child stays inside during peak-sun hours, n(%) | 172 (41.0) | 85 (40.1) | 87 (41.8) | 0.72 |
| Child uses long or short sleeve shirt, n(%) | 169 (40.2) | 86 (40.6) | 83 (39.9) | 0.89 |
| Child uses protective swimwear, n(%) | 134 (31.9) | 66 (31.1) | 68 (32.7) | 0.73 |
| Parent always or usually wears sunscreen when outside, n(%) | 198 (47.5) | 97 (46.0) | 101 (49.0) | 0.53 |
| Secondhand smoke | ||||
| Smoking not allowed anywhere in home, n(%) | 399 (95.7) | 201 (95.3) | 198 (96.1) | 0.67 |
| Smoking not allowed anywhere in car, n(%) | 372 (89.4) | 189 (89.6) | 183 (89.3) | 0.92 |
| Family Disaster Plan | ||||
| Family has fire escape plan, n(%) | 182 (43.7) | 91 (43.3) | 91 (44.0) | 0.90 |
| Family has plan for other disasters, n(%) | 277 (66.9) | 134 (64.4) | 143 (69.4) | 0.28 |
| Family has disaster emergency kit, n(%) | 89 (21.5) | 47 (22.6) | 42 (20.4) | 0.58 |
| Family has emergency meeting place, n(%) | 171 (41.2) | 82 (39.2) | 89 (43.2) | 0.41 |
| Gun safety a | ||||
| Gun in home, n(%)d | 127 (30.5) | 66 (31.3) | 61 (29.6) | 0.71 |
| BB gun or other type of gun in home, n(%)d | 77 (18.7) | 38 (18.5) | 39 (18.9) | 0.92 |
| Any type of gun in home, n(%)d | 154 (37.5) | 81 (39.3) | 73 (35.6) | 0.44 |
| Gun kept locked, n(%)c | 73 (57.0) | 37 (55.2) | 36 (59.0) | 0.67 |
| Gun kept unloaded, n(%)c | 114 (89.8) | 58 (87.9) | 56 (91.8) | 0.47 |
| Gun kept taken apart or with trigger lock, n(%)c | 81 (63.3) | 43 (64.2) | 38 (62.3) | 0.83 |
| Ammunition kept separate and locked, n(%)c | 33 (28.5) | 14 (23.3) | 19 (33.4) | 0.21 |
| Parent talked about gun safety with child, n(%) | 185 (45.1) | 95 (46.3) | 90 (43.9) | 0.62 |
| Internet and computer safety | ||||
| Child has internet access in home, n(%) | 348 (82.9) | 178 (84.0) | 170 (81.7) | 0.54 |
| Parent set child privacy settings on social networking sets, n(%)a,c | 25 (37.9) | 13 (40.6) | 12 (35.3) | 0.66 |
| Parental controls on home computer, n(%)a,c | 152 (38.5) | 77 (38.5) | 75 (38.5) | 0.99 |
| Parent talked to child about internet safety, n(%)a,c | 160 (64.8) | 81 (60.5) | 79 (69.9) | 0.12 |
| Child aware of “phishing,” n(%) | 53 (13.0) | 23 (11.2) | 30 (14.8) | 0.28 |
| Child understands to be careful sharing personal info online, n(%)c | 129 (31.6) | 60 (28.9) | 69 (34.5) | 0.22 |
| Antivirus software installed on home computer, n(%)a,c | 352 (88.7) | 180 (87.8) | 172 (89.6) | 0.58 |
| Computer in home, n(%) | 406 (96.7) | 206 (97.2) | 200 (96.2) | 0.56 |
| Computer in main living area, n(%) | 263 (62.6) | 137 (64.6) | 126 (60.6) | 0.39 |
| Computer in home office or computer room, n(%) | 171 (40.7) | 86 (40.6) | 85 (40.9) | 0.95 |
| Computer in adult bedroom, n(%) | 55 (13.1) | 20 (9.4) | 35 (16.8) | 0.02 |
| Computer in child bedroom, n(%) | 13 (3.1) | 6 (2.8) | 7 (3.4) | 0.75 |
Comparisons are made between participants for whom the question was applicable; not applicable is coded as missing.
P-value derived from Fisher's Exact Test.
Response “don't know” is coded as no (i.e. does not engage in behavior).
Response “don't know” is coded as yes (i.e. does engage in behavior).
Discussion
The Healthy Homes/Healthy Kids 5-10 study is a randomized trial examining the efficacy of a pediatric primary care-based intervention that integrates brief counseling by pediatric primary care providers and phone coaching conducted by health behavior change specialists designed to support parents in making changes in the home environment to promote healthy growth among children at risk for obesity. A secondary goal of the trial is to examine the efficacy of the contact control condition that focuses on general health and safety. This paper describes the study design, measurement and intervention protocols, and statistical analysis plan for the HHHK 5-10 trial. Additionally, the recruitment process and outcomes and the baseline characteristics of the study sample are described.
Pediatric primary care is an important setting in which to address obesity prevention, yet work in this area has been limited by barriers such as time, training, and participant burden. We designed the HHHK 5-10 study with these barriers in mind, from the design of the recruitment and evaluation process to the intervention approach. Strengths of the HHHK trial include demonstration of the ability to conduct a large scale randomized controlled trial within the normal operations of a clinic system without altering the length or timing of the regularly scheduled clinic. The design also allows for augmenting this clinic contact with useful information consistent with recommended preventative goals. Additionally, we designed the intervention to leverage the strength of the provider/patient relationship and provider expertise in a way that adds value and credibility to the concept of obesity prevention without putting an added burden on limited provider time. Moreover, delivering the second component of the intervention by telephone reduces burden on parents and allows for the tailoring of intervention messages and strategies to each families situation. The phone-based coaching component incorporates key elements of several powerful intervention approaches, including behavioral techniques, motivational interviewing, and adherence enhancement strategies. Moreover, the phone-based approach may increase the likelihood of dissemination, should the intervention be successful. To this end, we will quantify intervention costs, including program development, materials/supplies and program implementation which will inform the feasibility of implementation in other settings.
The study design and measurement protocol also include important strengths. First, the measurement protocol includes multiple outcome measures which assess for changes in all prevention goals of both study arms. In addition to comprehensive assessment of relevant obesity prevention and safety and injury prevention behaviors, parenting, and home environmental factors, a comprehensive intervention tracking system, including tape recordings of phone coaching sessions has been put in place. This detailed information will allow for assessment of intervention fidelity, coaching quality, and the relationship between these intervention content and process measures and study outcomes. Moreover, the study design includes a one-year post-intervention follow-up. Thus, we will be able to assess efficacy post-intervention and maintenance one year later in the absence of continued intervention contact. Several study limitations should be acknowledged, however. First, study participants are recruited from a pediatric primary care setting and results may not be generalizable to a broader population of children and their parents. Additionally, participants are not blinded to intervention condition. Thus, parent self-report measures in particular may be subject to self-presentation bias. It is possible that participants may report more favorable responses related to their respective condition. Alternatively, participants who would have preferred the other treatment condition may be influenced by a tendency toward compensatory rivalry.
Medical care settings are a challenging environment in which to do behavioral interventions for many reasons, including limited time medical personnel can commit to such activities. The HHHK 5-10 study will provide useful scientific and practical information in several areas, including the feasibility of delivering such interventions in a real world practice setting, the attractiveness of such a program to parents and providers, and the efficacy and cost of the programs. Stated most broadly, the long-range goal of the HHHK 5-10 study is to develop an intervention program for addressing behavioral contributors to children's health and illness in medical care settings which can be widely utilized across a variety of settings, is acceptable to health care providers, parents and children, and will have the effect of reducing health risk factors in families. We conceptualize the first steps in developing this treatment model as testing an intervention that has a measurable positive influence on child and family health and that is potentially usable within the constraints of primary medical practice. Study results will serve as a springboard for future aims to: 1) test these components across a range of clinical settings, populations and behavior issues, 2) measure the long-term stability of intervention effects, and 3) examine the generalizability of effects when implemented by practitioners in usual medical practice.
Acknowledgments
This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases including 1R01DK084475, as well as P30DK050456 and P30DK092924.
Footnotes
ClinicalTrials.gov Identifier: NCT01084590
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo AA, Etzel RA, Chilton LA, Watson C, Gorski PA. Primary Care Pediatrics and Public Health: Meeting the Needs of Today's Children. Am J Public Health. 2012;102:e17–e23. doi: 10.2105/AJPH.2012.301013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taveras EM, Blackburn K, Gillman MW, Haines J, McDonald J, Price S, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217–27. doi: 10.1007/s10995-010-0696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slusser W, Frankel F, Robison K, Fischer H, Cumberland WG, Neumann C. Pediatric overweight prevention through a parent training program for 2-4 year old Latino children. Child Obes. 2012;8:52–9. doi: 10.1089/chi.2011.0060. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor TM, Hilmers A, Watson K, Baranowski T, Giardino AP. Feasibility of an obesity intervention for paediatric primary care targeting parenting and children: Helping HAND. Child: care, health and development. 2013;39:141–9. doi: 10.1111/j.1365-2214.2011.01344.x. [DOI] [PubMed] [Google Scholar]
- 6.Nemet D, Barkan S, Epstein Y, Friedland O, Kowen G, Eliakim A. Short- and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics. 2005;115:e443–9. doi: 10.1542/peds.2004-2172. [DOI] [PubMed] [Google Scholar]
- 7.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10:22–32. doi: 10.1038/oby.2002.4. [DOI] [PubMed] [Google Scholar]
- 8.Dolinsky DH, Armstrong SC, Walter EB, Kemper AR. The effectiveness of a primary care-based pediatric obesity program. Clin Pediatr (Phila) 2012;51:345–53. doi: 10.1177/0009922811425232. [DOI] [PubMed] [Google Scholar]
- 9.Weigel C, Kokocinski K, Lederer P, Dotsch J, Rascher W, Knerr I. Childhood obesity: concept, feasibility, and interim results of a local group-based, long-term treatment program. J Nutr Educ Behav. 2008;40:369–73. doi: 10.1016/j.jneb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Eliakim A, Friedland O, Kowen G, Wolach B, Nemet D. Parental obesity and higher pre-intervention BMI reduce the likelihood of a multidisciplinary childhood obesity program to succeed--a clinical observation. Journal of pediatric endocrinology & metabolism : JPEM. 2004;17:1055–61. doi: 10.1515/jpem.2004.17.8.1055. [DOI] [PubMed] [Google Scholar]
- 11.Cotton B, Smith A, Hansen I, Davis C, Doyle A, Walsh A. Physician-directed primary care intervention to reduce risk factors for type 2 diabetes in high-risk youth. Am J Med Sci. 2006;332:108–11. doi: 10.1097/00000441-200609000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gillis D, Brauner M, Granot E. A community-based behavior modification intervention for childhood obesity. Journal of pediatric endocrinology & metabolism : JPEM. 2007;20:197–203. doi: 10.1515/jpem.2007.20.2.197. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RP, Hamre R, Dietz WH, Wasserman RC, Slora EJ, Myers EF, et al. Office-based motivational interviewing to prevent childhood obesity: a feasibility study. Arch Pediatr Adolesc Med. 2007;161:495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- 14.Kubik MY, Story M, Davey C, Dudovitz B, Zuehlke EU. Providing obesity prevention counseling to children during a primary care clinic visit: results from a pilot study. J Am Diet Assoc. 2008;108:1902–6. doi: 10.1016/j.jada.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Young PC, West SA, Ortiz K, Carlson J. A pilot study to determine the feasibility of the low glycemic index diet as a treatment for overweight children in primary care practice. Ambul Pediatr. 2004;4:28–33. doi: 10.1367/1539-4409(2004)004<0028:apstdt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Ford BS, McDonald TE, Owens AS, Robinson TN. Primary care interventions to reduce television viewing in African-American children. Am J Prev Med. 2002;22:106–9. doi: 10.1016/s0749-3797(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 17.Ewing LJ, Cluss P, Goldstrohm S, Ulrich R, Colborn K, Cipriani L, et al. Translating an evidence-based intervention for pediatric overweight to a primary care setting. Clin Pediatr (Phila) 2009;48:397–403. doi: 10.1177/0009922808330109. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson D, Melnyk BM. A primary care healthy choices intervention program for overweight and obese school-age children and their parents. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2012;26:126–38. doi: 10.1016/j.pedhc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Korsten-Reck U, Kromeyer-Hauschild K, Wolfarth B, Dickhuth HH, Berg A. Freiburg Intervention Trial for Obese Children (FITOC): results of a clinical observation study. Int J Obes (Lond) 2005;29:356–61. doi: 10.1038/sj.ijo.0802875. [DOI] [PubMed] [Google Scholar]
- 20.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Efficacy of Family-Based Weight Control Program for Preschool Children in Primary Care. Pediatrics. 2012 doi: 10.1542/peds.2012-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wake M, Baur LA, Gerner B, Gibbons K, Gold L, Gunn J, et al. Outcomes and costs of primary care surveillance and intervention for overweight or obese children: the LEAP 2 randomised controlled trial. BMJ. 2009;339:b3308. doi: 10.1136/bmj.b3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCallum Z, Wake M, Gerner B, Baur LA, Gibbons K, Gold L, et al. Outcome data from the LEAP (Live, Eat and Play) trial: a randomized controlled trial of a primary care intervention for childhood overweight/mild obesity. Int J Obes (Lond) 2007;31:630–6. doi: 10.1038/sj.ijo.0803509. [DOI] [PubMed] [Google Scholar]
- 23.Kalavainen MP, Korppi MO, Nuutinen OM. Clinical efficacy of group-based treatment for childhood obesity compared with routinely given individual counseling. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803628. [DOI] [PubMed] [Google Scholar]
- 24.Savoye M, Shaw M, Dziura J, Tamborlane WV, Rose P, Guandalini C, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. Jama. 2007;297:2697–704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 25.Taveras EM, Gortmaker SL, Hohman KH, Horan CM, Kleinman KP, Mitchell K, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165:714–22. doi: 10.1001/archpediatrics.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brambilla P, Bedogni G, Buongiovanni C, Brusoni G, Di Mauro G, Di Pietro M, et al. “Mi voglio bene”: a pediatrician-based randomized controlled trial for the prevention of obesity in Italian preschool children. Italian journal of pediatrics. 2010;36:55. doi: 10.1186/1824-7288-36-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veldhuis L, Struijk MK, Kroeze W, Oenema A, Renders CM, Bulk-Bunschoten AM, et al. ‘Be active, eat right’, evaluation of an overweight prevention protocol among 5-year-old children: design of a cluster randomised controlled trial. BMC Public Health. 2009;9:177. doi: 10.1186/1471-2458-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare ME, Coday M, Williams NA, Richey PA, Tylavsky FA, Bush AJ. Methods and baseline characteristics of a randomized trial treating early childhood obesity: the Positive Lifestyles for Active Youngsters (Team PLAY) trial. Contemp Clin Trials. 2012;33:534–49. doi: 10.1016/j.cct.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalton WT, 3rd, Schetzina KE, Holt N, Fulton-Robinson H, Ho AL, Tudiver F, et al. Parent-Led Activity and Nutrition (PLAN) for healthy living: design and methods. Contemp Clin Trials. 2011;32:882–92. doi: 10.1016/j.cct.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 31.van Horn LV, Stumbo P, Moag-Stahlberg A, Obarzanek E, Hartmuller VW, Farris RP, et al. The Dietary Intervention Study in Children (DISC): dietary assessment methods for 8- to 10-year-olds. J Am Diet Assoc. 1993;93:1396–403. doi: 10.1016/0002-8223(93)92241-o. [DOI] [PubMed] [Google Scholar]
- 32.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–65. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 33.American Academy of Pediatrics Children, adolescents, and television. Pediatrics. 2001;107:423–6. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 34.Sherwood NE, Crain AL, Martinson BC, Hayes MG, Anderson JD, Clausen JM, et al. Keep it off: a phone-based intervention for long-term weight-loss maintenance. Contemp Clin Trials. 2011;32:551–60. doi: 10.1016/j.cct.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennison BA, Russo TJ, Burdick PA, Jenkins PL. An intervention to reduce television viewing by preschool children. Arch Pediatr Adolesc Med. 2004;158:170–6. doi: 10.1001/archpedi.158.2.170. [DOI] [PubMed] [Google Scholar]
- 36.Raynor HA, Polley BA, Wing RR, Jeffery RW. Is dietary fat intake related to liking or household availability of high- and low-fat foods? Obes Res. 2004;12:816–23. doi: 10.1038/oby.2004.98. [DOI] [PubMed] [Google Scholar]
- 37.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Boutelle KN, Fulkerson JA, Neumark-Sztainer D, Story M, French SA. Fast food for family meals: relationships with parent and adolescent food intake, home food availability and weight status. Public Health Nutr. 2007;10:16–23. doi: 10.1017/S136898000721794X. [DOI] [PubMed] [Google Scholar]
- 39.Hughes SO, Power TG, Orlet Fisher J, Mueller S, Nicklas TA. Revisiting a neglected construct: parenting styles in a child-feeding context. Appetite. 2005;44:83–92. doi: 10.1016/j.appet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36:201–10. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- 41.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42:963–70. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 42.Taveras EM, Mitchell K, Gortmaker SL. Parental confidence in making overweight-related behavior changes. Pediatrics. 2009;124:151–8. doi: 10.1542/peds.2008-2892. [DOI] [PubMed] [Google Scholar]
- 43.Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self-administered version of the Active Australia physical activity survey in a sample of mid-age women. Australian and New Zealand journal of public health. 2008;32:535–41. doi: 10.1111/j.1753-6405.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 44.Trost SG, Sallis JF, Pate RR, Freedson PS, Taylor WC, Dowda M. Evaluating a model of parental influence on youth physical activity. Am J Prev Med. 2003;25:277–82. doi: 10.1016/s0749-3797(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 45.The Henry J. Kaiser Family Foundation. In: Rideout V, Foehr U, Roberts D, Generation M, editors. [superscript 2]: Media in the Lives of 8- to 18-Year Olds. Kaiser Family Foundation; Menlo Park, CA: 2010. [Google Scholar]
- 46.Neumark-Sztainer D. The social environments of adolescents: associations between socioenvironmental factors and health behaviors during adolescence. Adolesc Med. 1999;10:41–55, v. [PubMed] [Google Scholar]
- 47.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 48.Robinson C, Mandleco B, Olsen SF, Hart C. The parenting styles and dimensions questionnaire (PSDQ). In: Touliatos J, Perlmutte B, editors. Handbook of family measurement techniques. 2 ed. Sage; Thousand Oaks: 2001. pp. 319–21. [Google Scholar]
- 49.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 50.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children's health-related quality of life: an analysis of 13,878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health and quality of life outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan KS, Mangione-Smith R, Burwinkle TM, Rosen M, Varni JW. The PedsQL: reliability and validity of the short-form generic core scales and Asthma Module. Medical care. 2005;43:256–65. doi: 10.1097/00005650-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Caplan A, Walker L, Rasquin A. Development and preliminary validation of the questionnaire on pediatric gastrointestinal symptoms to assess functional gastrointestinal disorders in children and adolescents. Journal of pediatric gastroenterology and nutrition. 2005;41:296–304. doi: 10.1097/01.mpg.0000172748.64103.33. [DOI] [PubMed] [Google Scholar]
- 53.Caplan A, Walker L, Rasquin A. Validation of the pediatric Rome II criteria for functional gastrointestinal disorders using the questionnaire on pediatric gastrointestinal symptoms. Journal of pediatric gastroenterology and nutrition. 2005;41:305–16. doi: 10.1097/01.mpg.0000172749.71726.13. [DOI] [PubMed] [Google Scholar]
- 54.Vaca F, Anderson CL, Agran P, Winn D, Cheng G. Child safety seat knowledge among parents utilizing emergency services in a level I trauma center in Southern California. Pediatrics. 2002;110:e61. doi: 10.1542/peds.110.5.e61. [DOI] [PubMed] [Google Scholar]
- 55.Laberge-Nadeau C, Maag U, Bellavance F, Lapierre SD, Desjardins D, Messier S, et al. Wireless telephones and the risk of road crashes. Accident; analysis and prevention. 2003;35:649–60. doi: 10.1016/s0001-4575(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Questionnaire. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia: 2009. [Google Scholar]
- 57.Robertson AS, Rivara FP, Ebel BE, Lymp JF, Christakis DA. Validation of parent self reported home safety practices. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2005;11:209–12. doi: 10.1136/ip.2005.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Posner JC, Hawkins LA, Garcia-Espana F, Durbin DR. A randomized, clinical trial of a home safety intervention based in an emergency department setting. Pediatrics. 2004;113:1603–8. doi: 10.1542/peds.113.6.1603. [DOI] [PubMed] [Google Scholar]
- 59.Watson M, Kendrick D, Coupland C. Validation of a home safety questionnaire used in a randomised controlled trial. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention. 2003;9:180–3. doi: 10.1136/ip.9.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee LK, Thompson KM. Parental survey of beliefs and practices about bathing and water safety and their children: guidance for drowning prevention. Accident; analysis and prevention. 2007;39:58–62. doi: 10.1016/j.aap.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Adams P, Marano M. Current estimates from the National Health Interview Survey, 1994. National Center for Health Statistics. Vital Health Stat. 1995:10. [PubMed] [Google Scholar]
- 62.Next Generation Learning Challenges. Internet Safety Quiz.
- 63.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 64.Bandura A. Social Foundations of Thought and Action: A social cognitive theory. Prentice Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 65.Rollnick S, Miller WR, Butler CC, Aloia MS. Motivational Interviewing in Health Care: Helping Patients Change Behavior. Copd. 2008;5:203. [Google Scholar]
- 66.Miller W, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. Guilford Press; New York: 2002. [Google Scholar]
- 67.Miller W, Rollnick S. Motivational interviewing: Preparing people to change addicitve behavior. Guilford Press; New York: 1991. [Google Scholar]
- 68.Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72:1050–62. doi: 10.1037/0022-006X.72.6.1050. [DOI] [PubMed] [Google Scholar]
- 69.Levy R. Compliance and medical practice. In: Blumenthal J, McKee D, editors. Applications in Behaviorial Medicine. Professional Resource Exchange; Sarasota: 1987. [Google Scholar]
- 70.Levy RL, Feld AD. Increasing patient adherence to gastroenterology treatment and prevention regimens. Am J Gastroenterol. 1999;94:1733–42. doi: 10.1111/j.1572-0241.1999.01200.x. [DOI] [PubMed] [Google Scholar]
- 71.Levy RL, Finch EA, Crowell MD, Talley NJ, Jeffery RW. Behavioral intervention for the treatment of obesity: strategies and effectiveness data. Am J Gastroenterol. 2007;102:2314–21. doi: 10.1111/j.1572-0241.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- 72.Davison KK, Birch LL. Childhood overweight: a contextual model and recommendations for future research. Obes Rev. 2001;2:159–71. doi: 10.1046/j.1467-789x.2001.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stokols D. Establishing and maintaining healthy environments. Toward a social ecology of health promotion. Am Psychol. 1992;47:6–22. doi: 10.1037//0003-066x.47.1.6. [DOI] [PubMed] [Google Scholar]
- 74.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 75.Little RJ, Rubin DB. Statistical analysis with missing data. 2nd ed. John Wiley & Sons; New York: 2002. [Google Scholar]
- 76.Hox JJ. Multilevel analysis: techniques and applications. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2002. [Google Scholar]
- 77.Muthen B, Kaplan D, Hollis M. On structural equation modeling with data that are not completely missing at random. Psychometrika. 1987;52:431–62. [Google Scholar]
- 78.Raudenbush S, Byrk A. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 79.Rubin DB. Multiple Imputation of Nonresponses in Survey Data. Wiley; New York: 1987. [Google Scholar]
- 80.Ratitch B, O'Kelly M, Tosiello R. Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Pharmaceutical statistics. 2013 doi: 10.1002/pst.1549. [DOI] [PubMed] [Google Scholar]