Abstract
It has been two decades since 1993 when research on the biology of rotifer aging was last reviewed by Enesco. Much has transpired during this time as rotifer biologists have adapted to the “omics” revolution and incorporated these techniques into the experimental analysis of rotifers. Rotifers are amenable to many of these approaches and getting adequate quantities of DNA, RNA, and protein from rotifers is not difficult. Analysis of rotifer genomes, transcriptomes, and proteomes is rapidly yielding candidate genes that likely regulate a variety of features of rotifer biology. Parallel developments in aging biology have recognized the limitations of standard animal models like worms and flies and that comparative aging research has essentially ignored a large fraction of animal phylogeny in the lophotrochozoans. As experimentally tractable members of this group, rotifers have attracted interest as models of aging. In this paper, I review advances over the past 20 years in the biology of aging in rotifers, with emphasis on the unique contributions of rotifer models for understanding aging. The majority of experimental work has manipulated rotifer diet and followed changes in survival and reproductive dynamics like mean lifespan, maximum lifespan, reproductive lifespan, and mortality rate doubling time. The main dietary manipulation has been some form of caloric restriction, withholding food for some period or feeding continuously at low levels. There have been comparative studies of several rotifer species, with some species responding to caloric restriction with life extension, but others not, at least under the tested food regimens. Other aspects of diet are less explored, like nutritional properties of different algae species and their capacity to extend rotifer lifespan. Several descriptive studies have reported many genes involved in rotifer aging by comparing gene expression in young and old individuals. Classes of genes up or down-regulated during aging have become prime targets for rotifer aging investigations. Alterations of gene expression by exposure to specific inhibitors or RNAi knockdown will probably yield valuable insights into the cellular mechanisms of rotifer life extension. I highlight major experimental contributions in each of these areas and indicate opportunities where I believe additional investigation is likely to be profitable.
Rotifers as aging models
Aging research could benefit from new invertebrate models that can identify new genes/pathways associated with human aging and identify interventions capable of life extension (Austad 2009). The traditional non-vertebrate metazoan model systems C. elegans and D. melanogaster are more closely related to each other than originally thought (both belong to the Ecdysozoa superphylum) (Dunn et al. 2008), and both have undergone extensive gene loss since they and humans shared a common ancestor. In contrast, more than 10% of the genes identified in the phylum Cnidaria have clear human homologs not found in the worm and fly genomes (Kortschak et al. 2003). It is likely that new genes with relevance to human aging are yet to be identified in non-ecdysozoan animals.
Among lophotrochozoans, rotifers are among the most experimentally tractable, with a rich body of natural history and ecological research going back hundreds of years. They make up one of the largest micro-invertebrate phyla in terms of biomass, ecological importance, and number of species, and are major components of inland and coastal aquatic ecosystems throughout the world (Wallace et al 2006). Most rotifers are smaller than 1mm, but have ganglia, muscles, photo-, chemo-, and tactile sensory organs; structures for crawling, feeding, and swimming; digestive and secretory organs; and ovaries. Development is eutelic and direct, and like C. elegans most rotifers contain about 1,000 nuclei.
In addition to their short generation times and ease of culturing monogonont rotifers have specific features that make them attractive models for aging studies, including: 1) a history of aging research extending back nearly a century; 2) asexual propagation of clonal cultures, so that experiments can take place in the same genetic background, without the potential inbreeding depression imposed on isogenic lines; 3) sexual and asexual reproduction in the same genetic background; 4) haploid males, allowing direct expression of alleles and simplifying crosses in the absence of complex marker chromosomes; 5) production of highly stable diapausing embryos; 5) many closely related strains and species that differ in life history traits; and 6) a well developed tool box of genetic resources including partially sequenced genomes and transcriptomes, and a working RNAi protocol.
A prominent hypothesis in aging biology is that aging rate (the rate of mortality due to aging) and longevity (a combination of the aging rate and the time until onset of age-related mortality) is regulated directly and indirectly by signaling networks, including nutrient-sensing mitogen-activated, stress-responsive, and DNA damage signaling pathways. Fundamental processes underlying aging include damage and repair of macromolecules, regulation of cell proliferation, differentiation and programmed cell death, control of cellular bioenergetics, and control of genomic stability. Identifying pathways regulating aging will likely provide molecular targets for intervention to slow aging and extend lifespan (Hadley et al. 2005). Moreover, the expression of genes in these pathways could be modified by diet, stress, reproductive mode, and diapause, or directly altered through RNAi or inhibitors, thereby changing the aging rate and lifespan. Comparing gene expression in closely related species which differ in their aging rates will yield insight into molecular targets for intervention.
Quantifying aging rates in rotifers
There are several ways to quantify aging rates in rotifers. Many rotifer species have long been used in laboratory cohort life table experiments (e.g. Gilbert 1963, Snell and King 1977), mostly to examine ecological and evolutionary questions about diet, temperature, salinity, adaptation, and speciation. Life table statistics like mean lifespan, age-specific mortality and reproduction rates important in aging studies also were calculated in most these studies. Consequently, we know a fair amount about how survival and reproduction rates of several rotifer species vary in different environments. In addition to the life table statistics, there are structural, behavioral, and metabolic changes that are associated with rotifer aging (Snell et al. 2012). An important tissue is the rotifer vitellarium, a yolk gland that supplies yolk to developing embryos (Gilbert 1983). Female brachionids are born with their lifetime supply of oocytes and initiate the maturation of these sequentially throughout their lifetime. Metabolic activity of the vitellarium is therefore essential for rotifer reproduction and likely declines with age, following the age-specific decline in reproduction. Identification of genes that are effective biomarkers of vitellarium metabolic activity might be useful in quantifying rotifer aging.
Genes involved in aging
It is now widely believed that aging is regulated by specific genes conserved from yeast to mice (Bartke 2008). Key genes have been identified in several signaling pathways, including the insulin growth factor and insulin-activated pathways, mitogen-activated and stress-activated pathways, the DNA damage response, and the FOXO, Sirtuin, TOR, P13-K, and AMPK pathways. These have become prime targets in RNAi experiments to examine whether knock down can extend life in C. elegans (Lee et al. 2002, Hamilton et al. 2005). Many of these same genes have been found in rotifer transcriptomes (http://forest.mbl.edu/cgi-bin/site/bmanjavacas4), enabling us to examine whether RNAi knock down of these genes extends rotifer lifespan (Snell et al. 2010). Identifying evolutionarily conserved genes in ecdyszoan and lophotrochozoan phyla that regulate organismal aging could provide attractive targets for pharmacological intervention in mammalian aging.
Dietary restriction and aging in model systems
While the search for genes that regulate aging has made remarkable progress, dietary restriction (DR) remains the most reliable and consistent way to extend lifespan in animals (Sinclair 2005, Bordone and Guarente 2005, Mair and Dillin 2008). The evolutionary survival of organisms depends on their ability to adapt to changes in food availability. One such adaptation is the ability to store energy as glycogen and lipids for use when food supply is scarce and organisms are faced with starvation (Bordone and Guarente 2005). Energy stores can be mobilized for short-term use by changes in gene expression that are adaptive. Regulatory proteins can sense scarcity and generate an appropriate physiological response to produce adequate supplies of nutrients to maintain metabolism. Overnight starvation of cultured cells causes important metabolic changes, including glycogen mobilization, fat mobilization, gluconeogenesis, and ketogenesis (Bordone and Guarente 2005). In addition, there are changes in hormone levels like insulin, glucagon, adipokines, and glucocorticoids that are coupled with changes in the concentration of regulatory proteins like peroxisome proliferator-activated receptor (PPAR)γ, forkhead protein (FOXO), and silent information regulator-1 (SIRT1). The SIRT1 gene belongs to the sirtuin gene family that is involved in nutrient sensing, DNA damage sensing and responses, and cancer in mammals (Bordone and Guarente 2005). Most sirtuins act in the same pathway that is regulated by TOR (target of rapamycin) genes and extend life by similar mechanisms as dietary restriction (Medvedik et al. 2007).
The mechanisms underlying life extension through dietary restriction are still highly uncertain (Merry, 2002). The hormesis hypothesis (Sinclair 2005) proposed that DR works in most animals because low caloric intake is mildly stressful and provokes a survival response based on the up- and down-regulation of particular genes. This altered pattern of gene expression helps the organism to survive starvation by altering metabolism. The increased defenses against the damages of inadequate nutrition also confer resistance to the causes of aging. One line of supporting evidence comes from the role of reactive oxygen species (ROS) in aging.
ROS damage accumulates in older animals, which contain higher quantities of oxidized lipids and proteins as well as more damaged/mutated DNA, particularly in the mitochondrial genome (Droge 2003, Dufour and Larsson 2004). DR restricted animals have less ROS mediated damage, including lipid peroxidation and loss of membrane fluidity, and oxidatively damaged proteins and DNA (Merry 2002; Barja 2004). There is ongoing debate as to whether DR works primarily by decreasing ROS production or increasing ROS defenses and repair. It is known that DR up-regulates classic stress response genes like SOD, HSP60, and HSP70 due to hormesis (Sinclair 2005). A clear prediction of the hormesis hypothesis is that DR animals also should be more resistant to many types of stressors (heat, osmotic stress, UV, starvation, metals, pesticides) because their stress response genes are induced.
Dietary restriction in rotifers
Dietary restriction in many rotifer species extends mean lifespan (Kirk 2001). He argued that in natural rotifer populations, episodic periods of starvation are common and rotifers respond by reallocating their reproductive effort. Species that reduced reproduction, extended their mean lifespan, maximum lifespan, reproductive lifespan, and mortality rate doubling time, in contrast to those that continued reproducing and experienced reductions in these parameters. The rotifer response to DR is therefore associated with changes in allocation of reproductive effort. Kirk (2001) used cohort life table data to compare the responses to DR in 10 rotifer species. Most were herbivores fed unialgal diets, but two Asplanchna species were carnivorous. DR caused >100% increase in mean lifespan of A girodi and Philodina acuticornis. The extension of lifespan in response to DR was more modest (13–29%) in B. calyciflorus, Euchlanis dilitata and A brightwelli. In contrast, DR reduced mean lifespan in B. plicatilis, Synchaeta pectinata, and Keratella testudo. Mortality rate doubling time (MRDT) is a good estimator of senescence rate (Ricklefs 2008) and changes in this variable also differed markedly among rotifer species. DR produced a 20% MRDT decline in S. pectinata (faster senescence) and a 147% increase in A girodi (slower senescence). Yoshinaga et al. performed a series of experiments examining the effects of dietary restriction on asexual reproduction of Brachionus plicatilis. Diet restricted rotifers were fed 1 or 3 hours daily, and then starved until the following day, whereas control animals were fed throughout their life (Yoshinaga et al. 2000). Diet restricted rotifers matured later and produced their first offspring at an older age than control animals. Diet restriction decreased lifetime fecundity to less than half that of the non-starved control. The reproductive period and lifespan were 2–3 times longer in the starved animals than in the control animals. A trade-off between lifetime fecundity and lifespan was interpreted as an adaptive response to starvation.
Yoshinaga et al. (2003) applied dietary restriction in a different way. Dietary restricted rotifers were fed until the ages of 1–4 days, and then starved the rest of their lives. The control group was fed continuously. Rotifers fed 1 or 2 days and then starved lived 48% and 56% longer than controls, respectively, but those fed 3 or 4 days and then starved lived a shorter lifespan. Generally, the more offspring produced before food deprivation, the shorter the subsequent survival under starvation, which they interpreted as the cost of reproduction. In a second experiment, newborns were starved until age 1–5 days and then subsequently fed. The lifespan of rotifers starved up to 3 days were similar to non-starved controls, so no life extension was observed with this dietary restriction method. Although the starved rotifers began reproducing once feeding resumed, their lifetime fecundity was significantly lower than controls.
The role of PI3-kinase in regulating the insulin/IGF-1 pathway and lifespan of B. plicatilis was examined by Yoshinaga at al. (2005). They exposed rotifers to 1–100 nM of LY294002, a morpholine derivative of quercetin and a reversible inhibitor of PI3-kinase. Exposure to 1 nM resulted in life extension of 30% compared to unexposed controls, but 10 and 100 nM exposures had no effect on lifespan. No effects on lifetime fecundity were observed, so life extension could not be attributed to a reproduction-lifespan trade-off. These results suggest that PI3-kinase and the insulin/IGF-1 pathway is somehow involved in regulation of lifespan in B. plicatilis.
Weithoff (2007) examined effect of DR on Elosa worallii and Cephalodella sp., rotifers collected from acidic mining lakes (pH 2.7) in Germany. Both rotifers were fed Chlamydomonas algae for 2 days, then transferred to starvation conditions for varying lengths of time and age-specific survivorship and fecundity was recorded until death. Dietary restriction did not produce life extension in Cephalodella, but did so in E. worallii. Fifteen days of food deprivation increased mean lifespan about 45% compared to the continuously fed controls. Reproduction was suspended during dietary restriction and resumed once food was restored.
Dietary restriction increased lifespan of Brachionus calyciflorus (Ozdemir 2009). B. calyciflorus was fed Chlorella at 1.5×106 cells/ml intermittently at intervals of 12, 24, 36, 48, 60 and 72 h. Mean lifespan increased 75% to 14 days in the 36 h treatment, whereas reproductive lifespan increased by 52% to 11 d, although lifetime fecundity decreased by 33%.
Brachionus plicatilis on a DR diet by feeding every other day lived 53% longer than controls fed daily, with a mean DR lifespan of 13.5 days versus controls with a 8.8 day lifespan (Kaneko et al. 2011). The lifetime fecundity of DR treated females was 13.5 daughters, 73% lower than the 23.3 daughters produced by controls. DR treated females had 31% greater oxidative stress resistance to 10mM paraquat exposure than controls. DR also produced elevated mRNA levels of catalase and Mn SOD compared to controls. Perhaps most interesting was the demonstration of mother to daughter transmission of DR-induced life extension for the first time in any animal. Daughters from the DR-treated mothers lived 20% longer than those from control mothers, regardless of the feeding regime for the daughters. Daughters from the DR-treated mothers also had greater resistance to oxidative stress from birth and higher mRNA levels of catalase, but not Mn SOD.
Genes differentially expressed genes in dietary restricted B. plicatilis were identified by Oo et al. (2010). Controls were fed the alga Nannochloropsis oculata continuously whereas the DR treatment was fed only 3 h per day. A subtracted cDNA library between these two treatments revealed 163 differentially expressed sequence tags,109 were putative gene sequences identified in public databases and 54 were unknown ESTs. Assembly of the 109 ESTs revealed 38 differentially expressed genes, 29 of which were up-regulated on a calorically restricted diet. These genes represented a variety of classes including transcription, RNA biosynthesis, metabolism, DNA synthesis, and cell structure, transport and division.
Food limitation in stationary phase of population growth mimics dietary restriction (Ozaki et al. 2010). Working with B. plicatilis populations, these authors showed that rotifers dietary restricted for 3 or 4 days, then exposed to hypoxia (<0.1% normal oxygen levels) for 11 or 7.5 h survived as much as 52% better than controls fed ad libitum. They argued that DR causes a shift from aerobic to anaerobic metabolism, contributing to hypoxia tolerance. They quantified the expression of three genes in the glycolytic pathway under DR: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), enolase (ENO), and phosphoglycerate mutase (PGM). Relative mRNA levels of GAPDH under DR were 1.7–3.6X greater than ad libitum controls. Similarly, ENO mRNA levels were 1.5–3X higher, but PGM levels under DR were not significantly different from controls. A variety of animals up-regulate expression of glycolysis genes under anaerobic conditions, and B. plicatilis apparently also possesses this metabolic flexibility.
Gribble and Mark Welch (2012) discriminated the effects of different ways of applying dietary restriction to B. manjavacas: chronic caloric restriction (CCR, chronic low food levels) and intermittent fasting (IF, alternating days of feeding and starvation). IF increased lifespan for both amictic and unfertilized mictic females. In contrast, CCR (10%–50% of ad libitum feeding) significantly increased lifespan of amictic females, but not unfertilized mictic females. This suggests a threshold of caloric restriction of at least 50% ad libitum feeding before life extension is observed. The greatest life extension of 150–171% occurred with IF of both amictic and mictic females. DR of amictic females delayed death at young ages, rather than preventing death in middle ages or extending maximum lifespan. DR of mictic females greatly increased lifespan of a few long-lived individuals. No tradeoff was observed between lifetime fecundity and lifespan with DR, suggesting no resource allocation conflict between growth, maintenance and reproduction.
The conclusion to be taken from this work on DR in rotifers is that different levels of DR can lead to different results, even in the same species. Moreover, different populations of the same species can respond differently to the same levels of DR. Thus it is difficult to categorically say that a rotifer species does or does not respond to DR with lifespan extension. Future progress will require a better understanding of the mechanism of DR before we can understand this variability in DR response among species and feeding regime.
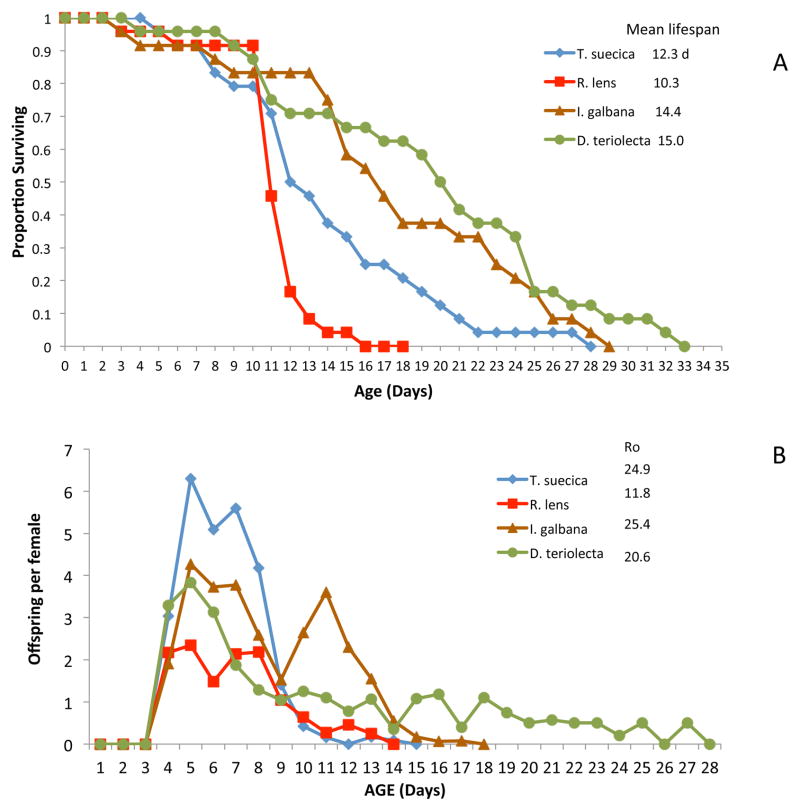
Life extension from DR results from manipulating the quantity of food available to test animals. There could be similarly interesting lifespan effects from manipulating food quality. A facile way to compare the qualitative effects of food on rotifer lifespan is to feed different species of microalgae. Using the methods of Snell et al. (2012), we fed B. manjavacas four different algal diets of equal mass (300 μg dry wt/ml) and compared survival and fecundity patterns (Figure 1). Mean lifespan at 22°C ranged from 10.3 days on a diet of Rhodomonas lens to 15.0 days for Dunaliella tertiolecta. Rotifer lifespan on this latter diet was consistently 20–25% longer than on the control diet of Tetraselmis suecica. Reproduction patterns were markedly different on different algae diets as well. On a T. suecica diet, reproduction peaked at age 5 days and rapidly declined so that by day 12, it was finished. On a D. tertiolecta diet, reproduction likewise peaked on day 5, but at only about 2/3 of the Tetraselmis maximum, and reproduction continued until day 28. This suggests that some algae diets can extend rotifer lifespan primarily by allowing reproduction to continue longer. We are currently engaged in biochemical analyses trying to answer the key question of why rotifers are longer lived on a Dunaliella diet than Tetraselmis.
Figure 1. Comparative rotifer lifespan on different algae diets.
Effects of different algae diets on the survival (A) and reproduction (B) of Brachionus manjavacas. Algae tested: Tetraselmis suecica, Rhodomonas lens, Isochrysis galbana, Dunaliella tertiolecta. Ro is mean offspring produced by a female throughout her life.
Increasing the renewal rate of microalgal chemostat cultures leads to increased nutrient and light availability, which in turn causes changes in the biochemical composition of microalgae cells (Ferreira et al. 2011). Changes in the biochemical composition of algae could have marked effects on rotifer survival and reproduction. For example, the productivity of Brachionus plicatilis cultures increased more than two-fold with the increase of Isochrysis galbana renewal rate from 10% to 50% of culture volume per day (Ferreira et al. 2011). I. galbana protein content more than doubled to 36% when renewal rate was increased from 10% to 40%. Carbohydrate content was reduced by half to 15% and lipid content remained steady at about 45%. Maximum content of polyunsaturated fatty acids was reached in rotifers fed I. galbana from the renewal rate of 40%. These are dramatic changes in algae nutritional quality with changes in culture renewal rate, but their effect on rotifer lifespan was not determined in this work. Similar increases in protein content and decreases in carbohydrate content were observed when renewal rates of Dunaliella tertiolecta cultures were manipulated (Fábregas et al. 1995). D. tertiolecta protein content more than doubled from 30% to 70% when culture renewal rate was increased from 10% to 50%. Carbohydrate content was reduced from 60% to 10% and lipid content remained steady at about 15%. In Drosophila, protein:carbohydrate ratio was more important for life extension than overall calories consumed (Lee et al. 2008). Fly lifespan increased as P:C ratios decreased and as caloric intake decreased. They concluded that nutritional composition of diets, rather than restriction of dietary intake, was the main controlling variable for lifespan extension.
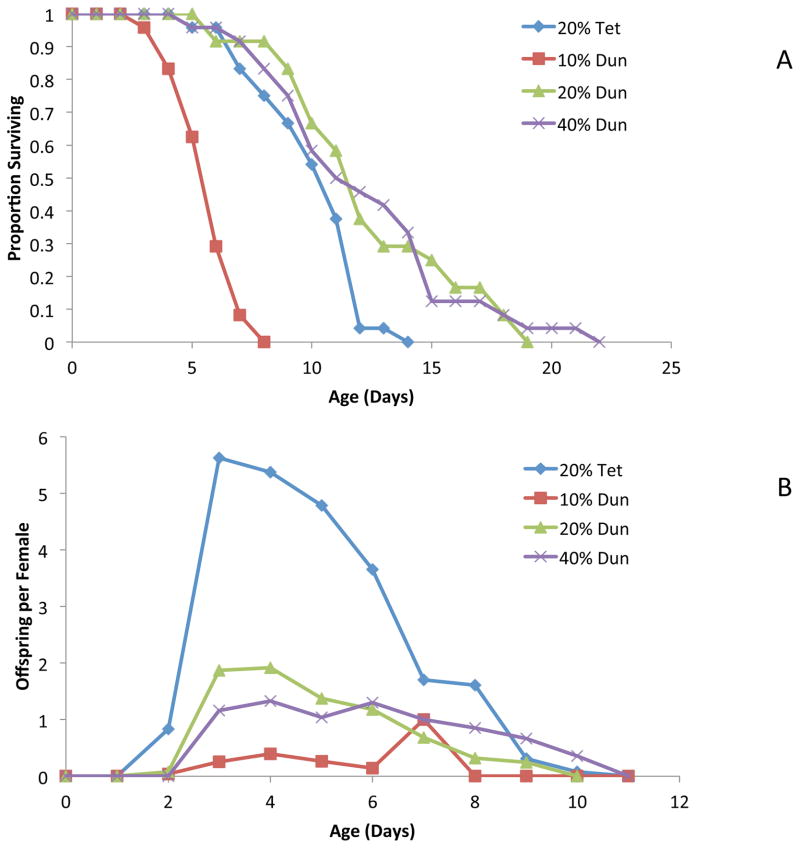
As might be predicted, dramatic changes in algae biochemical composition with culture replacement rate could cause marked changes in rotifer survival. We fed B. manjavacas a diet of D. tertiolecta (300 μg dry wt/ml) cultured in chemostats with daily medium replacement rates of 10–40% (Figure 2). Rotifer lifespan was compared to lifespan on a control diet of T suecica cultured with a 20% replacement rate. As we reported above, a rotifer diet of Dunaliella cultured at 20% replacement rate produced 25% longer lifespan than a Tetraselmis diet. Moreover, when rotifers were fed Dunaliella from cultures with a 10% replacement rate, we observed a mean lifespan of 4.8 days, as compared to 11.4 days for Dunaliella from 20 and 40% replacement rate cultures. Rotifer offspring production on a diet of Dunaliella from 10% replacement cultures also was diminished by 87%. Rotifer reproduction was initiated on day 3 for all Dunaliella replacement rates, but peak reproduction occurred on day 3 for 20 and 40% replacement as compared to day 7 for the 10% replacement rate treatment. Clearly, manipulating algae culture conditions dramatically changes their nutritional quality for rotifers, affecting both survival and reproduction. More experimental analysis is needed to identify the causative agents of these changes.
Figure 2. Algae culture replacement rate affects rotifer survival and reproduction.
Effect of Dunaliella chemostat replacement rate on its nutritional quality for rotifer survival (A) and reproduction (B). 20% Tet – Tetraselmis suecica grown in a chemostat with 20% medium replacement per day, 10, 20, 40% Dun – Dunaliella tertiolecta grown in chemostats with 10, 20 or 40% medium replacement per day.
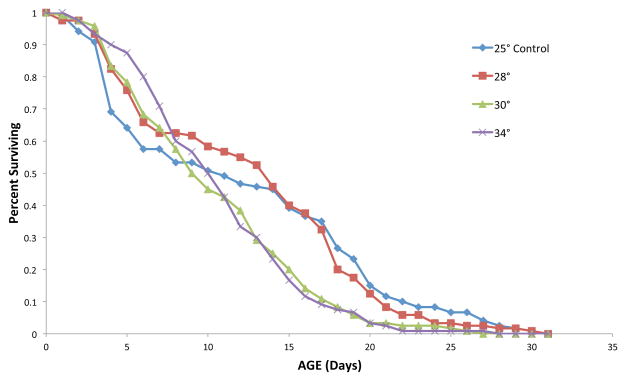
The temperature at which algae are grown affects rotifer lifespan (Figure 3). The mean rotifer lifespan when fed a diet of Tetraselmis suecica cultured at 25° or 28°C is 12.5 days. When Tetraselmis is cultured at 30° or 34°C, mean rotifer lifespan was reduced by 18%. Similarly, maximum lifespan (age when 5% of the initial cohort remain alive) was reduced by 31% on a diet of Tetraselmis grown at the two higher temperatures. Algae culture temperature affects their biochemical composition (Renaud et al. 2002) and therefore their nutritional quality for rotifers.
Figure 3. Algae culture temperature affects rotifer survival.
B. manjavacas survival on a diet of Dunaliella tertiolecta grown at various temperatures.
Effects of temperature on rotifer lifespan
Culture temperature has a big effect on rotifer lifespan. Kauler and Enesco (2011) investigated effects of 16, 22 and 29°C on longevity of B. calyciflorus. The expected inverse relationship with temperature was observed, with mean lifespans of 11.3, 6.4 and 4.1 days at 16, 22 and 29°C, respectively. Number of offspring produced was 23% lower at 16°C and the rate of reproduction (offspring/day) was only about 25% of that at 29°C. Analysis of long and short-lived rotifers at22°C revealed that long-lived rotifers had a 75% longer reproductive period and 3-fold longer post-reproductive period. Reproduction during the last 20% of reproductive effort had more negative effects on lifespan than earlier reproduction. They suggested that their data supported the rate of living/oxidative damage theory of aging (Brys et al. 2007) since fecundity can be considered a proxy for metabolic activity and reactive oxygen species (ROS) generation.
Effects of antioxidants on rotifer lifespan
Antioxidants are compounds that can reduce cellular damage caused by ROS, slowing aging and sometimes producing life extension in experimental animals (Halliwell 2011). Many researchers are searching for treatments that reduce oxidative damage to slow aging and neurodegenerative diseases. These are often associated with mitochondrial dysfunction which can be mitigated by amphiphilic antioxidants (Poeggler et al. 2005). These authors found that the nitrone amide, N-[4-(octa-O-acetyllactobionamidomethylene) benzylidene]-N-[1,1-dimethyl-2-(N-octanoyl)amido] ethylamine N-oxide (LPBNAH1) at 5 μM increased Philodina acuticornis size by 60% and extended mean lifespan by 150% to 57 days. Net fecundity of females also increased 2-fold by extending reproductive lifespan by 2.6-fold. The antioxidant capacity of LPBNAH1 was demonstrated by its protection of rotifers from exposure to 500 μM H2O2, a potent oxidant, which typically killed almost all animals in 24 h.
Poeggeler et al. (2010) identified the antioxidant indolepropionamide (IPAM), which is similar in structure to melatonin, as life extending in Philodina acuticornis. They suggested that IPAM binds to the rate-limiting component of oxidative phosphorylation in complex I of the respiratory chain and acts as a stabilizer of energy metabolism, thereby reducing ROS production. Treating rotifers with 30 μM IPAM increased mean lifespan from 24.6 days in the control to 90.5 days, a 2.7 fold increase. Treatment with IPAM also increased rotifer body length on day 15 by 47% over control and increased net fecundity 2.4 fold. The reproductive lifespan of P. acuticornis increased from 5 days in control to 18 days in the 30 μM IPAM treatment.
Both of these papers by Poeggeler et al. implicated ROS and antioxidants in rotifer aging, so Snell et al. (2012) examined a variety of antioxidants for their ability to extend the lifespan of Brachionus manjavacas. They found that exposing rotifers to certain combinations of antioxidant supplements can produce up to about 20% longer lifespan. They performed life table tests with 20 single antioxidants and none yielded significant rotifer life extension, including IPAM. They tested 60 two-way combinations of selected antioxidants and only seven (12%) produced significant rotifer life extension. None of the 20 three- and four-way antioxidant combinations tested yielded significant rotifer life extension. These observations suggest that dietary exposure to antioxidants can extend rotifer lifespan, but most antioxidants do not. They observed significant rotifer life extension only when antioxidants were paired with trolox, N-acetyl cysteine, L-carnosine, or EUK-8. Although some antioxidants extended rotifer lifespan, they likely did so by another mechanism than direct antioxidation. Swimming speed increased about 2.7 fold from 0.9 to 2.4 mm/s as B. manjavacas females mature and begin reproduction and then declined in older age classes. This age-dependent decline in swimming could be mitigated by treatment of rotifers with a mixture of 20 μM N-acetyl cysteine and 20 μM L-carnosine which maintained higher swimming speed in 10 day old rotifers.
Effects of gender and reproductive mode on rotifer lifespan
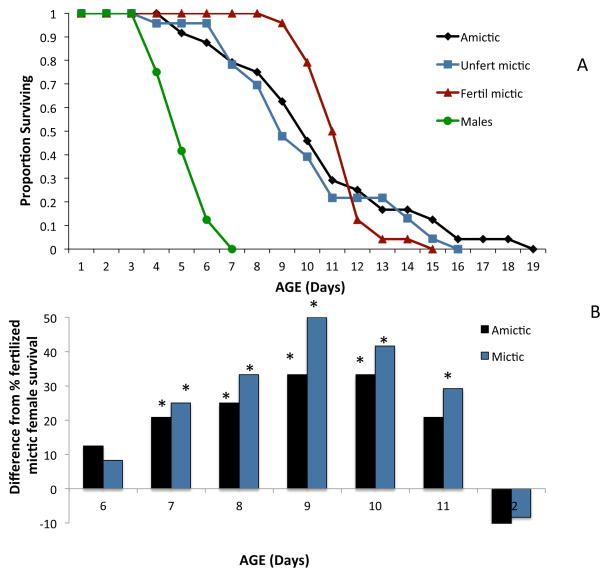
Gender and reproductive physiology of females affect rotifer lifespan (Figure 4a). Mean lifespan of B. manjavacas males at 22°C is 4.3 days and only half that of females. Compared to diploid females, monogonont males are haploid, never feed, and are seasonally ephemeral, appearing only when sexual reproduction can be completed (Serra et al. 2004). Amictic and unfertilized mictic females have similar survival curves, but fertilized mictic females have higher survival in the middle age classes. The fraction of fertilized mictic females surviving from days 7–11 ranged from 20 to 35% higher than amictic females and 25 to 50% higher than mictic females (Figure 4b). Differences in the schedule of reproduction is a factor likely contributing to this difference in survival. Unfertilized mictic females produce on average 25 male offspring concentrated in young age classes, so by age 3.8 days, 90% of their reproduction is finished. Amictic females likewise begin reproduction early, produce a mean of 25 female offspring and complete 90% of their reproduction by age 5.1 days. In contrast to both of these, fertilized mictic females produce an average of 2.2 resting eggs and do not complete 90% of their reproduction until age 7 days. Clearly, the length of reproductive lifespan, as determined by the mode of reproduction strongly influences overall lifespan.
Figure 4. a&b Reproductive mode and lifespan.
Comparative survival of rotifer males and different types of females. (A) Amictic – asexual females producing daughters, Unfertilized mictic – sexual females producing males, Fertilized mictic – sexual females producing resting eggs. (B) Percent difference between fertilized female survival and amictic or mictic female survival for days 6–12 of life. Asterisks indicate significant differences by Fisher’s Exact Test from fertilized mictic female survival.
Effects of diapause on rotifer lifespan
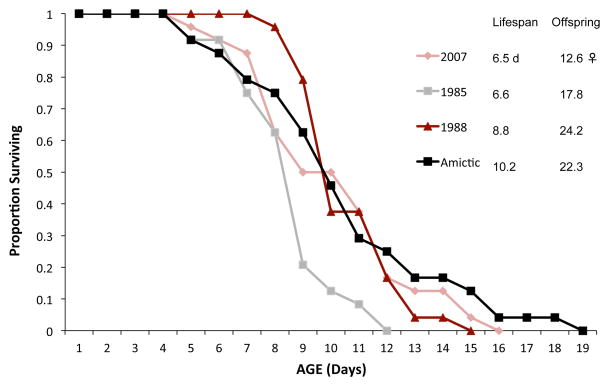
We tested whether emergence from diapause affects the lifespan of resting egg hatchlings as compared to amictic egg hatchlings (Figure 5). Our thinking was that perhaps the mechanisms that slow metabolism in diapause convey some residual protection against aging in the hatchlings. We found no significant differences in lifespan between hatchlings emerging from resting eggs that were in diapause for 23 or 26 years compared to 4 years. Likewise, there were no significant differences in lifespan between these resting egg hatchlings and females hatching from amictic eggs which never experienced diapause.
Figure 5. Dormancy and lifespan.
Comparative survival of hatchlings from resting eggs or amictic eggs. The dates 1985,1988, 2007 refer to the year when the resting eggs were produced and stored at −20°C. Amictic – hatchlings from amictic eggs which lack diapause. Lifespan is the mean lifespan in days of the cohort, Offspring is the total offspring produced by the average female over her lifetime (Ro).
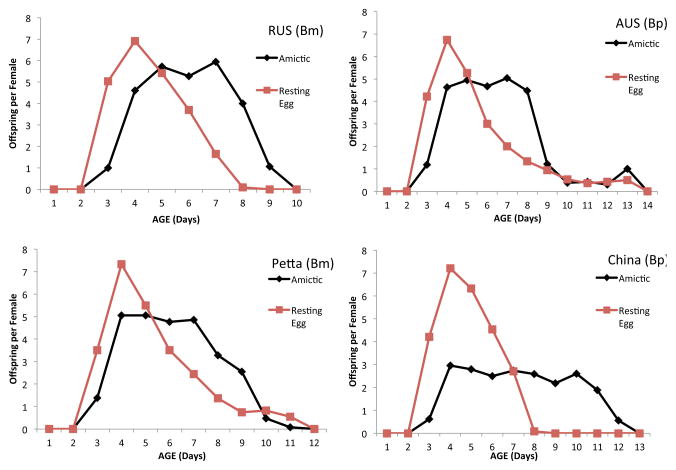
We also compared lifespan of amictic and resting egg hatchlings of two geographical isolates each of Brachionus plicatilis (Austria, China) and B. manjavacas (Russian, Pettaquamsett) (Suatoni et al. 2006). No significant differences in lifespan were observed among any of the four populations or between amictic and resting egg hatchlings. In contrast, we found significant differences in the schedule of reproduction of amictic and resting egg hatchlings in all four populations (Figure 6). In every case, the reproductive curve peaked earlier and higher for hatchlings from resting egg than amictic eggs. Comparing survival and reproduction statistics demonstrates this pattern. For example, the mean intrinsic rate of population increase (r) for resting egg hatchlings was 0.75 offspring per day, 25% higher than for amictic egg hatchlings. The mean generation time (T) for resting egg hatchlings (4.25 days) was 26% shorter than that for amictic egg hatchlings. The mean age when 50% of reproductive value has been achieved was 2.53 days for resting egg hatchlings, 65% younger than amictic egg hatchlings. In contrast to these three life table parameters, lifetime fecundity (Ro) and median lifespan (0.5lx) were not significantly different among amictic and resting egg hatchlings. Clearly, something in diapause causes reproduction of resting egg hatchlings to shift to earlier age classes than amictic egg hatchlings.
Figure 6. Reproductive schedule of amictic and resting egg hatchlings.
Comparative age-specific reproduction of amictic or resting egg hatchlings of Brachionus manjavacas (Bm) geographical isolates RUS (Russian) and Petta (Pettaquamsett), and B. plicatilis (Bp) isolates AUS (Austria) and China.
Effects of inhibiting DNA synthesis on rotifer lifespan
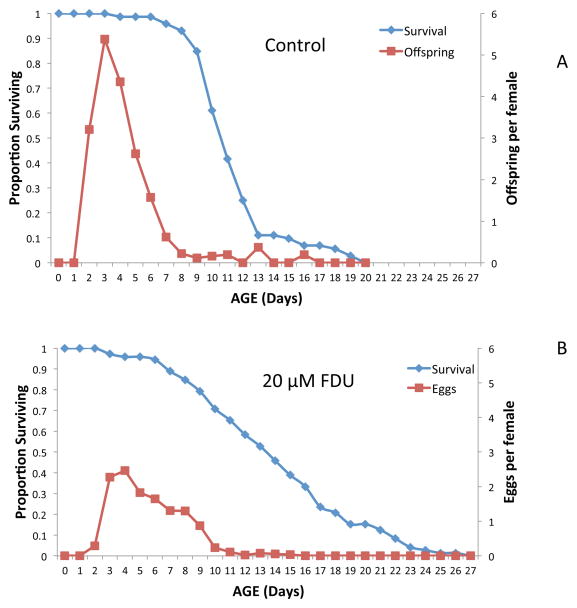
The cancer drug 5-fluorodeoxyuridine (FDU) is useful for inhibiting DNA synthesis in vivo in a variety of animals (Fischer et al. 2006). It is often used in C. elegans life table experiments (Hosano 1978) to prevent larval development and the birth of F1 offspring because they can easily be confused with parental animals. Birth of F1s necessitates frequent checking and removal from life table experiments, requiring more manpower and limiting the number of replicates and treatments that can be tested. FDU is likewise useful in monogonont rotifer life table experiments to prevent egg hatching and confusion of maternal females with F1 offspring (Snell et al. 2012). FDU inhibits thymidylate synthase, a key enzyme for maintaining sufficient TTP pools required for DNA synthesis (Fischer et al. 2006). We often employ 20 μM FDU in our life table experiments to inhibit amictic egg hatching. FDU has no obvious effect on maternal females who feed and swim like controls, but lifetime fecundity is diminished by 57% (Figure 7). In these experiments, untreated control females produce viable eggs, but FDU treated females produce amictic eggs that do not hatch. Since rotifers have a fixed cell number (eutelic) (Wallace et al. 2006), there is no cell division after birth (Pagani et al. 1993). Therefore FDU treatment has little effect on hatched rotifers that have completed development. In contrast, amictic eggs are extruded as single cells that undergo extensive DNA synthesis, cell division and development (Gilbert 1983). This is inhibited by FDU, so that eggs are extruded and dropped by females, but they do not hatch.
Figure 7. FDU and rotifer lifespan.
Effect of 5-fluorodeoxyuridine (FDU) on survival and reproduction of B. manjavacas. Control females (A) produced offspring normally, whereas FDU treated females (B) produced amictic eggs that were dropped, but did not hatch.
An interesting effect of inhibiting DNA synthesis with FDU is about a 25% life extension in treated B. manjavacas females relative to controls (Figure 7). It is not clear why inhibiting DNA synthesis in eutelic animals should produce life extension. Using H3-thymidine labeling in Asplanchna brightwelli, Birky et al. (1967) found DNA synthesis in adults only in the vitellarium and cytoplasm. They attributed the vitellarium synthesis to the polyploidization of this tissue and cytoplasmic DNA synthesis to DNA replication in mitochondria. If FDU restricts the vitellarium to the diploid state, this may account for the reduced reproduction of FDU treated females because they simply cannot produce enough egg yolk fast enough to support maximum reproduction. Another hypothesis is that FDU not only inhibits DNA synthesis in chromosome and mitochondria replication, but also general DNA repair. Clearly more investigation is required to explain the FDU-life extension observation.
Evolution of rotifer lifespan
Smith and Snell (2012) used experimental evolution in chemostat cultures to examine changes in lifespan and aging rate in B. plicatilis s.s. After 385 d, which was equivalent to up to 84 rotifer generations, asexual females lived 26% longer (mean lifespan 11 ± 0.2 days) with a 24% longer maximum lifespan and a 23% lower aging rate than the founding population. Lifespan extension is commonly observed when extrinsic mortality factors are removed from populations (Williams et al. 2006).
Longer rotifer lifespan was achieved by remaining reproductive longer and extending the post-reproductive period more than in earlier generations. These asexual females experiencing selection in chemostats also displayed a 56% increase in fecundity. This positive relationship between fecundity and longevity is inconsistent with the predictions of the antagonistic pleiotropy and disposable soma theories of aging (Hughes and Reynolds 2005, Monaghan et al. 2008). In contrast to asexual females, chemostat selection produced no significant change in the lifespan or fecundity of sexual (unfertilized mictic) females. The differences in evolvability between asexual and sexual females sharing the same chemostat environment emphasizes the importance of reproductive physiology in limiting the evolution of life history traits.
Conclusions
Rotifers have several advantages for aging studies including: short generation times, ease of culture, asexual propagation of clonal cultures, sexual and asexual reproduction in the same genetic background, haploid males, highly stable diapausing embryos, many closely related strains that differ in life history traits, and a well developed suite of genetic tools. Animal aging rate is regulated by nutrient-sensing mitogen-activated, stress-responsive, and DNA damage signaling pathways. Comparing gene expression in closely related species which differ in their aging rates will yield insight into molecular targets for intervention. There are clear structural, behavioral, and metabolic changes associated with rotifer aging and it is easily quantifiable with life table experiments.
Dietary restriction remains the most reliable and consistent way to extend animal lifespan, including most rotifer species. Rotifers are evolutionarily adapted to rapid changes in food availability, mobilizing glycogen and lipids when faced with starvation. Regulatory proteins can sense nutrient limitation and alter metabolism to maintain physiological homeostasis. Signals from altered cellular bioenergetics can produce hormesis and protection against aging processes. Some rotifer species are less responsive to dietary restriction than others, but how DR is applied is critical. In Brachionus manjavacas, intermittent fasting (alternating days of feeding and starvation) increased lifespan >150% for both amictic and unfertilized mictic females. However, chronically low food levels (50% of ad libitum feeding), significantly increased lifespan of amictic females, but not unfertilized mictic females. There is no tradeoff between lifetime fecundity and lifespan with DR.
Manipulating algal food quality also can produce significant rotifer life extension. B. manjavacas lives 20–25% longer on a Dunaliella diet than Tetraselmis. Altering the biochemical composition of Dunaliella by increasing culture renewal rate from 10% (low protein, high carbohydrate) to 40% (high protein, low carbohydrate) significantly increased rotifer lifespan. Exposing B. manjavacas to certain combinations of antioxidant dietary supplements can produce up to about 20% longer lifespan. However, none of the 20 single antioxidants tested yielded significant rotifer life extension, and only 12% of the 60 two-way combinations tested produced significant life extension.
Male B. manjavacas only live about ½ the lifespan of females. Length of diapause does not affect the lifespan of resting egg hatchlings, but females hatched from resting eggs begin reproduction earlier and complete it sooner than amictic egg hatchlings. B. plicatilis amictic females grown in chemostats for about 84 asexual generations evolved about 26% longer lifespans than the initial population. In contrast, unfertilized mictic females in the same environment did not evolve longer lifespans. Reproductive physiology somehow limits the evolvability of important life history traits like lifespan. The compound FDU inhibits DNA synthesis and extends B. manjavacas lifespan by 25%, perhaps by preventing polyploidization in the vitellarium or reducing DNA repair.
Many promising opportunities remain for employing rotifers in aging research. The role of diet in regulating animal lifespan is still not clearly understood. Intermittent fasting has big effects, but what is the minimum fasting period necessary to produce significant life extension? Are there dietary supplements that can reduce the level of fasting necessary for maximal life extension? Are there novel metabolic pathways yet to be discovered that regulate aging and have large effects on animal lifespan? Our observations from probing rotifer metabolic pathways using RNAi suggests that more investigation of the JNK/adipocytokine signaling pathway is warranted.
Acknowledgments
I acknowledge with gratitude grant support from the National Institute of Aging, grant R01AG037960-02 for this work. I also express my appreciation for discussions and insights provided by David Mark Welch, Hilary A. Smith, and Kristen E. Gribble. Rachel Johnston, Cody Zipperer, and Stephanie Teat provided expert technical assistance for the experiments.
Literature Cited
- Austad SN. Is there a role for new invertebrate models for aging research? - J Gerontol A Biol Sci Med Sci. 2009;64A:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends in Neuroscience. 2004;27:3602–3607. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Bartke A. Insulin and aging. Cell Cycle. 2008;7:3338–3343. doi: 10.4161/cc.7.21.7012. [DOI] [PubMed] [Google Scholar]
- Birky CW, Jr, Bignami RZ, Bentfield MJ. Nuclear and cytoplasmic DNA synthesis in adult and embryonic rotifers. Biol Bull. 1967;133:502–509. doi: 10.2307/1539913. [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Brys K, Vanficteren JR, Braeckman BP. Testing the rate-of-living/oxidative damage theory of aging in the ncmatode model Caenorhahditis elegans. Experimental Gerontology. 2007;42:845–851. doi: 10.1016/j.exger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Droge W. Oxidative stress and aging. Advances in Experimental Medicine and Biology. 2003;543:191–200. doi: 10.1007/978-1-4419-8997-0_14. [DOI] [PubMed] [Google Scholar]
- Dufour E, Larsson NG. Understanding aging: revealing order out of chaos. Biochem Biophys Acta. 2004;1658:122–132. doi: 10.1016/j.bbabio.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Dunn CW, Hejnol A, Matus DQ, Pang K, Brown WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sorensen MV, Haddock SHD, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–749. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- Fábregas J, Patino M, Arredondo-Vega BO, Tobar JL, Otero A. Renewal rate and nutrient concentration as tools to modify productivity and biochemical composition of cyclostat cultures of the marine microalgae Dunaliella tertiolecta. Applied Microbiology Technology. 1995;44:287–292. [Google Scholar]
- Ferreira M, Seixas P, Coutinho P, Fábregas J, Otero A. Effect of the nutritional status of semi-continuous microalgal cultures on the productivity and biochemical composition of Brachionus plicatilis. Mar Biotechnol. 2011;13:1074–1085. doi: 10.1007/s10126-011-9370-y. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Muller-Weeks S, Caradonna SJ. Fluorodeoxyuridine modulates cellular expression of the DNA base excision repair enzyme uracil-DNA glycosylase. Cancer Res. 2006;66:8829–8837. doi: 10.1158/0008-5472.CAN-06-0540. [DOI] [PubMed] [Google Scholar]
- Gilbert JJ. Contact chemoreception, mating behaviour, and sexual isolation in the rotifer genus Brachionus. J Exp Biol. 1963;40:625–641. [Google Scholar]
- Gilbert JJ. Rotifera. In: Adiyodi KG, Adiyodi RG, editors. Reproductive biology of invertebrates, vol I. Oogenesis, oviposition,and oosorption. Wiley; New York: 1983. pp. 181–209. [Google Scholar]
- Gribble KE, Mark Welch DB. Lifespan extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera) J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley EC, Lakatta EG, Morrison-Bogorad M, Warner HR, Hodes RJ. The future of aging therapies. Cell. 2005;120:557–567. doi: 10.1016/j.cell.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants—Quo vadis? - Trends Pharmacol Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS. A systematic RNAi screen for longevity genes in C. elegans. Genes and Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Exp Gerontol. 1978;13:369–374. doi: 10.1016/0531-5565(78)90047-5. [DOI] [PubMed] [Google Scholar]
- Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annual Review of Entomology. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S. Calorie restriction-induced mother’s longevity is transmitted to the daughter’s generation in the rotifer Brachionus plicatilis. Functional Ecology. 2011;25:209–216. [Google Scholar]
- Kauler P, Enesco HE. The effect of temperature on life history parameters and cost of reproduction in the rotifer Brachionus calyciflorus. J Freshwater Ecol. 2011;26:399–408. [Google Scholar]
- Kirk K. Dietary restriction and aging: Comparative tests of evolutionary hypotheses. J Gerontology. 2001;56A:B123–B129. doi: 10.1093/gerona/56.3.b123. [DOI] [PubMed] [Google Scholar]
- Kortschak RD, Samuel G, Saint R, Miller DJ. EST analysis of the cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Current Biology. 2003;13:2190–2195. doi: 10.1016/j.cub.2003.11.030. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Ahn B, Choi IS, Koo HS. The gene expression and deficiency phenotypes of Cockayne syndrome B protein in Caenorhabditis elegans. FEBS Letters. 2002;522:47–52. doi: 10.1016/s0014-5793(02)02880-6. [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and Survival: The Genetics of Life Span Extension by Dietary Restriction. Ann Rev Biochemistry. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 Link Calorie Restriction and TOR to Sirtuin-Mediated Lifespan Extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry BJ. Molecular mechanisms linking calorie restriction and longevity. International Journal of Biochemistry and Cell Biology. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Monaghan P, Charmantier A, Nussey DH, Ricklefs RE. The evolutionary ecology of senescence. Functional Ecology. 2008;22:371–378. [Google Scholar]
- Oo AKS, Kaneko G, Hirayama M, Kinoshita S, Watabe S. Identification of genes differentially expressed by calorie restriction in the rotifer (Brachionus plicatilis) J Comp Physiol B. 2010;180:105–116. doi: 10.1007/s00360-009-0389-6. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Kaneko G, Kanagawa Y, Watabe S. Calorie restriction in the rotifer Brachionus plicatilis enhances hypoxia tolerance in association with the increased mRNA levels of glycolytic enzymes. Hydrobiologia. 2010;649:267–277. [Google Scholar]
- Ozdemir N. The effect of caloric restriction on the lifespan and reproduction of freshwater rotifer (Brachionus calyciflorus) J Animal and Veterinary Advances. 2009;8:669–673. [Google Scholar]
- Pagani M, Ricci C, Redi CA. Oogenesis in Macrotrachela quadricornifera (Rotifera, Bdelloidea) Hydrobiologia. 1993;255/256:225–230. [Google Scholar]
- Poeggeler B, Durand G, Polidori A, Pappolla MA, Vega-Naredo I, Coto-Montes A, Boker J, Hardeland R, Pucci B. Mitochondrial medicine: neuroprotection and life extension by the new amphiphilic nitrone LPBNAH1 acting as a highly potent antioxidant agent. J Neurochemistry. 2005;95:962–973. doi: 10.1111/j.1471-4159.2005.03425.x. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Sambamurti K, Siedlak SL, Perry G, Smith MA, Papolla MA. A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PLoS ONE. 2010;5:e10206. doi: 10.1371/journal.pone.0010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud SM, Thinh LV, Lambrinidis G, Parry DL. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture. 2002;211:195–214. [Google Scholar]
- Serra M, Snelland TW, King CE. The timing of sex in monogonont rotifers. In: Moya A, Font E, editors. Evolution: From molecules to ecosystems. Oxford University Press; 2004. pp. 135–146. [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Smith HA, Snell TW. Evolvability of lifespan and reproduction. BMC Ecology. 2012 submitted. [Google Scholar]
- Snell TW, Fields AM, Johnston RK. Antioxidants can extend lifespan of Brachionus manjavacas (Rotifera), but only in a few combinations. Biogerontology. 2012;7 doi: 10.1007/s10522-012-9371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, King CE. Lifespan and fecundity patterns in rotifers: the cost of reproduction. Evolution. 1977;31:882–890. doi: 10.1111/j.1558-5646.1977.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Snell TW, Shearer TL, Smith HA. Exposure to dsRNA elicits RNA interference in Brachionus manjavacas (Rotifera) Marine Biotechnology. 2011;13:264–274. doi: 10.1007/s10126-010-9295-x. [DOI] [PubMed] [Google Scholar]
- Suatoni E, Vicario S, Rice S, Snell TW, Caccone A. Phylogenetic and biogeographic patterns in the salt water rotifer Brachionus plicatilis. Molecular Phylogenetics & Evolution. 2006 doi: 10.1016/j.ympev.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Wallace RL, Snell TW, Ricci C, Nogrady T. Rotifera. Volume 1. Biology, ecology and systematics. 2 SPB Academic Publishing; The Hague, Belgium: 2006. [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Williams PD, Day T, Fletcher Q, Rowe L. The shaping of senescence in the wild. Trends in Ecology & Evolution. 2006;21:458–463. doi: 10.1016/j.tree.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T, Hagiwara A, Tsukamoto K. Effect of periodical starvation on the life history of Brachionus plicatilis O.F. Müller (Rotifera): a possible strategy for population stability. J Exp Mar Biol Ecol. 2000;253:253–260. doi: 10.1016/s0022-0981(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T, Kaneko G, Kinoshita S, Furukawa S, Tsukamoto K, Watanabe S. Insulin-like growth factor signaling pathway involved in regulating longevity of rotifers. Hydrobiologia. 2005;181:347–352. [Google Scholar]
- Yoshinaga T, Hagiwara A, Tsukamoto K. Life history response and age-specific tolerance to starvation in Brachionus plicatilis O.F. Muller (Rotifera) J Exp Mar Biol Ecol. 2003;287:261–271. doi: 10.1016/s0022-0981(00)00268-9. [DOI] [PubMed] [Google Scholar]