Abstract
The diversity of nanomaterials in terms of size, shape, and surface chemistry poses a challenge to those who are trying to characterize the human health and environmental risks associated with incidental and unintentional exposures. There are numerous products that are already commercially available that contain solid metal and metal oxide nanoparticles, either embedded in a matrix or in solution. Exposure assessments for these products are often incomplete or difficult due to technological challenges associated with detection and quantitation of nanoparticles in gaseous or liquid carriers. The main focus of recent research has been on hazard identification. However, risk is a product of hazard and exposure, and one significant knowledge gap is that of the target organ dose following in vivo exposures. In order to reach target organs, nanoparticles must first breech the protective barriers of the respiratory tract, gastrointestinal tract, or skin. The fate of those nanoparticles that reach physiological barriers is in large part determined by the properties of the particles and the barriers themselves. This article reviews the physiological properties of the lung, gut, and skin epithelia, the physicochemical properties of metal and metal oxide nanoparticles that are likely to affect their ability to breech epithelial barriers, and what is known about their fate following in vivo exposures.
Keywords: Nanoparticles, Respiratory tract, Skin, Gastrointestinal tract, Epithelial barriers
Nanomaterials are objects with at least one external dimension or internal structure on the order of 100 nm or less and which may have properties that differ significantly from those without nanoscale features [20]. Nanoparticles (NPs) – which can have morphologies ranging from spherical to chain-like to fibrous – are a subset of nanomaterials and are the focus of this review. A characteristic of NPs is that their surface area per unit mass increases as size decreases, as does the percentage of atoms that can be found at the surface of the material [98]. This may partly explain some of their unique properties.
The Woodrow Wilson Institute maintains a database of the currently-available consumer products that contain NPs, of which there are more than 580 that are composed of Ag, Zn, silica, TiO2, Au, or graphite (including carbon nanotubes and fullerenes) [59]. Many of these are poorly soluble materials. Some consumer products are expected to release NPs such that human exposures are likely during use and/or disposal. Of concern, too, are potential occupational exposures to these materials during product manufacturing and packaging. There are gaps in current knowledge regarding the safety of NPs, some of the most significant of which include exposure assessment, target organ (internal) dose, identification of mechanisms of toxicity and sensitive subpopulations, and the physicochemical characteristics of NPs that correlate with health outcome [87]. This review focuses attention on one of these knowledge gaps – that of internal dose – by exploring the conditions under which NPs may breach epithelial barriers, be distributed in the body, and reach target organs.
Assuming that the majority of NP exposures will occur in air or from the food chain/drinking water, the most likely routes of entry into the body are the respiratory and gastrointestinal (GI) tracts and skin. Unfortunately, current understanding regarding the passage of NPs across epithelial tissue and mechanisms of distribution and elimination is poor. Furthermore, there is inconsistent and incomplete reporting of NP physicochemical properties such as particulate core and outer shell chemical composition, surface oxidation state, surface charge, singlet and agglomerate sizes in relevant carriers (gas, liquid), shape, solubility, and surface area. Most if not all of these properties are likely to impact the NP dose that reaches target organs. However, surface properties, in particular, are likely to change when NPs enter the body and during transport.
This review begins with a focus on the structural and functional similarities and differences amongst the physiological barriers at the likely portals of entry. This naturally leads to a discussion of the physicochemical NP properties that are considered to be most important in breeching those barriers. Lastly, a review of what is currently known about the fate of poorly-soluble NPs that are delivered via skin and the GI and respiratory tracts is provided, focusing mainly on results from in vivo studies.
3. Epithelial Barrier Structure and Function at Portals of Nanoparticle Entry
3.1. Microanatomy and Mechanisms of Nanoparticle Deposition in Lung
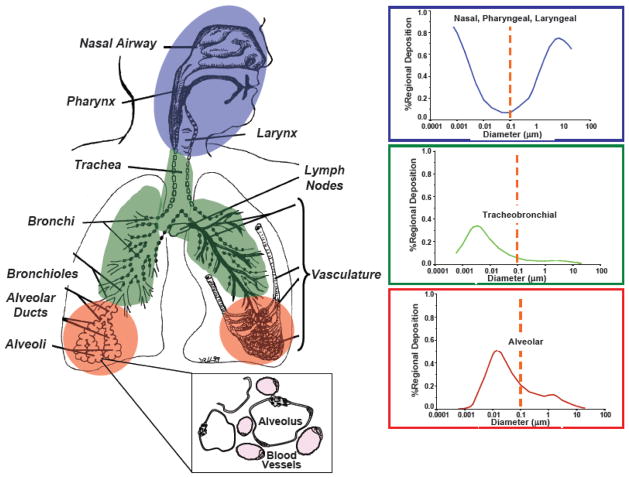
To understand how inhaled NPs might interact with lung cells, it is critical to understand where and how they deposit in the respiratory tract. The International Commission on Radiological Protection developed a model that predicts fractional deposition efficiencies for aerosolized particulates [8,65]. This model divides the respiratory tract into three regions: the nasopharyngeal-laryngeal, tracheobronchial, and alveolar regions. NPs that are suspended in air are predicted to efficiently deposit in the alveolar regions of the lung; however, significant amounts also deposit in the other regions, resulting in high total deposition (Figure 1). The nature of the respiratory tract epithelial barrier varies in that the cell type distribution changes from the proximal (nose) to distal (alveoli) end. Ciliated pseudostratified columnar epithelial cells and mucous-producing goblet cells line the conducting airways down to the small bronchi, whereas the epithelium in the alveoli is one squamous cell thick in most places [71,120].
Figure 1.
Deposition of particles in the respiratory tract as a function of their size, with inset illustrating the proximity of the air spaces (alveoli) to the vasculature (in pink). Used with permission and adapted from Oberdörster et al., Environ. Health Perspect., 2005 [110].
Although NPs are predicted to deposit efficiently throughout the respiratory tract, there are some key anatomical features of the alveolar epithelial barrier that may contribute significantly to their subsequent fate. These features include: 1) the large alveolar surface area (80–140 m2 in humans [130]) that facilitates gas exchange; and 2) the large extent of vascularization. The alveoli are lined by squamous (type I) and cuboidal (type II) epithelial cells. Whereas these two cell types have similar number distributions in the alveolus, the type I cells have a larger surface area than type II cells and, therefore, cover ~95% of the alveolar epithelial surface [120]. The type II cells proliferate to repair injured areas of the alveolus and also differentiate into type I cells [1]. Gas exchange occurs through the thin, extended filipodia of the type I cells, which form zonulae occludens with other type I cells. The basement membrane of the type I epithelial cell is continuous with that of the endothelial cells lining the pulmonary capillaries, except for a thin interstitium, so the total distance through which gases (or NPs) have to travel to reach the blood is 0.36–2.5 μm [117]. The pulmonary capillaries form a dense, intertwining network in the parenchymal region of the lung (Figure 1, inset). A small fraction of the applied dose of nanosized particles can pass from the epithelial surface of the air space into blood, but the fraction increases if the barrier is disrupted, for example, by an inflammatory stimulus. The amount that gets into blood has also been shown to be size-dependent, with smaller (~55 nm) particles having greater fractional penetration than larger particles (~200 nm) [26].
Selective permeability and active transport of ions through tight junctions give rise to a transepithelial potential difference such that the lung mucosa has a net negative charge. Hence, NPs containing high positive surface potential might experience stronger interactions with the lung mucosa and be more prone to interaction with cell membranes if the particles reach the cell surface, as has been demonstrated using cultured cells [55]. However, early in vivo work demonstrated that the distribution of functional surface charge is cell type-specific: type I alveolar epithelial cells were found to have no or few anionic sites, while the type II cell surface is largely anionic [135]. Recent studies with surface functionalized quantum dots suggest that carboxylation of the NP surface promotes enhanced retention by lung tissue, which may reflect interactions of the negatively-charged particle with the large available surface area of the type I alveolar epithelial cells that are devoid of anionic sites (unpublished data).
The microenvironment, namely the lipids and proteins in lung lining fluid, is likely to alter NP-cell surface charge interactions. However, the nature of the lining fluid changes as a function of location in the respiratory tract. The lining fluid of the conducting airways is a complex mixture of mucous substances and aqueous components and varies in depth from ~5–100 μm [102]. The combined activity of phagocytic cells and the movement of mucous from the airways towards the oropharynx represents the main mechanism by which particulate matter is cleared. The alveolar lining fluid consists of surfactants and an overlying aqueous phase. Pulmonary surfactant contains ~90% lipid and 10% protein. The lipid component is composed largely of disaturated dipalmitoylphosphatidylcholine and phosphatidylglycerol with smaller amounts of cholesterol. Surfactant proteins, which are secreted by type II alveolar epithelial and Clara cells [57], join the lipid fraction to keep the alveoli and bronchioles patent during respiration. The alveolar lining fluid also contains plasma-derived proteins (e.g. albumin, transferrin, immunoglobulins) that are critical to host defense functions [73].
3.2. Microanatomy and Mechanisms of Particle Translocation in GI Tract
NP uptake across the gastrointestinal mucosa is determined by the complex structure, function, and segmental heterogeneity of the epithelium covering the gut. The oral cavity, pharynx, and esophagus are lined by stratified squamous epithelia, but the intestinal mucosa is covered by only a single cell layer. Because mucosal tissues face the outside world, it should come as little surprise that they are extremely immunologically active. Indeed, it has been established that the lamina propria of the intestine contains more antibody-producing B-cells than any other organ in the body, including the spleen, thymus, and lymph nodes [19]. The acidic environment of the stomach and upper small intestine is an effective sterilizer and lymphoid follicles are relatively rare in these regions except in certain infectious disease states. Single, scattered lymphoid follicles increase in frequency from the stomach to the distal ileum, where the microbial flora becomes more abundant and diverse. Lymphoid follicles are grouped in large patches (Peyer’s patches, PP) that are visible to the naked eye [76].
Intestinal epithelial cells are continuously shed either through high rates of mechanical attrition or as a result of the terminal differentiation of cells with a short lifespan. Intestinal epithelial cells, therefore, must be replaced at an extraordinary rate that matches their rate of loss for efficient fluid and electrolyte absorption under both normal and stressed conditions, outpacing all other epithelia in the body (3–5 day life span). Mature and terminally differentiated intestinal epithelium is continuously replaced by progenitor cells located within the lower poles of the crypts of Lieberkuhn – invaginations of the epithelium into the underlying connective tissue. Each new progeny cell will undergo four to six rounds of cell division as it migrates out of the crypt and into the villus – large finger-like protrusions into the gut lumen [7]. As the cells move up from the base of the crypt to the villus, they undergo maturation and differentiation.
There are four distinct types of terminally differentiated cells in the intestine: absorptive villus epithelial cells (enterocytes), goblet cells, enteroendocrine cells, and Paneth cells. Only the villus epithelial cells are absorptive; the other three cell types are all secretory. This has important implications for the passage of NPs from the gut lumen to underlying tissues. Villus epithelial cells are seen only in small intestine and perform the function of sodium, chloride, and nutrient absorption, the latter of which is confined to the small intestine. Goblet cells secrete mucus into the lumens of the small intestine and colon and their apical cytoplasm is generally distended with mucus-filled secretory granules. Enteroendocrine cells (of which there are many subtypes) are smaller and secrete various gut hormones (peptides and catecholamines). Paneth cells in the small intestine contain large apical secretory granules and express specific proteins, including lysozyme, tumor necrosis factor, and defensins. Another less common cell lineage is the M (microfold) cell.
The follicle-associated epithelium (FAE) covers the intestinal PPs and is comprised of enterocytes and the highly-specialized M cells. Antigen and microorganism transport through the FAE to underlying lymphoid tissue in the PP occurs via the transcytosis-competent M cells (Figure 2), so they have an important role in mucosal and systemic immune responses [14,15,21]. Numerous studies have been done in the past to understand the ultra-structure [21], histochemistry [29,53,113], transport function, and interaction of murine M cells with microorganisms [30,31,70,151]. Particles smaller than 1 μm are taken up by the M cells and transported across the full thickness of the PP, whereas particles >5 μm remain trapped in the PP [28,42]. Certain conditions, such as Crohn’s disease, increase the size and number of PP [33,67,128] and have as a feature increased antigen transcytosis. The FAE that covers the PP differs from that of the villi in that there is a paucity of goblet cells and the enterocytes there participate in very little antigen binding and transcytosis as compared to normal enterocytes. Thus, M cells may represent a unique site in the GI tract for NP binding and uptake.
Figure 2.
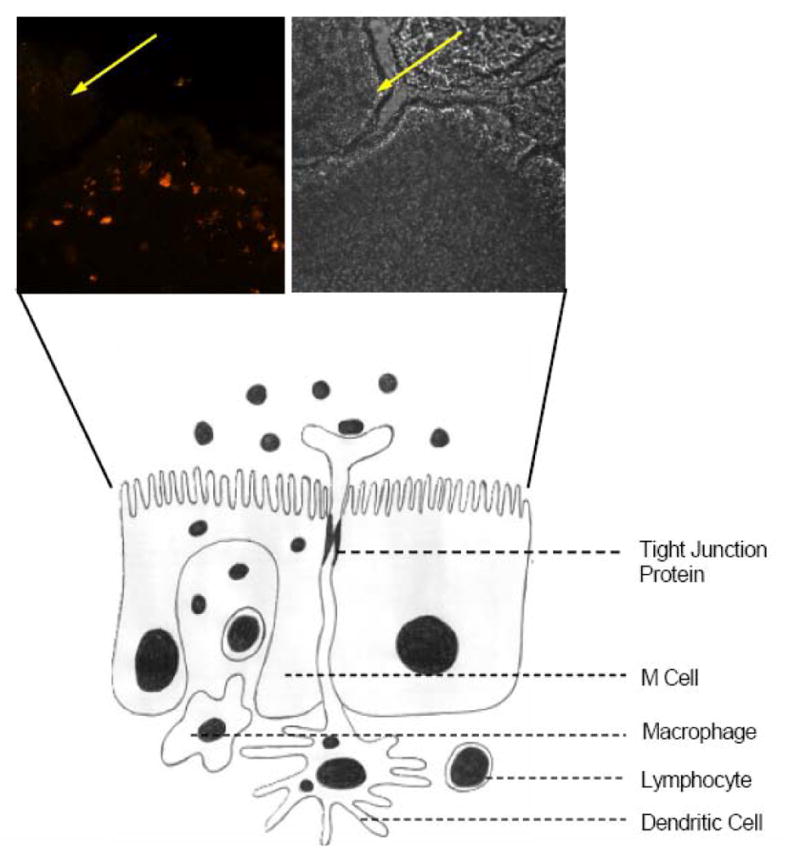
Possible routes of NP uptake in GI tract include tight junctions (paracellular), dendritic cells, and transcytosis. Inset shows phase contrast (left) and confocal (right) images of FAE, with only the M cells taking up and transporting antigen (Ulex europeaus), in this case bound to 0.5 μm Fluoresbrite microspheres. Enterocytes (yellow arrows) do not take up antigen.
The paracellular permeability of the GI tract is another critical factor that contributes to host exchange with the outside environment. Under physiological conditions, paracellular uptake of NPs would be limited by the smaller surface area of the intercellular spaces as compared to that of the cells themselves and as a result of the tightness of the junctions in these spaces. Hydrophilic polymers, though, such as chitosan, starch, and ones that are thiolated, are reported to traverse the paracellular space [23,124]. The tight junction between mucosal epithelial cells prevents passive loss of fluids and/or electrolytes as well as the invasion of pathogens from the lumen. The open tight junction has a pore radius of 5 nm and allows the passage of small macromolecules (4000–5500 Da) [32,114]. In the material exchange between the host and environment, both transcellular and paracellular transport are orchestrated in a synchronized way. Transepithelial Resistance (TER), measured during routine Ussing chamber experiments, is a composite of transcellular and paracellular resistance. There is considerable segmental heterogeneity in paracellular resistance and it is much lower than the transcellular resistance [9,54,139]. The two pathways are arranged in parallel, 1/TER=(1/Rtranscellular)+(1/Rparacellular); hence, the measured TER essentially reflects paracellular resistance.
A key determinant for transcellular transport of electrolytes and nutrients – and possibly NP – is the presence of an epithelial microclimate, a cell membrane surface mucous coat that is also referred to as the unstirred layer [38,127,137]. The mucous coat is produced by goblet cells and helps to maintain epithelial surface pH. Just like there is segmental heterogeneity in the epithelial surface and in paracellular resistance, there is also heterogeneity in regards to surface pH. These differences directly affect absorption rates for various compounds. Molecules passing from the bulk phase of the intestine (i.e., the gut contents) to the epithelial cell apex encounter two specific regions: the unstirred layer and the acid microclimate. The unstirred layer is not a distinctive layer on the mucosal surface, but rather a diffusion barrier in which molecules diffuse at a rate different from that predicted by the diffusion coefficient of water [126]. It is known to be a significant barrier to the passage of highly lipid-soluble molecules. However, fats are absorbed, for example, via micelle formation, after which the lipids are taken up directly into the epithelial cell. These observations are likely to be useful in predicting NP uptake.
Enterocyte apical surfaces are covered by rigid, closely placed microvilli that greatly increase the absorptive surface area. The tips of microvilli contain large, negatively charged, carbohydrate side chains of glycoproteins that form a continuous, filamentous brush border glycocalyx. The exact composition of the carbohydrate side chains varies greatly between animal species, within regions of the intestinal tract, and during development. The negative charge of the carbohydrate side chains prevents the diffusion of hydrogen ions, which are released due to the action of the Na-H exchanger. These trapped hydrogen ions contribute to the acidic microclimate through which any material must pass to be absorbed. Changes in the epithelial surface pH are less variable than those in the bulk phase. In the stomach, the pH at the epithelial cell surface is higher than in the bulk phase. In the proximal small intestine, the pH of the microclimate is significantly more acidic than in the bulk phase. These features of the GI tract work together to form a dynamic barrier between the host and macromolecular aggregates, particles, viruses, and bacteria in the gut lumen.
3.3. Microanatomy and Mechanisms of Particle Translocation in Skin
The skin is the largest organ in the body, exhibiting a surface area of ~1–2 m2 in human adults [144]. Skin provides many functions, the most vital being maintenance of a two-way barrier: inside-out to prevent water loss and outside-in to protect the body from external environmental insults. Skin has a multilayered architecture grossly consisting of the innermost dermis, the viable epidermis and the outermost stratum corneum (SC) layers (Figure 3). Each layer consists of different cell types, biomolecules, and appendages (hair follicles and glands) that function synergistically to maintain the two-way barrier. To understand how NP may penetrate skin, it is useful to consider how the barrier is formed on a cellular level to provide mechanistic insight into permeation pathways. For detailed descriptions of skin structure and function, readers may consult texts or reviews [45,92].
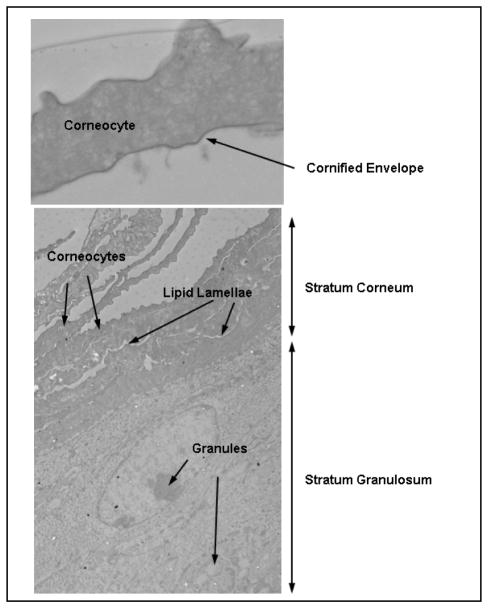
Figure 3.
TEM images of (top) a corneocyte, illustrating the dense cornified envelope feature and (bottom) a skin section, illustrating corneocytes in the stratum corneum and granules in the stratum granulosum (extends beyond what is shown). Black spots are silver- enhanced quantum dots that penetrated the stratum corneum following application to UV-irradiated mouse skin (see Mortensen et al., 2008, for details).
3.3.1. Skin Architecture and Primary Barrier Formation
The dermis is the innermost portion of the skin; it is vascularized and provides nutrients to and waste transport from the epidermis. It is comprised of collagen-glycosaminoglycan complexes, loose connective tissue, and elastin proteins that provide mechanical strength and thermal insulation due to a structural association with the subcutis – the layer below the dermis containing adipose tissue. The dermis is usually not considered in the context of the primary skin barrier; however, it is important to note that appendages reside in the dermis (follicles, sweat and sebaceous glands) and these play a secondary role in barrier function, discussed below.
The skin basement membrane exhibits an invaginated structure and separates the dermis from the viable layers of the epidermis. It consists of closely packed collagen, laminin, fibronectin and other cell adhesion molecules. The basement membrane is directly under the stratum basale, which consists of melanocytes (pigment producing cells) and keratinocyte stem cells that are responsible for maintaining the proliferative potential of the epidermis. Stem cells divide at the stratum basale to produce suprabasal daughter cells called transiently amplifying cells [72]. Transiently amplifying cells can undergo several cycles of division; however, once keratinocytes loose their integrin attachment to basement membrane, they undergo terminal differentiation (~28 days to complete). This results in the formation of the stratum spinosum and stratum granulosum (SG) in viable epidermis and the nonviable outermost skin layer, the SC, which is comprised of dead keratinocytes (corneocytes) (Figure 3). Keratinocytes in the SG layer produce granules that contain lipids (e.g. ceramides, fatty acids and cholesterol) and proteins (e.g. filaggrin, involucrin, loricrin) that form the lipid lamellae between corneocytes. It is plausible that the outward movement of differentiating keratinocytes contribute to barrier function by clearing substances that have breeched the barrier back out towards the skin surface. However, the prevailing view is that the structural composition of the thin (~10–20 μm) outer SC layer (12–16 cell layers thick) provides the primary skin barrier [155]. The SC presents a long, torturous, interdigitated paracellular pathway (Figure 3). The lipid lamella is a highly organized self-assembled structure exhibiting orthorhombic lateral packing of polar head groups [118]. The lipids inhibit inside-out water loss and outside-in permeation of hydrophilic substances >500 MW [18]. The corneocyte cytosol is comprised of dense, highly cross-linked keratin filaments arranged with a cubic rod packing [104]. The keratin filaments and thick cornified cell envelope provide a strong physical barrier to outside-in penetration via a transcellular route. It is widely accepted that transdermal penetration of hydrophilic substances occurs via a transcellular polar pathway, as the majority of the water in the SC is associated with corneocyte proteins [13]. However, polar channels exist in the lipid lamella between the oriented polar head groups, which can support paracellular transport of hydrophilic substances [10,35]. Overall, skin is far more permeable to hydrophobic materials, which follow a paracellular route between corneocytes via interactions with the lipid lamellae.
3.3.2. Secondary Barrier Function of Skin
In recent years, there has been growing recognition that additional features of skin physiology are essential for maintaining healthy barrier function. Some important secondary components include maintenance of tight junction complexes between keratinocytes in the SG layer, SC hydration, skin immune function, and secretory function of sebaceous glands associated with hair follicles. The spacing between tight (1–4 nm) junctions and adherens junctions (10–20 nm) in healthy tissue [103] should restrict penetration of NP into the viable epidermis. However, barrier dysfunction associated with skin diseases (atopic dermatitis, psoriasis) or environmental damage (mechanical trauma, UV radiation, pathogen exposure) can enhance both the inside-out and outside-in permeation of substances. For example, the absence of claudin-1 (a tight junction protein functional in the SG layer of skin) is lethal: knock-out mice die as newborns due to dehydration [46]. This result provided awareness that tight junctions play a role in maintaining the inside-out skin barrier. More recent studies have linked degradation of tight junction complexes by microbial toxins with exacerbation of symptoms in patients with eczema [6,111]. Mitigating the effects of microbes and eliminating substances that breech the SC skin barrier requires proper immune function (innate and adaptive). Keratinocytes produce cytokines in response to penetration of injurious substances. A common skin repair response is cellular proliferation and differentiation that over a few days thickens the SC to resist further penetration [146]. Proper immune function requires, however, that substances be recognized as invaders. Langerhans cells are professional antigen presenting cells and, so, phagocytize foreign substances (microbial invaders, particulates as large as 0.5–3.5μm diameter [143] and present antigens to T-cells [119]. Studies show however, as discussed below, that phagocytic cells are less effective at recognizing submicron-sized particulates [61,90]. This raises questions about the fate of NP that may breech the skin barrier.
Hair follicles cover about 0.5–2% of the skin surface area [112]. The average follicular diameter is ~100 μm and the follicular volume is ~0.2 mm3; however, racial and gender differences exist [93]. Follicles play an important role in skin permeation. Their physical invaginated structure provides a niche for mechanical accumulation and storage of substances [80–82]. The SC brick and mortar barrier of the skin surface does not extend down into the hair follicle. Rather, the hair follicle barrier is a combination of an inner and outer root sheath, sebum production, and hair anagen (growth cycle). The fact that the base of the hair follicle is located in the dermis and is fed by the blood and lymph systems makes the follicle a potential portal for NP systemic access [95,145]. Langerhans cells are highly concentrated around hair follicles [149], presumably to compensate for the reduced SC-like barrier. Studies comparing efficacy of transdermal drug delivery through normal and scarred tissue (hair follicles and glands do not regenerate in deep tissue injury, although these are not the only key differences) confirm the importance of follicles in skin permeation [63,64].
4. Nanoparticle Physicochemical Properties
The above sections have described the microanatomy of epithelial tissues and the nature of the lung, gut, and skin barriers. The present section considers the physicochemical characteristics of metal and metal oxide NP that may affect epithelial barrier penetration. Some key properties that have emerged as important determinants of NP penetration include size, surface charge, and surface energy (hydrophobicity/hydrophilicity) [83,84,115]. This is not, however, an exhaustive list. Properties such as composition as it relates to NP solubility and protein binding capacity are also likely to play important roles in the passage of NPs across epithelial barriers, cellular uptake, cytotoxicity, and biodistribution.
4.1. Nanoparticle Size
Paracellular penetration of NPs larger that a few nanometers (~4 nm diameter) may be physically blocked by tight junctions [5] unless they are leaky due to physical damage or disease. However, paracellular transport is not the only mechanism by which NPs can breech an epithelial barrier. NPs may be able to diffuse through cell membranes if the size and surface chemistry allows partitioning in the lipid-rich microenvironment of the lipid bilayer. Additional uptake routes include clathrin-coated pits and caveolae, both of which are lipid-enriched regions of the membrane. Although there is some controversy about whether or not the latter participate in endocytosis per se, the engagement of molecules in these regions and subsequent internalization and transcytosis are energy-dependent. Clathrin-coated pits themselves are lipid rafts of diameters slightly smaller than 100 nm up to ~300 nm, although they get slightly larger as they pinch off the membrane to form vesicles. Their participation in receptor-mediated endocytosis alludes to the concentration of various receptors in the pits, such as for LDL and transferrin [44,152]. Thus, the adsorption of transferrin, for example, to the NP surface may promote internalization via clathrin-coated pits. Caveolae are involved in intracellular signaling, as growth factor and other receptors co-localize with these structures. They are also lipid-rich domains in the membrane – though not present in all cell types – and are vase-shaped with openings of ~20–40 nm [101. Caveolae also co-localize with receptors for albumin, an abundant serum protein that is likely to interact with NP surfaces. Uptake and transport of NPs via these two pathways is, thus, likely to be dependent on particle size and surface chemistry.
NP size is also likely to play a role in the clearance of those particles that end up in the circulation, as NPs smaller than ~5.5 nm hydrodynamic diameter are filtered by the kidneys [27]. Some studies show that quantum dot NPs (~13 nm) can be retained in tissues on the order of months [154] if they are not rapidly filtered into urine. NPs may also be cleared by uptake into phagocytic cells in the organs of the reticuloendothelial system (RES) [43]. However, effective clearance by the RES also depends on size [90]. Studies have found that larger particles (radius 250 nm) are phagocytosed faster than smaller particles (radius 25 nm) [61]. This has implications for NPs that are delivered as agglomerates or for those that agglomerate as a result of their interactions with biomolecules.
4.2. Nanoparticle Chemical Composition
NP chemistry (surface and core) is likely to influence cellular uptake, clearance, and biocompatibility. Probably one of the most important factors is the NP surface chemistry, either that of the particle itself or of a coating material. Surface charge and surface energy deserve separate consideration in this discussion about chemistry and are addressed below. It is not clear from the existing literature whether or not core chemistry is a determinant of NP fate; this is the subject of ongoing research. However, NP surface chemistry will affect agglomeration behavior and biomolecule adsorption and, thus, possibly dominates in the issue of in vivo fate. For example, hydrophilic coatings like polyethylene glycol (PEG) potentiate the circulatory half-times of NPs [3,156]. Surface chemistry also determines in vivo dissolution rates and, therefore, contributes to distribution, retention, and toxicity. Furthermore, intracellular trafficking into acidic vesicles may potentiate cytotoxicity if the milieu leads to NP degradation and release of toxic compounds (e.g. the Cd core of some quantum dots) [25,88,89,123]. NP surface composition also affects the generation of reactive oxygen species via redox or catalytic activity [66,86,89,99,129,147,153]. It is worth pointing out here that NPs with high oxidant and cytotoxic potential may have more significant effects at the initial site of deposition and may also be cleared more rapidly due to an influx of inflammatory cells.
4.2.1. Nanoparticle Surface Charge
In biological tissues, NP surface charge will influence nonspecific interactions with proteins that are present in the milieu. Cell membranes are typically negatively charged, although positive domains exist [52]. At physiological pH (~7.3), aqueous pore channels are also anionically charged, which would slightly favor the penetration of positively-charged NPs via electrostatic attraction [5]. A positive surface charge has been shown to enhance phagocytosis by mouse macrophages (of 200 nm polymer beads) as well as non-specific uptake of larger particles (~5 μm microcapsules) by epithelial cells, possibly due to greater adhesive forces between the cell membrane and the oppositely-charged particle surface [69,156]. These data are contradicted, however, by findings that phagocytosis of hydrophilic polystyrene particles (~1 μm) by mouse macrophages increased with negative charge [51]. These observations illustrate the difficulty in predicting NP uptake and fate as a function of a single property, like surface charge. Further complicating such generalizations is the fact that particle surface charge is likely to change in vivo as a result of exposure to pH gradients (e.g. in the GI tract) and of protein adsorption/deadsorption processes [91].
4.2.2. Nanoparticle Surface Energy
Surface energy has also been found to greatly impact how NPs interact with biomolecules and tissues [24]. In an aqueous environment, low energy surfaces (hydrophobic) are particularly prone to nonspecific adsorption as proteins unfold to expose their hydrophobic core. Such surfaces also have surfactant-like properties, which may disorganize lipid components of cell membranes and enhance epithelial penetration [11]. High energy surfaces (hydrophilic), particularly those that carry a weakly negative or neutral charge, are ideal for resisting protein adsorption and cell uptake [37,156]. It is important to note that biomolecule adsorption is a competitive and dynamic process. Known as the Vroman effect and initially described for plasma proteins [148], nonspecific binding begins with the adsorption of abundant low molecular weight species (e.g. albumin) that diffuse more quickly to the surface. In time, high molecular weight species (e.g. fibrogenin) of lower concentration accumulate on the surface. As such, NPs engineered with coatings to affect a specific function (e.g. protect against degradation, target delivery, resist cellular uptake or clearance) may, in fact, change with time in vivo depending upon the route of administration and transport processes [24,75,91].
5. Nanoparticle Fate Following Contact with Epithelial Barriers
5.1. Nanoparticle Interactions with the Respiratory Tract
Some important lessons that are likely to be applicable to NPs have been learned by studying the health effects of ambient nanosized particles (ultrafine particles, UFP). One important point, as discussed previously, is that the deposition of particles in the alveolar region of the lung is size-dependent, with a peak ~20 nm [65]. In addition, alveolar macrophages – like RES phagocytes – do not efficiently take up singlet UFP [2,58]. The high deposition efficiencies and escape from macrophage-mediated clearance lead to the potential for increased interaction of NPs with epithelial structures in the alveoli, increased retention in the lung, and passage from the epithelial surface into the blood. The results of years of research with ambient air and industrially-relevant UFP support the conclusion that nanosized particles produce greater adverse effects as compared to larger particles with similar chemistry [85,106,109,116,142]. Effects of UFP outside of the respiratory tract have also been documented, including enhanced venous thrombus and atherosclerotic lesion formation, alterations in circulating thrombin-anti-thrombin complexes and fibrinogen, inflammatory mediator production in cortical neurons and olfactory bulb, and alterations in heart rate and heart rate variability [22,39–41,78,100,134,142]. These extrapulmonary effects may be due either to direct transport to sensitive target tissues or to the generation of inflammatory mediators (or a combination of these two processes).
An important first site of accumulation for NPs is the lung interstitium. In vivo studies have demonstrated that nanosized particles, in comparison to larger particles with the same chemistry, accumulate to a higher degree in the lung interstitium [105,133]. In terms of extrapulmonary tissues, the liver, kidneys, and spleen demonstrate significant and rapid accumulation of NPs that cross the alveolar epithelial barrier [77,108,132,140]. Ongoing research is aimed at understanding how NP size and chemistry might contribute to overall biodistribution. Because the chemical traces of NPs can be detected very rapidly in liver and blood following respiratory tract exposures [140] and the kinetics are more rapid than would be expected from predicted in vivo solubility, this suggests that at least a small fraction of the total amount deposited in the lung is transported as solid particulate.
The nose filters large volumes of air containing both small and large particles and it absorbs gases. The olfactory epithelium has ciliated olfactory receptor cells, which are bioploar neurons that are continuous with the olfactory bulb inside the skull. These receptor cells are potential portals of entry for NPs that deposit on the olfactory epithelium (Figure 1). Transport of NPs along the olfactory nerve into the olfactory bulb has been demonstrated via electron microscopy by using Polio virus that was applied intranasally and with silver-coated colloidal (50 nm) gold NPs [16,17,36]. Studies with inhaled insoluble Mn oxide and 13C UFP also showed that about 11% and 20%, respectively, of the inhaled amount that deposited in the nose traveled to the olfactory bulb (41,107). Whether or not translocation away from the original site of exposure occurs for all tissues and the extent to which the process is dependent on key physicochemical properties of the NPs are issues being addressed by current research.
5.2. Nanoparticle Interactions with GI Tract
The GI tract is potentially a primary route of exposure to NPs via ingestion. Exposure may also occur via respiratory tract clearance mechanisms that propel mucous with trapped particles or those that have been taken up by macrophages to the oropharynx, where they are swallowed. However, the barrier function of the GI tract with respect to NPs, as addressed by in vivo studies, is somewhat equivocal.
The extreme shifts in acidity along the GI tract and the negatively charged mucous layer in the small intestine, as described in Section 1.2, are likely to significantly influence the uptake of NPs from the bulk flow into enterocytes, absorption into blood, and tissue distribution. For NPs that still carry a surface charge when they reach the small intestine, this mucous layer may act as a trap and potentiate fecal clearance. Micron-sized insoluble particles can be transported from the intestinal lumen to the blood via paracellular pathways in a process known as persorption [150]. In vivo studies have shown intestinal absorption of particles to be size-dependent, with smaller particles (polystyrene microspheres, colloidal gold) being absorbed to a greater degree than larger ones [60,68]. Studies with highly insoluble radioactive metal NPs have shown extremely low transfer into blood following GI tract exposures [77,138], but with some evidence that a negative surface charge promotes the absorption of the smallest particles [12]. Electron microscopy with elemental analysis has identified nanosized particulates in liver, kidney, and colon tissue samples from humans [48–50]. Although it is possible that these particulates were derived from combustion processes or food and gained access to the organs via the digestive and/or respiratory tracts, it is not clear how the accumulation occurred, whether they accumulated as NPs (as opposed to in situ dissolution), and what role the RES played (as opposed to free particle transport).
5.3. Nanoparticle Interactions with Skin
In recent years, studies of NP interactions with skin have escalated owing to their increasing use in commercial products. For example, the antimicrobial properties of silver NPs have been widely exploited in wound care products (bandages, masks), food containers, and refrigerators [96]. Metal oxide NPs (TiO2, ZnO) are used in sunscreens and cosmetic products [34,125,136]. Most in vitro studies have been conducted with keratinocytes and fibroblasts – cell types that reside in the epidermis and dermis – to investigate NP-induced toxicological responses related to inflammation and oxidative stress [99,121,147]. Information regarding NP uptake by these cells is also often provided. However, the relevance of these findings to the issue of skin penetration by NPs is questionable, as the underlying assumption is that the SC is breeched to allow NP penetration to the viable epidermis and in the dermis. Such studies are also typically done over a 24–48 hr period at dose levels that far exceed what might be expected to occur from incidental exposure. It is, therefore, important to consider the conditions under which NP penetrate the SC and what constitutes a “typical” skin exposure to NPs.
Ex vivo studies using human and pig skin have sought to elucidate this needed insight, yet results are difficult to interpret due to inconsistencies in the processing of skin tissue samples and in the formulation and application of the NP. Important trends have emerged from existing literature, though, with regard to the role of hair follicles, which have been found to serve as efficient reservoirs for NPs. Follicular accumulation and penetration depth depend on NP size [4]. In addition, mechanical flexion enhances follicular accumulation [82,121,141,143,145], and substances are retained longer in the hair follicle when they are NP-associated [80]. These findings raise the issue of NP penetration into the viable epidermis. Studies using rigid iron oxide core NPs (~5 nm to 23 nm) applied to ex vivo human skin demonstrated NP accumulation throughout the SC and occasionally in the uppermost strata of the viable epidermis [11]. Flow cytometry studies on Langerhans cells (LC) recovered from human skin exposed to 40 nm polymer NP found 24% of LC contained NP [149]. Ex vivo studies with quantum dots [122] show evidence for penetration into viable epidermal layers depending on the NP size, surface chemistry, and shape (e.g. PEG-amine quantum dots penetrated to the dermis, whereas PEG- and carboxylic acid-coated quantum dots localized in the epidermis). Studies measuring the distribution profile of elastic and rigid vesicles (~115 nm diameter) in human skin found that elastic particles penetrate further than the rigid ones under identical conditions [62]. These studies using ex vivo systems highlight some of the NP material properties that affect skin penetration.
Few in vivo studies [82,94,136] have been conducted to address the issue of NP penetration through skin and absorption into blood. Those that have been done to assess NP penetration (mainly with metal oxides) have produced findings that are similar to ex vivo studies in that there is accumulation in hair follicles, the SC, and in skin defects [11,34,47,79–81,131,143]. However, there is negligible penetration to viable epidermal layers. A possible explanation is that the metal oxide NPs used in cosmetics, including sun screens, span a range of sizes, shapes, and surface coatings and are typically formulated in oil/water emulsions. Other factors beyond the pure NP physicochemical properties may be important in mitigating NP skin penetration, such as the vehicle formulation, skin surface pH, and the presence of salts and oily secretions. All of these factors may affect dispersion after NPs come into contact with skin. Interestingly, more recent studies have shown that when the skin barrier is impaired via mechanical abrasion [157] or UV irradiation [97,136], NP penetration through the SC into viable epidermal layers is potentiated. These results bring to light the importance of the barrier integrity with respect to NP skin penetration.
Having established that under certain conditions, NP exposure can result in penetration, albeit slight, there is a clear need for further research to develop methods to quantify penetration, to understand translocation mechanisms through skin, and NP fate. In vitro studies show that NP can be toxic to cells if taken up in sufficiently high amounts [84]; however, the relevance of these findings to realistic exposure conditions and concentrations is unclear. NPs that penetrate skin might also gain access to circulation and then distribute throughout the body. In vivo studies of intradermally injected quantum dots show that the NP migrate to local lymph tissue and rapidly accumulate in liver, kidney, and spleen [55,74]. NPs must be <5–6 nm to be efficiently cleared from the body via the kidneys into urine [27], so the possibility of long-term retention of NPs after crossing epithelial barriers – as has been recently demonstrated for intravenously injected quantum dots [154] – must be investigated.
6. Conclusion
This review concludes by summarizing some of the key similarities and differences of the three physiological barriers that NPs must cross to gain entry to the body and its target organs, namely the respiratory and gastrointestinal tracts and the skin. Predictions are then offered regarding the likelihood of NPs to breech these barriers.
First, the skin and gut epithelia have in common a rapid cell turn-over rate – although gut is faster than skin – and an outward progression of cycling cells, which may result in clearance of NPs that penetrate these two barriers. Skin and gut also have progenitor cell pools that reside in invaginated areas (“crypts”) at the border between layers of the respective barriers. In lung, progenitor pools lie at airway branching points and in specific populations of cells (e.g. Clara cells, type II alveolar cells). A unique feature of the alveolar region of the respiratory tract is the short space between a predicted site for initial deposition of NPs and the vasculature. These features of the barrier epithelia allow some predictions regarding the potential for breeching by solid, poorly-soluble metal/metal oxide NPs. With respect to the respiratory tract, the most vulnerable sites are likely to be the olfactory mucosa and the alveolar region, due to architectural considerations and high deposition efficiencies for airborne NPs. For the GI tract, the small intestine and the FAE, in particular, are likely to be sites of NP absorption from ingesta because these regions are specialized for nutrient uptake and antigen transcytosis (M cells). The skin appears to be a fairly solid barrier with respect to this class of NPs, but small amounts may get to immune-competent cells or the microcirculation over time following repeated exposure of skin to NPs. A key point to emphasize, though, is that the health of the epithelial barrier is a determinant of penetration and absorption potential of NPs.
An important NP elimination mechanism for both skin and gut is the outward movement of rapidly turning over cells. For the respiratory tract, a significant elimination pathway for NPs is likely to be translocation to other tissues. However, there is evidence for re-entrainment of interstitialized material through the bronchus-associated lymphoid tissue back into the airways. It is possible the M cells play a similar role in the GI tract, albeit in a much shorter time frame.
Lastly, all three epithelial surfaces are anionic in nature. However, microenvironments – surfactant in lung, the unstirred layer in gut, and lipid lamellae and hair follicles in skin – are likely to have a larger overall impact on NP penetration through the barriers in the process of absorption into blood. The surface of the NP, though, also plays a significant role in epithelial interactions and the surface itself is likely to change during uptake and distribution processes, e.g. oxidation state of metals, surface charge, adsorbed proteins and other biomolecules. It is the combination of all of these factors that will determine the ultimate fate of NPs that come into contact with barrier epithelia, which is an obviously complicated set of issues to sort out in future health impact-related research.
Contributor Information
Alison Elder, Email: Alison_Elder@urmc.rochester.edu, University of Rochester, Department of Environmental Medicine.
Sadasivan Vidyasagar, Email: Sadasivan_Vidyasagar@urmc.rochester.edu, University of Rochester, Department of Radiation Oncology.
Lisa DeLouise, Email: Lisa_DeLouise@urmc.rochester.edu, University of Rochester, Departments of Dermatology and Biomedical Engineering.
References
- 1.Adamson IY, Bowden DH. Derivation of type I epithelium from type II cells in the developing rat lung. Lab Invest. 1975;32(6):736–745. [PubMed] [Google Scholar]
- 2.Ahsan F, Rivas IP, Khan MA, Suárez-Torres AI. Targeting to macrophages: role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 3.Åkerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. PNAS. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Roma R, Naika A, Kaliaa YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99(1):53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JM. Molecular Structure of Tight Junctions and Their Role in Epithelial Transport. News Physiol Sci. 2001;16:126–130. doi: 10.1152/physiologyonline.2001.16.3.126. [DOI] [PubMed] [Google Scholar]
- 6.Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002;119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 7.Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogen. 2000;21:469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- 8.Bailey MR. The new ICRP model for the respiratory tract. Radiat Prot Dosimetry. 1994;53:107–114. [Google Scholar]
- 9.Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbero AM, Frasch HF. Transcellular route of diffusion through stratum corneum: results from finite element models. J Pharma Sci. 2006;95(10):2186–2194. doi: 10.1002/jps.20695. [DOI] [PubMed] [Google Scholar]
- 11.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, Lopez-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127(7):1701–1712. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 12.Behnke M, Lipka J, Takenaka S, Parak W, Simon U, Schmid G, Brandau W, Kreyling WG. Strong size dependency of biodistribution of gold nanoparticles (NP) between 1.4 and 200 nm administered either to the lungs, blood or gut of rats. Nanotox 2008 conference abstract; Zürich, Switzerland. September, 2008. [Google Scholar]
- 13.Benson HAE. Transdermal drug delivery: penetration enhancement techniques. Cur Drug Deliv. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 14.Bhalla DK, Owen RL. Cell renewal and migration in lymphoid follicles of Peyer’s patches and cecum--an autoradiographic study in mice. Gastroenterology. 1982;82:232–242. [PubMed] [Google Scholar]
- 15.Bjerknes M, Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981;199:565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- 16.Bodian D, Howe HA. Experimental studies on intraneural spread of poliomyelitis virus. Bull Johns Hopkins Hosp. 1941a;68:248–267. [Google Scholar]
- 17.Bodian D, Howe HA. The rate of progression of poliomyelitis virus in nerves. Bull Johns Hopkins Hosp. 1941b;69:79–85. [Google Scholar]
- 18.Bos JD, Meinardi MMHM. The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P. Regionalized immune function of tonsils and adenoids. Immunol Today. 1999;20:383–384. doi: 10.1016/s0167-5699(99)01498-x. [DOI] [PubMed] [Google Scholar]
- 20.British Standards Institute Publicly Available Specification, “Vocabulary – Nanoparticles”. PAS. 2005;71:2005. [Google Scholar]
- 21.Bye WA, Allan CH, Trier JS. Structure, distribution, and origin of M cells in Peyer’s patches of mouse ileum. Gastroenterology. 1984;86:789–801. [PubMed] [Google Scholar]
- 22.Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicol. 2005;26:133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Carino GP, Mathiowitz E. Oral insulin delivery. Adv Drug Deliv Rev. 1999;35:249–257. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 24.Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. PNAS. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of Qdot cytotoxicity based on intracellular uptake. Small. 2006;2(12):1412–1417. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Tan M, Nemmar A, Song W, Dong M, Zhang G, Li Y. Quantification of extrapulmonary translocation of intratracheal-instilled particles in vivo in rats: effect of lipopolysaccharide. Toxicol. 2006;222(3):195–201. doi: 10.1016/j.tox.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark MA, Blair H, Liang L, Brey RN, Brayden D, Hirst BH. Targeting polymerised liposome vaccine carriers to intestinal M cells. Vaccine. 2001;20:208–217. doi: 10.1016/s0264-410x(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 29.Clark MA, Jepson MA, Simmons NL, Hirst BH. Differential surface characteristics of M cells from mouse intestinal Peyer’s and caecal patches. Histochem J. 1994a;26:271–280. [PubMed] [Google Scholar]
- 30.Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994b;145:543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 31.Clark MA, Reed KA, Lodge J, Stephen J, Hirst BH, Jepson MA. Invasion of murine intestinal M cells by Salmonella typhimurium inv mutants severely deficient for invasion of cultured cells. Infect Immun. 1996;64:4363–4368. doi: 10.1128/iai.64.10.4363-4368.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cleary JE, Burke WM, Baxi LV. Pregnancy after avascular necrosis of the femur complicating Gaucher’s disease. Am J Obstet Gynecol. 2001;184:233–234. doi: 10.1067/mob.2001.107203. [DOI] [PubMed] [Google Scholar]
- 33.Colombel JF, Grandbastien B, Gower-Rousseau C, Plegat S, Evrard JP, Dupas JL, Gendre JP, Modigliani R, Bélaïche J, Hostein J, Hugot JP, van Kruiningen H, Cortot A. Clinical characteristics of Crohn’s disease in 72 families. Gastroenterology. 1996;111:604–607. doi: 10.1053/gast.1996.v111.pm8780563. [DOI] [PubMed] [Google Scholar]
- 34.Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles. Skin Pharmacol Physiol. 2007;20(3):148–154. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- 35.Dayan N. Pathways for skin penetration. Cosmetics & Toiletries Magazine. 2005;120(6):67–76. [Google Scholar]
- 36.DeLorenzo AJD. The Olfactory Neuron and the Blood-Brain Barrier. In: Wolstenholme GEW, Knight J, editors. Taste and Smell in Vertebrates. Vol. 1970. J&A Churchill; London: pp. 151–76. [Google Scholar]
- 37.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of Qdots. Nano Lett. 2004;4(1):11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeSimone JA. Diffusion barrier in the small intestine. Science. 1983;220:221–222. doi: 10.1126/science.6828892. [DOI] [PubMed] [Google Scholar]
- 39.Elder A, Couderc JP, Gelein R, Eberly S, Cox C, Xia X, Zareba W, Hopke P, Watts W, Kittelson D, Frampton M, Utell M, Oberdörster G. Effects of on-road highway aerosol exposures on autonomic responses in aged, spontaneously hypertensive rats. Inhal Toxicol. 2007;19:1–12. doi: 10.1080/08958370600985735. [DOI] [PubMed] [Google Scholar]
- 40.Elder A, Gelein R, Finkelstein J, Phipps R, Frampton M, Utell M, Topham D, Kittelson D, Watts W, Hopke P, Jeong CH, Kim E, Liu W, Zhao W, Zhou L, Vincent R, Kumarathasan P, Oberdörster G. On-road exposure to highway aerosols. 2. Exposures of aged, compromised rats. Inhal Toxicol. 2004;16:41–53. doi: 10.1080/08958370490443222. [DOI] [PubMed] [Google Scholar]
- 41.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdörster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, Tic TR. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release. 1990;11:205–214. [Google Scholar]
- 43.Fischer HC, Liu L, Pang KS, Chan WCW. Pharmacokinetics of nanoscale quantum dots: in vivo distribution, sequestration, and clearance in the rat. Adv Funct Mat. 2006;16(10):1299–1305. [Google Scholar]
- 44.Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 45.Freedberg IM, Eisen AD, Wolff K, Austen KF, Goldsmith LA, Katz S, editors. Fitzpatrick’s Dermatology in General Medicine. 7. McGraw-Hill; New York: 2007. [Google Scholar]
- 46.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gamer AO, Leibold E, van Ravenzwaay B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol in Vitro. 2006;20(3):301–307. doi: 10.1016/j.tiv.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Gatti AM, Montanari S. Nanopathology: the health impact of nanoparticles. Pan Stanford; Singapore: 2008. [Google Scholar]
- 49.Gatti AM, Rivasi F. Biocompatibility of micro- and nanoparticles (Part I) in liver and kidney. Biomater. 2002;23(11):2381–2387. doi: 10.1016/s0142-9612(01)00374-x. [DOI] [PubMed] [Google Scholar]
- 50.Gatti AM. Biocompatibility of micro- and nanoparticles in the colon (Part II) Biomater. 2004;25(3):385–392. doi: 10.1016/s0142-9612(03)00537-4. [DOI] [PubMed] [Google Scholar]
- 51.Gbadamosi HJ, Hunter AC, Moghimi SM. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett. 2002;532(3):338–344. doi: 10.1016/s0014-5793(02)03710-9. [DOI] [PubMed] [Google Scholar]
- 52.Ghitescu L, Fixman A. Surface charge distribution on the endothelial cells of liver sinusoids. J Cell Biol. 1984;99:639–647. doi: 10.1083/jcb.99.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108–1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Mariscal L, Chavez de Ramirez B, Lazaro A, Cereijido M. Establishment of tight junctions between cells from different animal species and different sealing capacities. J Membr Biol. 1989;107:43–56. doi: 10.1007/BF01871082. [DOI] [PubMed] [Google Scholar]
- 55.Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Warbritton AR, Yu WW, Colvin VL, Walker NJ, Howard PC. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol Sci. 2007;98(1):249–257. doi: 10.1093/toxsci/kfm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grenha A, Seijo B, Remuñán-López C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur J Pharm Sci. 2005;25(4–5):427–437. doi: 10.1016/j.ejps.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Griese M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur Resp J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 58.Hahn FF, Newton GJ, Bryant PL. In vitro phagocytosis of respirable-sized monodisperse particles by alveolar macrophages. In: Sanders CL, Schneider RP, Dagle GE, Ragen HA, editors. Pulmonary Macrophages and Epithelial Cells. Vol. 43. Oak Ridge: Technical Information Center, Energy Research and Development Administration; 1977. pp. 424–435. [Google Scholar]
- 59.Hansen SF, Michelson ES, Kamper A, Borling P, Stuer-Lauriddsen F, Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicol. 2008;17(5):438–447. doi: 10.1007/s10646-008-0210-4. [DOI] [PubMed] [Google Scholar]
- 60.Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001;90(12):1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 61.Holmberg SB, Forssell-Aronsson E, Gretarsdottir J, Jacobsson L, Rippe B, Hafstrom L. Vascular clearance by the reticuloendothelial system--measurements using two different-sized albumin colloids. Scand J Clin Lab Invest. 1990;50(8):865–871. doi: 10.3109/00365519009104954. [DOI] [PubMed] [Google Scholar]
- 62.Honeywell-Nguyen PL, Gooris GS, Bouwstra JA. Quantitative assessment of the transport of elastic and rigid vesicle components and a model drug from the vesicle formulation into human skin in vivo. J Invest Dermatol. 2004;123:902–910. doi: 10.1111/j.0022-202X.2004.23441.x. [DOI] [PubMed] [Google Scholar]
- 63.Hueber F, Wepierre J, Schaefer H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: In vivo study in the hairless rat. Skin Pharmacol. 1992;5:99–107. doi: 10.1159/000211026. [DOI] [PubMed] [Google Scholar]
- 64.Illel B. Formulation for transfollicular drug administration: some recent advances. Crit Rev Ther Drug Carrier Syst. 1997;14(3):207–219. [PubMed] [Google Scholar]
- 65.International Committee on Radiological Protection. Human Respiratory Tract Model for Radiological Protection. A Report of Committee 2 of the ICRP. Oxford: Pergamon Press; 1994. [Google Scholar]
- 66.Ipe BI, Lehnig M, Niemeyer CM. On the generation of free radical species from quantum dots. Small. 2005;1:706–709. doi: 10.1002/smll.200500105. [DOI] [PubMed] [Google Scholar]
- 67.Ishimoto H, Isomoto H, Shikuwa S, Wen CY, Suematu T, Ito M, Murata I, Ishibashi H, Kohno S. Endoscopic identification of Peyer’s patches of the terminal ileum in a patient with Crohn’s disease. World J Gastroenterol. 2004;10:2767–2768. doi: 10.3748/wjg.v10.i18.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;442(12):821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 69.Javier AM, Kreft O, Alberola AP, Kirchner C, Zebli B, Susha AS, Horn E, Kempter S, Skirtach AG, Rogach AL, Rädler J, Sukhorukov GB, Benoit M, Parak WJ. Combined atomic force microscopy and optical microscopy measurements as a method to investigate particle uptake by cells. Small. 2006;2(3):394–400. doi: 10.1002/smll.200500282. [DOI] [PubMed] [Google Scholar]
- 70.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Junquiera CL, Carneiro J, Kelley RO. Basic Histology. 7. 1992. [Google Scholar]
- 72.Kaur P, Li A. Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000;114:413–420. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 73.Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol. 2003;284(2):L247–L259. doi: 10.1152/ajplung.00235.2002. [DOI] [PubMed] [Google Scholar]
- 74.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein J. Probing the interactions of proteins and nanoparticles. PNAS. 2007;104:2029–2030. doi: 10.1073/pnas.0611610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Ann Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 77.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 78.Künzli N, Jerret M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lademann J, Ilgevicius A, Zurbau O, Liess HD, Schanzer S, Weigmann HJ, Antoniou C, Pelchrzim RV, Sterry W. Penetration studies of topically applied substances: optical determination of the amount of stratum corneum removed by tape stripping. J Biomed Opt. 2006b;11(5):054026-1–054026-6. doi: 10.1117/1.2359466. [DOI] [PubMed] [Google Scholar]
- 80.Lademann J, Richter H, Schaefer UF, Blume-Peytavi U, Teichmann A, Otberg N, Sterry W. Hair follicles - a long-term reservoir for drug delivery. Skin Pharmacol Physiol. 2006c;19(4):232–236. doi: 10.1159/000093119. [DOI] [PubMed] [Google Scholar]
- 81.Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, Weiss B, Schaefer UF, Lehr CM, Wepf R, Sterry W. Nanoparticles - an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2006a;66(2):159–164. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Lademann J, Weigmann H, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W. Penetration of titanium dioxide microparticles in the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol. 1999;12(5):247–256. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 83.Lee S, Lee J, Choi YW. Skin permeation enhancement of ascorbyl palmitate by liposomal hydrogel (Lipogel) formulation and electrical assistance. Biol Pharm Bull. 2007;30(2):393– 396. doi: 10.1248/bpb.30.393. [DOI] [PubMed] [Google Scholar]
- 84.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Hamilton RF, Jr, Kirichenko A, Holian A. 4-Hydroxynonenal-induced cell death in murine alveolar macrophages. Toxicol Appl Pharmacol. 1996;139:135–143. doi: 10.1006/taap.1996.0152. [DOI] [PubMed] [Google Scholar]
- 86.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linkov I, Steevens J, Adlakha-Hutcheon G, Bennet E, Chappell M, Covin V, Davis M, Davis T, Elder A, Hansen SF, Hakkinen P, Hussain S, Karkan D, Korenstein R, Lynch I, Metcalfe C, Ramadan A, Satterstrom FK. Emerging methods and tools for environmental risk assessment, decision-making, and policy for nanomaterials: Summary of NATO advanced research workshop. J Nanopart Res. 2008 doi: 10.1007/s11051-008-9514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lovrić J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005a;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 89.Lovrić J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005b;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Lutz O, Meraihi Z, Mura JL, Frey A, Riess GH, Bach AC. Fat emulsion particle size: influence on the clearance rate and the tissue lipolytic activity. Am J Clin Nutr. 1989;50(6):1370–1381. doi: 10.1093/ajcn/50.6.1370. [DOI] [PubMed] [Google Scholar]
- 91.Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity: a complex fluids and surface science challenge for the 21st Century. Adv Colloid Inter Sci. 2007;134–135:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Madison KC. Barrier function of the skin: la raison d’être of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 93.Mangelsdorf S, Otberg N, Maibach HI, Sinkgraven R, Sterry W, Lademann J. Ethnic variation in vellus hair follicle size and distribution. Skin Pharmacol Physiol. 2006;19:159–167. doi: 10.1159/000093050. [DOI] [PubMed] [Google Scholar]
- 94.Mavon A, Miquel C, Lejeune O, Payre B, Moretto P. In vitro percutaneous absorption and iv vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharm Phys. 2007;20:10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- 95.Meidan VM, Bonner MC, Michniak BB. Transfollicular drug delivery - Is it a reality. Int J Pharm. 2006;306(1–2):1–14. doi: 10.1016/j.ijpharm.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 96.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnol. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 97.Mortensen L, Oberdörster G, Pentland AP, DeLouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: Effect of UVR. Nano Lett. 2008;8(9):2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nanda KK, Maisles A, Kruis FE, Fissan H, Stappert S. Higher surface energy of free nanoparticles. Phy Rev Lett. 2003;91(10):106102-1–106102-4. doi: 10.1103/PhysRevLett.91.106102. [DOI] [PubMed] [Google Scholar]
- 99.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(3):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 100.Nemmar A, Hoylaerts M, Hoet PHM, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med. 2002;166(7):998–1004. doi: 10.1164/rccm.200110-026OC. [DOI] [PubMed] [Google Scholar]
- 101.Newman GR, Campbell L, von Ruhland C, Jasani B, Gumbleton M. Caveolin and its cellular and sub-cellular immunolocalization in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res. 1995;295:111–120. doi: 10.1007/s004410051217. [DOI] [PubMed] [Google Scholar]
- 102.Ng AW, Bidani A, Heming TA. Innate host defense of the lung: Effects of lung-lining fluid pH. Lung. 2004;182:297–317. doi: 10.1007/s00408-004-2511-6. [DOI] [PubMed] [Google Scholar]
- 103.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 104.Norlén L, Al-Amoudi A. Stratum corneum keratin structure, function, and formation: the cubic rod-packing and membrane templating model. J Invest Dermatol. 2004;123:715–732. doi: 10.1111/j.0022-202X.2004.23213.x. [DOI] [PubMed] [Google Scholar]
- 105.Oberdörster G, Ferin J, Gelein R, Soderholm S, Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oberdörster G, Ferin J, Lehnert B. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 108.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 109.Oberdörster G. Toxicology of ultrafine particles: In vivo studies. Phil Trans Royal Soc London. 2000;358:2719–2740. [Google Scholar]
- 110.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(71):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohnemus U, Kohrmeyer K, Houdek P, Rohde H, Wladykowski E, Vidal S, Horstkotte MA, Aepfelbacher M, Kirschner N, Behne MJ, Moll I, Brandner JM. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative staphylococcus strains. J Invest Dermatol. 2008;128:906–916. doi: 10.1038/sj.jid.5701070. [DOI] [PubMed] [Google Scholar]
- 112.Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 2004;122:14–19. doi: 10.1046/j.0022-202X.2003.22110.x. [DOI] [PubMed] [Google Scholar]
- 113.Owen RL, Bhalla DK. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer’s patch M cells. Am J Anat. 1983;168:199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- 114.Pappenheimer JR, Dahl CE, Karnovsky ML, Maggio JE. Intestinal absorption and excretion of octapeptides composed of D amino acids. Proc Natl Acad Sci U S A. 1994;91:1942–1945. doi: 10.1073/pnas.91.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parisel C, Saffar L, Gattegno L, Andre’ V, Abdul-Malak N, Perrier E, Letourneur D. Interactions of heparin with human skin cells: Binding, location, and transdermal penetration. J Biomed Mat Res Part A. 2003;67(2):517–523. doi: 10.1002/jbm.a.10085. [DOI] [PubMed] [Google Scholar]
- 116.Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- 117.Phalen RF, Yeh H, Prasad SB. Morphology of the respiratory tract. In: McClellan RO, Henderson RF, editors. Concepts in Inhalation Toxicology. Taylor & Francis; Washington, D.C: 1995. pp. 129–149. [Google Scholar]
- 118.Pilgram GSK, Engelsma-van Pelt AM, Bouwstra JA, Koerten HK. Electron diffraction provides new information on human stratum corneum lipid organization studied in relation to depth and temperature. J Invest Dermatol. 1999;113:403–409. doi: 10.1046/j.1523-1747.1999.00706.x. [DOI] [PubMed] [Google Scholar]
- 119.Proksch E, Brasch J. Influence of epidermal permeability barrier disruption and Langerhans’ cell density on allergic contact dermatitis. Acta Derm Venereol. 1997;77(2):102–104. doi: 10.2340/0001555577102104. [DOI] [PubMed] [Google Scholar]
- 120.Ross MH, Romrell LJ. Histology: A Text and Atlas. 2. 1989. [Google Scholar]
- 121.Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monterio-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7(1):155–160. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]
- 122.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by QDots with diverse physicochemical properties. Toxicol Sci. 2006;91(1):159–165. doi: 10.1093/toxsci/kfj122. [DOI] [PubMed] [Google Scholar]
- 123.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127(1):143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 124.Salamat-Miller N, Johnston TP. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294:201–216. doi: 10.1016/j.ijpharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 125.Salleh A. Nano sunblock safety under scrutiny. News in Science, ABC Science Online. 2004 Jul; http://www.abc.net.au/science/news/stories/s1165709.htm.
- 126.Sanderson IR. The physicochemical environment of the neonatal intestine. Neoreviews. 2003;4:e117–120. doi: 10.1542/neo.4-5-e117. [DOI] [PubMed] [Google Scholar]
- 127.Sanderson IR. The physicochemical environment of the neonatal intestine. Am J Clin Nutr. 1999;69:1028S–1034S. doi: 10.1093/ajcn/69.5.1028s. [DOI] [PubMed] [Google Scholar]
- 128.Satsangi J, Grootscholten C, Holt H, Jewell DP. Clinical patterns of familial inflammatory bowel disease. Gut. 1996;38:738–741. doi: 10.1136/gut.38.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26(36):7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 130.Scheuch G, Kohlhaeufl MJ, Brand P, Siekmeier R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv Drug Deliv Rev. 2006;58:996–1008. doi: 10.1016/j.addr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 131.Schulz J, Hohenberg H, Pflücker F, Gärtner E, Will T, Pfeiffer S, Wepf R, Wendel V, Gers-Barlag H, Wittern KP. Distribution of sunscreens on skin. Adv Drug Deliv Rev. 2002;54 (Suppl 1):S157–163. doi: 10.1016/s0169-409x(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 132.Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdörster G, Kreyling WG. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 133.Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdörster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect. 2007;115(5):728–733. doi: 10.1289/ehp.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silva VM, Corson N, Elder A, Oberdörster G. The rat ear vein model for investigating in vivo thrombogenicity of ultrafine particles (UFP) Toxicol Sci. 2005;85:983–989. doi: 10.1093/toxsci/kfi142. [DOI] [PubMed] [Google Scholar]
- 135.Simionescu D, Simionescu M. Differentiated distribution of the cell surface charge on the alveolar-capillary unit. Characteristic paucity of anionic sites on the air-blood barrier. Microvasc Res. 1983;25(1):85–100. doi: 10.1016/0026-2862(83)90045-6. [DOI] [PubMed] [Google Scholar]
- 136.Sincai M, Argherie D, Ganga D, Bica D, Vekas L. Application of some magnetic nanocompounds in the protection against sun radiation. J Magnet Magnet Mat. 2007;311(1):363–366. [Google Scholar]
- 137.Smithson KW, Millar DB, Jacobs LR, Gray GM. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981;214:1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- 138.Stather JW, Harrison JD, Rodwell P, David AJ. The gastrointestinal absorption of plutonium and americium in the hamster. Phys Med Biol. 1979;24(2):396–407. doi: 10.1088/0031-9155/24/2/015. [DOI] [PubMed] [Google Scholar]
- 139.Stefani E, Cereijido M. Electrical properties of cultured epithelioid cells (MDCK) J Membr Biol. 1983;73:177–184. doi: 10.1007/BF01870440. [DOI] [PubMed] [Google Scholar]
- 140.Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109(Suppl 4):547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tan M, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australas J Dermatol. 1996;37(4):185–187. doi: 10.1111/j.1440-0960.1996.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 142.Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef B, Gold DR, Heinrich J, Hoek G, Lanki T, Peters A, Tarkiainen T, Tiittanen P, Kreyling W, Pekkanen J. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease: The ULTRA study. J Expo Anal Environ Epidemiol. 2005;16(4):332–341. doi: 10.1038/sj.jea.7500460. [DOI] [PubMed] [Google Scholar]
- 143.Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, Adkins EJ. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ Health Perspect. 2003;111(9):1202–1208. doi: 10.1289/ehp.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tobin DJ. Biochemistry of human skin--our brain on the outside. Chem Soc Rev. 2006;35(1):52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 145.Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting terminal hair follicles. J Invest Dermatol. 2004;123:168–176. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 146.Tripp CS, Blomme EAG, Chinn KS, Hardy MM, LaCelle P, Pentland AP. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J Invest Dermatol. 2003;121:853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 147.Tsay JM, Michalet X. New light on quantum dot cytotoxicity. Chem Biol. 2005;12:1159–1161. doi: 10.1016/j.chembiol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Turbill P, Beugeling T, Poot AA. Proteins involved in the Vroman effect during exposure of human blood plasma to glass and polyethylene. Biomaterials. 1996;17(13):1279–1287. [PubMed] [Google Scholar]
- 149.Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 2006;126:1316–1322. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 150.Volkheimer G. Hematogenous dissemination of ingested polyvinyl chloride particles. Ann NY Acad Sci. 1975;246:164–171. doi: 10.1111/j.1749-6632.1975.tb51092.x. [DOI] [PubMed] [Google Scholar]
- 151.Wolf JL, Rubin DH, Finberg R, Kauffman RS, Sharpe AH, Trier JS, Fields BN. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- 152.Wu X, Zhao X, Baylor L, Kaushal S, Eisenberg E, Greene LE. Clathrin exchange during clathrin-mediated endocytosis. J Cell Biol. 2001;155(2):291–300. doi: 10.1083/jcb.200104085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 154.Yang RS, Chang LW, Wu JP, Tsai MH, Wang HJ, Kuo YC, Yeh TK, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ Health Perspect. 2007;115(9):1339–43. doi: 10.1289/ehp.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yu B, Dong CY, So PTC, Blankschtein D, Langer R. In vitro visualization and quantification of oleic acid induced changes in transdermal transport using two-photon fluorescence microscopy. J Invest Dermatol. 2001;117:16–25. doi: 10.1046/j.0022-202x.2001.01353.x. [DOI] [PubMed] [Google Scholar]
- 156.Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22:8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 157.Zhang LW, Monteiro-Riviere NA. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol Physiol. 2008;21(3):166–80. doi: 10.1159/000131080. [DOI] [PubMed] [Google Scholar]