Abstract
Sex differences in Ca2+-dependent signaling and homeostasis in the vasculature of hypertensive rats are well characterized. However, sex-related differences in store-operated calcium entry (SOCE) have been minimally investigated. We hypothesized that vascular protection in females, compared to male, reflects decreased Ca2+ mobilization due to diminished activation of Orai1/ stromal interaction molecule 1 (STIM1). In addition, we investigated if female ovariectomy affects the activation of Orai1/STIM1 pathway. Endothelium-denuded aortic rings from male and female Stroke Prone Spontaneously Hypertensive (SHRSP) and Wistar-Kyoto (WKY) rats and from ovariectomized (OVX) or sham SHRSP and WKY female rats were used to functionally evaluate Ca2+ influx-induced contractions. Compared to female, aorta from male SHRSP displayed: 1) increased contraction during Ca2+ loading period; 2) similar transient contraction during Ca2+ release from the intracellular stores; 3) increased activation of STIM1 and Orai1, as evidenced by blockade of STIM1 and Orai1 with neutralizing antibodies, which reversed sex differences in contraction during Ca2+ loading period and 4) increased expression of STIM1 and Orai1. Additionally, we found that aortas from ovariectomized SHRSP showed increased contraction during the Ca2+ loading period and increased Orai1 expression but no changes in the SR buffering capacity or STIM1 expression. These data suggest that augmented activation of STIM1/Orai1 in aortas from male SHRSP represents a mechanism that contributes to sex-related impaired control of intracellular Ca2+ levels. Furthermore, female sex-hormones may negatively modulate the STIM/Orai1 pathway, contributing to vascular protection observed in female rats.
Keywords: aorta, Ca2+, hypertension, STIM1, Orai1
INTRODUCTION
The coupling process between the endoplasmic reticulum (ER) and plasma membrane to mediate store-operated calcium entry (SOCE) remained a mechanistic mystery until the recent discovery of the stromal interaction molecule 1 (STIM1). The calcium (Ca2+) sensor STIM1 was identified by two independent research groups, as an essential component of the Ca2+ store depletion-triggered Ca2+ influx [1, 2]. Later, it was demonstrated that, in addition to being an ER Ca2+ sensor, STIM1 functions within the plasma membrane to control operation of the Ca2+ entry through Ca2+ release activated Ca2+ (CRAC) channels [3]. These observations were better understood after the report of a new plasma membrane protein, Orai1 (also known as CRACM1). It was shown that Orai1 is essential for store-operated Ca2+ entry [4]. Accordingly, STIM1 and Orai1 accumulate and colocalize in specific areas where the ER comes in close proximity to the plasma membrane, the so called puncta formations [5]. Orai1 gathers at discrete sites in the plasma membrane directly opposite to STIM1, resulting in local CRAC channel activation [6]. Mutation of Orai1 in patients with a hereditary severe combined immune deficiency syndrome results in defective CRAC channel function [7].
Calcium plays a central role in vascular contraction. Abnormalities in Ca2+ handling have been implicated in the increased response to constrictor stimuli and augmented myogenic tone in vascular smooth muscle cells (VSMC) in hypertension [8-11]. We recently reported that augmented activation of STIM/Orai is a contributing mechanism that leads to impaired control of intracellular Ca+2 levels in hypertension [12].
Sex-associated differences in hypertension have been repeatedly observed in epidemiological studies. However, the mechanisms conferring vascular protection in females, compared to males, are not totally elucidated. Because Ca+2 triggers VSMC contraction and its regulation is highly controlled, differences in Ca+2 handling mechanisms have been proposed to explain sex-related differences in vascular function in hypertension [13, 14].
Therefore, we hypothesized that vascular protection in females reflects decreased Ca2+ mobilization due to lower activation of Orai1/CRAC channels, via its interaction with the intracellular Ca2+ sensor STIM1. In addition, we investigated whether ovariectomy affects activation of the Orai1/STIM1 pathway.
METHODS
Animals
Five to six month-old male and female stroke-prone spontaneously hypertensive rats (SHRSP) were obtained from the breeding colony at Michigan State University. Age-matched male and female Wistar-Kyoto (WKY) rats were purchased from Harlan (Indianapolis IN). Rats were maintained on a 12-hour light dark cycle, housed two per cage and allowed access to normal chow and free water intake. Systolic blood pressure (SBP) was measured in non-anesthetized animals by tail cuff using a RTBP1001 blood pressure system (Kent Scientific Corporation, Connecticut, MA, USA). All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education.
Ovariectomy
Ovariectomy was performed in six month-old WKY and SHRSP. Briefly, under aseptic conditions, rats were anesthetized with isoflurane (5% in O2). A small incision was made in the lower abdomen, and both ovaries were removed (OVX rats) or not (SHAM rats). After 12 weeks, systolic blood pressure (SBP) was assessed. Body and uterus (dry) weight were evaluated and blood was collected to measure hormone levels by radioimmunoasssay (Diagnostic Systems Laboratories - Webster, Texas), in order to confirm the effectiveness of ovariectomy.
Vascular functional studies
After euthanasia with CO2, thoracic aortas were rapidly excised and placed in ice-chilled (≈4 °C) physiological salt solution (PSS). Segments of thoracic aorta were carefully dissected and the endothelium was removed by gently rubbing the lumen side of the vessels with a metallic pin. Aortic rings (4 mm in length) were mounted on two stainless steel wires in standard organ chambers (model 610M; Danish MyoTech, Aarhus, Denmark) for isometric force recording, as previously described [15, 16]. After stabilization, arterial integrity was assessed first by stimulation of vessels with 120mM potassium chloride (KCl) and, after washing and a new stabilization period, by contracting the segments with phenylephrine (PE; 1μM). Endothelium denudation was assessed by the absence of a relaxation response to acetylcholine (ACh; 1μM) during PE-induced contraction. At the end of the experimental protocol, aortas were allowed to stabilize in normal buffer for 10 minutes and then contracted to 120mM of KCl in order to evaluate membrane integrity. No significant differences between initial and final KCl-induced contractions were observed.
Experimental protocol
Force development in response to a Ca2+ handling experimental protocol was evaluated in aortas from both rat groups, as described in Figure 1. Briefly, aortic rings were contracted with PE (1μM). When the contraction reached a plateau, aortas were washed in Ca2+ free-EGTA buffer, in order to deplete intracellular Ca2+ stores (depletion period), for 20 minutes. During the depletion period, aortas were incubated with vehicle or thapsigargin (10μM, selective Ca2+-ATPase inhibitor) and some rings were treated with non-selective CRAC channel blockers, 2-aminoethoxydiphenyl borate (2-APB, 100μM) and gadolinium (Gd3+; 100μM). After Ca2+ depletion, intracellular Ca2+ stores were loaded (Ca2+ loading period) by placing the aortas in 1.6mM Ca2+ buffer, for 15 minutes, and contractile responses were determined. The bathing medium was then replaced with Ca2+ -free buffer and after 2 minutes the aortic segments were stimulated with caffeine (20mM), in order to release intracellular Ca2+ stores, which resulted in a transient contraction [15, 16].
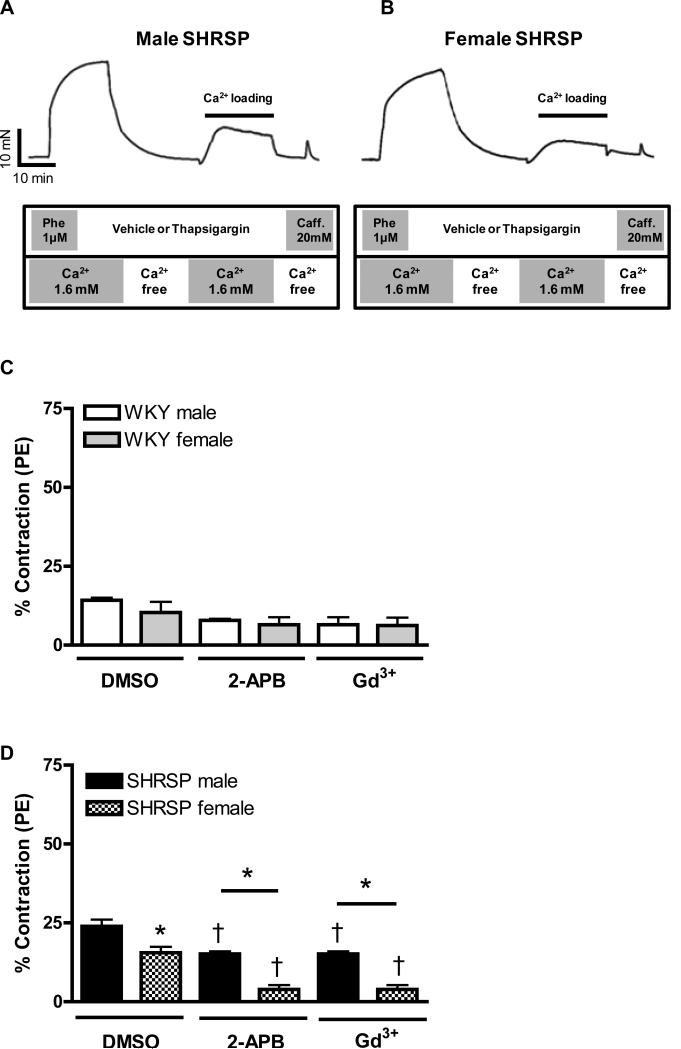
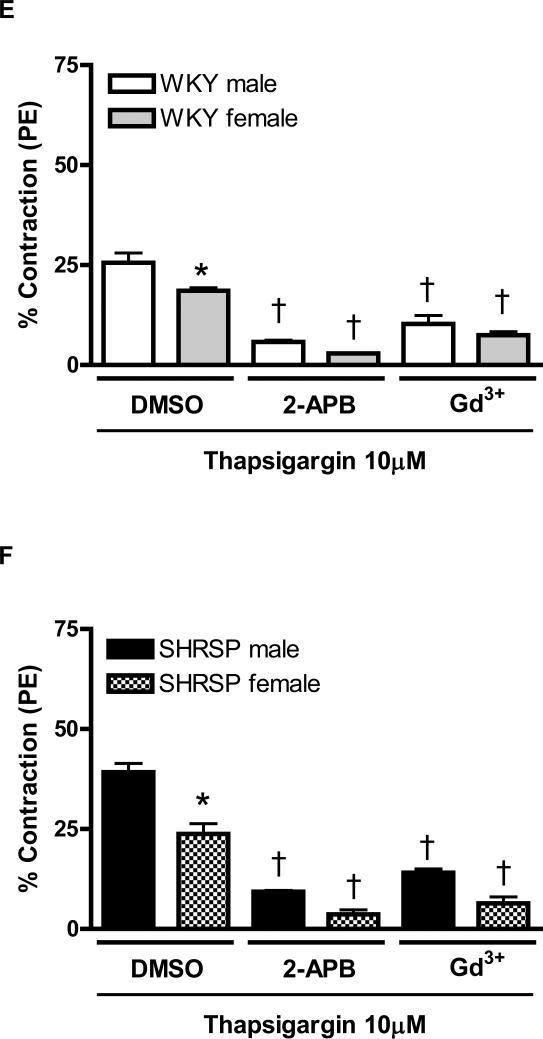
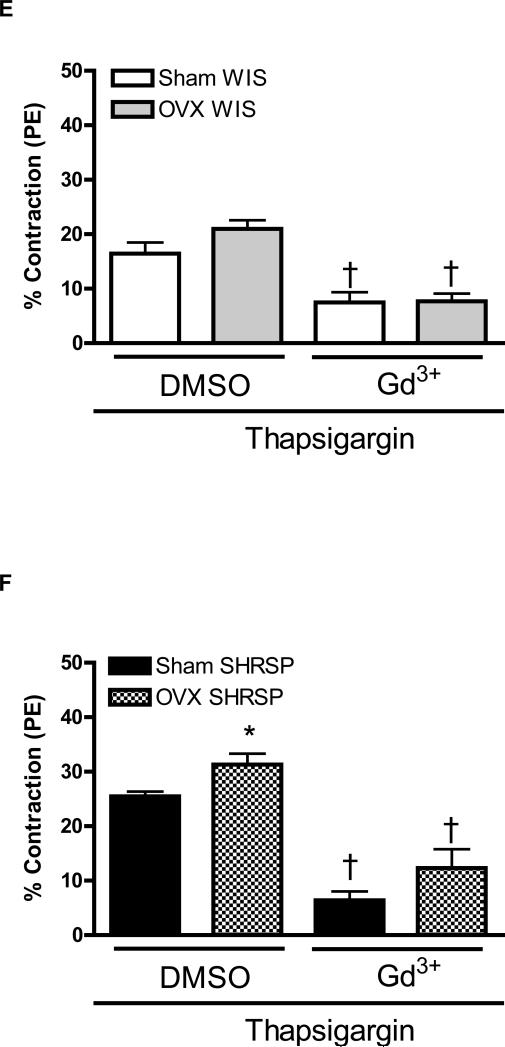
Figure 1. Increased contraction upon Ca2+ loading period, in aortas from male compared to female SHRSP, is normalized after Orai1/CRAC channel inhibition.
The tracings illustrate the protocol used to evaluate force in response to Ca2+ influx after depletion of intracellular Ca2+ stores (loading period) in aortas from (A) male and (B) female SHRSP rats. Aortic rings were contracted with PE (1μM) and then, washed in Ca2+ free -EGTA buffer, in the presence or absence of thapsigargin (10μM). After 20 minutes, 1.6mM Ca2+ buffer was added and aortic rings were incubated for an additional 15 minutes with or without thapsigargin. Aortic rings were washed with Ca2+ free-EGTA buffer for 2 minutes and then stimulated with caffeine (20mM). This protocol was performed in aortas from (C, E) male (white bars) and female (grey bars) WKY rats, or in aortas from (D, F) male (black bars) or female (hatched bars) SHRSP rats, in the (C, D) absence or in the (E, F) presence of thapsigargin. Note that the contractions were significantly inhibited after CRAC channel blockade with 2-APB and Gd3+. Values are expressed as mean ±SEM. * P< 0.05 vs. respective male; † P< 0.05 versus respective DMSO.
Antibody delivery by the chariot technique
Antibodies against STIM1 and Orai1 (ProSci, Poway – CA, USA) were intracellularly delivered by the Chariot technique (Chariot™ Protein Delivery Reagent, Active Motif, Carlsbad, CA). This transfection reagent is able to deliver antibodies into cells whilst preserving their ability to localize to the proper cellular compartment and to recognize antigens within the cell [17-19]. Chariot/antibody complexes were prepared and used according to the manufacturer's instructions. Briefly, aortic rings were incubated in Eagle's minimum essential medium (EMEM) containing L-glutamine (1%), fetal bovine serum (10%), penicillin and streptomycin (0.5%), for 30 minutes at 37°C. For each transfection, 12μl Chariot in 100 μl 40% dimethyl sulphoxide was mixed with 6μg antibody in 100μl phosphate buffered saline (PBS) and incubated at room temperature for 30 min to allow the complex to form. Aortas were transferred to a sterile 24-well cell culture plate, overlaid with 200μl Chariot/antibody complex and mixed gently. EMEM medium (400μl) was added and the tissues were incubated for 1 hr at 37°C. Then, EMEM medium (750μl) was added and tissues were further incubated for 2 hr at 37°C. After this period, rings were mounted in the myograph and functional studies were performed. Chariot-anti-mouse IgG complexes were incubated with aortas and used as additional controls, but they did not produce any significant effects, compared to empty chariot (data not shown).
Western blotting
Aortas from hypertensive and control rats were isolated, cleaned of fat, and endothelium denuded prior to freezing in liquid nitrogen. Extracted proteins (40 μg) were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween (0.1%) for 1 hour at 24°C. Membranes were then incubated with antibodies overnight at 4°C. Antibodies were as follows: anti-STIM1 and anti-Orai1; (1:1000, ProSci, Poway – CA, USA); and β-actin (1:1000, Sigma-Aldrich, MO, USA). Human ovary and mouse thymus tissue lysate (ProSci, Poway – CA, USA) were used as positive controls for STIM1 and Orai1, respectively. After incubation with secondary antibodies, signals were revealed with chemiluminescence and quantified densitometrically. Results were normalized by β-actin expression and expressed as units of change from the control.
Drugs and solutions
Physiological salt solution of the following composition was used: 130mM NaCl, 14.9mM NaHCO3, 4.7mM KCl, 1.18mM KH2PO4, 1.17mM MgSO4·7H2O, 5.5mM glucose, 1.56mM CaCl2·2H2O and 0.026mM EDTA. 2-APB, gadolinium, phenylephrine hydrochloride (PE), thapsigargin and acetylcholine chloride (ACh) were purchased from Sigma Chemical Co. (St. Louis, MO). All reagents were of analytical grade. Stock solutions were prepared in deionized water. Control solutions containing vehicle (DMSO) were also used through the experimental protocols.
Data Analysis
Results are presented as mean±SEM (standard error of the mean). Contractions during de Ca2+ loading period and upon caffeine stimulation were recorded as changes in the displacement (mN) from baseline, and were expressed as percentage from the initial PE-induced contraction. Statistically significant differences were calculated by 2-way ANOVA using Bonferroni- post hoc test. P<0.05 was considered significant.
RESULTS
Sex differences in systolic blood pressure
At 24 weeks, no differences were observed in the systolic blood pressure (mmHg) between male WKY rats and female WKY rats (116±3, n=8 vs. 110±2 n=8; respectively). However, male SHRSP displayed higher systolic blood pressure in comparison with female SHRSP (234±10, n=8 vs. 168±4, n=8; respectively). Additionally, both male and female SHRSP showed higher blood pressure, when compared to male and female WKY, respectively.
Sex differences in force development during the Ca2+ loading period
Figure 1A and B illustrates the protocol used to evaluate first, the contractile response during the Ca2+ loading, generated as a consequence of the intracellular Ca2+ stores depletion; and second, the transient contraction induced by caffeine, which reflects the functional capacity of the sarcoplasmic reticulum (SR) to release Ca2+ (please, refer to legend of figure 1 for further details).
PE-induced contraction was similar between aortas from male WKY compared to female WKY (20.2±0.9mN vs. 16.9±1.8mN, respectively; n=6). However, contraction to PE was increased in aortas from male SHRSP compared to female SHRSP (24.4±1.2mN vs. 18.5±2.3mN, respectively; n=6). Contractile-responses induced during Ca2+ loading period or upon caffeine stimulation were represented as percentage from the initial PE-induced contraction.
During the Ca2+ loading period, contraction was not changed in aortas from WKY male (11.3±2.4%, n=6) compared to WKY female (11.78±0.3%, n=6). Pre-incubation with 2-APB (100μM) or Gd3+ (100μM), used to inhibit CRAC channel, did not affect contraction induced during Ca2+ loading period either in aortas from male or female WKY (Figure 1C).
Contraction-induced by Ca2+ loading was greater in aortas from male SHRSP (23.9±2.1%, n=6), compared to female SHRSP (15.9±2.2%, n=6). CRAC channel blockade with 2-APB or Gd3+ significantly inhibited contractions both in aortas from male and female SHRSP. However, sex-differences observed before CRAC channel inhibition, persisted even after 2-APB and Gd3+ incubation (Figure 1D).
Thapsigargin (10μM) was used to inhibit the SR Ca2+-ATPase and promote depletion of intracellular Ca2+ stores. This pharmacological approach resulted in continuous stimulation of the SR Ca2+ sensor, STIM1 and, consequently, amplified the activation of SOCE through CRAC channels. Accordingly, after thapsigargin incubation, increased contraction was observed during the Ca2+ loading period in all experimental groups.
After thapsigargin incubation, augmented contraction was observed in aorta from male WKY (25.6±2.4%, n=6), compared to female WKY (18.6±0.7%, n=6). In the presence of thapsigargin, CRAC channel-blockade with 2-APB or Gd3+ resulted in significant reduction of contraction both in aorta from male and female WKY (Figure 1E).
Thapsigargin resulted in greater contractile response in aortas from male SHRSP, compared to female SHRSP, during Ca2+ loading period (39.2± 2.2% vs. 23.8±2.5%). Simultaneous incubation of thapsigargin with 2-APB or Gd3+ reduced contraction during the Ca2+ influx period in aortas from male and female SHRSP and abolished sex-differences within this groups (Figure 1F).
Because 2-APB or Gd3+ can be used to block other store operated Ca2+ channels, we used transfection with Orai1 or STIM1 antibodies, as an strategy to neutralize these enzymes and to better evaluate the role of Orai1-CRAC/STIM1, during Ca2+ loading period.
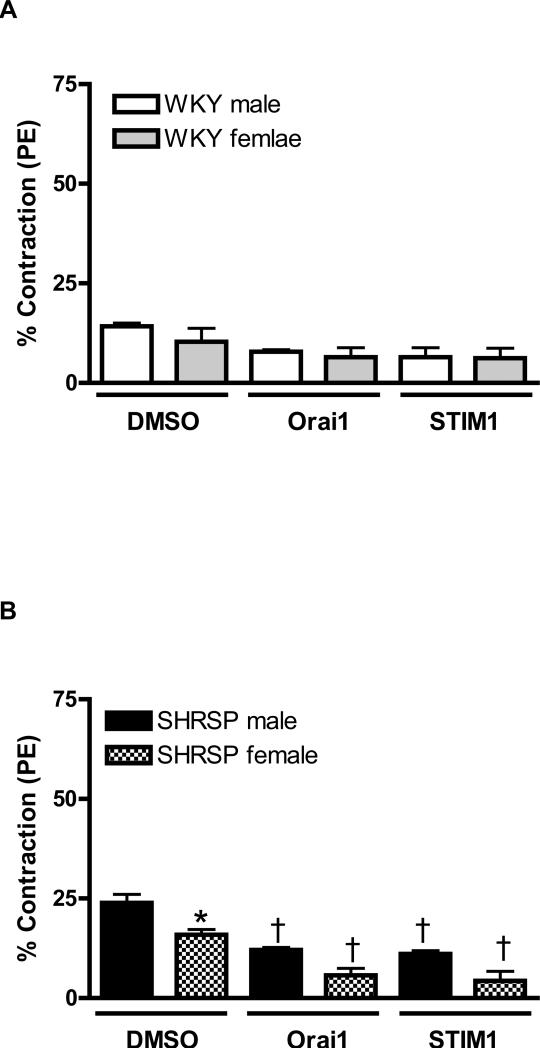
Neutralizing antibodies against Orai1 or STIM1 did not modify contractile-response in aortas from male or female WKY, during Ca2+ loading period (Figure 2A). However, antibodies against Orai1 and Stim1 resulted in decreased contraction during the Ca2+ loading period in aortas from male and female SHRSP. In addition, after antibodies transfection, sex-differences, observed in aortas from SHRSP, were abolished (Figure 2B).
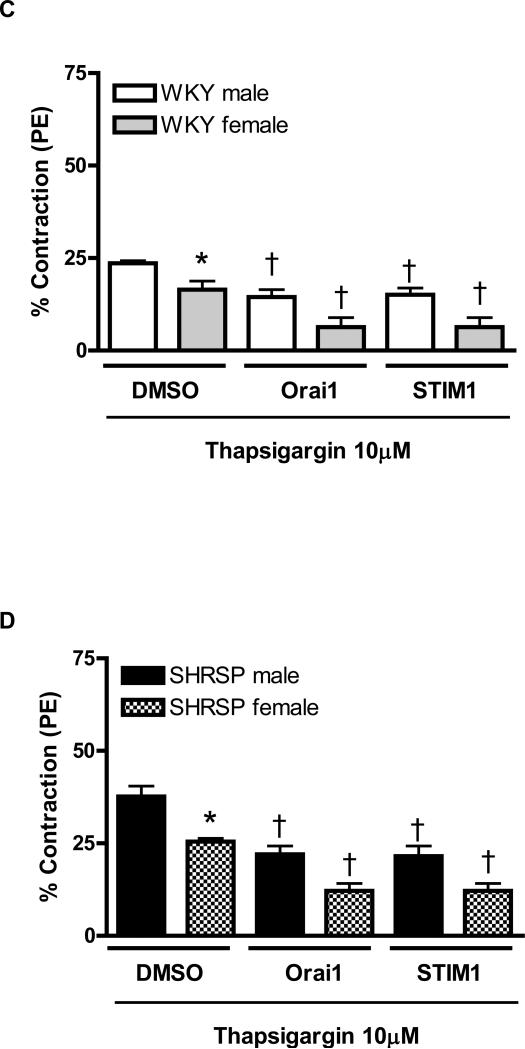
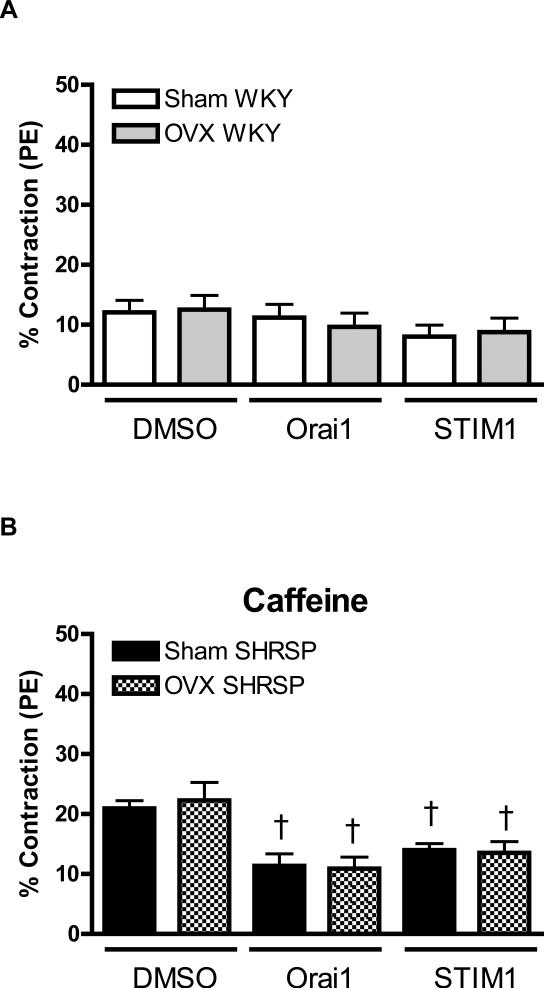
Figure 2. Neutralizing antibody against STIM1 and Orai1, by prevented sex-differences in contractile response during Ca2+ loading period, in aortas from SHRSP.
Contractions during the Ca2+ loading period were evaluated in aorta from (C, E) male (white bars) and female (grey bars) WKY rats, or in aortas from (D, F) male (black bars) or female (hatched bars) SHRSP rats, in the (C, D) absence or in the (E, F) presence of thapsigargin. Values are expressed as mean ± SEM. * P< 0.05 vs. respective male; † P< 0.05 versus respective DMSO.
Simultaneous incubation of thapsigargin with anti-Orai1 or anti-STIM1 reduced contraction during Ca2+ loading period, in aortas from male and female WKY. Furthermore, sex-differences in aortas from WKY, observed after thapsigargin-incubation, were abolished by neutralizing antibodies (Figure 2C). Similar results were observed when aortas from male and female SHRSP were simultaneously incubated with thapsigargin and transfected with neutralizing antibodies (Figure 2D).
Intracellular Ca2+ stores depletion in male and female SHRSP
Caffeine-stimulation (20mM) was used to evaluate the SR Ca2+-buffering capacity. Aortas from male (13.2±0.5%, n=6) and female WKY (12.1±2.0%, n=6) displayed similar contractile-response upon caffeine stimulation. Neutralizing antibodies did not affect caffeine-induced contraction, either in aortas from male or female WKY (Figure 3A).
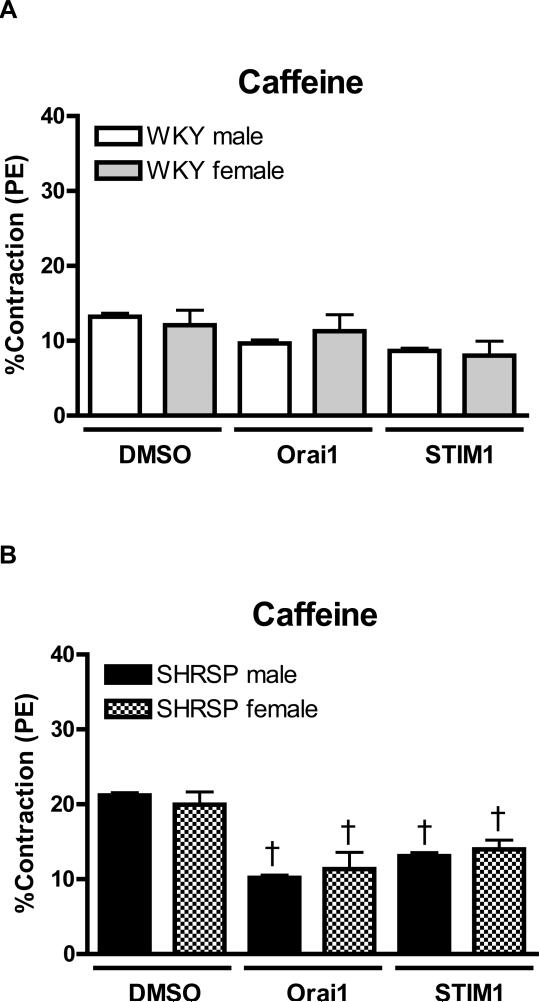
Figure 3. There are no sex-differences in Ca2+ release from the SR, upon caffeine stimulation, in aortas from SHRSP or WKY rats.
Transiente contractions upon caffeine stimulation, after incubation in Ca2+ free buffer, were measured in aortas from (C) male (white bars) and female (grey bars) WKY rats, or in aortas from (D) male (black bars) or female (hatched bars) SHRSP rats. Values are expressed as mean ± SEM. † P< 0.05 versus respective DMSO.
No sex differences were observed in the contractile response induced by caffeine in aorta from SRHSP (male: 21.2±0.3% vs. female: 20.0±1.7%, n=6). Neutralizing antibodies against Orai1 and STIM1 resulted in reduced contractions upon caffeine-stimulation, both in arteries from male and female SHRSP (Figure 3B).
When caffeine-stimulation was performed in the presence of thapsigargin, contractions were almost abolished in all experimental groups and no differences among the groups were observed. In this condition, transfection of Orai1 or STIM1 antibodies had no further effect (data not shown).
Sex-differences in Orai1 and STIM1 expression
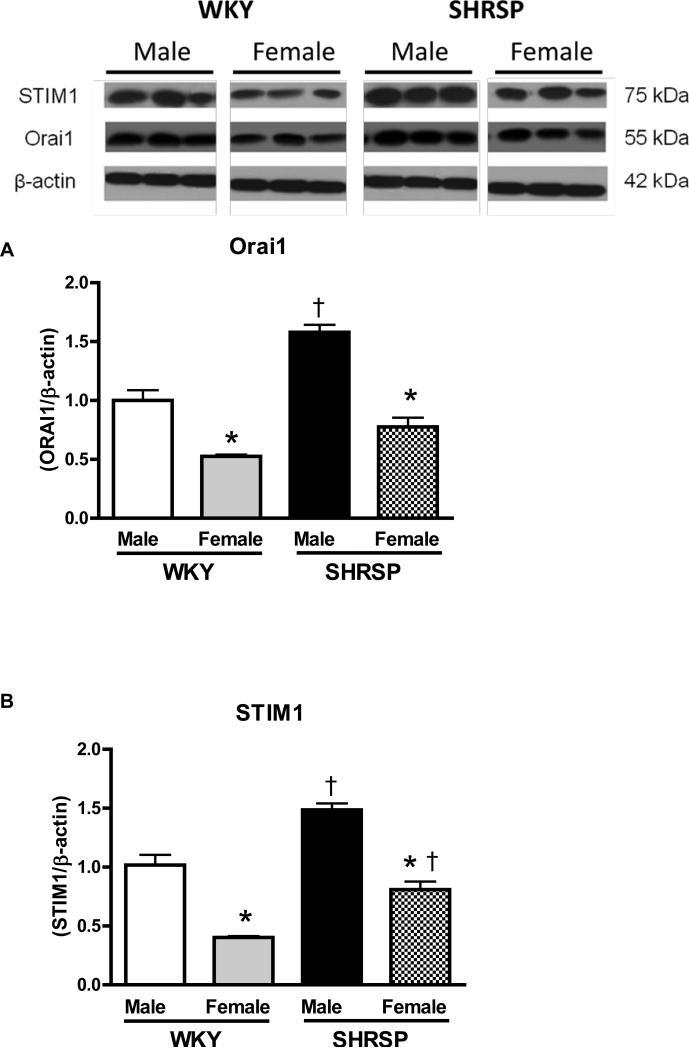
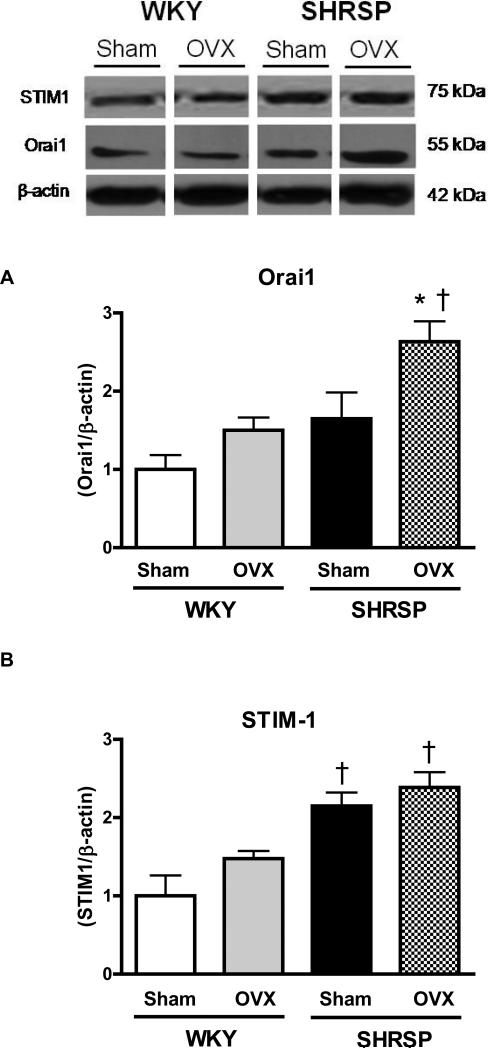
Aortas from male SHRSP and male WKY displayed augmented levels of Orai1 when compared to their respective female groups. Additionally, Orai1 protein expression is augmented in aortas from male SHRSP, compared to male WKY (Figure 4A).
Figures 4. Aorta from male SHRSP display increased Orai1 and STIM1 protein levels, compared to arteries from female SHRSP.
Top, representative western blot images and bottom, corresponding bar graphs demonstrating that (A) Orai1 and (B) STIM1 protein are augmented in male SHRSP aortas, compared to aortas from male WKY or female SHRSP. Densitometric analyses were performed and values were normalized to β-actin protein expression. * P< 0.05 vs. respective control; ‡ P<0.05 vs. respective male.
Augmented levels of STIM1 were observed in male SHRSP and male WKY, when compared to their respective female groups. Hence, STIM1 protein expression was increased in aortas from male and female SHRSP compared to male and female WKY (Figure 4B).
Effect of ovariectomy on systolic blood pressure, body weight and sex-hormones
After 12 weeks of ovariectomy, systolic blood pressure (mmHg) was increased in female WKY OVX, compared to female WKY sham (128±3, n=6 vs. 113±3, n=6, respectively), as well as in female SHRSP OVX (206±2, n=6) compared to female SHRSP sham (184±4, n=6).
Body weight (grams) was augmented after ovariectomy (OVX) both in female WKY (256±3, n=6) and female SHRSP OVX (238±5, n=6), compared to their respective sham groups, WKY (238±5, n=6) and SRSHP (218±5, n=6 – p<0.05).
After ovariectomy, uterus weight (grams) was reduced in female SHRSP OVX (0.053±0.009, n=6), compared to female SHRSP Sham (0.118±0.009, n=6 – p<0.001) and in female WKY OVX (0.004±0.005, n=6) compared to female WKY Sham (0.087±0.003, n=6 – p<0.01).
The efficiency of ovariectomy was also determined by hormonal levels. After ovariectomy, 17-beta-estradiol, progesterone and testosterone plasma levels were reduced both in female WKY and SHRSP rats (Table 1).
Table 1.
Plasma sex-hormones levels in sham- or OVX-WKY and SHRSP.
| WKY | SHRSP | |||
|---|---|---|---|---|
| Sham | OVX | Sham | OVX | |
| 17-beta-Estradiol | 16.1±1.4 | 5.7±0.7† | 10.0±0.2* | 4.9±1.2† |
| Progesterone | 27.2±2.3 | 16.4±0.8† | 35.2±7.3* | 10.9±3.7† |
| Testosterone | 0.16±0.03 | 0.02±0.01† | 0.22±0.02 | 0.02±0.001† |
Values (pmol) are expressed as mean ±SEM; n=5-6.
P< 0,05 vs. respective WKY.
P< 0,05 vs. respective Sham.
Effect of ovariectomy in force development during the Ca2+ loading period
Our previous results showed sex-differences in vascular responses during the Ca2+ influx period, which were enhanced after thapsigargin incubation. Therefore, female rats were ovariectomized in order to investigate if sex-hormones play a protective role in the vasculature of female hypertensive rats.
PE-induced contraction was similar between aortas from sham- and OVX-WKY rats (17.2±.0.4mN and 18.7±2.8mN and, respectively, n=6) as well as in aortas from sham- and OVX-SHRSP (19.1±1.8 and 21.7±1.6mN, respectively, n=6).
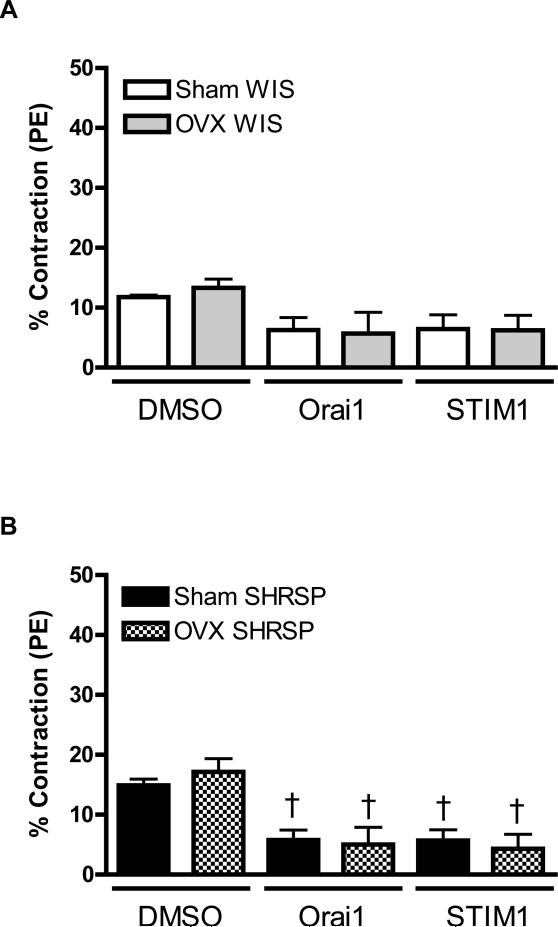
During the Ca2+ loading period, contractions were similar in aortas from sham- or OVX-WKY and neutralizing antibodies against Orai1 and STIM1 did not affect the contractile response (Figure 5A).
Figures 5. Upon thapsigargin stimulation, ovariectomy increased contractile-response during Ca2+ influx, in aortas from SHRSP.
Contractions during the Ca2+ loading period were evaluated in aorta from (A, C, E) Sham (white bars) and OVX (grey bars) WKY rats, or in aortas from (B, D, F) Sham (black bars) or OVX (hatched bars) SHRSP rats, in the (A, B) absence or in the (C-F) presence of thapsigargin. Values are expressed as mean ± SEM. * P< 0.05 vs. respective Sham; † P< 0.05 versus respective DMSO.
Aortas from sham- and OVX-SHRSP displayed similar contractile response during the Ca2+ loading period. Neutralizing antibodies against Orai1 and STIM1 significantly reduced vascular contractions during Ca2+ loading period both in aortas from sham- and OVX-SHRPS (Figure 5B).
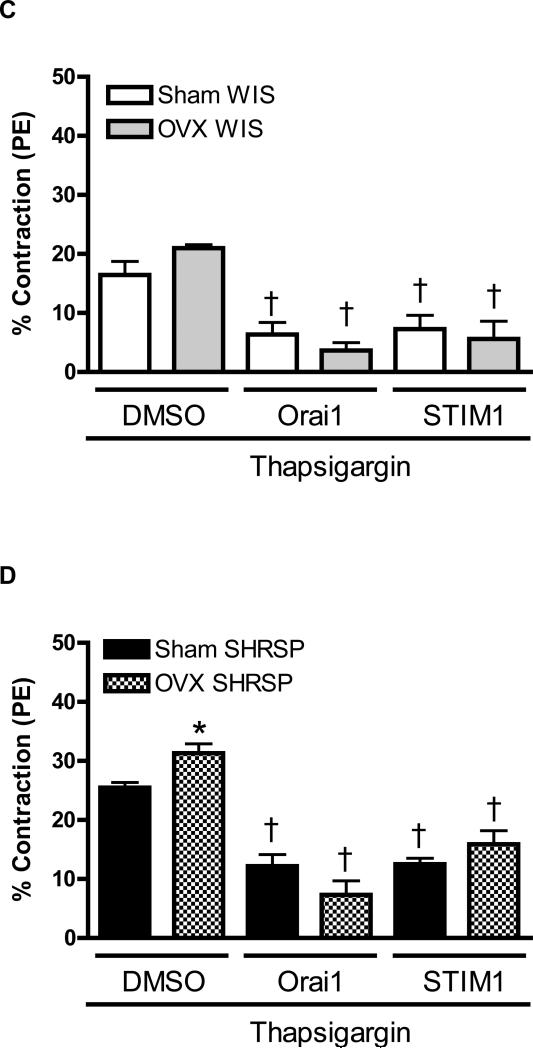
After thapsigargin incubation all experimental groups displayed increased contractile-response during Ca2+ loading period. No significant differences were observed between aortas from sham- and OVX-WKY (16.4±2.3%, n=6 and 20.96±0.6%, n=6; respectively). Simultaneous thapsigargin-incubation and antibodies transfection reduced contractile responses in aortas from sham- and OVX-WKY, during Ca2+ loading period (Figure 5C and 5E).
However, under thapsigargin incubation, OVX-SHRSP aortas displayed increased contraction during the Ca2+ loading period (25.5±0.8%, n=6), when compared do sham-SHRSP (31.3±1.9%, n=6). When thapsigargin was performed in aortas transfected with neutralizing antibodies against Orai1 and STIM1or with Gd3+ resulted in reduced contraction response during Ca2+ loading period (Figure 5D and 5F). In these conditions, differences in the contractile response observed between aortas from sham- and OVX-SHRSP were abolished.
Effect of ovariectomy on intracellular Ca2+ stores
Caffeine-induced contraction was similar between aortas from sham- and OVXWKY (12.08±2% vs. 12.5±2%, n=6; respectively). Transfection with neutralizing antibodies did not affect the caffeine response in aortas from sham- and OVX-WKY rats (Figure 6A).
Figures 6. Ovariectomy does not interfere with Ca2+ release from the SR.
Contractions in response to caffeine after incubation in Ca2+ free buffer were evaluated in aorta from (A) Sham (white bars) and OVX (grey bars) WKY rats, or in aortas from (B) Sham (black bars) or OVX (hatched bars) SHRSP rats. Values are expressed as mean ± SEM.; † P< 0.05 versus respective DMSO.
Aortas from female sham- and OVX-SHRSP displayed increased contractile responses upon caffeine stimulation (19.3±3%, n=6 and 19.93±2%, n=6; respectively). No differences between OVX and sham groups were observed (Figure 6B).
Sex-differences in Orai1 and STIM1 expression
Ovariectomy did not change Orai1 expression in aortas from WKY rats. After ovariectomy, Orai1 expression levels were increased in SHRSP aortas, compared to aortas from sham-SHRSP and OVX-WKY rats (Figure 7A).
Figures 7. Ovariectomy increases expression of Orai1 protein levels in aorta from SHRSP.
Top, representative western blot images and bottom, corresponding bar graphs demonstrating (A) Orai1 and (B) STIM1 protein levels in aortas from sham- or OVX-WKY and SHRSP. * P< 0.05 vs. respective sham; † P< 0.05 versus respective WKY.
Aorta from sham- or OVX-SHRSP expressed increased STIM1 expression. Ovariectomy did not affect expression of STIM1 in aortas from WKY or SHRSP rats (Figure 7B).
DISCUSSION
The main findings of the present study are as follows: compared to female, aortas from male SHRSP display: 1) increased contraction during the Ca2+ loading period; 2) similar transient contraction upon Ca2+ release from the intracellular stores; 3) increased activation of STIM1 and Orai1, (as evidenced by blockade of STIM1 and Orai1 with neutralizing antibodies, which reversed sex differences in contraction during the Ca2+ loading period) and 4) increased expression of STIM1 and Orai1. Additionally, we found that aortas from ovariectomized SHRSP exhibit increased contraction during the Ca2+ loading period and increased Orai1 expression, without changes in the SR buffering capacity or STIM1 expression.
In experimental models of hypertension, compared to females, males develop an earlier and more severe alteration in the vascular function, as evidenced by increased vascular response upon contractile stimuli, which is a hallmark of hypertension [20, 21]. It is widely known that increased Ca2+ concentration is a key marker of hypertension and deregulation of Ca2+ homeostasis is a major contributor to increased vascular contraction observed during hypertension [8-11, 22]. In fact, abnormal handling of Ca2+ by vascular myocytes has been previously suggested to account for sex differences in vascular contractions [13, 23, 24] and for intracellular Ca2+ [14, 25-28]. Some of these studies have discussed the importance of sex-hormones as modulators of vascular functional differences during the Ca2+ loading period observed between male and female hypertensive rats, but the mechanisms leading to abnormal Ca2+ homeostasis are not completely understood.
In the present study, we used an adapted protocol to indirectly evaluate Ca2+ loading and SR buffer capacity, associated with their influence on the contractile response in arteries from male and female rats [15, 16]. The contractions triggered by Ca2+ influx were increased in aortas from male hypertensive rats. Sex differences were observed both in the presence and absence of thapsigargin indicating that intracellular Ca2+ concentration is different between vascular myocytes from males and females, both in conditions where the SR is full or empty. These results are in agreement with previous reports showing that Ca2+ influx is decreased both in isolated VSMC and aorta from female rats compared to male rats [25-27]. It has been shown that VSMC stimulated with angiotensin II display augmented Ca2+ influx, resulting in increased intracellular Ca2+ levels in male SHR, when compared to female SHR [13, 14, 28]. These data show that impaired Ca2+ loading is an important contributor to increased vascular contraction in male hypertensive rats, as well as to sex-related vascular dysfunction.
Caffeine-induced contractions, used here as an indicator of Ca2+ levels in the SR, were similar between the groups, suggesting that the SR in myocytes from females might have a higher buffering capacity [14, 25-27]. In addition, ovariectomy increased contractions during the Ca2+ loading period in aortas from female SHRSP, which is in agreement with previous reports [25-27].
This suggests that SR Ca2+-ATPase is less functional in male SHRSP vascular myocytes. Accordingly, agonist-stimulated intracellular Ca2+ responses were augmented in VSMC isolated from male hypertensive rats, resulting in greater contractile-responses, reinforcing the suggestion that the SR Ca2+-ATPase is less effective in removing Ca2+ after the stimulation of these cells [14].
Therefore, sex-hormones seem to play a role on sex differences during Ca2+ loading, but these differences are not due to Ca2+ release-dependent mechanisms.
Recently, it has been shown that in conditions where the intracellular Ca2+ stores are depleted, SOCE in smooth muscle cells via CRAC channels involves functional STIM1 proteins. Upon activation, STIM1 translocates to plasma membrane domains and activates Ca2+ entry, through CRAC/Orai1 channels, similar to the observations made in other tissues [29-32].
Neutralizing antibodies against STIM1 and Orai1 were intracellularly delivered using a protein deliverer reagent. This approach was used to complement pharmacological protocols and to directly inhibit STIM1 and Orai1, making it possible to better understand their contribution to sex differences during the Ca2+ influx period. Our data demonstrate that the inactivation of STIM1 and Orai1 proteins resulted in smaller contractions triggered by Ca2+ loading. Accordingly, STIM1 and Orai1 vascular expression was augmented in arteries from male hypertensive rats, compared to female SHRSP.
Additionally, we observed that up-regulation of expression and function of STIM1 and Orai1 contribute to augmented contractile responses in hypertension and plays a role in sex differences in vascular function.
We propose that not only the differential expression, but also the differential activation of CRAC/Orai1 channels and the SR Ca2+ sensor STIM1, contribute to sex differences observed during the Ca2+ loading period in arteries from SHRSP. In this regard, some mechanisms have been investigated suggesting that S-glutathionylation [33], O-glyconacylation [34], phosphorylation [35, 36] and lipid rafts [37] may play a role regulating STIM1 activation.
Hence, ovariectomy appears to influence the activation of both Orai1 and STIM1 in arteries from female SHRSP rats. It is conceivable that female sex-hormones may exert a protective role in the vasculature from female hypertensive rats.
Although these are exciting data, we do not wish to conclude that this is the only mechanism contributing to sex differences in increased vascular contractile responses observed in male SHRSP.
An additional point to be addressed is whether increased contractile responses in vessels from male SHRSP are exclusively associated with increased blood pressure levels in these animals. Previous work from Watts and colleagues indicate that this is not the case. These investigators demonstrated that aortas from SHRSP display increased contractile oscillations to cyclopiazonic acid (a SR Ca2+-ATPase inhibitor). Treatment of SHRSP with ramipril, an angiotensin converting enzyme inhibitor, effectively decreased blood pressure, but did not alter the increased contractile oscillations to cyclopiazonic acid [38]. Therefore, these data suggest that augmented contractile responses, upon depletion of intracellular Ca2+, in vascular smooth muscle from SHRSP, may not be primarily associated with elevated blood pressure. In the future, it will be interesting to address whether STIM1/Orai1 represents a direct contributor to blood pressure regulation, by evaluating this system in resistance size vessels or by treating animals, in vivo, with STIM1/Orai1 blockers. Currently, we do not have available drugs to perform such studies. In addition, studies in cell lines may be useful to further evaluate the importance of sex hormones in the STIM1/Orai1 pathway, as well as the effect of them on intracellular Ca2+ level.
In accordance with other studies [39, 40], we observed that ovariectomy increased systolic blood pressure in female rats, which reveal the protective effect of sex-hormones in the blood pressure control. It is known that estrogen has protective vascular effects. It has been shown that vessels from both normotensive and hypertensive ovariectomized female rats display increased Ca2+ influx, compared to control (sham-operated) female rats [27], and that estrogen replacement normalized Ca2+ influx [25].
In conclusion, compared to female, aortas from male SHRSP display increased contraction during Ca2+ influx, indicating augmented intracellular Ca2+, due to up-regulation of Ca2+ influx mechanisms including greater activation of STIM1 and Orai1, which results in augmented vascular contraction. In addition, sex-hormones seem to negatively modulate the STIM1/Orai1 pathway, contributing to vascular protection observed in female rats.
Considering that the STIM1 and Orai1 pathway participates in the Ca2+ loading process, it seems plausible that alterations in these proteins may explain differences in Ca2+ loading observed in arteries from male and female hypertensive rats. Therefore, the STIM/Orai system may represent an important contributor to the pathophysiology of cardiovascular diseases and sex-related differences in hypertension.
Acknowledgment and Funding
This study was funded by a grant from the American Heart Association (AHA 09GRNT2250383).
Footnotes
Author contribution:
This work was performed as part of the Ph.D thesis of Fernanda R. Giachini, who performed the vascular reactivity studies, blood pressure analysis, in addition of being responsible for written the manuscript. Victor V. Lima performed western blot studies. Fernando P. Filgueira ovariectomized the rats and performed some of the blood pressure measurements. Anne M. Dorrance provide the animals used in the study and, along with Zuleica B. Fortes and Maria Helena C. Carvalho, continuously provided ideas and expertise for the project and revisions for the manuscript. R.Clinton Webb and Rita C. Tostes designed the hypothesis and supervised the entire study. All of the authors had full access to the data and take responsibility for its integrity and the accuracy of the analysis. All authors have read and agree to the manuscript as written.
Authors’ disclosures: none
REFERENCES
- 1.Roos J, Digregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc Natl Acad Sci U S A. 2006;103:4040–5. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–76. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 6.Wu MM, Luik RM, Lewis RS. Some assembly required: constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–72. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 8.Noon JP, Rice PJ, Baldessarini RJ. Calcium leakage as a cause of the high resting tension in vascular smooth muscle from the spontaneously hypertensive rat. Proc Natl Acad Sci U S A. 1978;75:1605–7. doi: 10.1073/pnas.75.3.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlov S, Resink TJ, Bernhardt J, Ferracin F, Buhler FR. Vascular smooth muscle cell calcium fluxes. Regulation by angiotensin II and lipoproteins. Hypertension. 1993;21:195–203. doi: 10.1161/01.hyp.21.2.195. [DOI] [PubMed] [Google Scholar]
- 10.Bendhack LM, Sharma RV, Bhalla RC. Altered signal transduction in vascular smooth muscle cells of spontaneously hypertensive rats. Hypertension. 1992;19(II):142–8. doi: 10.1161/01.hyp.19.2_suppl.ii142. [DOI] [PubMed] [Google Scholar]
- 11.Cortes SF, Lemos VS, Stoclet JC. Alterations in calcium stores in aortic myocytes from spontaneously hypertensive rats. Hypertension. 1997;29:1322–8. doi: 10.1161/01.hyp.29.6.1322. [DOI] [PubMed] [Google Scholar]
- 12.Giachini FR, Chiao CW, Carneiro FS, Lima VV, Carneiro ZN, Dorrance AM, Tostes RC, Webb RC. Increased Activation of Stromal Interaction Molecule-1/Orai-1 in Aorta From Hypertensive Rats. A Novel Insight Into Vascular Dysfunction. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devynck MA. Gender and vascular smooth muscle cells: a direct influence on Ca2+ handling? J Hypertens. 2002;20:2139–40. doi: 10.1097/00004872-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Loukotova J, Kunes J, Zicha J. Gender-dependent difference in cell calcium handling in VSMC isolated from SHR: the effect of angiotensin II. J Hypertens. 2002;20:2213–9. doi: 10.1097/00004872-200211000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Karaki H, Kubota H, Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979;56:237–45. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- 16.Perry PA, Webb RC. Agonist-sensitive calcium stores in arteries from steroid hypertensive rats. Hypertension. 1991;17:603–11. doi: 10.1161/01.hyp.17.5.603. [DOI] [PubMed] [Google Scholar]
- 17.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–6. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 18.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, Pitson S, Spiegel S, Pohl U, Bolz SS. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 2006;20:702–4. doi: 10.1096/fj.05-4075fje. [DOI] [PubMed] [Google Scholar]
- 19.Clarke CJ, Forman S, Pritchett J, Ohanian V, Ohanian J. Phospholipase C-delta1 modulates sustained contraction of rat mesenteric small arteries in response to noradrenaline, but not endothelin-1. Am J Physiol Heart Circ Physiol. 2008;295:H826–34. doi: 10.1152/ajpheart.01396.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cambotti LJ, Cole FE, Gerall AA, Frohlich ED, Macphee AA. Neonatal gonadal hormones and blood pressure in the spontaneously hypertensive rat. Am J Physiol. 1984;247:E258–64. doi: 10.1152/ajpendo.1984.247.2.E258. [DOI] [PubMed] [Google Scholar]
- 21.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–9. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 22.Bohr DF, Webb RC. Vascular smooth muscle membrane in hypertension. Annu Rev Pharmacol Toxicol. 1988;28:389–409. doi: 10.1146/annurev.pa.28.040188.002133. [DOI] [PubMed] [Google Scholar]
- 23.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci (Lond) 2008;114:85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 24.Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36:1143–58. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- 25.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34:931–6. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JG, Khalil RA. Gender-specific reduction in contractility and [Ca(2+)](i) in vascular smooth muscle cells of female rat. Am J Physiol Cell Physiol. 2000;278:C834–44. doi: 10.1152/ajpcell.2000.278.4.C834. [DOI] [PubMed] [Google Scholar]
- 27.Crews JK, Khalil RA. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin Exp Pharmacol Physiol. 1999;26:707–15. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- 28.Loukotova J, Kunes J, Zicha J. Cytosolic free calcium response to angiotensin II in aortic VSMC isolated from male and female SHR. Physiol Res. 1998;47:507–10. [PubMed] [Google Scholar]
- 29.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–9. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietrich A, Kalwa H, Storch U, Mederos Y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–77. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- 31.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, Nobori K, Iijima T, Ito H. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun. 2007;361:934–40. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- 33.Hawkins BJ, Irrinki KM, Mallilankaraman K, Lien YC, Wang Y, Bhanumathy CD, Subbiah R, Ritchie MF, Soboloff J, Baba Y, Kurosaki T, Joseph SK, Gill DL, Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatham JC, Marchase RB. The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses. Biochim Biophys Acta. 1800:57–66. doi: 10.1016/j.bbagen.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 123:3084–93. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- 36.Smyth JT, Petranka JG, Boyles RR, Dehaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–72. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galan C, Woodard GE, Dionisio N, Salido GM, Rosado JA. Lipid rafts modulate the activation but not the maintenance of store-operated Ca(2+) entry. Biochim Biophys Acta. 1803:1083–93. doi: 10.1016/j.bbamcr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Watts SW, Traub O, Webb RC. Effects of ramipril on contractile oscillations in arteries from genetically hypertensive rats. Clin Exp Hypertens. 1994;16:881–98. doi: 10.3109/10641969409078032. [DOI] [PubMed] [Google Scholar]
- 39.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–93. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Gimenez J, Garcia PM, Bonacasa B, Carbonell LF, Quesada T, Hernandez I. Effects of oestrogen treatment and angiotensin-converting enzyme inhibition on the microvasculature of ovariectomized spontaneously hypertensive rats. Exp Physiol. 2006;91:261–8. doi: 10.1113/expphysiol.2005.032060. [DOI] [PubMed] [Google Scholar]