Abstract
Emerging evidence suggests that in addition of being the “power houses” of our cells, mitochondria facilitate effector responses of the immune system. Cell death and injury result in the release of mitochondrial DNA (mtDNA) that acts via Toll-like receptor 9 (TLR9), a pattern recognition receptor of the immune system, which detects bacterial and viral DNA but not vertebrate DNA. The ability of mtDNA to activate TLR9 in a similar fashion with bacterial DNA stems from evolutionary conserved similarities between bacteria and mitochondria. Mitochondrial DNA may be the trigger of systemic inflammation in pathologies associated with abnormal cell death. Preeclampsia (PE) is a hypertensive disorder of pregnancy with devastating maternal and fetal consequences. The etiology of PE is unknown and removal of the placenta is the only effective cure. Placentas from women with PE show exaggerated necrosis of trophoblast cells and circulating levels of mtDNA are higher in pregnancies with PE. Accordingly, we propose the hypothesis that exaggerated necrosis of trophoblast cells results in the release of mtDNA, which stimulate TLR9 to mount an immune response and to produce systemic maternal inflammation and vascular dysfunction that lead to hypertension and intrauterine growth restriction. The proposed hypothesis implicates mtDNA in the development of PE via activation of the immune system and may have important preventative and therapeutic implications, because circulating mtDNA may be potential markers of early detection of PE and anti-TLR9 treatments may be promising in the management of the disease.
INTRODUCTION
Preeclampsia (PE) is a pregnancy syndrome that is defined by the onset of hypertension and proteinuria after 20 weeks of gestation [1]. It affects every maternal organ and fetal development, is an important cause of preterm delivery in developed countries and a leading cause of maternal and fetal morbidity and mortality in developing countries. One of the main characteristics of the syndrome is an inability of the trophoblasts to invade the decidual arteries, causing defective placentation, reduced placental perfusion and nutrient supply [2]. Other features of the disease include placental and systemic oxidative stress and dysfunction of the maternal vasculature [3–5]. These are also associated with reduced placental perfusion. As PE progresses to a clinical stage the mother presents with symptoms such as hypertension, proteinuria, coagulopathy and/or hepatic dysfunction [6]. In most cases, removal of the placenta alleviates the clinical symptoms of the disease, indicating that placenta-derived factors are likely responsible for the pathogenesis and/or manifestation of PE.
Components of the immune system have been detected at the maternal-fetal interface [7–8] and their function in pregnancy has recently become an emerging field of investigation in an effort to understand the role of the immune system in defending the fetus and the mother from infections. Bacterial and viral infections are often responsible for pregnancy complications such as preterm labor and PE [9–10]. Consequently, several investigations have addressed the question of how exogenous (viral and bacterial) products induce poor pregnancy outcomes. In this paper, we address the question of how endogenous molecules released by the placenta induce clinical symptoms of PE, such as maternal vascular dysfunction and hypertension, as well as insufficient fetal growth.
Toll-like receptors (TLRs) are cellular components of the immune system that detect conserved sequences known as pathogen-associated molecular patterns (PAMPs) [11]. Our main knowledge regarding the role of TLR signaling in pregnancy derives from studies in placental explants and trophoblast cells. Human placenta expresses transcripts for TLR1-TLR10 [7–12] and placentas from patients with PE show greater expressions of TLR2, TLR3, TLR4, and TLR9 compared to controls [7–13], indicating that TLR signaling may be involved in the development of placenta deficiencies and the pathogenesis of PE.
Preeclampsia is characterized by exaggerated trophoblast apoptosis and necrosis [14–15] and increased expression of TLR9 in placental [13] and dendritic cells [16]. Further, pregnancies complicated with intrauterine growth restriction (IUGR), a common feature of PE, show elevated levels of circulating mtDNA [17]. Interestingly, the highest mtDNA levels were found in the more severe IUGR subsets that were complicated with maternal PE [17]. Based on recent evidence that mtDNA induces an immune response via activation of TLR9 signaling pathway [18], we propose the hypothesis that abnormal trophoblast cell death (i.e., exaggerated necrosis) results in the release of mitochondrial products, including mtDNA, which stimulate TLR9 to mount an immune response and produce systemic maternal inflammation, vascular dysfunction and intrauterine growth restriction.
TLR SIGNALING
Toll-like receptors are type I integral membrane glycoproteins that contain leucine-rich repeats in their extracellular domain and a cytoplasic Toll/interleukin-1 receptor (TIR) signaling domain [19]. These receptors recognize pathogen-associated molecular patterns (PAMPs) associated with bacteria and viruses and induce signals, which are critical for eliciting innate and adaptive immune responses to invading microorganisms [11]. In addition to detecting molecular structures of microbial origin, TLRs respond to endogenous molecular structures known as damage-associated molecular patterns (DAMPs), which are released due to cell death and injury [18]. At least eleven TLRs have been reported in mammals (TLR1-11). Toll-like receptors that recognize constituents of bacterial and fungal cell wall are localized on the cell surface (TLR1, TLR2, TLR4, TLR5, TLR6), whereas those that recognize pathogen-specific nucleic acids are localized to intracellular membranes and bind their ligands in phagosomes or endosomes (TLR3, TLR7, TLR8, TLR9) [20–23].
Toll-like receptor 9 (TLR9) recognizes bacterial DNA containing the dinucleotide CG where the C is unmethylated (CpG-containing DNA) [24]. Toll-like receptor 9 resides in the endoplasmic reticulum and upon cell activation with CpG DNA the distribution of TLR9 changes, with a portion of the total protein translocating first into early endosomes and later into lysosomal compartments, where signal transduction is initiated [21]. Following CpG DNA binding, TLR9 associates with the intracellular adapter protein myeloid differentiation factor-88 (MyD88) [25] to activate signal transducing proteins such as members of the interleukin-1 receptor-associated kinase (IRAK) family, mitogen-activated kinases (MAPK), or interferon regulatory factors (IRF) [26]. These events initiate the synthesis and release of inflammatory cytokines and antimicrobial products, and regulate costimulatory molecules [25].
The ability of TLR9 to discriminate between foreign and self-DNA is due to the higher frequency and presence of unmethylated CpG dinocluoteides in bacterial and viral as compared to mammalian DNA [27]. Mitochondria, however, evolved from saprophytic bacteria to become intracellular organelles [28] and therefore, mitochondrial DNA (mtDNA) is structurally similar to bacterial DNA and share unmethylated CpG DNA repeats [29]. Consequently, mtDNA is a ligand for TLR9 [18]. In this paper, we propose that mtDNA released by necrotic trophoblasts induces a maternal immune response via TLR9 signaling activation leading to the development of PE and its associated clinical symptoms.
TLR ACTIVATION AND CLINICAL SYMPTOMS OF PREECLAMPSIA
Intrauterine infections are associated with PE in humans [10–30], and viral and bacterial ligands have been often used to induce PE-like symptoms in animals. For instance, pregnant rats infused with low concentrations of endotoxin (TLR4 ligand) [31] or treated with the viral mimetic polyinosinic:polycytidylic acid (poly I:C, TLR3 ligand) [32] developed maternal hypertension, vascular dysfunction and proteinuria. Endotoxin or poly I:C treatment had no effect in non-pregnant rats [32–33]. Thus activation of TLR3 and TLR4 causes PE-like symptoms in rats, providing compelling evidence that viral or bacterial infection may contribute to the development of the disease through TLR signaling.
During a systemic or intrauterine infection in pregnancy, invading microorganisms and their breakdown products provide an increased pathogenic load to the maternal-fetal environment. Hypomethylated CpG motifs presented by infectious agents may therefore over-stimulate the TLR9, mediating maternal immune activation [34]. Previous studies examined the role of the CpG-TLR9 axis in animal models of pregnancy focusing on pregnancy outcomes such as pup survival and development [34–35]. High doses of a synthetic CpG oligonucleotide (ODN) during mouse pregnancy, stimulated Th1-cytokine release and induced fetal resorptions, craniofacial and limb defects, placental cell necrosis, calcification and inflammation, suggesting that activation of TLR9 signaling may have adverse pregnancy outcomes [35]. Recently, Thaxton and colleagues confirmed these findings and also showed that anti-inflammatory cytokine proficiency protects against CpG-induced pregnancy complications [34]. These previous studies focused on pup survival and growth but did not examine maternal physiological functions, which may be compromised in the presence of an inflammatory environment.
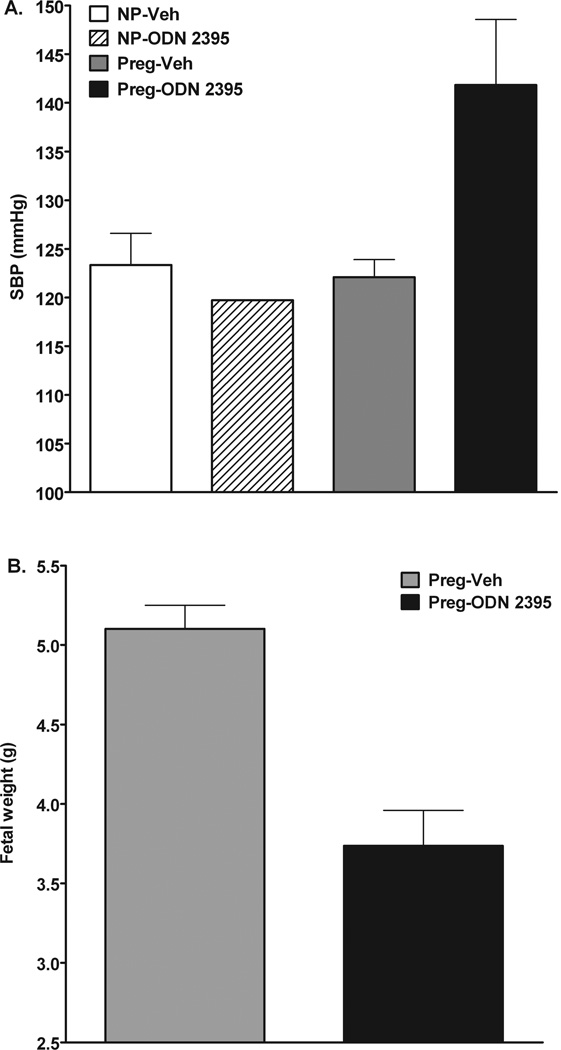
Preliminary observations in our laboratory suggest that activation of TLR9 via a synthetic CpG oligonucleotide elicits PE–like symptoms in pregnant rats. Figure 1 shows that continuous activation of TLR9 by exogenous synthetic oligonucleotides increases systolic blood pressure by ~20 mmHg in pregnant but not in non-pregnant rats (Fig. 1A). Further, treatment with the TLR9 agonist did not affect the number of pups/litter (Treated rats: 13.3 ± 1.3 vs. untreated rats: 12.7 ± 0.3)) but reduced fetal weights (Fig. 1B). These data suggest that activation of TLR9 during pregnancy not only affects fetal development as previously reported [34–35] but it also induces maternal hypertension, which is a main feature of pregnancies with PE. In contrast to pregnant rats, non-pregnant rats did not have a hypertensive response to TLR9 activation. Previous studies have shown that the intracellular localization of TLR9 determines the access of the receptor to different sources of DNA [20]. It is unknown, however, whether pregnancy affects TLR9 localization. An increase in TLR9 expression with gestation could also explain the differential responses to TLR9 in non-pregnant and pregnant rats. Accordingly, normal and complicated pregnancies may determine TLR9 responses to endogenous and/or exogenous threats by modifying TLR9 localization and expression. The effects of pregnancy on TLR9 localization and protein expression in different cell types are currently under investigation.
Figure 1.
A synthetic TLR9 ligand (ODN 2395; InvivoGen, San Diego, CA, USA) was administered by intraperitoneal injection (0.1 µg/rat) on days 14, 17, and 19 of gestation (term=21 days) in pregnant rats or corresponding days in non-pregnant rats. Blood pressure was measured via the tail cuff method on gestational day 20 and rats were euthanized on day 21. A) Systolic blood pressure of non-pregnant (NP) and late pregnant rats (Preg) treated with ODN 2395 (NP-ODN 2395, n=1; Preg-ODN 2395, n=2) or Vehicle (NP-Veh, n=3; Preg-Veh, n=2), B) Fetal weights from pregnant rats treated with ODN 2395 or Vehicle.
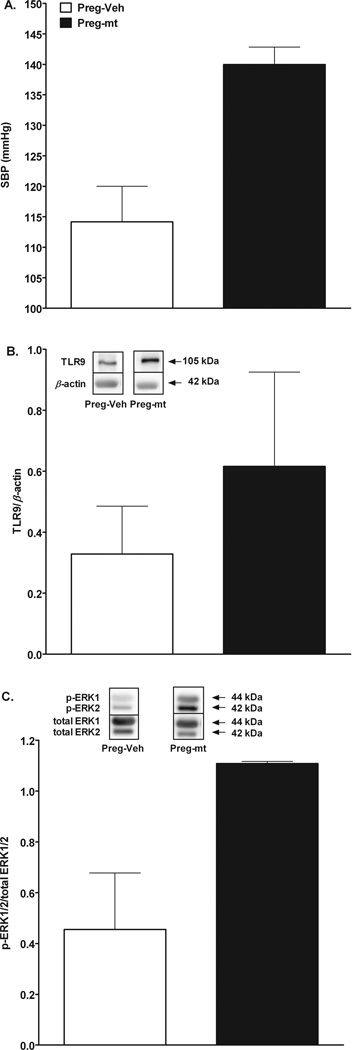
Both bacterial and mtDNA are ligands for TLR9 [18, 24]. Mitochondrial DNA is released from necrotic cells [18] and circulating levels of mtDNA are elevated in pregnancies complicated with maternal PE and IUGR [17]. Further, pregnancies with PE are characterized by exaggerated trophoblast cell apoptosis and necrosis [14–15], which may be the source of mtDNA released in the maternal circulation. Interestingly, smoking is associated with reduced risk of PE [36] and although the exact mechanism of this protection is unknown, it may relate to the fact that maternal smoking depletes mtDNA in the placenta [37]. To examine the effects of TLR9 activation by mitochondrial products on maternal cardiovascular responses, we injected mitochondria isolated from rat liver in pregnant rats. Dams injected with “damaged” mitochondria developed high blood pressure (Δ systolic blood pressure=26 mmHg) compared to controls (Fig. 2A).
Figure 2.
Mitochondria were isolated from rat liver (Mitochondria Isolation Kit, Pierce Biotechnology, Rockford, IL, USA) and their integrity was disrupted by sonication. Mitochondria solution (4 mg tissue/rat diluted in saline) was injected in pregnant rats on gestational day 15. Blood pressure was measured via the tail cuff method on gestational day 18 and rats were euthanized on day 19. A) Systolic blood pressure of pregnant rats injected with mitochondria (Preg-mt, n=2) or Vehicle (Preg-Veh, n=2), B) Densitometric intensity and representative Western blots for TLR9 protein) in relation to β-actin in second order mesenteric arteries from pregnant rats injected with mitochondria (Preg-mt, n=2) or Vehicle (Preg-Veh, n=2), C) Densitometric intensity and representative Western blots for phosphorylated ERK1/2 proteins in relation to total ERK1/2 in mesenteric arteries from pregnant rats injected with mitochondria (Preg-mt, n=2) or Vehicle (Preg-Veh, n=2).
Collectively, previous studies and our preliminary observations suggest that activation of TLR3, TLR4, and TLR9 lead to the development of PE-like symptoms in pregnant animals. Given that TLR9 can be activated by both bacterial and mtDNA, we suggest that both endogenous and exogenous DNA leads to PE via TLR9 signaling and we propose that the source of the endogenous DNA is mitochondria released by necrotic trophoblasts.
TLR9 ACTIVATION AND MATERNAL VASCULAR FUNCTION
Maternal vascular dysfunction is a hallmark of PE that may significantly contribute to the manifestations of the disease such as maternal hypertension and IUGR [38]. Further, PE poses a risk of future maternal cardiovascular disease [39], which may stem from alterations in the function of the vasculature during pregnancy. The exact mechanisms, by which placenta-derived factors cause maternal vascular dysfunction, are currently unknown.
Sustained maternal immune system activation via a TLR3 ligand (poly I:C) during rat pregnancy resulted in reduced endothelium-dependent conduit artery dilation [32], indicating that TLR signaling plays a role in the development of maternal vascular dysfunction in pregnancies with PE. A recent study showed that infusion of lipopolysaccharide (LPS, TLR4 ligand) on gestational day 15 decreased myogenic tone and increased wall thickness of posterior cerebral arteries in pregnant but not in non-pregnant rats [33]. These investigators did not examine the role of TLR4 signaling on LPS-mediated vascular effects but previous studies have provided compelling evidence that LPS-induced signal transduction is mediated by TLR4 [40]. Also, injection with poly (I:C) (TLR3 ligand) in pregnant rats [32] and mice [41], and infusion of LPS in pregnant rats [33] resulted in increased serum concentration and vascular mRNA levels of pro-inflammatory cytokines, respectively. Further, it has been reported that exogenous interleukin-10 treatment in pregnant mice injected with poly (I:C) prevented maternal endothelial dysfunction [41]. These data show that the effects of TLR signaling on maternal vascular function are likely mediated by induction of pro-inflammatory cytokines and can be regulated by anti-inflammatory cytokines, such as IL10.
Toll-like receptors are expressed on immune [42] and trophoblast cells [13] but have been also detected in the vascular endothelial [43] and smooth muscle cells [44]. Our laboratory has recently shown that treatment with anti-TLR4 antibody reduced blood pressure and small vessel contractility via a cyclooxygenase (COX)-dependent mechanism in a rat model of hypertension [45], implicating TLR signaling in hypertension-associated vascular dysfunction. Studies on TLR signaling in pregnancy suggest that the vascular effects of TLR activation are due to an increase in pro-inflammatory cytokines [32–33, 41]. There is a possibility, however, that exogenous and endogenous ligands directly act on TLRs in the vascular wall inducing a cytokine-independent signaling pathway that leads to vascular dysfunction.
In immune cells, activation of TLR9 via mitochondrial and bacterial DNA induces an immune and inflammatory response via activation of p38 MAPK [18]. Other studies suggest the involvement of other MAPKs, such as ERK and c-Jun N-terminal kinase (JNK), in the downstream TLR9 signaling pathway [46–47]. To examine the effects of TLR9 activation on the activity of ERK1/2 in maternal resistance vessels, we measured protein expression of TLR9 and phosphorylated ERK1/2 in mesenteric arteries from pregnant rats treated with mitochondria isolated from rat liver [18] and from rats treated with vehicle. Expression of TLR9 and phosphorylated ERK1/2 were greater in mitochondria-treated pregnant rats compared to controls (Figs. 2B–2C). Given that ERK1/2 plays a significant role in vascular responses to constrictor stimuli (i.e., phenylephrine, thromboxane mimetics) [48–49], we speculate that ERK1/2 is a downstream effector of the CpG-TLR9 axis in the maternal vascular tissue, mediating a direct effect of TLR9 ligation by mtDNA on maternal vascular function. Indeed, there are reports to suggest that activation of TLR9 leads to ERK1/2 activation in various cells [50].
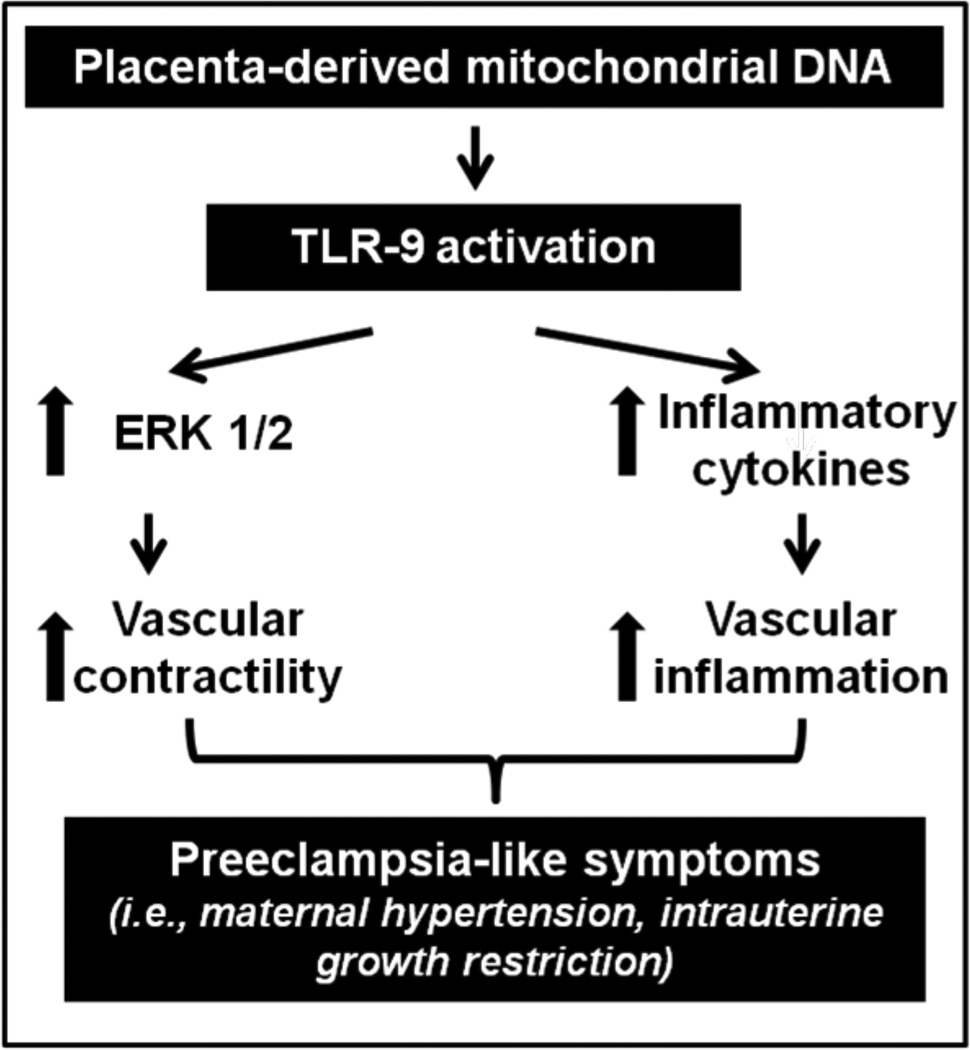
According to our preliminary data, we propose that during pregnancy activation of the CpG-TLR9 axis via mtDNA released by necrotic placental cells increases activation of ERK1/2, contributing to increased maternal vascular reactivity to constrictor stimuli, increased peripheral vascular resistance, maternal hypertension and insufficient uterine blood flow (Figure 3). This mechanism may be independent from the effects of pro-inflammatory cytokines released upon TLR9 activation on the maternal vasculature but this speculation warrants further investigation. Integrative approaches including physiological, pharmacological, biochemical, molecular and cellular techniques as well as translational studies are required to test the proposed hypothesis and to investigate the role of the innate immune system in maternal vascular inflammation and dysfunction and its contribution to the development of maternal hypertension and IUGR. Studies of maternal vascular reactivity, blood pressure responses, uterine blood flow, levels of maternal proteinuria and fetal development in pregnant animals treated with mtDNA isolated from placentas and use of tlr9 knock-out mice can establish a causal relationship between mtDNA and PE-like symptoms. Further, cell culture studies can provide information regarding the direct effects of mtDNA on TLR9 signaling in vascular smooth muscle and endothelial cells. Also, assessment of circulating mtDNA content in blood from women with PE is necessary to verify the relevance of the proposed hypothesis to pregnancies with PE.
Figure 3.
Diagram demonstrating the hypothesis that during pregnancy placenta-derived mitochondrial DNA, through TLR9 activation, increases vascular reactivity to constrictor stimuli via an ERK1/2-dependent signaling pathway and potentiates the release of pro-inflammatory cytokines, contributing to preeclampsia-like symptoms (i.e., maternal hypertension and intrauterine growth restriction).
PERSPECTIVES AND CLINICAL IMPLICATIONS
Mitochondrial DNA is released by necrotic cells inducing a systemic inflammatory response via activation of TLR9 [18]. Further, under certain pathophysiological conditions, the ability of TLR9 to discriminate between self and foreign DNA can be circumvented and this leads to immune pathologies and chronic inflammation [51]. In this paper, we propose the hypothesis that mtDNA is a placenta-derived factor with immunostimulatory properties that is secreted in the maternal circulation as a result of exaggerated trophoblast necrosis activating the maternal immune system via TLR9 signaling. These events lead to systemic maternal inflammation and vascular dysfunction, hypertension and intrauterine growth restriction. The proposed hypothesis implicates DNA in the development of PE via activation of the immune system and may have important preventative and therapeutic implications. For instance, circulating mtDNA may be potential markers of early detection of preeclampsia and anti-TLR9 treatments may be promising in the management of the disease.
Acknowledgements
This study was supported in part by the National Institutes of Health, USA (Grants: R01 HL071138, R01 DK083685, T32 HL066993-09), the Society for Women’s Health Research, and the Naito Foundation Japan.
Footnotes
The authors declare no conflict of interest.
References
- 1.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;1:S1–S22. [PubMed] [Google Scholar]
- 2.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;6:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 3.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;10:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;2:304–310. doi: 10.1161/HYPERTENSIONAHA.110.153163. [DOI] [PubMed] [Google Scholar]
- 5.Walsh SK, English FA, Johns EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension. 2009;2:345–351. doi: 10.1161/HYPERTENSIONAHA.109.132191. [DOI] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;5728:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;7:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 8.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;1:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;20:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CD, Witter FR. Urogenital infection in preeclampsia. Int J Gynaecol Obstet. 1995;3:271–275. doi: 10.1016/0020-7292(95)02373-k. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;10:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 12.Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;2:243–248. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- 13.Pineda A, Verdin-Teran SL, Camacho A, Moreno-Fierros L. Expression of toll-like receptor TLR-2, TLR-3, TLR-4 and TLR-9 is increased in placentas from patients with preeclampsia. Arch Med Res. 2011;5:382–391. doi: 10.1016/j.arcmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Stone P, Ching LM, Chamley L. A role for interleukin-6 in spreading endothelial cell activation after phagocytosis of necrotic trophoblastic material: implications for the pathogenesis of pre-eclampsia. J Pathol. 2009;1:122–130. doi: 10.1002/path.2425. [DOI] [PubMed] [Google Scholar]
- 15.Huppertz B, Kingdom JC. Apoptosis in the trophoblast--role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004;6:353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Panda B, Panda A, Ueda I, Abrahams VM, Norwitz ER, Stanic AK, Young BC, Ecker JL, Altfeld M, Shaw AC, Rueda BR. Dendritic cells in the circulation of women with preeclampsia demonstrate a pro-inflammatory bias secondary to dysregulation of TLR receptors. J Reprod Immunol. 2012 doi: 10.1016/j.jri.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni F, Lattuada D, Garretto A, Massari M, Mando C, Somigliana E, Cetin I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol. 2010;4:365 e361–365 e366. doi: 10.1016/j.ajog.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;7285:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;10:528–533. doi: 10.1016/s1471-4906(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 20.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;1:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 21.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;2:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 22.Nishiya T, Kajita E, Miwa S, Defranco AL. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem. 2005;44:37107–37117. doi: 10.1074/jbc.M504951200. [DOI] [PubMed] [Google Scholar]
- 23.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;6755:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;6813:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Hoshino K. Myeloid differentiation factor 88-dependent and - independent pathways in toll-like receptor signaling. J Infect Dis. 2003:S356–S363. doi: 10.1086/374749. [DOI] [PubMed] [Google Scholar]
- 26.Vollmer J. TLR9 in health and disease. Int Rev Immunol. 2006;3–4:155–181. doi: 10.1080/08830180600743107. [DOI] [PubMed] [Google Scholar]
- 27.Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, Sweet MJ, Hume DA. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol. 2003;7:3614–3620. doi: 10.4049/jimmunol.170.7.3614. [DOI] [PubMed] [Google Scholar]
- 28.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;3:255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 29.Gray MW, Burger G, Lang BF. The origin and early evolution of mitochondria. Genome Biol. 2001;6 doi: 10.1186/gb-2001-2-6-reviews1018. REVIEWS1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Dadelszen P, Magee LA. Could an infectious trigger explain the differential maternal response to the shared placental pathology of preeclampsia and normotensive intrauterine growth restriction? Acta Obstet Gynecol Scand. 2002;7:642–648. [PubMed] [Google Scholar]
- 31.Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol. 1994;1:158–164. doi: 10.1016/0002-9378(94)90463-4. [DOI] [PubMed] [Google Scholar]
- 32.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens. 2009;12:1314–1319. doi: 10.1038/ajh.2009.185. [DOI] [PubMed] [Google Scholar]
- 33.Cipolla MJ, Houston EM, Kraig RP, Bonney EA. Differential effects of low-dose endotoxin on the cerebral circulation during pregnancy. Reprod Sci. 2011;12:1211–1221. doi: 10.1177/1933719111410712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;2:1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine. 2006;3:263–271. doi: 10.1016/j.vaccine.2005.07.105. [DOI] [PubMed] [Google Scholar]
- 36.Lindqvist PG, Marsal K. Moderate smoking during pregnancy is associated with a reduced risk of preeclampsia. Acta Obstet Gynecol Scand. 1999;8:693–697. [PubMed] [Google Scholar]
- 37.Bouhours-Nouet N, May-Panloup P, Coutant R, de Casson FB, Descamps P, Douay O, Reynier P, Ritz P, Malthiery Y, Simard G. Maternal smoking is associated with mitochondrial DNA depletion and respiratory chain complex III deficiency in placenta. Am J Physiol Endocrinol Metab. 2005;1:E171–E177. doi: 10.1152/ajpendo.00260.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mishra N, Nugent WH, Mahavadi S, Walsh SW. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension. 2011;5:867–873. doi: 10.1161/HYPERTENSIONAHA.111.176602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;4:709–715. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 40.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;16:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 41.Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. 2011;3:489–496. doi: 10.1161/HYPERTENSIONAHA.111.172114. [DOI] [PubMed] [Google Scholar]
- 42.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;5:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Armas M, Simon-Santamaria J, Pettersen I, Moens U, Smedsrod B, Sveinbjornsson B. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J Hepatol. 2006;5:939–946. doi: 10.1016/j.jhep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;3:244–250. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 45.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;11:535–543. doi: 10.1042/CS20110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi AK, Krieg AM. Rapid induction of mitogen-activated protein kinases by immune stimulatory CpG DNA. J Immunol. 1998;9:4493–4497. [PubMed] [Google Scholar]
- 47.Yi AK, Yoon JG, Yeo SJ, Hong SC, English BK, Krieg AM. Role of mitogen-activated protein kinases in CpG DNA-mediated IL-10 and IL-12 production: central role of extracellular signal-regulated kinase in the negative feedback loop of the CpG DNA-mediated Th1 response. J Immunol. 2002;9:4711–4720. doi: 10.4049/jimmunol.168.9.4711. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, Tang S, Zhou S, Ware JA. The thromboxane A2 receptor activates mitogen-activated protein kinase via protein kinase C-dependent Gi coupling and Src-dependent phosphorylation of the epidermal growth factor receptor. J Pharmacol Exp Ther. 2001;2:426–433. [PubMed] [Google Scholar]
- 49.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2002;1:H292–H300. doi: 10.1152/ajpheart.2002.282.1.H292. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Wang J, An H, Zhou J, Zhang L, Cao X. Heat shock up-regulates TLR9 expression in human B cells through activation of ERK and NF-kappaB signal pathways. Immunol Lett. 2005;1:153–159. doi: 10.1016/j.imlet.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci. 2006:31–43. doi: 10.1196/annals.1348.005. [DOI] [PubMed] [Google Scholar]