Abstract
Purpose of review
Erectile dysfunction (ED) is recognized as a quality of life disorder that needs to be treated. Currently, it is estimated to affect as many as 30 million American men. Thirty percent of hypertensive patients complain of ED. The understanding of common mechanisms involved in the etiology of ED associated with hypertension, and the investigation of antihypertensive drugs that impact ED, will provide important tools toward indentifying new therapeutic targets that will improve the quality of life for patients in these conditions.
Recent findings
Hypertension and ED are closely intertwined diseases which have endothelium dysfunction as a common base. During hypertension and/or ED, disturbance of endothelium derived factors can lead to an increase in vascular smooth muscle (VSM) contraction. Hypertension can also lead to ED as a consequence of high blood pressure (BP) or due to antihypertensive treatment. However, growing evidence suggests ED as an early sign for hypertension. Also, some PDE-5 inhibitors used to treat ED can improve BP, but the link between these conditions has not been totally understood.
Summary
This review will discuss the interplay between hypertension and ED, exploring newest insights regarding hypertension-associated ED, as well as the effect of antihypertensive drugs in ED patients.
Keywords: Erectile dysfunction, hypertension, endothelial dysfunction, antihypertensive drugs
A-Introduction
Erectile dysfunction (ED) is a condition of increasing prevalence worldwide which has been estimated to affect 150 million individuals and is supposed to impact up to 50% of men between the ages of 40 and 70 (1). Also, it is expected that 322 million men will be suffering from ED by the year 2025 (2). ED and hypertension, a major factor for cardiovascular disease (CVD), are common conditions that possibly share pathophysiologic pathways. Compared to the general population, hypertensive patients have a higher prevalence of ED (3–4). However, an important point has been raised as to whether the higher prevalence of ED in those patients is the result of hypertension per se, of antihypertensive treatment, or as a combination of both. Also, studies in models of pre-clinical hypertension have suggested that high BP causes morphological modifications in the penile vascular bed, contributing to ED. Furthermore, much evidence suggests that vascular abnormalities such as endothelial dysfunction and atherosclerosis play a critical role in both conditions (3, 5). Importantly, the concept that ED is a prior indicator of CVD is growing stronger (6)*.
ED is often a disease of vascular origin. The penile endothelial bed is considered a specialized extension of the peripheral vascular system, responding similarly to various stimuli in order to maintain homeostasis, playing a particular regulatory role in the modulation of VSM tone which is crucial for normal erectile function (7)*. The small diameter of the cavernosal arteries plus the high content of endothelium and VSM may make the penile vascular bed a sensitive indicator of systemic vascular disease (8). Thus, the penis is a vascular organ that is sensitive to changes in oxidative stress and systemic NO levels. It is also sensitive to local modifications in the vasculature, making the penis an organ supposed to precede vascular systemic alterations. Therefore, ED has a higher incidence in patients with hypertension, a disease which it often precedes. In addition, atherosclerotic disease associated with hypertension can interrupt normal erectile physiology, and it has been proposed that ED is not only significantly correlated with but is also strongly predictive of subsequent atherosclerosis (9)*. Atherosclerosis is characterized by the cross-talk between excessive inflammation and lipid accumulation. Development of atherosclerosis and vascular inflammation involves Rho-kinase pathway, which the main function is the regulation of VSM tone (10)*. Also, Rho-kinase signaling is desregulated in hypertension, as well as ED. Finally, both conditions are interconnected by many common agents, such as endothelin (ET-1), angiotensin II (AngII) and reactive oxygen species (ROS), and its pathways.
B- Mechanisms of erection
ED is defined as the regular inability to reach or maintain a penile erection of sufficient quality to perform satisfactory sexual intercourse; it has been progressively associated with many comorbities. There are two main intracellular mechanisms for relaxing the cavernosal VSM leading to normal erectile function: the guanylate cyclase (GS)/cGMP and adenylate cyclase (AC)/cAMP pathways. Both pathways results in NO release, which is the main factor initiating erection. NO is produced by endothelial (eNOS) or neuronal (nNOS) nitric oxide synthase via acetylcholine or neuronal stimulation. Upon its release, NO diffuses locally into adjacent VSMC of the corpus cavernosum and binds to GC, which catalyzes the conversion of guanosine trisphosphate (GTP) to cGMP. Consequently, protein kinase G (PKG) is activated, leading to a decrease in cytosolic Ca2+ by various mechanisms. cGMP also blocks Rho-kinase activation. The decay in cytosolic Ca2+ concentration induces relaxation of the VSMC in the penis, leading to dilation of arterial vessels, increased blood flow into the corpora cavernosa, allowing penile erection. Contributing to penile relaxation and reduction of intracellular Ca2+, other substances activate the enzyme AC, leading to cAMP production, which in turn activates protein kinase A (PKA) (7). The erectile process is completely dependent on relaxation and intact endothelium function, which is also true for vascular homeostasis and normal BP maintenance. cGMP and cAMP levels are modulated by phosphodiesterase (PDE) enzymes, which cleave these signaling molecules to 5’GMP and 5’AMP, respectively. Phosphodisterase-5 (PDE-5) is a key enzyme in the NO/cGMP signal transduction pathway and functions to restrain VSM relaxation and thus the erectile process. Another mechanism involved in maintenance of the erectile process is the phosphatidylinositol 3-kinase (PI3-kinase) pathway that activates the serine/threonine protein kinase Akt (also known as PKB). PKB causes direct phosphorylation of eNOS, reducing the enzyme's Ca+2 requirement and causing increased production of NO. It has been suggested that rapid, brief activation of nNOS initiates the erectile process, whereas PI3-kinase/Akt-dependent phosphorylation and activation of eNOS by augmented blood flow and endothelial shear stress lead to sustained NO production and maximal erection (7).
C- ED in hypertension
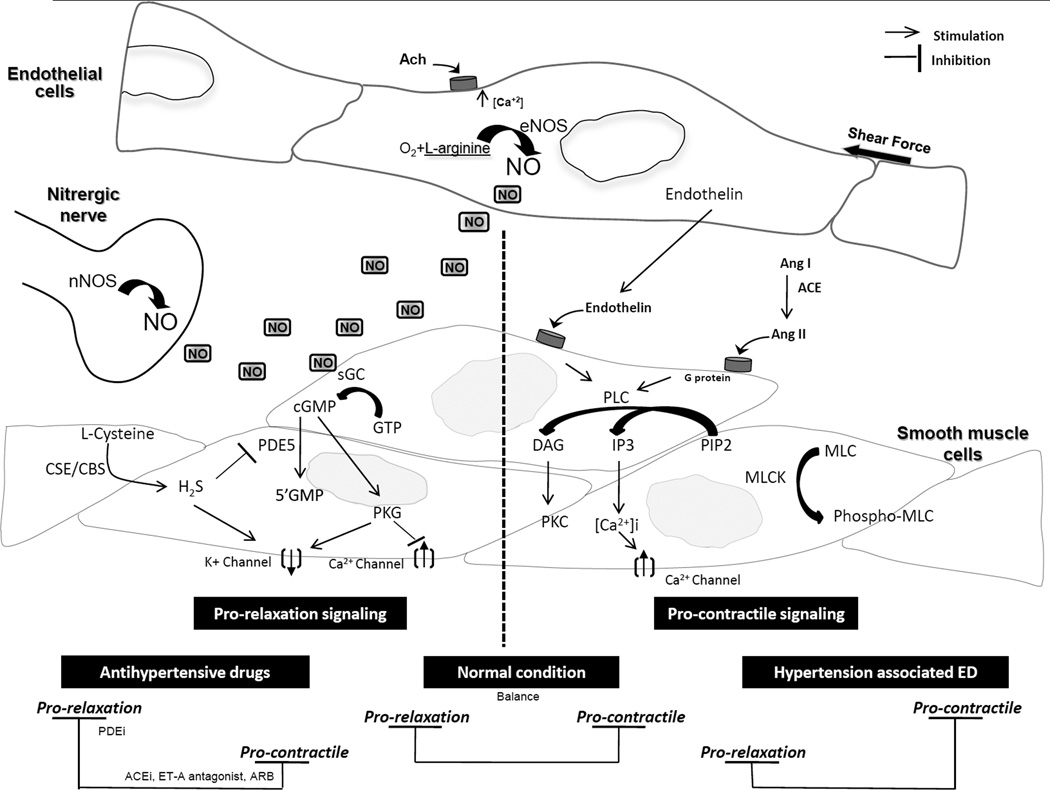
In the vasculature, as well in the penile tissue, circulating neurotransmitters, hormones and endothelium derived factors play a critical role in modulating the VSM tone (Figure 1). During hypertension, disturbance of these factors can lead to an increase in VSM tone which favors contraction.
Figure 1. Pathways involved in erectile function.
On the right, signaling pathways involved in mediating cavernosal smooth muscle cell (CSMC) relaxation. On the left, signaling pathway involved in mediating CSMC contraction. During normal conditions, there is a balance between pro-erectile and pro-relaxation signaling pathways resulting in a normal erectile function. During hypertension, there is an increase in pro-contractile signaling and/or decrease in pro-relaxation signaling resulting in increased contractility and decrease relaxation of CSMC, therefore, resulting in ED. Treatment with hypertensive drugs resulting in either increasing CSMC relaxation (i.e. PDEi), or decreasing CSMC contractility (i.e. ACEi), which will result in improvement of CSMC function and improving or restoring erectile function. Ach: acetylcholine, ACE: angiotensin converting enzyme, ACEi: ACE inhibitors, CBS: cystathionine-β-synthase, CSE: cystathionine gamma-lyase, DAG: diacylglycerol, H2S: hydrogen sulfide, IP3: inositol 1,4,5-trisphosphate, PIP2: phosphatidylinositol 4,5-bisphosphate, MLC: myosin light chain, MLCK: myosin light chain kinase, NO: nitric oxide, PLC: phospholipase C, PDE: phosphodiesterase, PDEi: PDE inhibitors.
1-Vasoconstrictors in hypertension and erectile function
Angiotensin II (AngII)
the predominate bioactive component of the renin-angiotensin system (RAS) plays a role in the maintenance of systemic BP through various mechanisms in the cardiovascular and renal systems. AngII also plays an important role in penile erection. However, an increase in circulating levels of AngII can lead to an increase vascular smooth muscle contraction and sodium retention, which contributes to the pathogenesis of hypertension. Some studies have shown that excessive production of AngII is associated with ED. AngII levels were also shown to be increased in the cavernous blood of men with ED as compared to healthy subjects (11–12). AngII exerts its biologic effect via activation of either one of its receptors: AT1 or AT2. While the AT2 receptor has been shown to modulate vasodilation (13), AT1 receptor activation is known to modulate vasoconstriction, induce VSM cell proliferation, inflammation, activation of sympathetic nervous system and aldosterone secretion. This is in addition to its effects on salt and water retention. In the penis, AngII modulates the tone of human penile arteries and trabecular VSM (14–15) *. AT1 receptor blockade was shown to improve erectile function (16). Jin et al. has shown that AngII infusion in rats caused ED through the activation of the NADPH oxidase, resulting in increased ROS (17). ROS are known to be detrimental to the endothelium and smooth muscle due to the direct scavenging of available NO necessary for vasorelaxation. ROS can also stimulate the RhoA/Rho-kinase pathway (11). Stimulation of the RhoA/Rho kinase pathway results in phosphorylation of the myosin-binding subunit of MLC phosphatase and inhibits its activity, thus promoting the phosphorylated state of MLC that leads to contraction and penile flaccidity.
Endothelin 1(ET-1)
ET-1 is an endothelium-derived peptide and one of the most potent endogenous vasoconstrictors. ET-1 cellular signaling in the vasculature is similar to that of AngII. In mammalian cells, ET-1 exerts its biologic effect through the activation of its receptors: ET-A and ET-B. The typical receptor found in the smooth muscle cells is ET-A receptor which mediates a vasoconstrictor effect. Penile VSM cells are able to synthesize ET-1 as well as respond to stimulation with ET-1(18). In addition, both ET-A and ET-B have been reported to be expressed in cavernosal tissue (19–22). In the penis, ET-1 induces corpus cavernosum vasoconstriction through the ETA receptor and subsequent activation of the inositol triphosphate (IP3) / Ca2+ signaling pathway (Ca2+sensitive) and the RhoA/Rho-kinase signaling pathway (Ca2+ independent) (23). On the other hand, ETB receptor activation was shown to induce vasodilation by means of NO release from the cavernosal endothelial cells (24).
In some forms of hypertension, ET-1 has been demonstrated to contribute to its pathogenesis i.e. mineralocorticoid hypertension. It was shown that salt-sensitive hypertensive animals display abnormal vascular responses to ET-1 as well as increased tissue ET-1 expression. As mentioned previously, ROS are produced during hypertension; these ROS have been shown to also stimulate ET-1 production by endothelial cells, producing a cycle leading to further increases in vasoconstriction. Concurrently, ET-1 was shown to increase ROS generation via NADPH oxidase activation. Data from our laboratory has shown that ET-1 mediates not only penile vasoconstriction, but also contraction of the internal pudendal artery, the major artery providing blood flow to the penis (22, 25). We have also revealed that the corpora cavernosum from DOCA-salt hypertensive rats exhibit increased contractile responses to ET-1when compared with their normotensive counterpart controls (22).
2-Morphological changes in hypertension and erectile dysfunction
As blood vessels undergo remodeling during hypertension, the penile tissue also undergoes morphological changes (26–27). During hypertension, increases in wall thickness and collagen deposition, with a decrease in lumen diameter are common structural changes which can affect the vasculature. Studies have shown that hypertension was associated with ED which might have resulted from a decrease in elastic fibers, increase of collagen fiber of the sinusoid, and a thinning of the tunica albugina in the penis of hypertensive animals. Hypertension was associated also with endothelial cells and VSM cells damage and degenerated Schwann cells (26). Also shown, an association of hypertension with increase vascular smooth muscle and cavernous VSM proliferation and fibrosis, moreover, hypertensive animals exhibited increase in surrounding connective tissue in the amyelinated nerves, suggesting that the increase in extracellular matrix seems to affect not only the interstitium but also the neural structure of the penis resulting in ED (28). In addition, the vascular changes in hypertension may affect the penile vasculature as well as the pudendal arteries resulting in a decrease in blood supply to the penis (29).
3-Vasodilators in hypertension and erectile dysfunction
ED can result from either an increase in the production of or an abnormal response to contractile stimuli such as AngII and ET-1, or from a decrease in production or response to stimuli that favor penile VSM relaxation. These are similar hallmarks of endothelial dysfunction, which is defined as a decreased responsiveness to vasodilatory mediators and an increased sensitivity to vasoconstrictor molecules. These abnormal responses to mediators can affect the normal regulatory role of peripheral vascular endothelium, including the cavernosal arterial and venous systems.
Nitric Oxide (NO)
During hypertension, there is a decrease in NO bioavailability, which may be due to decreased eNOS expression or uncoupling, as well as a decrease or impairment of the NO signaling pathway (30). The decrease in NO bioavailability can result from NO scavenging by ROS, of which is increased as result of NADPH activation and an alteration of intracellular antioxidant enzymes, including SOD (5, 17, 31). Other studies using hypertensive animal models also showed an impairment of relaxation mediated by neurogenic NO (32).
During hypertension, there is an imbalance between pro-oxidant and anti-oxidant mechanisms in the endothelial cells resulting in an increase in ROS generation. ROS are known to be very important in the pathophysiology of vascular disease. An interaction between ROS and NO has been implicated in many vascular diseases, such as atherogenesis and has been suggested to play an important role in ED (33). One of the most detrimental ROS is superoxide (O2−·), which interacts with NO decreasing NO bioavailability and resulting in the formation of peroxynitrite (ONOO−). In turn ONOO− was shown to cause damage to the mitochondria which may contribute to apoptotic and necrotic cell death (34). Although ONOO− was shown to mediate relaxation of rabbit corpus cavernosum, the relaxation to NO was short lived with the contractile tension returning to its original level, whereas relaxation to ONOO− was less potent, of a slower onset and more prolonged with tissues unable to recover tension (35). These will result in an ineffective relaxation in cavernosal tissue and may contribute to the pathogenesis of ED.
Hydrogen sulfide (H2S)
H2S deficiency contributes to hypertension. H2S is produced from L-cysteine through cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). CBS is mainly expressed in the brain, peripheral nervous system, liver and kidney, whereas CSE is mostly found in liver, vascular and nonvascular smooth cells. Pharmacological inhibition or genetic deletion of the gene responsible for H2S synthesis, CSE, resulted in hypertension and a decrease in endothelium- mediated relaxation (36). Recent studies have also shown that H2S can facilitate erectile function (37). Interestingly, a recent study revealed that human penile tissue possesses both CBS and CSE. Both enzymes were found to be localized in the muscular trabeculae and the smooth muscle component of penile artery. They also showed that human corpus cavernosum relaxes to exogenous H2S as well as L-cysteine in dose-dependent manner (38). Pharmacological inhibition of the H2S producing enzymes was shown to decrease intracavernosal pressure in non-human primates, as well as increase electric field stimulation-mediated contraction in human corpus cavernusom (38–39). The mechanisms through which H2S may mediate erection and penile smooth muscle relaxation are still not well understood and further investigation will be necessary to elucidate the role that H2S may play in erectile function and/or dysfunction (40–41)*. H2S represent a potential and promising therapeutic aimed for the treatment of ED.
D- Antihypertensive pharmacology
Despite the availability of more than 75 antihypertensive agents, blood pressure control in the general population is, at best, inadequate, making hypertension a public health problem with estimated direct and indirect costs of more than $93.5 billion per year (42). In addition, combination pharmacotherapy is often required in order to reach the currently recommended BP goals in the majority of hypertensive patients. Drugs used to treat hypertension are classified as: diuretics, sympathoplegic agents, direct vasodilators, angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptors antagonists (ARB). ED is considered a common side effect of various antihypertensive, such as central-acting, β blockers and diuretics (43). However, newer-generation antihypertensive drugs, for example calcium antagonists and ACE inhibitors, seem to have neutral effects (44). The first line of drugs for hypertension is β-blockers and diuretics. β-Blockers are a complex class of drugs involving several compounds that differ from each other by pharmacological characteristics, such as β1/β2 selectivity, intrinsic sympathomimetic activity and vasodilatory capacity. In general, they lower blood pressure by reducing cardiac output, inhibiting renin release, Ang II and aldosterone production, and decreasing adrenergic outflow from central nervous system (CNS) (45)*. β-Blockers are indicated as a treatment for hypertensive patients with some specific organ damage, but they have been outlined as one of the leading causes of drug-related ED (46). Diuretics act by depleting body sodium stores, which in the beginning causes a reduction in total blood volume, and consequently cardiac output. In turn, this initially causes an increase in peripheral vascular resistance, which is normalized after 6–8 weeks.
Direct vasodilators act by relaxing smooth muscle of arterioles and sometimes veins, reducing systemic vascular resistance. Some of them, such as hydralazine and minoxidil have rarely been reported to cause ED. Calcium channels blockers, which dilate arteries by reducing calcium influx into cells, effectively lower BP. The currently available ones inhibit l-type channels in humans and seem to have a neutral effect on erectile function (47). Possibly this is because this channel is linked with nNOS activation from cholinergic nerve endings into the penis, which is important for NO release and consequently erection. However, although this channel is inhibited, nNOS from nitrergic nerves will be activated, allowing the erectile process to begin. Another class of antihypertensive drugs is the ACE inhibitors. They act by mimicking the structure of the ACE substrate, directly blocking Ang II formation and at the same time increasing bradykinin levels. The net results are reduced vasoconstriction, decreased sodium and water retention, and increased vasodilation through bradykinin.
As a future antihypertensive treatment, there is theoretical evidence that gene therapy may produce long-lasting antihypertensive effects by influencing the genes associated with hypertension. Nevertheless, the treatment of human essential hypertension requires sustained over-expression of genes and identification of target genes can be a challenging task, which is necessary for successful results. Also, many other problems are encountered to reach the promising gene therapy. Among them is low efficiency for gene transfer into vascular cells, difficulties in determining how to prolong and control transgenic expression or antisense inhibition, lack of selectivity and minimization of adverse effects of viral vectors. Despite these concerns, animal studies showed that gene therapy may be feasible to treat human hypertension. Another possibility is the utilization of vaccines as a treatment, albeit not in the near future (48).
E- Treatment of ED in hypertensive patients
Although ED is often a result of hypertension, some studies have suggested that ED can also result from hypertension treatment. Whereas some studies did not find a significant influence, other suggested that anti-hypertension drugs, in particular, diuretics and β-blockers are associated with ED. Given that AngII plays an important role in the etiology of ED during hypertension, the use of ARB and ACE inhibitors were studied for their effects on erectile function. Animal studies showed that treatment with ARBs improves endothelial function in the cavernosal tissue. In humans, ARBs were shown to increase cavernosal relaxation mediated by NANC stimulation and sodium nitroprusside (15). Other studies have shown that treatment of hypertensive patients with ARBs resulted in improved sexual activity and erectile function. Whereas the use of ACE inhibitors in hypertensive animals showed improvement of erectile function, studies conducted in human showed neutral to negative effects on sexual activity (44).
Since it was shown that ET-1 is a potent vasoconstrictor of corporal SMCs, some studies have focused on ET-A antagonism and its effect on erectile function. Current studies did show a significant alteration of the erectile response despite inhibition of contraction to exogenous ET-1 using ET-1 receptor antagonists. ET-1 plays an important role in mineralocorticoid hypertension and is directly involved in end-organ damage, so ET-1 receptor blockade may provide a therapeutic approach to this hypertensive condition, or other clinical condition where ET-1 levels are increased such as in diabetes and systemic sclerosis (49)*.
The phosphodiesterase type 5 inhibitors (PDE5I) are approved treatment for ED. PDE5I act by inhibiting PDE5, the enzyme that catalyses the breakdown of the second messenger cGMP. cGMP is the downstream effector in the NO signaling pathway, so, PDE5I are dependent upon the integrity of the NO generating system. In patients where the NO pathway is compromised, the benefits of such drugs will be lower when compared to others. The PDE5I are used also for the treatment of pulmonary hypertension (PAH). However, patients treated with nitrates or nitrate-donors or vasodilators such as α-blockers should not take PDE5I, otherwise, combining these drugs will result in hypotension (50).
Treatment with β-blockers is considered to cause ED. In fact, studies have shown that treatment of hypertensive patients with β-blockers is associated with sexual dysfunction and impotence. However, the new generation β-blocker nebivolol may have positive effects on erectile function. Recent studies showed that nebivolol treatment improves endothelial function and reverses ED in animals through the activation of the NO/cGMP pathway (51). In humans, nebivolol was shown to dilate penile arteries and treatment of hypertensive patients with nebivolol significantly improved erectile function (44). More studies of the new generation of the β-blockers are necessary to elucidate their effects on the erectile function. Another treatment that was shown to be associated with ED is diuretic therapy. Treatment of patients with diuretics alone or with diuretics added to their therapy was shown to worsen sexual dysfunction (44).
Conclusion
A common denominator in hypertension-associated ED is endothelial dysfunction. Even though ED and hypertension are both vascular diseases, various aspects regarding the disruption and modifications in the pathways (Figure 1) leading to hypertension and ED remain to be elucidated. However, it is undeniable that many of the factors leading to hypertension also have contributed significantly to the ED process, and the opposite is also true. Also, ED can be a condition prior to hypertension or antihypertensive drug associated. It is expected that pathophysiological modifications resulting in ED can be more useful in the future to predict systemic vascular disorders such as hypertension.
Key Points.
Is ED an early sign for hypertension?
Hypertension and ED are related diseases with a common etiology: endothelium dysfunction
Are antihypertensive drugs a solution for hypertension and yet a problem for sexual function?
Could ED be used to predict systemic vascular disorders such as hypertension?
Acknowledgments
This work was supported by NIH and Dr. Nunes is supported by AHA.
Footnotes
The authors declare no conflict of interest.
References
- 1.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000 Feb;163(2):460–463. [PubMed] [Google Scholar]
- 2.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999 Jul;84(1):50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005 Dec 21;294(23):2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 4.Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000 Oct;164(4):1188–1191. [PubMed] [Google Scholar]
- 5.Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med. 2009 Sep;6(9):2390–2404. doi: 10.1111/j.1743-6109.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 6.Shin D, Pregenzer G, Jr, Gardin JM. Erectile dysfunction: a disease marker for cardiovascular disease. Cardiol Rev. 2011 Jan-Feb;19(1):5–11. doi: 10.1097/CRD.0b013e3181fb7eb8. [DOI] [PubMed] [Google Scholar]
- 7.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010 Jan;7(1 Pt 2):445–475. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 8.Billups KL. Erectile dysfunction as an early sign of cardiovascular disease. Int J Impot Res. 2005 Dec;17(Suppl 1):S19–S24. doi: 10.1038/sj.ijir.3901425. [DOI] [PubMed] [Google Scholar]
- 9.Chew KK, Finn J, Stuckey B, Gibson N, Sanfilippo F, Bremner A, et al. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med. 2010 Jan;7(1 Pt 1):192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- 10.Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci. 2010 Nov;67(22):3823–3836. doi: 10.1007/s00018-010-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin LM. Angiotensin II signaling and its implication in erectile dysfunction. J Sex Med. 2009 Mar;6(Suppl 3):302–310. doi: 10.1111/j.1743-6109.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 12.Becker AJ, Uckert S, Stief CG, Scheller F, Knapp WH, Hartmann U, et al. Plasma levels of angiotensin II during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology. 2001 Nov;58(5):805–810. doi: 10.1016/s0090-4295(01)01312-7. [DOI] [PubMed] [Google Scholar]
- 13.Wynne BM, Chiao CW, Webb RC. Vascular Smooth Muscle Cell Signaling Mechanisms for Contraction to Angiotensin II and Endothelin-1. J Am Soc Hypertens. 2009 Mar-Apr;3(2):84–95. doi: 10.1016/j.jash.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comiter CV, Sullivan MP, Yalla SV, Kifor I. Effect of angiotensin II on corpus cavernosum smooth muscle in relation to nitric oxide environment: in vitro studies in canines. Int J Impot Res. 1997 Sep;9(3):135–140. doi: 10.1038/sj.ijir.3900261. [DOI] [PubMed] [Google Scholar]
- 15.Ertemi H, Mumtaz FH, Howie AJ, Mikhailidis DP, Thompson CS. Effect of angiotensin II and its receptor antagonists on human corpus cavernous contractility and oxidative stress: modulation of nitric oxide mediated relaxation. J Urol. 2011 Jun;185(6):2414–2420. doi: 10.1016/j.juro.2011.02.2645. [DOI] [PubMed] [Google Scholar]
- 16.Yang R, Yang B, Wen Y, Fang F, Cui S, Lin G, et al. Losartan, an Angiotensin type I receptor, restores erectile function by downregulation of cavernous renin-angiotensin system in streptozocin-induced diabetic rats. J Sex Med. 2009 Mar;6(3):696–707. doi: 10.1111/j.1743-6109.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. NADPH oxidase activation: a mechanism of hypertension-associated erectile dysfunction. J Sex Med. 2008 Mar;5(3):544–551. doi: 10.1111/j.1743-6109.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- 18.Granchi S, Vannelli GB, Vignozzi L, Crescioli C, Ferruzzi P, Mancina R, et al. Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells. Mol Hum Reprod. 2002 Dec;8(12):1053–1064. doi: 10.1093/molehr/8.12.1053. [DOI] [PubMed] [Google Scholar]
- 19.Saenz de Tejada I, Carson MP, de las Morenas A, Goldstein I, Traish AM. Endothelin: localization, synthesis, activity, and receptor types in human penile corpus cavernosum. Am J Physiol. 1991 Oct;261(4 Pt 2):H1078–H1085. doi: 10.1152/ajpheart.1991.261.4.H1078. [DOI] [PubMed] [Google Scholar]
- 20.Holmquist F, Kirkeby HJ, Larsson B, Forman A, Alm P, Andersson KE. Functional effects, binding sites and immunolocalization of endothelin-1 in isolated penile tissues from man and rabbit. J Pharmacol Exp Ther. 1992 May;261(2):795–802. [PubMed] [Google Scholar]
- 21.Dai Y, Pollock DM, Lewis RL, Wingard CJ, Stopper VS, Mills TM. Receptor-specific influence of endothelin-1 in the erectile response of the rat. Am J Physiol Regul Integr Comp Physiol. 2000 Jul;279(1):R25–R30. doi: 10.1152/ajpregu.2000.279.1.R25. [DOI] [PubMed] [Google Scholar]
- 22.Carneiro FS, Giachini FR, Lima VV, Carneiro ZN, Nunes KP, Ergul A, et al. DOCA-salt treatment enhances responses to endothelin-1 in murine corpus cavernosum. Can J Physiol Pharmacol. 2008 Jun;86(6):320–328. doi: 10.1139/Y08-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingard CJ, Husain S, Williams J, James S. RhoA-Rho kinase mediates synergistic ET-1 and phenylephrine contraction of rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2003 Nov;285(5):R1145–R1152. doi: 10.1152/ajpregu.00329.2003. [DOI] [PubMed] [Google Scholar]
- 24.Ari G, Vardi Y, Hoffman A, Finberg JP. Possible role for endothelins in penile erection. Eur J Pharmacol. 1996 Jun 20;307(1):69–74. doi: 10.1016/0014-2999(96)00172-0. [DOI] [PubMed] [Google Scholar]
- 25.Allahdadi KJ, Hannan JL, Tostes RC, Webb RC. Endothelin-1 induces contraction of female rat internal pudendal and clitoral arteries through ET(A) receptor and rho-kinase activation. J Sex Med. 2010 Jun;7(6):2096–2103. doi: 10.1111/j.1743-6109.2010.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang R, Chen JH, Jin J, Shen W, Li QM. Ultrastructural comparison of penile cavernous tissue between hypertensive and normotensive rats. Int J Impot Res. 2005 Sep-Oct;17(5):417–423. doi: 10.1038/sj.ijir.3901329. [DOI] [PubMed] [Google Scholar]
- 27.Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, et al. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005 Jan;288(1):R276–R283. doi: 10.1152/ajpregu.00040.2004. [DOI] [PubMed] [Google Scholar]
- 28.Toblli JE, Stella I, Inserra F, Ferder L, Zeller F, Mazza ON. Morphological changes in cavernous tissue in spontaneously hypertensive rats. Am J Hypertens. 2000 Jun;13(6 Pt 1):686–692. doi: 10.1016/s0895-7061(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 29.Hale TM, Hannan JL, Carrier S, deBlois D, Adams MA. Targeting vascular structure for the treatment of sexual dysfunction. J Sex Med. 2009 Mar;6(Suppl 3):210–220. doi: 10.1111/j.1743-6109.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 30.Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep. 2010 Aug;12(4):267–275. doi: 10.1007/s11906-010-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ushiyama M, Kuramochi T, Yagi S, Katayama S. Antioxidant treatment with alpha-tocopherol improves erectile function in hypertensive rats. Hypertens Res. 2008 May;31(5):1007–1113. doi: 10.1291/hypres.31.1007. [DOI] [PubMed] [Google Scholar]
- 32.Ushiyama M, Morita T, Kuramochi T, Yagi S, Katayama S. Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens Res. 2004 Apr;27(4):253–261. doi: 10.1291/hypres.27.253. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006 May-Jun;27(3):335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 34.Packer MA, Scarlett JL, Martin SW, Murphy MP. Induction of the mitochondrial permeability transition by peroxynitrite. Biochem Soc Trans. 1997 Aug;25(3):909–914. doi: 10.1042/bst0250909. [DOI] [PubMed] [Google Scholar]
- 35.Khan MA, Thompson CS, Mumtaz FH, Mikhailidis DP, Morgan RJ, Bruckdorfer RK, et al. The effect of nitric oxide and peroxynitrite on rabbit cavernosal smooth muscle relaxation. World J Urol. 2001 Jun;19(3):220–224. doi: 10.1007/s003450000162. [DOI] [PubMed] [Google Scholar]
- 36.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008 Oct 24;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srilatha B, Adaikan PG, Li L, Moore PK. Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med. 2007 Sep;4(5):1304–1311. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 38.d'Emmanuele di Villa Bianca R, Sorrentino R, Maffia P, Mirone V, Imbimbo C, Fusco F, et al. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4513–4518. doi: 10.1073/pnas.0807974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srilatha B, Adaikan PG, Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction--a pilot study. Eur J Pharmacol. 2006 Mar 27;535(1–3):280–282. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Liaw RL, Srilatha B, Adaikan PG. Effects of hydrogen sulfide on erectile function and its possible mechanism(s) of action. J Sex Med. 2011 Jul;8(7):1853–1864. doi: 10.1111/j.1743-6109.2011.02279.x. [DOI] [PubMed] [Google Scholar]
- 41.d'Emmanuele di Villa Bianca R, Sorrentino R, Mirone V, Cirino G. Hydrogen sulfide and erectile function: a novel therapeutic target. Nat Rev Urol. 2011 May;8(5):286–289. doi: 10.1038/nrurol.2011.45. [DOI] [PubMed] [Google Scholar]
- 42.Vital signs: prevalence, treatment, and control of hypertension--United States 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011 Feb 4;60(4):103–108. [PubMed] [Google Scholar]
- 43.Papatsoris AG, Korantzopoulos PG. Hypertension, antihypertensive therapy, and erectile dysfunction. Angiology. 2006 Jan-Feb;57(1):47–52. doi: 10.1177/000331970605700107. [DOI] [PubMed] [Google Scholar]
- 44.Doumas M, Douma S. The effect of antihypertensive drugs on erectile function: a proposed management algorithm. J Clin Hypertens (Greenwich) 2006 May;8(5):359–364. doi: 10.1111/j.1524-6175.2005.05285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Caterina AR, Leone AM. The role of Beta-blockers as first-line therapy in hypertension. Curr Atheroscler Rep. 2011 Apr;13(2):147–153. doi: 10.1007/s11883-010-0157-9. [DOI] [PubMed] [Google Scholar]
- 46.Cordero A, Bertomeu-Martinez V, Mazon P, Facila L, Bertomeu-Gonzalez V, Conthe P, et al. Erectile dysfunction in high-risk hypertensive patients treated with beta-blockade agents. Cardiovasc Ther. 2010 Spring;28(1):15–22. doi: 10.1111/j.1755-5922.2009.00123.x. [DOI] [PubMed] [Google Scholar]
- 47.Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens (Greenwich) 2011 Sep;13(9):687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Israili ZH, Hernandez-Hernandez R, Valasco M. The future of antihypertensive treatment. Am J Ther. 2007 Mar-Apr;14(2):121–134. doi: 10.1097/01.pap.0000249915.12185.58. [DOI] [PubMed] [Google Scholar]
- 49.Ritchie R, Sullivan M. Endothelins & erectile dysfunction. Pharmacol Res. 2011 Jun;63(6):496–501. doi: 10.1016/j.phrs.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Montani D, Chaumais MC, Savale L, Natali D, Price LC, Jais X, et al. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther. 2009 Sep;26(9):813–825. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 51.Angulo J, Wright HM, Cuevas P, Gonzalez-Corrochano R, Fernandez A, Cuevas B, et al. Nebivolol dilates human penile arteries and reverses erectile dysfunction in diabetic rats through enhancement of nitric oxide signaling. J Sex Med. 2010 Aug;7(8):2681–2697. doi: 10.1111/j.1743-6109.2010.01710.x. [DOI] [PubMed] [Google Scholar]