Abstract
Background
Difficulties with the direct antiglobulin test (DAT) and its apparent lack of sensitivity and specificity for immune‐mediated hemolytic anemia (IMHA) in dogs have raised skepticism regarding its diagnostic value.
Objective
To compare different DATs and other hematologic parameters in dogs.
Animals
Anticoagulated blood samples from 59 nonanemic and 46 anemic dogs (± IMHA) from a research colony and veterinary clinics.
Methods
Prospective observational study: Immunochromatographic strip, gel microcolumn, and capillary techniques were compared with standard microtiter DAT using 2 polyvalent antiglobulins. Spherocytosis, autoagglutination, osmotic fragility, and clinical data were assessed.
Results
Blood samples from all 59 nonanemic dogs were DAT‐. Among 46 anemic dogs, 33 were suspected of IMHA, but only 20 were DAT+. Old and new DAT methods yielded comparable and consistent results even after storage of chilled blood samples for 1 week. Spherocytosis and autoagglutination (that did not persist after washing) were noted in 15 and 16 DAT+ dogs, respectively. The other 26 anemic dogs, including 21 previously transfused dogs and 4 with autoagglutination, tested DAT‐ by the other methods. Osmotic fragility was increased in 70% (19/27) of anemic and all 15 DAT+ dogs tested. Limited follow‐up testing revealed DAT+ results for 3–70 days.
Conclusions and Clinical Importance
The novel strip and capillary DAT methods are promising adjunct in‐clinic tools. Despite prior immunosuppressive treatment and presence of autoagglutination, the DAT was positive in anemic dogs with IMHA. Transfusion did not cause false DAT+ results. Our results support DAT as a cornerstone in the diagnosis of canine IMHA.
Keywords: Autoantibodies, Direct antiglobulin test, Immune‐mediated hemolytic anemia, Osmotic fragility
Abbreviations
- AT
antiglobulin testing band
- Capillary
capillary tube method
- C
control lectin band
- DAT
direct antiglobulin test
- DEA
dog erythrocyte antigen
- EDTA
ethylenediaminetetraacetate
- Gel
gel microcolumn method
- Hb
hemoglobin
- Ig (G, M)
immunoglobulin (G, M)
- IMHA
immune‐mediated hemolytic anemia
- Microtiter
microtiter plate method
- min
minutes
- n
number
- OF(T)
osmotic fragility (test)
- PBS
phosphate‐buffered saline
- RBC(s)
red blood cell(s)
- Reagent M
reagent MP Bio
- Reagent V
reagent VMRD
- SD
standard deviation
- Strip
immunochromatographic strip method
Dr Robin Coombs first introduced the antiglobulin test, referred to as the Coombs' test, into human clinical practice in 1945.1 This immunohematologic technique has proven invaluable, specific, and sensitive in the detection of erythrocytic auto‐ and alloantibodies documenting immune‐mediated hemolytic anemia (IMHA), hemolytic transfusion reactions, and hemolysis of the newborn.1, 2 The direct antiglobulin test (DAT) detects immunoglobulin (Ig), complement, or both, bound to the surface of red blood cells (RBCs).3, 4 Since the initial conventional tube Coombs' test, several additional methods using microtiter plates, capillary tubes, gel microcolumns, and flow cytometry have been developed. Also, a variety of reagents from polyvalent to specific Ig and complement reagents, under various incubation conditions, have been applied.5
The DAT has also been used with species‐specific reagents in veterinary medicine, mainly in the diagnosis of IMHA in dogs.6, 7, 8, 9, 10 However, difficulties in performing the DAT, subjective result interpretation, and its apparent lack of sensitivity and specificity for IMHA have overshadowed its clinical diagnostic usefulness.8, 10, 11 Anecdotally, clinicians speak of “Coombs'‐negative IMHA” dogs7, 12, 13, and some skip the Coombs' test altogether as DAT+ results are rarely received from clinical pathology laboratories. Furthermore, the autoagglutination observed in ethylenediaminetetraacetate (EDTA) tubes or on microscopic slides (with or without adding a drop of saline) is commonly considered sufficient for the diagnosis for IMHA and is also thought to interfere with DAT performance.9, 14 Moreover, there is a sense that immunosuppressive treatment will immediately convert DAT+ dogs to “false‐negative DAT” results and that even 1 transfusion will cause a “false‐positive DAT” result.14, 15, 16, 17 Finally, the erythrocytic osmotic fragility (OF) test (OFT) at specific saline concentrations (5, 50, and 90%) is by some considered a diagnostic test for IMHA.7, 12
In light of the perceived uncertainties of the value of the Coombs' test and the introduction of newer techniques, a prospective study aimed at comparing various laboratory and in‐clinic immunohematologic DAT methods to detect warm allo‐ and autoantibodies in anemic and nonanemic dogs before treatment, after treatment, or both and before transfusion, after transfusion, or both was undertaken. The presence of autoagglutination, spherocytosis, and increased OF was also assessed, and clinicopathologic and therapeutic information of dogs was evaluated where available.
Materials and Methods
Animals and Samples
From April to October 2012, a free‐of‐charge extended laboratory assessment of anemic dogs, in particular, those suspected of having IMHA, was offered as part of their diagnostic evaluation by notifying ACVIM diplomates and other referral clinicians in the United States via e‐mail. Small (1–3 mL) EDTA‐anticoagulated blood samples from 24 anemic dogs, freshly collected, chilled, and shipped on ice, were received by overnight mail along with similarly prepared samples from 17 healthy nonanemic dogs, to control for shipping artifacts. Leftover EDTA blood samples from 22 anemic and 11 nonanemic dogs submitted to the Clinical Pathology Laboratory at the Veterinary Hospital of the University of Pennsylvania (VHUP) were also assessed, as well as samples from 31 nonanemic dogs in the research colony at the University of Pennsylvania. All samples were stored at 4°C for less than 48 hours until processing. Lipemic samples that could compromise OF testing were not used.
To investigate effects of sample storage, 34 blood samples (11 anemic DAT+ samples and 23 nonanemic DAT‐ colony dogs) were repeatedly tested by DAT methods on subsequent days after blood collection. In addition, 9 DAT+ and 8 DAT‐ anemic dogs were followed up by repeat testing during monitoring of disease progression and treatment response. Signalment (Data S1), routine clinicopathologic and therapeutic information from each dog were also reviewed. Distinction between primary and secondary IMHA was not made. The authors, who established methods and routinely performed these immunohematologic techniques, conducted all testing. The Institutional Animal Care and Use Committee at the University of Pennsylvania approved these studies.
Laboratory Methods
Routine Hematology Studies—If not already included with the shipped, received samples, blood smears were prepared and stained with Wright Giemsa1 on arrival for manual microscopic examination. Spherocytosis was recorded, if there were at least 20 spherocytes per 100× microscopic field (all others had <2 per 100× field).11
Autoagglutination was assessed first by visual examination of the EDTA tube and by microscopy of the blood smear; if positive, saline agglutination of EDTA blood was performed by adding a drop of physiologic saline (0.9% NaCl) to a drop of blood on a slide.11, 14 Finally, after washing the RBC pellet 3 times by adding 4–6 parts of phosphate‐buffered saline (PBS2 ) to 1 part of packed RBCs, mixing, centrifuging for 5 minutes at 1000 × g,3 and each time discarding the supernatant to remove any remaining plasma proteins, agglutination was evaluated macro‐ and microscopically. The washed packed RBC samples that retained aggregates were said to exhibit persistent or true agglutination.9, 10, 18 The PCV of EDTA blood samples was determined by standard microcentrifugation; whole blood and plasma hemoglobin (Hb) concentrations were measured by HemoCue4; DEA 1.1 blood type was determined by the immunochromatographic strip method.19
To technically validate various antiglobulin tests for this study, conditions for positive and negative reactions were established (Data S1). For this prospective study, 3 DAT methods based on RBC agglutination were compared: microtiter5 plate (Microtiter) using canine antiglobulin reagent M6 (raised in rabbit) and reagent V7 (goat), capillary8 tube (Capillary, Fig 1A) and gel9 microcolumn (Gel) technique. In addition, all samples were tested using a novel immunochromatographic strip10 method (Strip, Fig 1B), where antibody‐coated RBCs migrate and bind to a band impregnated with antiglobulin.
Figure 1.
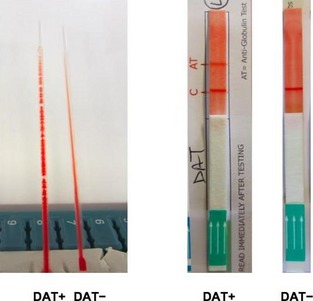
Capillary (A) and immunochromatographic strip (B) method for direct antiglobulin testing showing positive (+) and negative (−) DAT results. Note capillaries are positioned in 60° angle. Legend: C, control band; AT, antiglobulin testing band.
For the various DAT methods, washed RBC suspensions were stored at 4°C for <2 hours, microscopically examined for autoagglutination, and tested according to the manufacturers' instructions and as published and briefly described in Data S1. A DAT+ result indicates the presence of erythrocytic autoantibodies and was used as a criterion for immune‐related erythrocyte destruction. A standard OFT was performed, as described in Data S1.
Assessment of Various DAT Techniques
The various DAT methods applied in the subsequent studies were optimized such that (1) RBCs from DEA 1.1+, but not DEA 1.1‐ dogs strongly reacted with polyclonal anti‐DEA 1.1 alloantibodies, (2) RBCs from DEA 4+ RBCs reacted positively with anti‐DEA 4 alloantibodies, and (3) RBCs from 4 known DAT+ dogs with IMHA, but not those from 18 healthy control dogs gave DAT+ results with both reagent M and V (Data S1).
Statistical Analysis
Data were entered into a spreadsheet11 and grouped into nonanemic and anemic dogs based upon diagnostics according to spherocytosis, agglutination, and DAT results. Data were examined for normal distribution. To determine differences between results obtained with various methods, 2 × 2 tables were used. Kappa (k), the percentage agreement between results of Microtiter‐M and other methods beyond chance, was calculated using a clinical research calculator.12 Based upon OF curves, mean values of hemolysis at OF 5, 50, and 90% were statistically compared with an independent‐sample t‐test.13 Probability values P < .05 were considered statistically significant. Descriptive statistics for the relationships are expressed as mean ± SD (PCV, Hb, age, OF).
Results
Direct Coombs' Test Results in Nonanemic Dogs
Of 105 dogs studied, blood samples from the 59 nonanemic dogs gave uniformly DAT‐ results by all methods used. None of these dogs showed any evidence of anemia, hemolysis, agglutination, or spherocytosis (Table 1).
Table 1.
Hematology results of 105 anemic and nonanemic dogs with negative or positive DAT results (based upon the Microtiter‐M method).
| Anemia | Nonanemic | Anemic | |
|---|---|---|---|
| DAT result | DAT‐ | DAT‐ | DAT+ |
| Dogs tested, n | 59 | 26 | 20 |
| PCV (%, mean ± SD) | 51.1 ± 4.1 | 24.4 ± 6.4 | 17.5 ± 5.6 |
| Median (range) | 53 (40–56) | 25 (11–36) | 16 (9–31) |
| Plasma Hb (g/dL, mean ± SD) | 0.2 ± 0.3 | 0.3 ± 0.2 | 0.5 ± 0.8 |
| Median (range) | 0.0 (0–1.3) | 0.2 (0.1–0.5) | 0.3 (0–3.3) |
| Spherocytosis, n (%) | 0 | 0 | 15 (75) |
| Autogglutination prewashing, n/n tested (%) | 0/59 (0) | 4/26 (15) | 16/20 (80) |
| Increased osmotic fragility, n/n tested (%) | 0/28 (0) | 4/12 (33) | 15/15 (100) |
n, number of dogs; SD, standard deviation; Hb, hemoglobin
Direct Coombs' Test Results in Anemic Dogs
Among the 46 anemic (PCV ≤37%) dogs, 20 were DAT+ and 26 DAT‐ by Microtiter‐M (titer ≥8, used as reference method). Lowest and highest positive titers observed were 8 and 2,048, respectively. The failure to agglutinate at low antiserum dilutions, caused by a relative excess of antiglobulin in relation to antigen, called the prozone effect, was observed in 4 DAT+ dogs. In 2 dogs, the prozone effect was observed up to a titer of 16 with agglutination seen beyond a titer of 64 and 128, and in the 2 other dogs, the prozone effect was seen until a titer of 64 with final agglutination titer seen at 512 and 1,024, respectively.
The degree of DAT positivity (Fig 2), where gradable, varied, but correlated well between techniques: Reagent M gave uniformly equal to twice as strong titers compared with reagent V by Microtiter (Fig 2A). All DAT methods used in this comparative study gave similar results when performed at 37°C, 22°C, or 4°C, but agglutination with Microtiter was more readily interpretable after incubation at 37°C and 4°C. Among 20 DAT+ dogs determined by Microtiter‐M, the Strip revealed weakly positive (1+ to 2+) bands in 12 dogs, strongly positive bands (3+ to 4+) in 7 dogs, and missed 1 dog as positive (Fig 2B). In contrast, the Capillary and Gel (Fig 2C) results of the DAT+ dogs gave uniformly strong agglutination reactions, which were easy to read and interpret. Also, in the Capillary assay, reagent M subjectively gave more and larger beads (agglutination) than reagent V and thus concordant DAT+ results with the following exception: A 4‐year‐old female spayed Newfoundland with anemia and spherocytosis was only transiently (3 days) weakly to moderately positive by the Microtiter‐M (titer 8 and 64) and Capillary test, while all other DAT results were persistently negative. The dog recovered from anemia within 5 days.
Figure 2.
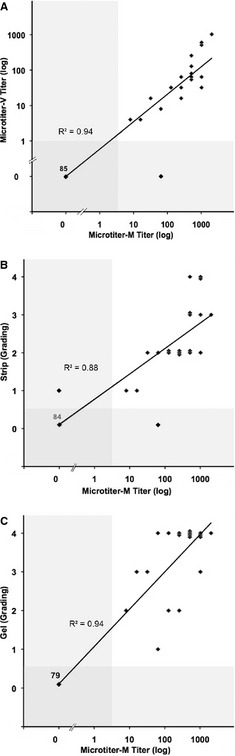
Comparison of DAT results of Microtiter‐M with Microtiter‐V (A), Strip (B) and Gel (C) methods for 105 dogs. Microtiters are expressed logarithmically; Strip and Gel grading is shown linearly. Each bullet (♦) represents results from both methods compared for each sample with a linear regression (—). The shaded area refers to the range of DAT‐ results. Numbers of samples tested are varying, depending on the applied method.
The DAT‐ samples, as determined by Microtiter‐M, from 26 anemic dogs were also DAT‐ by all other methods, with the following 2 exceptions: A 13‐year‐old castrated male Labrador Retriever had an isolated positive Capillary result and was later found to have intestinal leiomyosarcoma, but no hemolysis. A 7‐year‐old spayed female Bernese Mountain Dog had a weakly positive Strip (1+) result with all other DAT results negative and was later diagnosed with malignant histiocytosis and secondary erythrophagocytosis.
Most notably, results obtained using various DAT methods results were highly concordant: 19 of 20 DAT+ dogs by Microtiter‐M also had DAT+ results with every other method. Agreements among Microtiter‐M and V, Gel, Strip, or Capillary had kappa values of ≥0.94 and a 95% confidence interval of 0.85–1, which represent strong agreements between various DAT results (Table S1).
Association between Clinically Suspected IMHA Diagnosis, Spherocytosis, Autoagglutination and DAT Results
The PCV and plasma Hb values of DAT+ dogs were significantly lower and insignificantly higher than those of DAT‐ dogs, respectively, reflecting intravascular hemolytic anemia in some cases (Table 1).
Among 33 anemic dogs considered by clinicians to probably have IMHA (based on clinical impression, autoagglutination on slide, and review of blood smear, but rarely performing a Coombs' test), 13 dogs (39%) were DAT‐ by Microtiter‐M and also by other methods (see 2 singular exceptions above). Further clinical follow‐up, when available, revealed that these DAT‐ dogs had other reasons for anemia, including neoplasia (7), pancreatitis (1), iron deficiency anemia (1), pyometra with surgical complications (1), and cholelithiasis with surgical complications (1). None of these dogs had any evidence of spherocytosis or hemolysis, but 3 of these dogs showed agglutination that did not persist after washing. None of these had any DAT+ results from any outside laboratories.
In addition, 13 of 46 anemic dogs that were not suspected of having IMHA and were DAT‐ had neoplasia (lymphoma [3], liver mass [2], spindle cell sarcoma [1]), surgical complications (4; lung torsion, cruciate ligament, liver mass surgery, trauma), chronic osteoarthritis (1), hemophagocytic syndrome (1), and pure red cell aplasia (1).
None of the 26 DAT‐ dogs had spherocytosis, and 4 dogs exhibited agglutination that was not persistent after washing.
All 15 anemic dogs with marked spherocytosis were DAT+ by Microtiter‐M and all but one also DAT+ by all other DAT methods; 13 also showed autoagglutination that was not persistent.
Autoagglutination was noted in 20 anemic dogs, but was not persistent and thus DAT could be performed and interpreted: 16 agglutinating samples were DAT+ and 4 were DAT‐ by all methods. None of the latter had any evidence of IMHA, and were later diagnosed with pure red cell aplasia, malignant histiocytosis, intestinal leiomyosarcoma, and multisystemic organ failure, respectively.
Effect of Transfusion on DAT Results
Among 46 anemic dogs, 37 dogs received DEA 1.1‐matched RBC transfusions: 11 anemic dogs not suspected of having IMHA were transfused 1–21 days before Coombs' test performance; all were DAT‐. Another 10 dogs clinically suspected of having IMHA, transfused 1 day to 5 years before testing, also had DAT‐ results; they had neither spherocytosis nor hemolysis. Among 20 DAT+ dogs, 16 were transfused: 12 were tested before receiving a transfusion and 4 had been transfused just 1–2 days before testing.
Effect of Immunosuppressive Treatment on Results
Immunosuppressive treatment (glucocorticosteroids and other immunosuppressives) was administered to 22 anemic dogs: 12 of 16 DAT+ and 10 DAT‐ anemic dogs were treated with immunosuppressive drugs for 4 days to 2 years (details of dates, drugs, and dosage were not available) before performing DAT, whereas 4 dogs were immunosuppressed after obtaining DAT+ results.
Follow‐up DAT Results to Monitor IMHA
Retesting of 8 DAT‐ and 9 DAT+ anemic dogs for days to weeks revealed that all DAT‐ dogs persistently remained negative in all DAT analyses. In contrast, all 9 DAT+ dogs remained positive for at least 3 days, and 6 were DAT+ for ≥10 days. Two dogs followed beyond 10 days became DAT‐ by 26 and 96 days (Table 3), respectively, and remained DAT‐. Concomitantly, the anemia resolved and other hematologic parameters normalized. In addition, 34 DAT‐ nonanemic research dogs assessed multiple times (6–96 days; average time between tests 40 days) remained DAT‐. Finally, samples stored at 4°C from 11 DAT+ dogs tested were consistently positive by all DAT methods for 7 days, while all 23 DAT‐ samples tested remained negative over the same time span.
Erythrocytic OFT Results
In contrast to 28 nonanemic dogs, which all had normal OF curves, erythrocytic fragility was increased in 70% of 27 anemic dogs tested, including 15/15 DAT+ and 4/12 DAT‐ dogs (Table 2). Two of the 4 DAT‐ dogs with increased OF had lymphoma and in the other 2, the diagnosis remained unknown. All 12 spherocytic DAT+ dogs tested had increased OF with a right shift of the sigmoid OF curve toward physiologic saline concentrations (Fig 3A). Values of OF 5 and 50% from DAT+ dogs showed a significantly increased degree of lysis compared with values from healthy nonanemic dogs (Table 2). In the 5 DAT+ dogs that also had spherocytosis and agglutination, the OF curve was not only shifted to the right but also flattened. This specific pattern with slightly decreased lysis around OF 90% and increased lysis at near physiologic saline concentration (OF 5%) was not observed in any DAT‐ anemic dogs (Fig 3B).
Table 2.
Osmotic fragility test results: Grouping according to shape of osmotic fragility curves and average saline concentrations for defined lysis values in 55 dogs.
| Osmotic Fragility Curves | Anemia | DAT | n | Osmotic Fragility (mean ± SD) | ||
|---|---|---|---|---|---|---|
| 5% | 50% | 90% | ||||
| Normal | No | − | 28 | 0.50 ± 0.06a | 0.40 ± 0.05a | 0.29 ± 0.10 |
| Yes | − | 8 | 0.54 ± 0.05b | 0.42 ± 0.04b | 0.30 ± 0.08b | |
| Right‐shifted | Yes | − | 4 | 0.65 ± 0.02b, c | 0.52 ± 0.01b | 0.44 ± 0.04b, c |
| Right‐shifted or flattened | yes | + | 15 | 0.76 ± 0.07a, c | 0.57 ± 0.11a | 0.30 ± 0.13c |
n, number of dogs; SD, standard deviation; OF, osmotic fragility.
Difference between mean hemolysis at OF 5% and 50% of 28 nonanemic DAT‐ dogs with a normal OF and 15 anemic DAT+ dogs with an increased OF (P = .002).
Difference between mean hemolysis at OF 5%, 50%, 90% of 8 anemic DAT‐ dogs with a normal OF and 4 anemic DAT‐ dogs with an increased OF (P < .001).
Difference between mean hemolysis at OF 5% (P = .002) and 90% (P = .034) of 4 anemic DAT‐ and 15 DAT+ dogs with an increased OF.
Figure 3.
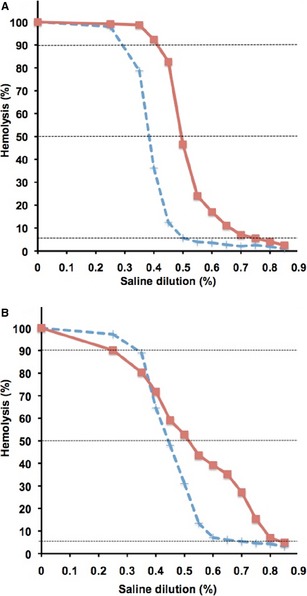
Result curves of Osmotic fragility tests (A) DAT+ ( ) and DAT‐ (
) and DAT‐ ( ) control dog. Note right‐shifted curve for DAT+ dog. (B) Note right‐shifted and flattened curve, same as above.
) control dog. Note right‐shifted curve for DAT+ dog. (B) Note right‐shifted and flattened curve, same as above.
Discussion
Accelerated destruction of RBCs induced by autoantibodies (and complement) is the hallmark of IMHA.10, 20, 21 While autoagglutination and spherocytosis suggest an immune process, only the DAT can specifically document the presence of autoantibodies on RBCs.2, 3, 4, 5, 8 Because of concerns regarding the methodologies, validity, sensitivity, and specificity of the DAT in canine medicine,6, 8, 9, 11 we compared various DAT techniques, including novel in‐clinic tests, using polyclonal canine antiglobulins and other parameters in a select group of anemic and nonanemic dogs prospectively. After establishing and optimizing each technique and confirming that the assays specifically detected erythrocyte‐bound allo‐ and autoantibodies, DAT+ results were observed in 20 of 46 anemic dogs, but not in any of the 59 nonanemic dogs. Moreover, observed DAT results correlated between various methods used as well as with the patients' manifestations and other parameters (eg, spherocytosis) suggestive of IMHA, thereby re‐establishing the DAT's clinical usefulness in the diagnosis of IMHA.
Although there is no DAT gold standard in human and veterinary immunohematology,8, 16, 21 the tube assay6 was the standard laboratory DAT technique in the past. However, this is a tedious procedure prone to varied test execution and interpretation of test results.11 For the studies presented here, we applied a commonly used, but often poorly described, 96‐well microtiter plate technique (with reagent M) as a reference method.2, 11, 13
In the present study, canine‐specific polyclonal antiglobulin M (rabbit) was used as reference reagent and results were nearly identical to reagent V (goat) thereby corroborating their specificity. Specific anti‐IgG, IgM, and complement reagents were not used to further characterize results of DAT+ samples in this study.
While warm autoantibodies generally cause hemolysis, the best temperature at which to perform the DAT is debatable.8, 11 The similarity in DAT+ results might be partly attributable to sequential performance from 22°C to 37°C and 4°C and extended duration of incubation at 4°C. Others22, 23 also observed that DAT sensitivity could improve after a period of cold incubation: optimal performance was found at both 4°C and 37°C, with both polyvalent and monovalent antiglobulins. Thus, incubation at room temperature may be sufficient to screen for DAT+ anemic dogs, which could simplify and quicken the procedure.
Extended titration of antiglobulin to 2,048 not only allowed determination of the strength of the agglutination reaction but also overcame the previously described interfering prozone effect at lower titers.8, 11, 16, 23 However, other DAT methods used here were set up with 1 fixed antiglobulin concentration, which, interestingly, gave very similar results and did not seem to affect sensitivity and specificity of DAT results. In a clinical setting, DAT methods with a single reagent concentration could greatly simplify the process and interpretation of results.
The gel microcolumn method uses a standardized procedure to specifically detect antibodies, offers a grading scale for objective interpretation, and has been widely used in immunohematology in humans2, 24 and more recently in veterinary medicine.8 This technique became the standard method for canine and feline blood typing.19, 25 Initial experiences from our laboratory14 and others16 with the reagent M‐containing gel columns used as DAT were very encouraging. Similarly, in the study reported here, the DAT results with the Gel correlated well with the Microtiter‐M and all other techniques, but unfortunately the Gel is no longer commercially available for dogs.
The capillary microtube test, introduced in the 1950s26 to detect antiglobulins, is still being used in human blood banking as a screening test for auto‐ and alloantibodies.5 The Capillary was quick and easy to perform, requiring no special equipment and only small quantities of reagent M (or V), and results were readily interpretable. Moreover, the obtained Capillary results corresponded very well with all other methods used. Thus, the Capillary method may be well suited to become the first in‐clinic method to detect the presence of autoantibodies on RBCs in dogs.
The Strip is an innovative and entirely new approach to immunohematology, which recently has already proven invaluable for canine and feline blood typing19 and is being developed for in‐clinic or laboratory DAT by the same manufacturer. It utilizes an immunochromatographic strip with impregnated reagent M to bind antiglobulin‐coated RBCs and thus is not an agglutination‐based test. In our experience, the Strip was easy to perform, but the resulting band strengths were frequently weak, which could make interpretation a little difficult. Our DAT+ Strip results, which included the weak bands, correlated well with those of other DAT methods and thus can readily be used as an in‐clinic screening test.
While various DAT method results correlated well among the techniques used, DAT+ results also seemed to be restricted to those dogs with evidence of hemolysis, suspected of having IMHA, or both. Of the 46 anemic dogs, 33 were suspected by clinicians to have clinically IMHA and 20 were found to be DAT+. Further evaluation of the 13 DAT‐ anemic dogs did not reveal any specific features of IMHA, and 11 of these DAT‐ dogs had either evidence of another underlying disorder, mechanism of anemia, or both. Thus, the DAT methods used appear to be sensitive and specific to detect dogs with IMHA. The high specificity for all DAT techniques described in this study was similar to that reported in a recent study comparing 2 methods.11 Most methods, including all used here detecting antiglobulins against RBCs, require previous washing of RBCs, which is a critical technical step, because plasma antibodies not specifically bound to RBCs in an assay can compete and negatively impact results. In humans with IMHA, autoantibodies are not typically removed by repeat washing,27 they are tightly bound to RBCs and special methods have to be used to elute off the autoantibodies from the red cell surface.28 However, there are no specific data on autoantibody binding in dogs and any low‐affinity antibodies being missed by the DAT and causing hemolysis. In the small study presented here, there were no cases of suspected IMHA and DAT‐ results, where there was any other evidence of an immune process causing hemolytic anemia.
Similarly, concerns have been raised that the DAT had to be performed immediately following blood collection. However, in this study, repeat testing of stored chilled EDTA samples from 11 DAT+ dogs gave identical results for 1 week. Hence, samples before emergency or acute treatment can be stored at 4°C and shipped later to a laboratory when deemed necessary to request a DAT without causing false‐negative DAT results.
Autoagglutination is frequently considered diagnostic for IMHA in dogs. In retrospective studies, it has been seen in 42–87% of IMHA cases, and is commonly used as a reason to not perform a DAT.13, 29, 30 However, the degree of agglutination varies, may be unspecific, and may not interfere with Coombs' testing as the agglutination is frequently not persistent. In this study, 20 anemic dogs exhibited autoagglutination in tube or on slide, but in all cases agglutination did not persist after washing, which permitted the performance of the DAT. Moreover, the DAT was positive in 80% (16/20) of agglutinating dogs; the other 4 DAT‐ dogs seemed to have no evidence of hemolysis and had other conditions. Thus, while agglutination suggests a diagnosis of IMHA, it should be followed up with washing and performance of a DAT to document autoantibody‐mediated autoagglutination when possible. Agglutination without washing is shown here to be not diagnostic on its own, with 4 agglutinating dogs having DAT‐ results; they also had no evidence of spherocytosis and hemolysis and were having other diseases. Besides the data in this prospective study, this has also been observed in many other cases.10, 18
Spherocytosis is considered a hallmark feature of IMHA in dogs.12 In this study, severe spherocytosis was seen in 75% of DAT+ and no DAT‐ dogs. Other studies also observed that 67–94% of dogs affected by IMHA had spherocytosis.9, 13, 18, 29, 30 However, spherocytes might also be seen with other acquired hemolytic conditions (only few spherocytes)12 and with hereditary spherocytosis.31 Moreover, in clinical practice, blood smears should be carefully reviewed for spherocytes in the appropriate fields on a slide and preferably analyzed by a veterinary diagnostic laboratory and experienced person or clinical pathologist to avoid false interpretations.32 Thus, while marked spherocytosis is highly suggestive of IMHA, additional cases are discovered by performing a DAT, which can also confirm the immune mechanism of partial intravascular lysis, phagocytosis leading to spherocytosis, or both.30
The sensitivity of DAT for IMHA in dogs is widely debated ranging from 50% to 89%.6, 8, 11, 12, 18, 22, 29, 30 False‐negative DAT results have been attributed to technical difficulties with the assay, such as inappropriate or inadequate strength of antisera, exclusive cold agglutinins, failing to adequately wash RBCs, prozone effect, low affinity or quantities of autoantibodies on RBCs, or presence of drug‐induced autoantibodies.6, 11, 14 However, the specific cause has generally not been determined.8, 14 Optimizing techniques in each laboratory and using standard methods and positive controls could help prevent false‐negative DAT results. Based upon analysis of available clinical information in the study reported here, the DAT detected probably all dogs with IMHA. Thus, DAT‐ dogs should be examined for other causes of anemia. For instance, nearly all anemic dogs found in the authors' laboratory to suffer from hereditary erythrocyte defects had been assumed to be having IMHA and were previously treated for weeks to years for IMHA and the DAT was either negative or never performed (data not shown). The efforts and costs of performing a DAT to confirm a diagnosis of IMHA seem negligible, when considering the costs for other tests, unnecessary treatment, and potential adverse effects of immunosuppressive agents in an anemic dog with other diseases.
Many clinicians believe that administration of immunosuppressive treatment will cause immediately false DAT‐ results,9, 14, 16 although the dose and duration of immunosuppressive treatment needed to affect DAT results are poorly documented.11, 14, 17, 20 This potential drug interference has been used as another reason for not performing a DAT. However, in this study, anemic dogs treated with immunosuppression had DAT+ results that remained positive for days to weeks, as previously observed by others.12, 17 Thus, contrary to common belief, Coombs' testing is recommended in anemic dogs even when already treated. Of course once an animal responds to treatment and goes into remission (normalizes its PCV and has no more evidence of hemolysis), the DAT becomes negative as shown here and elsewhere.11, 17
Various reports suggested that previous blood transfusions, especially 1–21 days before performing a DAT, could cause false‐positive results.14, 15 However, no effect of transfusion on DAT could be documented here in this and other studies.11, 30 This is clinically important, as probably over half of IMHA patients require transfusion during acute management.13, 29
The OFT has been developed as a screening test to detect RBC membrane defects, such as hereditary spectrin deficiency and stomatocytosis.31, 33 We also described cats with massively increased OF presumably because of a membrane defect.34 In light of the past perceived difficulties with the DAT, increased erythrocytic OF has been proposed as a diagnostic test result for canine IMHA. It was assumed that spherocytes or antibody‐coated erythrocytes could not withstand lower saline concentrations as well as normal canine RBCs.12 An increased OF was reported in 85% of dogs previously diagnosed with IMHA.6 In the prospective study reported here, all DAT+ dogs tested, but also some DAT‐ dogs had increased erythrocytic OF. Thus, together with previously reported hereditary and probably other acquired RBC membrane defects resulting in increased RBC fragility, the OFT is not specific for IMHA. Moreover, several DAT+ dogs with autoagglutination and spherocytosis had not only a right‐shifted but also a flattened sigmoid curve: resistant younger RBC population and fragile microspherocytes may explain this unique pattern. However, this complete OFT is fairly cumbersome to perform and may be difficult to interpret, particularly if baseline hemolysis exists; thus, we cannot recommend this test for the diagnosis of IMHA.
This study included only a small number of animals and cases tested under special laboratory conditions, blood smears were analyzed by clinical pathologists, and the person performing DAT was not blinded to other clinical results or other test outcomes. Therefore, further larger studies are needed, with specific protocols, to confirm these promising findings.
In conclusion, this study shows an excellent correlation among various DAT techniques with the reference Microtiter‐M method, clinical signs of hemolytic anemia, spherocytosis, and true agglutination. As in human medicine, the DAT remains the most sensitive and specific tool to specifically diagnose canine IMHA and seems resilient to storage, immunosuppression, and transfusion artifacts.10, 11, 35 The Capillary and Strip methods are novel and promising simple in‐clinic screening tools for IMHA in dogs.
Supporting information
Data S1. Detailed Methods.
Table S1. Agreement of DAT results among Microtiter, Gel, Strip, and Capillary method as well as spherocytosis, agglutination, and osmotic fragility test results, using the Microtiter‐M method as reference and interrater agreement with Cohen's Kappa value.
Table S2. Laboratory results of one DAT+ dog with IMHA monitored for 126 days.
Acknowledgments
Supported, in part, by the National Institutes of Health # OD 010939. Alvedia, Lyon, France, kindly provided the chromatographic DAT and DEA 1.1 typing strips for these studies. The Clinical Laboratory at the Veterinary Hospital of the University of Pennsylvania and referring clinicians are thanked for their assistance with submitting samples for these studies.
This study was carried out at the University of Pennsylvania, in part, as fulfillment of the doctoral thesis work of Ladina L. Caviezel at the University of Zürich, Switzerland.
Conflict of Interest Declaration: Urs Giger has been scientifically advising ALVEDIA, but without pay. The typing and Coombs' kits and reagents were received as a generous gift from ALVEDIA.
Footnotes
Wright Giemsa stain, Diff Quick Fix, Medion Diagnostics, Düdingen, Switzerland
DPBS (1x): Dulbecco's Phosphate Buffered Saline, 1000 mL, Gibco by Life Technologies Corporation, Grand Island, NY
Clay Adams Sero‐Fuge 2002 Series Centrifuge, BD Diagnostics, Franklin Lakes, NJ
HemoCue® AB‐ A Quest Diagnostics Company, Ängelholm, Sweden.
96‐well round bottom microtiter plate, Linbro, Flow Laboratories Inc., McLean, VA
MP Biomedicals website. Canine Coombs' Reagent 5 mL, MP Bio, Irvine, CA. Available at: http://www.mpbio.com/CH/Pages/Product.aspx?pid=08646351. Accessed April 22, 2012.
VMRD: Veterinary Medical Research and Development website. Canine Coombs' Reagent 5 mL, VMRD, Inc, Pullman, WA. Available at: http://www.vmrd.com/Pages/ProductDetail.aspx?productId=392-5&PI=0&RPP=25. Accessed April 22, 2012.
Capillary: Micro‐hematocrit capillary tubes‐ Fischer Scientific, Pittsburgh, PA
BioRad‐ DiaMed website. ID‐Gel canine antibody screening system and antiglobulin test, provided by DiaMed, Cressier‐sur‐Morat, Switzerland. Available at: http://www.diamed.ch/pdfs/B004024_50540_07.11_GEFISP.pdf. Accessed April 22, 2012.
Alvedia website. Blood typing quick test and immunochromatographic Antiglobulin Strip, provided by Alvedia, Lyon, France. Available at: http://www.alvedia.com/fr/QT. Accessed April 22, 2012.
Excel 2004, Microsoft Ltd, Reading, UK
IBM SPSS Statistics 21, Armonk, NY
Jackson KV, Withnall E, Giger U. Initial assessment of a novel gel column Coombs' test to detect auto‐ and alloantibodies in dogs. J Vet Intern Med 2007;21:623 (Abstract)
References
- 1. Coombs RR, Mourant AE, Race RR. A new test for the detection of weak and incomplete Rh agglutinins. Br J Exp Pathol 1945;26:255–266. [PMC free article] [PubMed] [Google Scholar]
- 2. Rumsey DH, Ciesielski DJ. New protocols in serologic testing: A review of techniques to meet today's challenges. Immunohematology 2000;16:131–137. [PubMed] [Google Scholar]
- 3. Rosse WF. The antiglobulin test in autoimmune hemolytic anemia. Annu Rev Med 1975;26:331–336. [DOI] [PubMed] [Google Scholar]
- 4. Bain BJ, Bates I, Laffan MA, Lewis SM. Dacie and Lewis Practical Haematology, 11th ed. Edinburgh: Elsevier Churchill Livingstone; 2012:229–244. [Google Scholar]
- 5. Widmann FK, Barnes A, Case J, et al. Technical Manual, 8th ed. Washington DC: AABB; 1981:422–516. [Google Scholar]
- 6. Slappendel RJ. The diagnostic significance of the direct antiglobulin test (DAT) in anemic dogs. Vet Immunol Immunopathol 1979;1:49–59. [DOI] [PubMed] [Google Scholar]
- 7. Piek CJ, Junius G, Dekker A, et al. Idiopathic immune‐mediated hemolytic anemia: Treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med 2008;22:366–373. [DOI] [PubMed] [Google Scholar]
- 8. Wardrop KJ. Coombs' testing and its diagnostic significance in dogs and cats. Vet Clin North Am Small Anim Pract 2012;42:43–51. [DOI] [PubMed] [Google Scholar]
- 9. Werner LL. Coombs' positive anemia in the dog and cat. Compend Cont Educ Pract Vet 1980;11:96–102. [Google Scholar]
- 10. Giger U. Regenerative anemia caused by blood loss or hemolysis In: Ettinger JE, Feldman EC, eds. Textbook of Veterinary Internal Medicine, vol. 2. St. Louis, MO: Elsevier Saunders; 2005:1886–1907. [Google Scholar]
- 11. Overmann JA, Sharkey LC, Weiss DJ, Borjesson DL. Performance of 2 microtiter canine Coombs' tests. Vet Clin Pathol 2007;36:179–183. [DOI] [PubMed] [Google Scholar]
- 12. Slappendel RJ. Interpretation of tests for immune‐mediated blood diseases. Curr Vet Ther 1986;IX:498–505. [Google Scholar]
- 13. Weinkle TK, Center SA, Randolph JF, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune‐mediated hemolytic anemia in dogs: 151 cases (1993–2002). J Am Vet Med Assoc 2005;226:1869–1880. [DOI] [PubMed] [Google Scholar]
- 14. Honeckman AL, Knapp DW, Reagan WJ. Diagnosis of canine immune‐mediated hematologic disease. Compend Cont Educ Pract Vet 1996;18:113–125. [Google Scholar]
- 15. Tan E, Bienzle D, Shewen P, et al. Potentially antigenic RBC membrane proteins in dogs with primary immune‐mediated hemolytic anemia. Vet Clin Pathol 2012;41:45–55. [DOI] [PubMed] [Google Scholar]
- 16. Piek CJ, Teske E, van Leeuwen MW, Day MJ. Good agreement of conventional and gel‐based direct agglutination test in immune‐mediated haemolytic anaemia. Acta Vet Scand 2012;54:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mason N, Duval D, Shofer FS, Giger U. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune‐mediated hemolytic anemia in dogs: A randomized controlled clinical trial. J Vet Intern Med 2003;17:206–212. [DOI] [PubMed] [Google Scholar]
- 18. Klag AR, Giger U, Shofer FS. Idiopathic immune‐mediated hemolytic anemia in dogs: 42 cases (1986‐1990). J Am Vet Med Assoc 1993;202:783–788. [PubMed] [Google Scholar]
- 19. Seth M, Jackson KV, Winzelberg S, Giger U. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am J Vet Res 2012;73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Day MJ. Serial monitoring of clinical, haematological and immunological parameters in canine autoimmune haemolytic anaemia. J Small Anim Pract 1996;37:523–534. [DOI] [PubMed] [Google Scholar]
- 21. Morley P, Mathes M, Guth A, Dow S. Anti‐erythrocyte antibodies and disease associations in anemic and nonanemic dogs. J Vet Intern Med 2008;22:886–892. [DOI] [PubMed] [Google Scholar]
- 22. Jones DR, Gruffydd‐Jones TJ, Stokes CR, Bourne FJ. Investigation into factors influencing performance of the canine antiglobulin test. Res Vet Sci 1990;48:53–58. [PubMed] [Google Scholar]
- 23. Warman SM, Murray JK, Ridyard A, et al. Pattern of Coombs' test reactivity has diagnostic significance in dogs with immune‐mediated haemolytic anaemia. J Small Anim Pract 2008;49:525–530. [DOI] [PubMed] [Google Scholar]
- 24. Lapierre Y, Rigal D, Adam J, et al. The gel test: A new way to detect red cell antigen‐antibody reactions. Transfusion 1990;30:109–113. [DOI] [PubMed] [Google Scholar]
- 25. Giger U, Stieger K, Palos H. Comparison of various canine blood‐typing methods. Am J Vet Res 2005;66:1386–1392. [DOI] [PubMed] [Google Scholar]
- 26. Chown B, Lewis M. The slanted capillary method of rhesus blood‐grouping. J Clin Pathol 1951;4:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petz LD, Garratty G. Immune Hemolytic Anemias, 2nd ed. Philadelphia, PA: Churchill Livingstone; 2004:204. [Google Scholar]
- 28. American Association of Blood Banks . AABB Technical Manual, 14th ed. Am Assoc Blood Banks, A. Brecher; Bethesda, MD; 2002:709. [Google Scholar]
- 29. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune‐mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med 2002;16:504–509. [DOI] [PubMed] [Google Scholar]
- 30. Burgess K, Moore A, Rand W, Cotter SM. Treatment of immune‐mediated hemolytic anemia in dogs with cyclophosphamide. J Vet Intern Med 2000;14:456–462. [DOI] [PubMed] [Google Scholar]
- 31. Slappendel RJ, van Zwieten R, van Leeuwen M, Schneijdenberg CT. Hereditary spectrin deficiency in Golden Retriever dogs. J Vet Intern Med 2005;19:187–192. [DOI] [PubMed] [Google Scholar]
- 32. Lanaux TM, Rozanski EA, Simoni RS, et al. Interpretation of canine and feline blood smears by emergency room personnel. Vet Clin Pathol 2011;40:18–23. [DOI] [PubMed] [Google Scholar]
- 33. Pinkerton PH, Fletch SM, Brueckner PJ, Miller DR. Hereditary stomatocytosis with hemolytic anemia in the dog. Blood 1974;44:557–567. [PubMed] [Google Scholar]
- 34. Kohn B, Goldschmidt MH, Hohenhaus AE, Giger U. Anemia, splenomegaly, and increased osmotic fragility of erythrocytes in Abyssinian and Somali cats. J Am Vet Med Assoc 2000;217:1483–1491. [DOI] [PubMed] [Google Scholar]
- 35. Piek CJ. Canine idiopathic immune‐mediated haemolytic anaemia: A review with recommendations for future research. Vet Q 2011;31:129–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Detailed Methods.
Table S1. Agreement of DAT results among Microtiter, Gel, Strip, and Capillary method as well as spherocytosis, agglutination, and osmotic fragility test results, using the Microtiter‐M method as reference and interrater agreement with Cohen's Kappa value.
Table S2. Laboratory results of one DAT+ dog with IMHA monitored for 126 days.


