Abstract
Vascular smooth muscle cell (VSMC) apoptosis and collagen synthesis contributes to aortic stiffening. A cellular signaling mechanism contributing to apoptotic and fibrotic events is endoplasmic reticulum (ER) stress. In this study we tested the hypothesis that induction of ER stress in a normotensive rat would cause pro-fibrotic and apoptotic signaling contributing to aortic stiffening. Furthermore, we hypothesized that inhibition of ER stress in an angiotensin II (Ang II) model of hypertension would improve aortic stiffening. Induction of ER stress with tunicamycin (TM) in normotensive Sprague Dawley rats (SD, 10 µg/kg/day, osmotic pump, 28 days) caused an increase in systolic blood pressure (mmHg; 160 ± 5) compared to vehicle-treated (127 ± 3) or TM-treated rats that were co-treated with ER stress inhibitor 4-phenylbutyic acid (PBA, 100 mg/kg/day, 28 days, (124 ± 6)). There was an increase in aortic apoptosis (fold; 3.0±0.3), collagen content (1.4±0.1) and fibrosis (2.0±0.1) in the TM-treated rats compared to vehicle-treated rats. Inhibition of ER stress in male SD rats given Ang II (60 ng/min, osmotic pump, 28 days) and treated with either tauroursodeoxycholic acid (TUDCA) or PBA (100 mg/kg/day, i.p., 28 days) led to a 20 mmHg decrease in blood pressure with either inhibitor, compared to Ang II treatment alone. Aortic apoptosis, increased collagen content and fibrosis in Ang II-treated rats were attenuated with ER stress inhibition. We conclude that ER stress is a new signaling mechanism contributing to aortic stiffening via promoting apoptosis and fibrosis.
Keywords: ER stress, vascular smooth muscle, aortic stiffness, fibrosis, apoptosis
1. Introduction
The endoplasmic reticulum (ER) is responsible for the integration of diverse intracellular signaling events. The ER is a key site where proteins are synthesized, folded and prepared for trafficking. A disruption in ER folding capacity which occurs following a variety of cellular stresses (oxidative, inflammatory and energy/calcium depletion) leads to the misfolding and aggregation of proteins within the ER lumen: a process known as ER stress. Following ER stress there is an initiation of the unfolded protein response (UPR), a complex signaling network, which acts through three main signaling pathways; protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1 (IRE1) and activating transcription factor 6 (ATF6)1. Short term ER stress activates adaptive, pro-survival signaling leading to the upregulation of ER chaperones, attenuation of translation and activation of ER-associated degradation of proteins in an attempt to restore ER homeostasis. However, prolonged ER stress, which is a feature of many cardiovascular diseases, causes the UPR to switch from a pro-survival signaling network into a pro-apoptotic pathway2.
Aortic stiffening is associated with increased vascular smooth muscle cell (VSMC) proliferation, migration and apoptosis, as well as, increased fibrosis3. While cell proliferation and migration have been extensively studied, apoptosis is becoming recognized as playing a major role in vascular stiffening4. Apoptosis within the aortic wall is critical in determining aortic structure and while beneficial in the early stages of stiffening, it later becomes detrimental. An interplay exists between apoptotic VSMCs and collagen synthesis where apoptotic VSMCs have been shown to promote collagen synthesis5. Increased collagen deposition and synthesis are critical contributors to aortic fibrosis6. Recent evidence suggests ER stress is involved in cardiac damage via an increase in apoptosis and fibrosis in hypertensive mice7. Indeed, in osteoblasts and gingival fibroblasts ER stress has been shown to trigger collagen synthesis8, 9, but it is unknown if ER stress can increase VSMC collagen synthesis, thereby, contributing to aortic fibrosis.
Therefore, the hypothesis of this study was ER stress contributes to aortic stiffening via increasing VSMC apoptosis and collagen synthesis. To elucidate the role of ER stress, a drug that induces ER stress, tunicamycin (TM), should cause a “pro-fibrotic”-like phenotype by increasing aortic VSMC apoptosis and collagen synthesis. Consequently, inhibition of ER stress through the use of chemical chaperones, tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (PBA) that work by increasing ER folding capacity10, 11 in an angiotensin II (Ang II) model of hypertension will attenuate apoptosis, collagen synthesis and improve aortic function.
2. Methods
Experimental Animals
Male Sprague-Dawley rats (SD, 12wks old, Harlan Laboratories) were used in these studies. They were maintained on a 12:12 hour light-dark cycle with both rat chow and water ad libitium. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee of the Georgia Regents University.
Rats were anesthetized via nose cone for mini-pump implantation with isoflurane at an initial concentration of 5% and then maintained at 2.5% in 100% oxygen. The anesthesia was verified by toe pinch and noting the absence of any physical movement. Osmotic mini pumps (28 days- model 2004, Alzet Co.) were implanted subcutaneously below the neck. For pharmacological induction of ER stress animals were divided into three groups: control group receiving sham surgery and injections of saline (vehicle, i.p., 28 days) (n=10) and two groups receiving tunicamycin (TM, 10 µg/kg/day, 28 days, osmotic pump, n=11) and saline (250 µl, i.p., 28 days) or injections of 4-phenylbutyric acid (PBA, 100mg/kg/day, i.p., 28 days) these doses were chosen based on previous reports12. For pharmacological inhibition of ER stress in a model of Ang II-induced hypertension, animals were divided into four groups: control group receiving sham surgery (n=9), angiotensin II group receiving (Ang II 60ng/min, osmotic pump) and phosphate buffered saline (PBS, 250 µl, i.p., 28 days, n=9), angiotensin II group receiving (Ang II 60ng/min, osmotic pump) and tauroursodeoxycholic acid (TUDCA,100mg/kg/day, i.p., n=9) and angiotensin II group receiving (Ang II 60ng/min, osmotic pump) and PBA (100mg/kg/day, i.p., n=9) doses that have been reported in the literature to decrease ER stress13–15.
For detailed methods on blood pressure measurements, vascular function, western blot, immunohistochemical studies, serum creatinine measurements and chemicals please see the online-only Data Supplement.
Data Analysis
The data are shown as mean ± standard error of the mean (SEM) and “n” represents the number of rats used in the experiments. Contractions are noted as changes in force (mN) from baseline. Relaxation is expressed as percent change from the PE contracted levels. Concentration-response curves were fitted using a nonlinear regression program (Graph Pad Prism 5.0; GraphPad Software Inc, San Diego, CA). The length-tension curve was fitted by a linear equation, the slope relates directly to the stiffness. Statistical analysis was performed using two-way analysis of variance with Bonferroni post hoc analysis to compare the responses between all groups. P values of less than 0.05 were taken as being statistically significant.
3. Results
Effect of ER stress induction on blood pressure
At the end of the treatment the rats treated with TM had a lower body weight ((grams) 315 ± 11) compared to the vehicle (397 ± 4) or TM-treated rats given PBA (398 ± 4.5). Serum Creatinine levels were increased in the TM-treated rats ((mg/dl) 2.0 ± 0.1) compared to vehicle or TM-treated rats given PBA (0.78 ± 0.2; 0.64 ± 0.1; respectively). The systolic blood pressure was elevated in the TM-treated rats (mmHg; 160 ± 5) compared to vehicle-treated (127 ± 3) or TM rats treated with PBA (124 ± 6).
Effect of ER stress induction on ER stress and apoptotic marker expression in the aorta
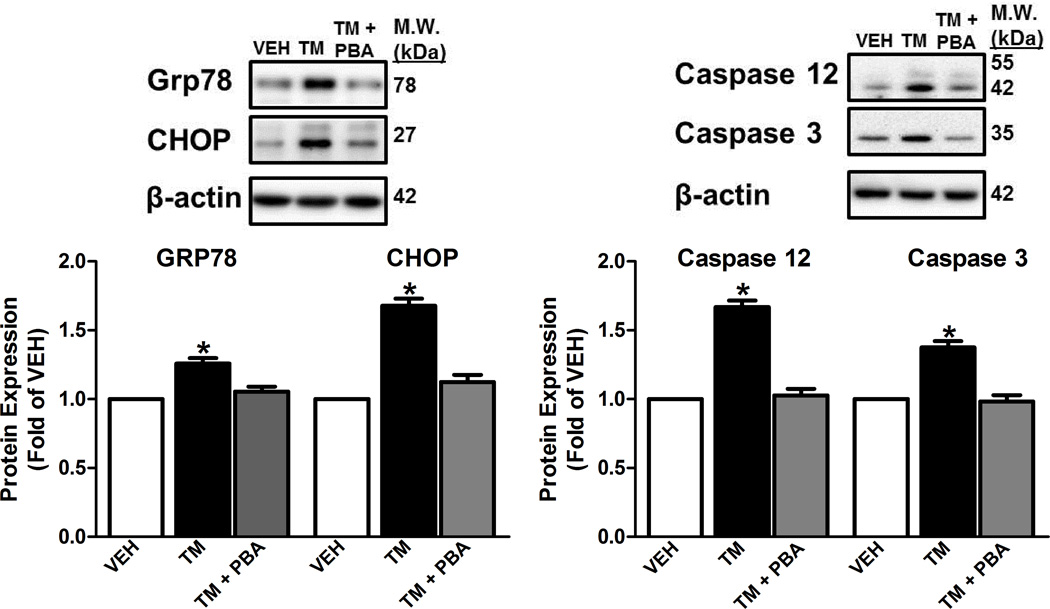
We observed increased expression of ER stress markers: 78 kDa glucose-regulated protein (GRP78), an ER protein chaperone and CCAAT/-enhancer-binding protein homologous protein (CHOP), a pro-apoptotic transcription factor in the aorta from TM-treated rats compared to the vehicle-treated animals or TM-treated rats given PBA (Figure 1A). Furthermore, prolonged ER stress is widely linked to apoptosis and in the aorta of TM-treated animals there was increased expression of cleaved Caspase 12 and Caspase 3 and this was abolished when animals were TM-treated animals were given PBA (Figure 1B).
Figure 1.
Induction of ER stress increases expression of aortic ER stress and apoptotic protein markers. A, Top, representative images of ER stress markers, GRP78 and CHOP. Bottom, Densitometry analysis. B, Top, representative images of immunoblots of Caspase 12 and Caspase 3. Bottom, Densitometry analysis. *P < 0.05 versus Vehicle- treated rats. n = 4–12.
Effect of ER stress induction on aortic apoptosis, fibrosis and collagen content the aorta
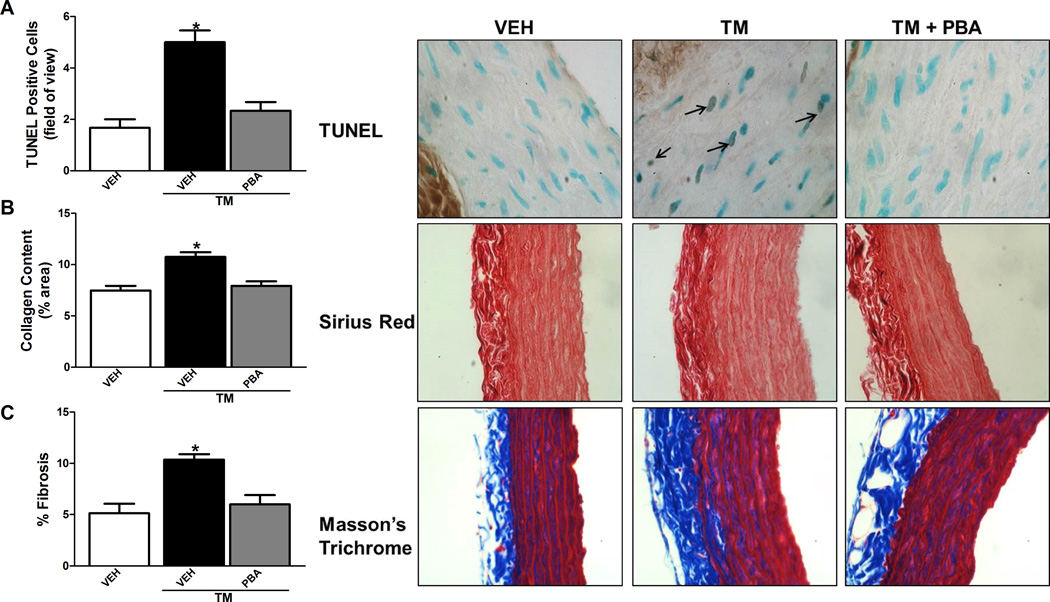
There was an increase in the number of TUNEL positive nuclei from aortic sections of the TM-treated rats compared to vehicle-treated rats which was prevented when TM-treated rats received PBA (Figure 2A). Interestingly, TM treatment caused an increase in collagen content and fibrosis in the aortic sections compared to vehicle-treated rats which was prevented with PBA administration (Figure 2B and C). Subsequently there was an increase in matrix metalloproteinase 2 (MMP2) activity in the aorta of the TM-treated rats compared to vehicle or TM-treated rats given PBA (S1).
Figure 2.
Induction of ER stress increased TUNEL positive nuclei, collagen content and fibrosis in the aorta. A, Top, representative TUNEL staining of aortic sections (100× magnification). Bottom, analysis of positive TUNEL stained cells per field of view. B, Top, representative Sirius Red staining of aortic sections (40× magnification). Bottom, analysis of % Sirius Red stain per total area. C, Top, representative Masson’s Trichrome staining of aortic sections (40× magnification). Bottom, analysis of % Masson’s Trichrome stain per total area. *p < 0.05 versus Vehicle-treated rats. n= 4–10.
Effect of ER stress inhibition on aortic contraction, relaxation and compliance
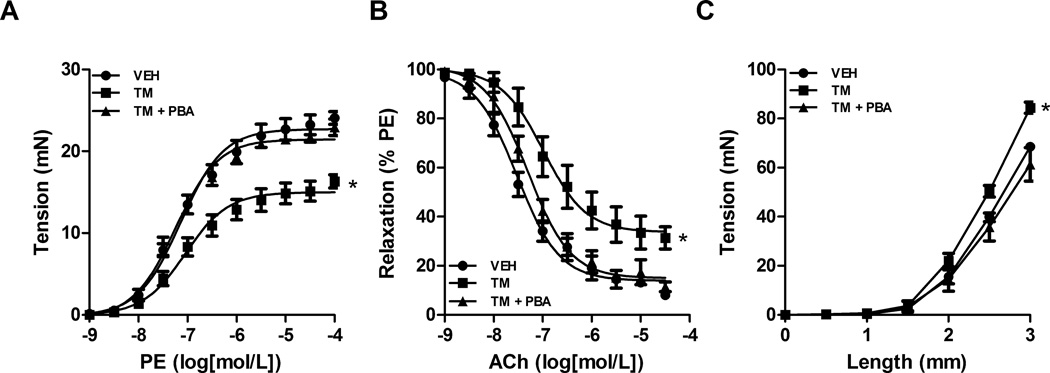
To assess ER stress induction on functional changes in the aorta vascular contractility, relaxation and compliance was assessed. Maximum contraction to phenylephrine (PE) was reduced in aortic segments from TM-treated animals (mN; 16 ± 1.2) compared to vehicle (24 ± 2.3) and this was prevented with PBA treatment (22 ± 2.1) (Figure 3A). Maximum relaxation to acetylcholine (ACh) in aortic segments from TM-treated rats was impaired (% relaxation; 31.5 ± 4.5) compared to vehicle-treated rats (8.1 ± 6.3) and TM-treated rats receiving PBA (11.1 ± 5.2) (Figure 3B). As a measurement of aortic compliance a length-tension curved was performed. A leftward shift in the maximum tension development following increasing the length of the aortic ring was seen in the aorta from TM-treated rats (mN; 84.4 ± 3.5) compared to vehicle-treated rats (61.2 ± 1.1) rats which was attenuated when TM-treated rats were given PBA (68.5 ± 2.7) (Figure 3C).
Figure 3.
Induction of ER stress decreases vasoconstriction, impairs relaxation and causes loss of compliance in the aorta. Concentration-response curves were performed in aortic rings to A, phenylephrine (PE, 1 nM – 10 µM) or B, acetylcholine (ACh, 1 nM – 10 µM). C, a length-tension curve was performed in aortic rings. *P < 0.05 versus Vehicle-treated rats. n= 4–8.
Effect of ER stress inhibition on blood pressure in the Ang II-induced hypertensive rat
No significant differences were observed in body weight amongst groups ((grams) vehicle, 407 ± 8; Ang II, 390 ± 8; Ang II TUDCA, 392 ± 7; Ang II PBA, 396 ± 5). Serum creatinine levels were increased in the Ang II-treated rats ((mg/dl) 1.79 ± 0.1) compared to vehicle-treated, TUDCA or PBA treated Ang II rats (0.77 ± 0.06; 0.61 ± 0.1; 0.78 ± 0.1; respectively). The systolic blood pressure was elevated in the Ang II treated group (mmHg; 186.5±3) compared to vehicle treated group (125±5). Additionally, a decrease in systolic blood pressure was seen in the TUDCA (165±3) and PBA (167±4) treated Ang II rats.
Effect of ER stress inhibition on ER stress and apoptotic marker expression in the Ang II-induced hypertensive rat
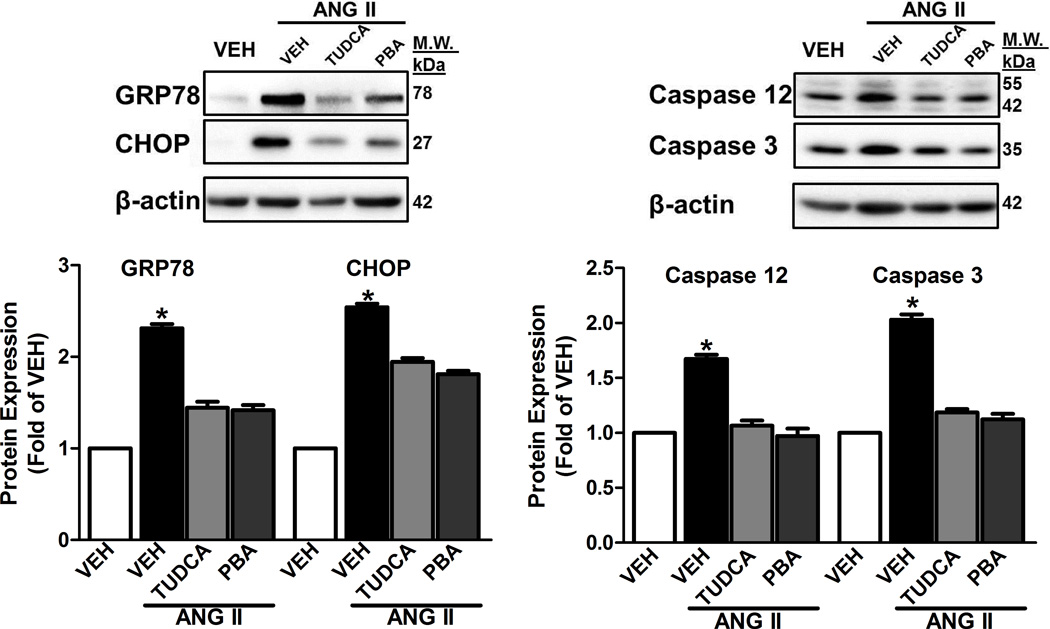
Increased expression of ER stress markers GRP78 and CHOP in the aorta from Ang II alone-treated rats was attenuated in the aorta from Ang II groups receiving either TUDCA or PBA (Figure 4A). Increased expression of aortic cleaved Caspase 12 and Caspase 3 in the Ang II alone-treated rats was abolished in aorta from the Ang II rats treated with either TUDCA or PBA (Figure 4B).
Figure 4.
ER stress inhibition decreased aortic ER stress and apoptotic marker expression in Ang II-induced hypertensive rats. A, Top, representative images of ER stress markers, GRP78 and CHOP. Bottom, Densitometry analysis. B, Top, representative images of immunoblots of Caspase 12 and Caspase 3. Bottom, Densitometry analysis. *P < 0.05 versus Vehicle- treated rats. n = 8–10.
Effect of ER stress inhibition on aortic apoptosis, fibrosis and collagen content in the Ang II hypertensive rat
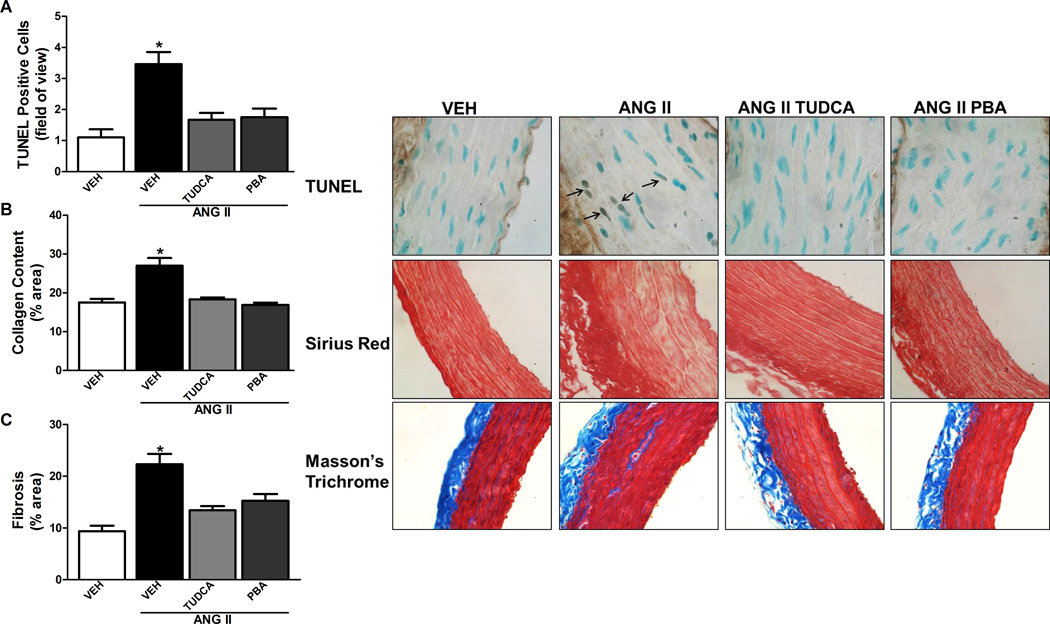
There was an increase in the number of TUNEL positive nuclei from aortic sections of the Ang II alone-treated rat compared to vehicle treated rats (Figure 5A). Treatment with either TUDCA or PBA significantly decreased the number of TUNEL positive nuclei in Ang II infused rats. As depicted in Figure 5B and 5C, Ang II caused an increase in aortic collagen content and fibrosis compared to vehicle-treated rats which was attenuated in the aorta from Ang II rats treated with either TUDCA or PBA. There was an increase in MMP2 activity in the aorta from Ang II-treated rats compared to sham, which was prevented by treatment with either TUDCA or PBA (S2).
Figure 5.
ER stress inhibition decreases TUNEL positive nuclei, collagen content and fibrosis in the aorta of Ang II-induced hypertensive rats. A, Top, representative TUNEL staining of aortic sections (100× magnification). Bottom, analysis of positive TUNEL stained cells per field of view. B, Top, representative Sirius Red staining of aortic sections (40× magnification). Bottom, analysis of % Sirius Red stain per total area. C, Top, representative Masson’s Trichrome staining of aortic sections (40× magnification). Bottom, analysis of % Masson’s Trichrome stain per total area. * P < 0.05 versus Vehicle-treated rats. n= 5–8.
Effect of ER stress inhibition on aortic contraction, relaxation and compliance
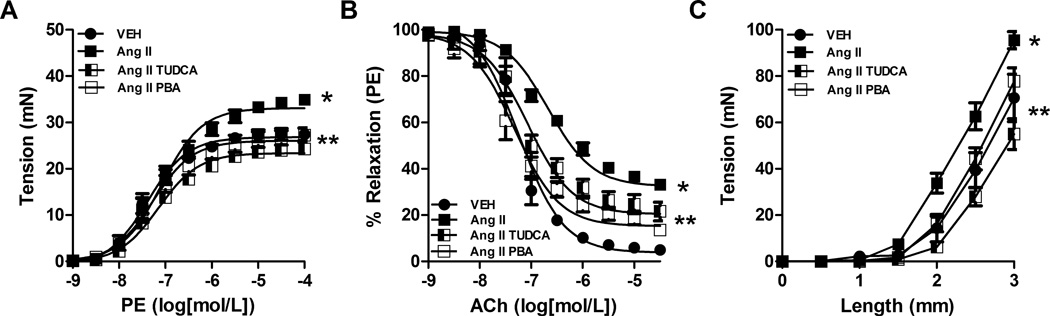
Maximum contraction to phenylephrine (PE) was enhanced in aortic segments from Ang II alone-treated rats ((mN), 34.9 ± 3.1) compared to vehicle (27.2 ± 1.6) and normalized in the Ang II rats treated with either TUDCA (26.9 ± 2.2) or PBA (24.3 ± 1.9) (Figure 6A). Maximum relaxation to acetylcholine (ACh) in aortic segments from Ang II alone-treated rats (% relaxation; 33.2 ± 2.5) was significantly reduced compared to sham rats (4.9 ± 4.1, Figure 6B). Treatment with either TUDCA (13.6 ± 3.8) or PBA (21.6 ± 3.6) improved ACh-induced vascular relaxation. A shift to the left in maximum tension development was seen in the Ang II alone-treated rats (EMAX mN; 95.6 ± 6.6) compared to sham (60.0 ± 4.5) rats which was attenuated in the Ang II rats treated with either TUDCA (77.8 ± 3.1) or PBA (70.1 ± 3.8) (Figure 6C).
Figure 6.
Inhibition of ER stress attenuated enhanced vasoconstriction, reduced relaxation and loss of compliance in the aorta of Ang II-induced hypertensive rats. Concentration-response curves were performed in aortic rings from all 4 groups to A, phenylephrine (PE, 1 nM – 10 µM) or B, acetylcholine (ACh, 1 nM – 10 µM). C, a length-tension curve was performed in aortic rings from all 4 groups. *P < 0.05 versus Vehicle treated rats. ** P < 0.05 versus vehicle treated Ang II rats. n= 6–12.
Discussion
The purpose of this study was to investigate the role of ER stress in the signaling mechanisms that occur in the aorta leading to VSMC apoptosis and fibrosis. We observed that (1) ER stress induction with TM leads to elevated blood pressure in rats, (2) TM led to increased aortic ER stress, apoptosis, collagen deposition and fibrosis and impairment of vascular function, (3) ER stress occurs in the aorta of Ang II-induced hypertensive rats, and (4) ER stress inhibition decreases blood pressure, aortic apoptosis, collagen content and fibrosis, while improving vascular function in an Ang II model of hypertension.
The administration of TM led to an elevation in blood pressure; moreover, the systemic treatment with chemical chaperones TUDCA or PBA decreased blood pressure in the Ang II hypertensive rat model. This presents a question of how these chemicals are acting systemically to cause changes in blood pressure. When directly infused into the subfornical organ (SFO), Ang II or ER stress inducer thapsigargin can cause ER stress, increased sympathetic outflow and hypertension which could be prevented with TUDCA16. Therefore the systemic treatments in the present study may be acting directly through the SFO to alter blood pressure. Furthermore, the role of the kidneys during the development of hypertension has identified and characterized by heightened nephron damage and tubular apoptosis. Rats treated with TM had higher serum creatinine levels, a characteristic of impaired renal function compared to sham or TM plus PBA treated-rats. The Ang II-treated rats receiving either TUDCA or PBA had normalized levels of serum creatinine compared to Ang II alone-treated rats, suggesting an attenuation of renal dysfunction in these rats. In this case, the systemic treatments could be altering kidney function and therefore affecting blood pressure. Lowering blood pressure could have consequences on aortic stiffening; however, in the SHR, minoxidil, a vasodilatory agent that lowered blood pressure did not ameliorate changes in the collagen content in the aorta of the SHR suggesting that prevention of vascular fibrosis may be independent of blood pressure lowering effects.
ER stress mediated apoptosis of VSMCs contributes to the disease pathology of many cardiovascular diseases17, 18. Following transverse aortic constriction in mice, prolonged ER stress in cardiomyocytes initiated apoptotic signaling that lead to heart failure19 and in human coronary arteries increased ER stress and VSMC apoptosis contribute to plaque vulnerability associated with acute coronary syndrome20. However, the role of ER stress-induced VSMC apoptosis and how it might contribute to aortic stiffening is still unclear. In this study, prolonged treatment with ER stress inducer, TM caused increased aortic ER stress, apoptotic proteins and aortic TUNEL positive nuclei. Several studies have reported that Ang II causes VSMC apoptosis and as a consequence can lead to vascular dysfunction, stiffening and fibrosis21. Here we found that in the Ang II-induced hypertensive rat has increased aortic ER stress marker expression, pro-apoptotic proteins and TUNEL positive nuclei and co-treatment with chemical chaperones, TUDCA and PBA prevented this from occurring.
During hypertension changes in the composition of the aortic wall through alterations in extracellular matrix (ECM) proteins led to vascular fibrosis and dysfunction22, 23. Interestingly, mice with genetic defects in ER chaperone proteins exhibit impaired collagen synthesis24. These findings corroborate our findings that after induction of ER stress aortic collagen content, fibrosis and MMP-2 activity were increased. Matrix metalloproteinases (MMPs) contribute to vascular fibrosis through the degradation of extracellular matrix proteins, and during Ang II-induced hypertension MMP2 has been demonstrated to be a major player25, 26. ER stress inhibition in the Ang II-induced hypertensive rat with either TUDCA or PBA attenuated Ang II-induced increases in aortic collagen levels, collagen content and fibrosis, as well as, MMP2 activity. Similar studies found that chemical chaperones can decrease collagen deposition and cardiac fibrosis following pressure-overload27, isoproterenol treatment28 and in Ang II-infused mice7.
Aortic stiffening, associated enhanced contractility of the vascular smooth muscle, endothelial dysfunction and a loss of vessel compliance, is an important contributor to heart failure during aging or hypertension29. Induction of ER stress led to impaired vascular function, as evidenced by decreased contraction to PE, reduced relaxation to ACh and loss of compliance in the aorta from TM-treated rats. In contrast, the enhanced PE-induced contraction, reduced relaxation to ACh and loss of compliance was attenuated in the aorta from Ang II rats treated with either TUDCA or PBA compared to Ang II alone treated rats demonstrating that inhibition of ER stress improves vascular function.
Perspectives
Aortic stiffening is a negative predictor of negative cardiovascular mortality. During aortic stiffening changes in vascular smooth muscle cell function and death play an important role during this process. The results suggest that the ER stress response in the aorta could be a new mechanism through which hypertensive stimuli, such as, Ang II mediate aortic stiffening. This study demonstrates that in the aorta ER stress activates pro-apoptotic and pro-fibrotic cellular signaling pathways. Through the elucidation of a signaling pathway linked to aortic apoptosis and fibrosis, a novel mechanism-based therapy can be developed for the treatment of cardiovascular diseases.
Supplementary Material
Novelty and significance”.
1) What is new?
ER stress led to increased VSMC apoptosis and fibrosis in the aorta
ER stress inhibition has beneficial outcomes on aortic stiffening and hypertension
2) What is relevant?
Aortic stiffening is an important target during hypertension and ER stress may provide to be a new target for the development of new therapies.
Summary
Induction of ER stress leads to increased aortic apoptosis, fibrosis and stiffening. Ang II causes an increase in pro-apoptotic and fibrotic signaling in the aorta contributing to stiffening. Administration of inhibitors of ER stress in the Ang II hypertensive rat decreased blood pressure and improved aorta function.
Acknowledgments
Funding: This study was supported by grants from National Institutes of Health [HL71138], [DK83685]; The Society for Women’s Health Research and a pre-doctoral fellowship from the American Heart Association.
Footnotes
Disclosures: NONE
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: Cell life and death decisions. The Journal of clinical investigation. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett MR. Apoptosis of vascular smooth muscle cells in vascular remodelling and atherosclerotic plaque rupture. Cardiovasc Res. 1999;41:361–368. doi: 10.1016/s0008-6363(98)00212-0. [DOI] [PubMed] [Google Scholar]
- 4.Mallat Z, Tedgui A. Apoptosis in the vasculature: Mechanisms and functional importance. Br J Pharmacol. 2000;130:947–962. doi: 10.1038/sj.bjp.0703407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Clarke MC, Figg N, Littlewood TD, Bennett MR. Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2402–2409. doi: 10.1161/ATVBAHA.111.235622. [DOI] [PubMed] [Google Scholar]
- 6.Briones AM, Arribas SM, Salaices M. Role of extracellular matrix in vascular remodeling of hypertension. Curr Opin Nephrol Hypertens. 2010;19:187–194. doi: 10.1097/MNH.0b013e328335eec9. [DOI] [PubMed] [Google Scholar]
- 7.Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1652–1661. doi: 10.1161/ATVBAHA.112.249318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee GH, Oh HW, Lim HD, Lee W, Chae HJ, Kim HR. 4-phenylbutyric acid regulates collagen synthesis and secretion induced by high concentrations of glucose in human gingival fibroblasts. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2011;15:345–351. doi: 10.4196/kjpp.2011.15.6.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. Atf4 is a substrate of rsk2 and an essential regulator of osteoblast biology; implication for coffin-lowry syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 10.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell stress & chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian X, Hou W, Zhengang L, Sha B. Direct interactions between molecular chaperones heat-shock protein (hsp) 70 and hsp40: Yeast hsp70 ssa1 binds the extreme c-terminal region of yeast hsp40 sis1. The Biochemical journal. 2002;361:27–34. doi: 10.1042/0264-6021:3610027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, Zou MH. Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of amp-activated protein kinase-alpha2 in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitler KM, Matsumoto T, Webb RC. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. American journal of physiology. Heart and circulatory physiology. 2013;305:H344–H353. doi: 10.1152/ajpheart.00952.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim do H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochemical and biophysical research communications. 2012;421:578–584. doi: 10.1016/j.bbrc.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes. 2009;58:2863–2872. doi: 10.2337/db09-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. Er stress in the brain subfornical organ mediates angiotensin-dependent hypertension. The Journal of clinical investigation. 2012;122:3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circulation research. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circulation research. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 19.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: Possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 20.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin ii in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 22.Lee KM, Tsai KY, Wang N, Ingber DE. Extracellular matrix and pulmonary hypertension: Control of vascular smooth muscle cell contractility. Am J Physiol. 1998;274:H76–H82. doi: 10.1152/ajpheart.1998.274.1.H76. [DOI] [PubMed] [Google Scholar]
- 23.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: Emerging concepts. Hypertension. 2010;55:9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464. [DOI] [PubMed] [Google Scholar]
- 24.Nagai N, Hosokawa M, Itohara S, Adachi E, Matsushita T, Hosokawa N, Nagata K. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. The Journal of cell biology. 2000;150:1499–1506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. Mmp-2 mediates angiotensin ii-induced hypertension under the transcriptional control of mmp-7 and tace. Hypertension. 2011;57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 26.Brassard P, Amiri F, Schiffrin EL. Combined angiotensin ii type 1 and type 2 receptor blockade on vascular remodeling and matrix metalloproteinases in resistance arteries. Hypertension. 2005;46:598–606. doi: 10.1161/01.HYP.0000176744.15592.7d. [DOI] [PubMed] [Google Scholar]
- 27.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim do H. The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun. 2012;421:578–584. doi: 10.1016/j.bbrc.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Ayala P, Montenegro J, Vivar R, Letelier A, Urroz PA, Copaja M, Pivet D, Humeres C, Troncoso R, Vicencio JM, Lavandero S, Diaz-Araya G. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol. 2012;92:97–104. doi: 10.1016/j.yexmp.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.