Abstract
The developmental origins of metabolic syndrome have been established through the consistent observation that small-for-gestational age and large-for-gestational age fetuses have an increased risk for hypertension and related metabolic disorders later in life. These phenotypes have been reproduced in various species subjected to a range of intrauterine insults and ongoing research is directed towards understanding the underlying molecular mechanisms. Current evidence suggests that the creation of a pro-inflammatory and pro-oxidant intrauterine milieu is a common thread among prenatal factors that impact upon fetal size. Furthermore, studies demonstrate that a shift in fetal redox status consequent to environmental cues persists after birth and drives the progression of vascular dysfunction and hypertension in postnatal life. Toll-like receptor signaling has emerged as a key link between inflammation and oxidative stress and pathogenic contributor to hypertension, insulin resistance and obesity, in both human patients and animal models of disease. Thus, Toll-like receptor activation and dysregulation of its signaling components represent potential molecular underpinnings of programmed hypertension and related disorders in those subjected to sub-optimal intrauterine conditions, yet their contributions to developmental programming remain unexplored. We propose that danger signals mobilized by the placenta or fetal tissues during complicated pregnancy activate the fetal innate immune system through TLRs and thereby potentiate the generation of reactive oxygen species and orchestrate fetal adaptive responses, including changes in gene expression which later translate to vascular dysfunction. Further, we suggest that after birth, continual activation of TLR signaling propagates vascular oxidative stress and thereby accelerates the advancement of hypertension and heart failure.
Keywords: Developmental programming, metabolic syndrome, Toll-like receptors
Introduction
Developmental origins of chronic disease
Improving maternal health was recently recognized at the United Nations High-level Meeting on Non-communicable Diseases as an important strategy in tackling the pandemic of chronic illness. This event heralds the evolution of a life-course approach to chronic disease, yet the molecular mechanisms that link intrauterine perturbations to later disease vulnerability remain elusive. With the progression of gestation, fetal growth and development become increasingly reliant on maternal provision of substrate and thus submissive to any deficiencies in maternal health and placental function. Numerous maternal and placental factors challenge human pregnancy, including placental insufficiency, preeclampsia, maternal malnutrition, anemia and drug use, all of which impact upon the materno-fetal delivery of critical nutrients and oxygen. In the face of such adverse conditions, the fetus holds a remarkable capacity to adapt and does so by suppressing its genetically-determined growth trajectory and preferentially distributing limited substrates to organs critical for survival. Yet, this short-term gain comes at a cost as aberrant changes in gene expression and organ structure become fixed going forward in postnatal life, eventually leading to dysfunction and disease. Indeed, adults and children born small are at increased risk for obesity, insulin resistance and hypertension, which together constitute the metabolic syndrome [1,2]. These phenotypes have been reproduced in various species subjected to a range of prenatal insults [3]. Interestingly, the metabolic syndrome tends to manifest not only in those exposed to prenatal deprivation but also in those born from pregnancies complicated by maternal obesity, excessive weight gain or high fat diet [4]. Accordingly, the correlation between metabolic syndrome and birth weight appears to be U-shaped, such that increased risk occurs in the smallest and largest babies [5]. Thus, there appears to be mechanistic commonalities among programmed deficits consequent to the array of intrauterine insults that impact upon fetal growth.
The pro-inflammatory and pro-oxidant nature of intrauterine stress
Universal among the antenatal conditions that have been studied in the context of developmental programming, is the creation of a pro-inflammatory and pro-oxidant intrauterine milieu. Increases in pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β, with concomitant reductions in anti-inflammatory cytokines, have been reported in the fetal circulation, placenta and amniotic fluid, when normative growth curves imply intrauterine growth restriction (IUGR) [6-8]. Likewise, abnormal umbilical Doppler waveforms indicative of placental insufficiency, the most common cause of IUGR in Western society, are associated with increased fetal levels of IL-6 [9]. This cytokine activation has been reproduced in animal models of placental insufficiency [10] and is related in a dose-dependent manner to the severity of fetal hypoxemia [11].
Given that inflammatory and redox pathways are intimately linked, it is probable that reciprocal or synergistic activation occurs in response to intrauterine stress. Lipid peroxidation and reduced antioxidant enzyme activity in umbilical cord blood [12] and increased markers of oxidative stress in the placenta [13] are evident in human IUGR pregnancies. On the other end of the spectrum, maternal obesity and gestational diabetes which increase the risk for macrosomic babies, also mediate their effects on the fetus in part through induction of placental nitrosative and oxidative stress and share with the IUGR pregnancy an increased risk for metabolic and cardiovascular disorders in the offspring [4,5]. This disruption in redox equilibrium consequent to poor maternal nutritional status may influence every stage of development, as demonstrated in a rat model of maternal high fat diet wherein markers of oxidative stress were increased in both the fetus and pre-implantation embryo [14]. Due to immature antioxidant defenses, the redox state of the embryo and fetus is readily shifted toward oxidative stress [15]. Vulnerability of the conceptus together with a high output capacity for reactive oxygen species (ROS) by the metabolically active placenta, amplify the impact of a pro-oxidant intrauterine insult. Interestingly, it appears that in utero disruptions in the oxidant-antioxidant balance persist into postnatal life, as increases in oxidant indices and decreases in antioxidant enzyme activities have been reported in both human [16] and animal IUGR offspring [17]. It is widely believed that in addition to acting as a trigger for aberrant changes in gene expression and organ development in utero, oxidative stress continually promotes organ dysfunction throughout postnatal life (Figure 1).
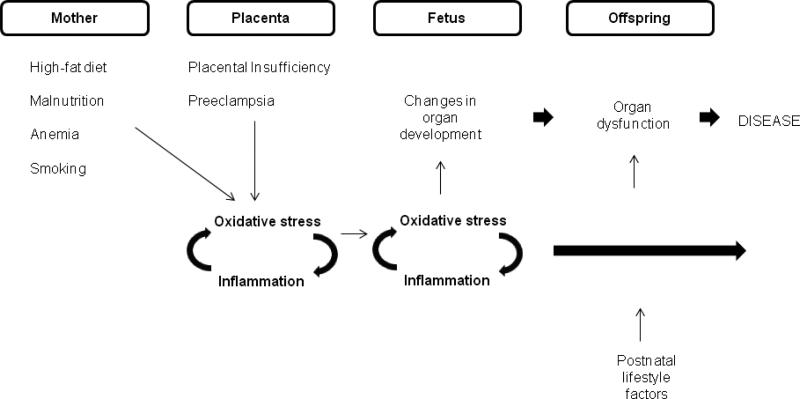
Figure 1.
The pro-inflammatory and pro-oxidant nature of complicated pregnancy. The various forms of intrauterine stress which impact upon fetal growth are thought to exert their influence on developmental processes largely through pro-inflammatory and pro-oxidant stimuli. These stimuli arise from a hypoxic/ischaemic placenta, which is a common correlate of poor maternal health and abnormal placental development, or derive directly from hypoxic fetal tissue. Redox and inflammatory signals share common pathways and may be activated in a synergistic fashion in response to prenatal perturbations. Although these responses likely play fundamental roles in fetal adaptation and survival, their impact on gene expression during critical developmental windows often leads to abnormalities in the structure and function of organs and in the set-points of endocrine axes, which become fixed with the loss of plasticity after birth. In fact, it appears that the redox pathway is itself programmed, as an imbalance in antioxidant/oxidant status is a common long-term outcome in offspring subjected to sub-optimal intrauterine conditions. This enduring oxidative stress together with functional disadvantage and the superimposition of postnatal risk factors, accelerate the path towards chronic illness.
ROS are mediators of programmed vascular dysfunction
Immune and redox signals serve critical regulatory functions in normal gestation and thus modulation of these pathways is a primary means by which intrauterine stress exerts its influence on placental and fetal development. During healthy pregnancy, increasing oxygen tensions that parallel invasion of the uterus and elaboration of the fetal villi drive both organogenesis and placental maturation. This transition in redox potential of the intrauterine environment leads to increased production of ROS which in turn mediate cellular differentiation, proliferation and gene expression, directly through activation of transcription factors and indirectly via epigenetic processes [18]. Recently, a study by Giussani and colleagues demonstrated that maternal administration of allopurinol during uncomplicated ovine pregnancy blunts constrictor responses of the fetal femoral artery and increases umbilical blood flow, suggesting a role for ROS in normal fetal vascular function [19]. Hence one may deduce that excess ROS release contributes to the hemodynamic adaptation to fetal hypoxemia characterized by peripheral vasoconstriction and redistribution of cardiac output. Although ROS likely serve important adaptive functions during an acute intrauterine insult, unremitting intrauterine stress and prolonged ROS production will lead to negative developmental outcomes. Indeed, there exists ample evidence to suggest that ROS are obligatory participants in programming of vascular dysfunction. For example, supplementation with the peroxidation inhibitor, Lazaroid, abrogated the abnormal vascular reactivity and blood pressure elevation in rat offspring born from mothers restricted to a low protein diet during pregnancy [17]. Using the same model, Yzydorczyk et al. showed that enhanced arterial responses to ANG II in IUGR offspring were normalized after treatment with Tempol, a superoxide dismutase analog [20]. In another study, rescue of postnatal vascular function was accomplished by treatment with apocynin but not with L-NAME or oxypurinol, thus identifying NADPH oxdiase as a primary source of superoxide after nutrient-restricted pregnancy [21]. Likewise, aortic thickening in utero and subsequent impairment in endothelium-dependent vascular responses consequent to hypoxic pregnancy were ameliorated with maternal administration of exogenous antioxidants [22]. Thus, accumulating evidence highlights oxidative stress as a causative factor in programming of arterial dysfunction under a variety of intrauterine insults.
A new link between oxidative stress and inflammation
The innate immune system, on which the sterile intrauterine environment heavily depends, has emerged as a key link between oxidative stress and inflammation and mediator of stress-induced organ damage and dysfunction. Although the pathogenic contributions of the innate immune system to the development of hypertension and related metabolic disorders have come to light over the past few years, it has rarely been studied in the context of fetal programming. This genetically coded defense program provides an immediate response to invading microbial organisms, independent of immunological memory. Responses are initiated upon activation of pathogen recognition receptors (PRRs) which detect foreign invasion upon interaction with conserved structural motifs released from microbes, known as pathogen associated molecular patterns (PAMPS) [23,24]. Seminal studies revealed that this innate agent of immunity not only responds to exogenous pathogens, but is activated to the same degree by damage associated molecular patterns (DAMPS) derived from injured, stressed or necrotic cells [25,26]. Uric acid, heat shock proteins and HMGB1 (DNA-binding nuclear protein) are among the DAMPS mobilized after oxidative stress-induced injury to DNA and proteins.
With respect to sterile inflammation, ongoing research has focused on the major family of PRRs known as Toll-like receptors (TLRs). Expression of TLRs is ubiquitous and diverse, now known to occur in cells of the musculo-skeletal, digestive and cardiovascular systems, in addition to primary immune cells. These type-1 trans-membrane proteins located intracellularly (TLR-7, TLR-9) or on the cell surface (TLR-2, TLR-4), contain leucine-rich repeats (LRRs) that recognize foreign microbes or host-derived danger signals, and a toll/interleukin 1 receptor (TIR) domain [27]. The TIR domain facilitates interaction with the cytoplasmic adapter protein MyD88, to initiate a signaling cascade culminating in activation of the mitogen activated protein kinase (MAPK) and IKK pathways, both of which contribute to the release of proinflammatory cytokines, chemokines and cell adhesion molecules [28,29]. Formation of the IKK complex triggers phosphorylation and subsequent proteasomal degradation of the intracellular inhibitors of κB (IκB), resulting in the liberation and translocation of the transcription factor, nuclear factor-κB (NF-κB) [30,31]. NF-κB orchestrates key cellular events such as inflammation, proliferation, differentiation and survival, through transcriptional regulation of numerous genes. Downstream molecules of the TLR-NF-κB pathway (mainly TNF-α) amplify inflammation through a positive feedback loop [32], yet others function as internal restraints on the duration and magnitude of the response [33]. Further, TLR-NF-κB signaling is redox-sensitive and can potentiate ROS generation through activation of TNF-α and NADPH oxidase [34]. In this light, dysregulation of TLR-NF-κB signaling may represent the molecular underpinnings of the unfettered cycle of inflammation and oxidative stress that drives evolution of the metabolic syndrome.
The role of TLR signaling in the development of disease characterized by low-grade chronic inflammation has been substantiated by recent human and animal studies. For instance, Dasu et al., reported increased TLR-2 and TLR-4 mRNA expression along with activation of downstream TLR signaling molecules, in monocytes isolated from type II diabetic patients [35]. Another study showed that increased TLR-4 mRNA and protein expression in skeletal muscle of type II diabetic patients were correlated with the severity of insulin resistance [36]. In agreement, mice fed an obesogenic diet exhibit increased TLR-2 expression in muscle and adipose tissue, while insulin signaling in these mice was improved with inhibition of TLR-2 signaling [37]. Insulin resistance and obesity are accompanied by hypertension and underlying vascular dysfunction, in 80 percent of patients [38]. Cumulating oxidative stress and inflammation drive the advancement of arterial dysfunction and current evidence suggests that TLR signaling may act to propagate this insidious process. Oxidized low density lipoprotein (LDL) induces inflammatory responses through TLR-4 activation and studies in murine models of atheroslcerosis have demonstrated that TLR-4 expression is increased in the early stages of foam cell formation and that lesion development is prevented with either deletion of TLR-2 or TLR-4 [39]. Using Cre/LoxP-mediated gene targeting, Polykratis et al., recently showed that endothelial-cell specific interference in TLR signaling improves atherosclerosis, whereas inhibition of TLR signaling in macrophages exacerbated vascular inflammation and lesion formation, thus highlighting the site-specific role of TLRs in vascular health. [40]. Lastly, studies in our laboratory recently revealed that inhibition of TLR-4 decreases blood pressure and arterial contractility in spontaneously hypertensive rats [41]. The above studies provide important insight into the frequent co-existence of cardiovascular and metabolic abnormalities.
Hypothesis: Toll-like receptors play a role in programming of hypertension and the metabolic syndrome
Components of the innate immune system, including TLRs, are present and functional very early in pregnancy. In addition to serving as the primitive artillery of the sterile womb, TLRs play a fundamental role in development. In fact, TLRs were originally identified as regulators of dorsal-ventral patterning in the fruit fly, Drosophilia melanogaster [42]. The influence of TLRs in organ hypertrophy and maturation which drive fetal growth in the second half of gestation is less characterized. However, the capacity of TLR activation to alter fetal development has been demonstrated by a number of studies using models of maternal infection whereby the TLR-4 agonist (lipopolysaccharide, LPS) is injected into the amniotic fluid. For instance, LPS administration induced inflammation and accelerated maturation of the ovine fetal lung [43] and in fetal mice led to severe cardiac dysfunction and cytokine activation in cardiomyocytes [44]. Still, the contribution of TLR signaling to non-infectious inflammatory responses and associated aberrations in organ development, under conditions of altered substrate availability, remains virtually unexplored. Nutritional insults of diverse nature transmit their deleterious effects on the fetus through oxidative and/or hypoxic placental injury. Thus, DAMPs mobilized by the placenta may serve to communicate the nutritional threat and direct the fetal inflammatory response. A recent study reported that maternal obesity leads to up-regulated expression of TLR-4/2 and NF-κB signaling in ovine fetal skeletal muscle [45]; to our knowledge, the first study to show TLR activation in fetal tissue in response to altered nutrient availability. Given the recent demonstration that ROS maintain fetal vascular tone under normal conditions [19], TLR activation in the fetal vasculature may contribute to hypoxic-induced hemodynamic adaptations through propagation of ROS generation. There is evidence to suggest that oxidative stress is present in the vasculature of fetuses growth restricted by oxygen deprivation [22]. Further, previous work conducted by one of the co-authors and colleagues, demonstrated that aberrant extracellular matrix remodeling in the aorta of hypoxic ovine fetuses was accompanied by intima hyperplasia and increased expression of cell adhesion molecules and pro-inflammatory cytokines [46]. Together, these data support the notion that oxidative stress and inflammation underlie aberrant arterial development in IUGR fetuses and it is possible that their synergistic activation is fed through TLR signaling.
Premature activation of TLR signaling in an attempt to overcome a prenatal challenge may not only render organ development receptive to the pro-inflammatory stimuli which are normally suppressed in healthy pregnancy, but may translate to abnormal signaling dynamics over time given that the pathway itself undergoes maturation in utero (Figure 2). Pro-inflammatory and pro-oxidant products of TLR may continuously generate DAMPs in a feed forward fashion, thus entrenching an unrestrained cycle of inflammation and oxidative stress in postnatal life. As mentioned above, there is strong evidence for an imbalance in antioxidant-pro-oxidant activity that persists in the vasculature of IUGR offspring [16,17]. Hence, the accelerated progression of arterial dysfunction and hypertension and associated risk for heart failure in offspring of complicated pregnancy, may be driven by enduring abnormalities in TLR signaling. In this light, hyper-active TLR responses may be responsible for the apparent vulnerability of IUGR offspring to a secondary insult. For instance, a recent study reported exaggerated remodeling and intima hyperplasia in response to arterial injury, in IUGR rat offspring compared to their normal birth weight counterparts [47]. Further, a study by Rueda-Clausen et al. demonstrated that IUGR later exacerbates the metabolic consequences of a high fat diet [48]. The same group showed that the heart of IUGR offspring is particularly sensitive to ischaemia/reperfusion (I/R) in adult life [49], while several studies have demonstrated that TLRs play a pivotal role in mediating cardiac injury under conditions of I/R [50]. Overall, TLRs represent a potential unifying factor in the constellation of phenotypes determined to have developmental origins. The involvement of TLRs in postnatal advancement of vascular disease may be evaluated through administration of TLR inhibitors in vivo. Such an approach has revealed the role of ROS in programmed hypertension, yet it is unknown whether TLRs underlie this festering oxidative stress. As well, characterizing the influence of TLRs on critical developmental events and the expression patterns of TLRs under various intrauterine insults would provide important information on the potential of TLRs as agents of programming.
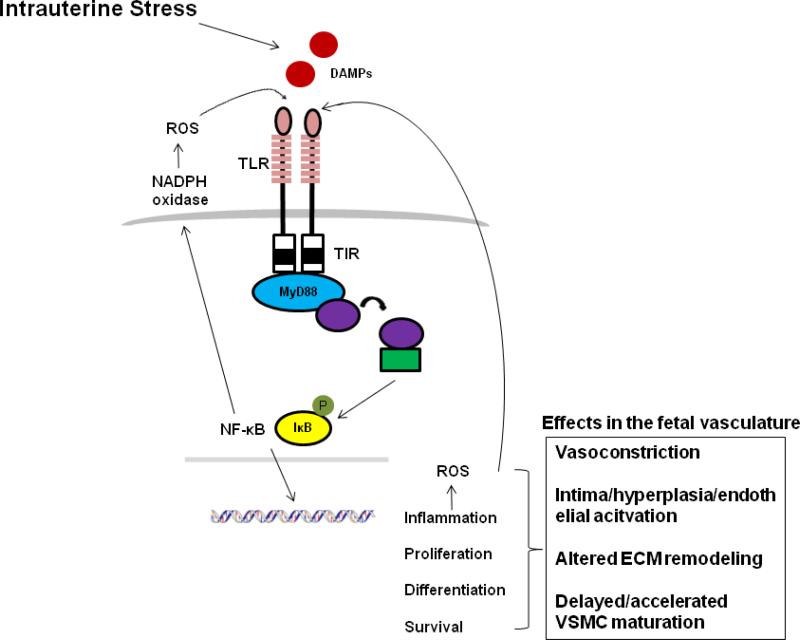
Figure 2.
Hypothesis. Various forms of intrauterine stress elicit the mobilization of DAMPs which direct fetal and placental responses through activation of TLRs of the innate immune system. TLR activation leads to the expression of genes involved in inflammation, cellular differentiation, proliferation and survival. TLR activation can account for all of the reported changes underlying programmed vascular dysfunction and hypertension, including vasoconstriction, intima hyperplasia, altered extracellular matrix (ECM) remodeling and altered phenotypic modulation of vascular smooth muscle cells (VSMC). Reactive oxygen species (ROS) are both products and inducers of TLR signaling and thus continual TLR activation during prolonged intrauterine stress may propagate the cycle of oxidative stress observed in IUGR fetuses and thereby exacerbate deleterious developmental aberrations.
Conclusion
Toll-like receptor signaling is an alluring, yet unexplored, candidate as a molecular mechanism of programmed hypertension and related metabolic disorders. Identifying the molecular underpinnings of developmental programming is essential for developing strategies aimed to decelerate or prevent the advancement of the metabolic syndrome in those born at risk. Such knowledge would facilitate an understanding of the utility and limitations of currently available lifestyle and medicinal interventions, as applied to this vulnerable population. Addressing intrauterine influences has become particularly salient with the realization that today's alarming rate of childhood hypertension and type II diabetes may be traced to poor maternal nutrition and weight control and the trans-generational transmission of programmed dysfunction. Taming the innate immune system may represent an effective strategy in enhancing health outcomes in infants surviving unfavourable intrauterine conditions.
Acknowledgments
Funding
NIH DK-83685, Society for Women's Health Research
Abbreviations
- DAMPS
damage associated molecular patterns
- ECM
extracellular matrix
- HMGB-1
high-mobility group protein B1
- IκB
inhibitor of kappa B
- IKK
IκB kinase
- IL-6
interleukin 6
- IL-1β
interleukin-1-beta
- IUGR
intrauterine growth restriction
- LPS
lipopolysaccharide
- LRRs
leucine rich repeats
- MAPK
mitogen-activated protein kinase
- MyD88
myeloid differentiation primary response gene (88)
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- PAMPS
pathogen associated molecular patterns
- PRRs
pathogen recognition receptors
- ROS
reactive oxygen species
- TIR
toll/interleukin-1 receptor
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-alpha
- VSMC
vascular smooth muscle cell
References
- 1.Bavdekar A, Yajnik C, Fall C, Bapat S, Pandit A, Deshpande V, Bhaye S, Kellingray S, Joglekar C. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years or both? Diabetes. 1999;48:2422–2429. doi: 10.2337/diabetes.48.12.2422. [DOI] [PubMed] [Google Scholar]
- 2.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JA, Regnault TRH. In utero origins of the metabolic syndrome. Semin. Reprod. Med. 2011;29(3):211–224. doi: 10.1055/s-0031-1275522. [DOI] [PubMed] [Google Scholar]
- 4.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity and gestational diabetes mellitus. Pediatrics. 2005;115:290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 5.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am. J. Epidemiol. 2007;165:849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 6.Hahn-Zoric M, Hagberg H, Kjellmer L, Ellis J, Wennergren M, Hanson LA. Aberrations in placental cytokine mRNA related to intrauterine growth retardation. Pediatr. Res. 2002;51(2):201–206. doi: 10.1203/00006450-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Heyborne KD, Witkin SS, McGregor JA. Tumor necrosis factor-alpha in midtrimester amniotic fluid is associated with impaired intrauterine fetal growth. Am. J. Obstet. Gynecol. 1992;167(4 Pt.1):920–925. doi: 10.1016/s0002-9378(12)80012-3. [DOI] [PubMed] [Google Scholar]
- 8.Lo Vasco VR, Cosmi R, Visentin S, Di Raimo T, Salmaso R, Zanardo V, Trevisanuto D, Businar R. IL-1β and IL-23 in amniotic fluids of ultrasound-detected aortic intima/media thickness and growth retardation. J. Reprod. Immunol. 2012;93:64–67. doi: 10.1016/j.jri.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Trudinger B, Wang J, Athayde N, Beutler L, Wang S. Association of umbilical placental vascular disease with fetal acute inflammatory cytokine responses. J. Soc. Gynecol. Investig. 2002;9(3):152–157. [PubMed] [Google Scholar]
- 10.Bertucci MC, Loose JM, Wallace EM, Jenkin G, Miller SL. Anti-inflammatory therapy in an ovine model of fetal hypoxia induced by single umbilical artery ligation. Reprod. Fertil. Dev. 2011;23(2):346–352. doi: 10.1071/RD10110. [DOI] [PubMed] [Google Scholar]
- 11.Guo R, Hou W, Dong Y, Yu Z, Stites J, Weiner CP. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod. Sci. 2010;17(6):540–548. doi: 10.1177/1933719110364061. [DOI] [PubMed] [Google Scholar]
- 12.Leduc L, Delvin E, Ouellet A, Garofalo C, Grenier E, Morin L, Dube J, Bouity-Voubou M, Moutquin JM, Fouron JC, Klam S, Levy E. Oxidized low-density lipoproteins in cord blood from neonates with intra-uterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;156:46–49. doi: 10.1016/j.ejogrb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Fujimaki A, Watanabe K, Mori T, Kimura C, Shinohara K, Wakatsuki A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta. 2011;32(5):367–372. doi: 10.1016/j.placenta.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010;59(12):3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J. Anat. 2009;215(1):27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohn A, Chiavaroli V, Cerruto M, Blasetti A, Giannini C, Bucciareli T, Chiarelli F. Increased oxidative stress in prepubertal children born small for gestational age. J. Clin. Endocrinol. Metab. 2007;92(4):1372–1378. doi: 10.1210/jc.2006-1344. [DOI] [PubMed] [Google Scholar]
- 17.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Oanh NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1236–R1245. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free. Radic. Biol. Med. 2007;43(7):1023–1036. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera A, Kane AD, Hansell JA, Thakor AS, Allison BJ, Niu Y, Giussani DA. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J. Physiol. 2012;590(Pt 8):1825–1837. doi: 10.1113/jphysiol.2011.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yzydorczyk C, Gobeil F, Jr., Cambonie G, Lahaie I, Le NL, Samarani S, Ahmad A, Lavoie JC, Oligny LL, Pladys P, Hardy P, Nuyt AM. Exaggerated vasomotor response to ANG II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(4):R1060–R1068. doi: 10.1152/ajpregu.00798.2005. [DOI] [PubMed] [Google Scholar]
- 21.Franco MCP, Akamine EH, Marco GSD, Casarini DE, Fortes ZB, Tostes RCA, Carvalho MHC, Nigro D. NADPH oxidase and enhanced superoxide generation in intrauterine undernourished rats: involvement of the rennin-angiotensin system. Cardiovas. Res. 2003;59:767–775. doi: 10.1016/s0008-6363(03)00461-9. [DOI] [PubMed] [Google Scholar]
- 22.Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM, Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PloS One. 2012;7(2):e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity:update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 25.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA. 2006;103:10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Israel A. The IKK complex, a central regulator of NF-κB activation. Cold. Spring. Harb. Perspect. Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matusmoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi W, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 32.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free. Radical. Biology. Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shembade N, Ma A, Harhaj EW. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33(4):861–888. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57(10):2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caricilli AM, Nascimento PH, Pauli JR, Tsukumo DML, Velloso LA, Carvalheira JB, Saad MJA. Inhibition of toll-like receptor 2 improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J. Endocrinol. 2008;199:399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 38.Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am.J.Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- 39.Cole JE, Georgiou E, Monaco C. The expression and functions of Toll-like receptors in atherosclerosis. Mediators. Inflamm. 2010;2010:393946. doi: 10.1155/2010/393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polykratis A, Loo GV, Xanthoulea S, Hellmich M, Pasparakis M. Conditional targeting of tumor necrosis factor receptor-associated factor 6 reveals opposing functions of Toll-like receptor signaling in endothelial and myeloid cells in a mouse model of atherosclerosis. Circulation. 2012;126:1739–1751. doi: 10.1161/CIRCULATIONAHA.112.100339. [DOI] [PubMed] [Google Scholar]
- 41.Bomfim GF, Dos Santos RA, Oliveira Ma., Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin. Sci. 2012;122(11):535–43. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. Thedorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potentantifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 43.Prince LS, Dieperink HI, Okoh VO, Fierro-Perez GA, Lallone RL. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev. Dyn. 2005;233:553–561. doi: 10.1002/dvdy.20362. [DOI] [PubMed] [Google Scholar]
- 44.Rounioja S, Rasanen J, Glumoff V, Ojaniemi M, Makikallio K, Hallman M. Intraamniotic lipopolysaccharide leads to fetal cardiac dysfunction. A mouse model for fetal inflammatory response. Cardiovasc. Res. 2003;60:156–64. doi: 10.1016/s0008-6363(03)00338-9. [DOI] [PubMed] [Google Scholar]
- 45.Yan X, Ahu MJ, Xu W, Tong JF, Ford SP, Nathanlelsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-κB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology. 2010;151:380–87. doi: 10.1210/en.2009-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson JA, Richardson BS, Gagnon R, Regnault TRH. Chronic intrauterine hypoxia interferes with aortic development in the late gestation ovine fetus. J. Physiol. 2011;589(Pt13):3319–3332. doi: 10.1113/jphysiol.2011.210625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menendez-Castro C, Cordasic N, Schmid M, Fahlbusch F, Rascher W, Amann K, Hilgers KH, Hartner A. Intrauterine growth restriction promotes vascular remodelling following carotid artery ligation in rats. Clin. Sci. 2012;123(7):437–444. doi: 10.1042/CS20110637. [DOI] [PubMed] [Google Scholar]
- 48.Reuda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JRB, Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60:507–516. doi: 10.2337/db10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc. Res. 2011;90(2):285–294. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 50.Mkaddem SB, Bens M, Vandewalle A. Differential activation of Toll-like receptor-mediated apoptosis induced by hypoxia. Oncotarget. 2010;1(8):741–750. doi: 10.18632/oncotarget.209. [DOI] [PMC free article] [PubMed] [Google Scholar]