Abstract
Pro-inflammatory cytokines, chemokines and the reactive oxygen species are excessively produced in endotoxemia. However, attempting to inhibit all of these inflammatory signaling pathways at the same time in order to prevent endotoxemia is difficult. In a previous study we observed that activation of P2X7 receptors elicited the release of interleukin (IL)-1β from lipopolysaccharide (LPS)-incubated vessels. In the present study we hypothesize that P2X7 receptor activation is the initial event leading to vascular dysfunction following LPS treatment. LPS-induced decreases in mean arterial blood pressure and pressor responses to norepinephrine were attenuated in P2X7 knockout (P2X7KO) mice. Hypo-reactivity to phenylephrine in isolated mesenteric arteries by LPS treatment was also observed in C57BL/6 (wild type, WT) mice, which was prevented by IL-1 receptor antagonist (IL1ra), L-NAME and indomethacin, and in P2X7KO mice. Additionally, treatment with IL1ra plus L-NAME produced an additive inhibition of LPS-induced vascular hypo-reactivity, suggesting different signaling pathways between IL-1β and nitric oxide synthase (NOS). LPS-induced plasma levels of IL-1β, tumor necrosis factor (TNF)-α, IL-10, vascular eNOS and cyclooxygenase (COX)2 protein expression, as determined by ELISA and western blot, observed in WT mice were inhibited by IL1ra and in P2X7KO mice. These results suggest that P2X7 receptor activation involves an initial upstream mechanism of LPS-induced vascular dysfunction, which is associated with IL-1β-mediated eNOS, COX2 activation and TNF-α release.
Keywords: P2X7 receptor, lipopolysaccharide, interleukin-1β, cyclooxygenase, tumor necrosis factor, nitric oxide synthase
INTRODUCTION
In sepsis, the immune system is initially hyper-reactive, releasing many pro-inflammatory factors and cytokines. Subsequently, a systemic inflammatory response is activated, leading to circulatory system collapse, multiple organ failure, septic shock and death [1]. Thus, it is understandable that most therapeutic strategies have targeted pro-inflammatory mediators, including cytokines, platelet-activating factor, oxygen radicals, coagulation factors, and complement system. [1]. However, the only severe sepsis therapy drug - Xigris has been removed from the US market in 2011, because it failed to replicate the initial positive findings. Consequently, a great effort has been directed to find new, and more effective therapeutic agents for sepsis/septic shock.
P2 purinoceptors mediate the actions of extracellular nucleotides [2]. Fifteen members have been cloned and classified into either the subfamilies of G protein-coupled P2Y receptors or cation-selective channels of P2X receptors [3]. The P2X7 receptor functions as an ATP-gated ion channel [4,5]. The receptor gene encodes a 595 amino acid polypeptide with two transmembrane domains, a bulky extracellular domain and N- and C-terminal residues, both on the cytoplasmic side of the plasma membrane [6,7]. The main structural distinctive feature of the P2X7 receptor is a long C-terminal tail that contains multiple protein- and lipid- interacting motifs, including a 90% homologous lipopolysaccharide (LPS) binding region [8], and a tumor necrosis factor (TNF) receptor 1 homology domain [7], which might be responsible for some of its pro-inflammatory effects.
Many studies have demonstrated that the P2X7 receptor up-regulates interleukin (IL)-1 processing and release in LPS-stimulated inflammatory cells [9-11] and vascular endothelial cells [12]. LPS acting via toll-like receptor (TLR) 4 potently induces the synthesis and accumulation of large quantities of pro-IL-1β (immature IL-1β) in intracellular inflammasomes. Activation of purinergic P2X7 receptors by extracellular ATP triggers potassium efflux, pro-caspase-1 cleavage, conversion of pro-IL-1β into mature IL-1β (bioactive IL-1β) and extensive release of this cytokine to the extracellular environment [7,13,14]. In vivo and in vitro studies indicate that IL-1β decreases blood pressure and vascular tone [15-17]. In addition, IL-1β increases vascular inducible nitric oxide synthase (iNOS) protein expression and decreases vascular reactivity to constrictor stimuli [12]. Our previous study demonstrated that P2X7 activation amplified LPS-induced vascular hypo-reactivity via IL-1β-mediated release of nitric oxide via iNOS. LPS signals through CD14/MD2/Toll-like receptor-dependent, as well as CD14/P2X7-dependent, pathways [18]. LPS is also a major trigger of sepsis-induced disseminated intravascular coagulation [19], and ATP release from dense granules during platelet activation [20], which activates P2X7 receptors. Therefore, a cross-talk between P2X7 receptor and LPS-dependent pathways is clearly evident.
In the early phase of endotoxemia and sepsis, excessive production of pro-inflammatory cytokines and chemokines and upregulations of adhesion molecules induce the release of large amounts of granular enzymes and the generation of reactive oxygen species. However, attempting to inhibit all of these inflammatory signaling pathways at the same time in order to prevent endotoxemia has been proved to be difficult. Thus, we hoped to find a suitable initial upstream signaling component for potential therapeutic purpose and hypothesized that the P2X7 receptor represents this character to mediate LPS-induced vascular dysfunction. To test our hypothesis, we performed in vivo, in vitro and ex vitro experiments in C57BL/6 and P2X7 knockout (P2X7KO) mice, with which to evaluate the levels of LPS-induced vascular dysfunction. Additionally, we also investigated downstream signaling pathways involved in P2X7-mediated vascular dysfunction under LPS treatment.
METHODS
In vivo experiments
This study was approved by the local Institutional Review Board according to the Helsinki recommendations and internationally accepted principles for the care and use of experimental animals. Male, twelve-week-old, C57BL/6 and P2X7KO mice were purchased from the Jackson Laboratory. They were maintained under a 12-hr light-dark cycle at a controlled temperature with free access to food and tap water. Mice were anesthetized by intraperitoneal (i.p.) injection of ketamine HCl (70 mg/kg) plus xylazine (10 mg/kg). The left carotid artery and right jugular vein were cannulated with polyethylene -10 tubes, which were exteriorized in the scapular region. Upon completion of the surgical procedure, mice were placed on a warm plate until they regained consciousness. Conscious mice received saline, LPS or IL-1receptor antagonist (IL1ra) via a catheter in the right jugular vein. A catheter from the left carotid artery was connected to a pressure transducer.
Arterial blood pressure was recorded in conscious animals. After recording baseline arterial blood pressure, mice were given norepinephrine (NE, 2 μg/kg i.v.), and 10 min later they received saline (vehicle) or Escherichia coli LPS (50 mg/kg i.v.). Blood pressure was then monitored continuously for 3 hours and pressor responses to NE were assessed every hour. In another experiment, mice received IL1ra (80 μg/kg i.v.), which was administered 30 minutes before the injection of vehicle or LPS.
Vascular function studies
Mice were killed by CO2 inhalation after the three hour-recording of hemodynamic function. First-order mesenteric arteries were cleaned of adhering periadventitial fat, cut into 2-mm length rings, and then mounted in a myograph (Danish Myo Technology A/S, Aarhus, Denmark) containing warmed (37°C), oxygenated (95% O2/5% CO2) physiological salt solution consisting of the following: 130 mM NaCl, 4.7 mM KCl, 1.18 mM KH2PO4, 1.18 mM MgSO4 7H2O, 1.56 mM CaCl2 2H2O, 14.9 mM NaHCO3, 5.6 mM glucose, and 0.03 mM EDTA. The preparations were equilibrated for at least 60 min under a passive tension of 2.5 mN. After the equilibration period, arteries were stimulated with phenylephrine (PE, 10 μM) followed by relaxation with acetylcholine (10 μM), which was used to test endothelial function. Cumulative concentration-response curves to PE (10−9-10−4 M) were performed to determine the impact of LPS treatment on vasoconstrictor activity. Contractile responses to PE were also determined in the presence of L-NAME (NOS inhibitor, 100 μM), 1400W (selective iNOS inhibitor, 10 μM), TFA (selective nNOS inhibitor, 50 and 100 μM) and indomethacin [cyclooxygenase (COX) inhibitor, 10 μM]. The contractile response to 120 mM KCl was also tested at the beginning and end of each experimental protocol to rule out the possibility of vascular damage.
Immunofluorescence microscopy analysis
P2X7 receptor and TLR4 expression in endothelium-intact aortas from C57BL/6 mice were determined by immunofluorescence staining technique. Aortas were frozen at optimal cutting temperature and sections were obtained. Aortic sections were washed with phosphate buffer saline (PBS) and 0.2% Triton X (PBS-T) for 15 minutes at room temperature, then fixed in acetone for 5 minutes at −20°C. Treatment with PBS plus 1% bovine serum albumin (BSA) for 10 minutes at room temperature was used to block nonspecific binding sites of aortic sections. Expression of P2X7 receptor, TLR4 and GAPDH was determined by incubating the aortic sections with anti-P2X7 (1:100, rabbit anti-mouse antibody, Alomone labs), anti-TLR4 (1:20, goat anti-mouse antibody, Santa Cruz) and anti-GAPDH (1:50, mouse anti-mouse antibody, Santa Cruz) antibodies overnight at 4°C. Sections were rinsed with PBST 5 times, probed with goat anti-rabbit Alexa fluor 546 (1: 300 dilution, Invitrogen), donkey anti-goat Alexa fluor 488 (1:300 dilution, Invitrogen) and goat anti-mouse Alexa fluor 488 (1:300 dilution, Invitrogen) secondary antibodies for 2 hours. For the P2X7 antibody specificity control, the antibody was pre-incubated with an antigen peptide before used for labeling the sections. Images were acquired by a confocal microscope (LSM 510 Meta 3.2 Zeiss) after aortic sections were rinsed.
Measurement of IL-1β, TNF-α and IL-10 levels by ELISA
Blood samples for the measurement of plasma IL-1β, TNF-α and IL-10 levels were obtained 3 hr after the injection of saline or LPS. Blood samples were collected from cannulated carotid arteries and were centrifuged at 14000 rpm for 4 min. Plasma samples were then analyzed using enzyme-linked immunosorbent assay (ELISA) kits following the manufactory's instruction (Pierce Biotechnology, Rockford IL).
Protein expression in mesenteric artery by western blot
Forty micrograms of extracted protein were loaded directly into sodium dodecyl sulphate (SDS) sample buffer for 10% SDS-polyacrylamide gel electrophoresis. After transfer onto a 0.45 μm pure nitrocellulose membrane (Trans-Blot Transfer Medium; Bio-Rad, Hercules, CA), the membranes were blocked with 5% defatted milk in Tris buffer solution containing 0.1% Tween 20, for 1 h, and then incubated with antibodies against iNOS, COX2 (BD Biosciences Transduction Laboratories), eNOS, and nNOS (Cell Signaling Technology) in Tris buffer solution containing 0.1% Tween 20, for 24 h, at 4°C. The membranes were washed and finally incubated with a 1:1000 dilution of sheep anti-mouse or sheep anti-rabbit IgG-horseradish peroxidase antibody (GE Healthcare, Chalfont St. Giles, UK) for 1 h at room temperature. After successive rinses, the immunocomplexes were developed using an enhanced peroxidase/luminol chemiluminescence reaction (ECL Western blotting detection reagents; Pierce Biotechnology) and exposed to X-ray film by autoradiography (Carestream Health, Rochester, NY).
Statistical analysis
All values in the figures and text are expressed as mean±S.E.M. of n observations, where n represents the number of animals studied. For measurement of NOS and COX2, three mesenteric arterial beds from the same group were pooled, and each pool was considered n=1. In the hemodynamic and vascular functional studies, statistical evaluation was performed by analysis of variance (ANOVA) followed by the Bonferroni's multiple comparisons test. Differences in cytokine production and protein expression were analyzed by ANOVA followed by Newman-Keuls Multiple Comparison Test. A P value less than 0.05 was considered to be statistically significant.
RESULTS
P2X7R and TLR4 co-localize in vascular cells of C57BL/6 mice
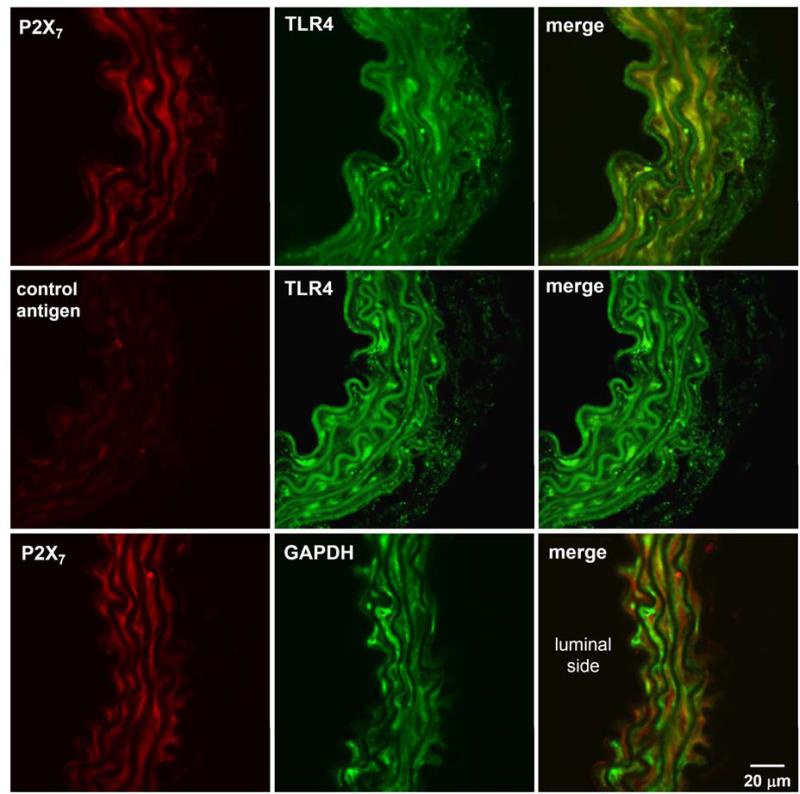
The expression of P2X7R and TLR4 proteins in thoracic aortas of C57BL/6 mice was detected by immunofluorescence microscopy. P2X7R and TLR4 were found co-localized in both endothelial and smooth muscle cells of the mouse aorta (Figure 1, top panel). Pre-incubation of P2X7R antibody with the control antigen peptide (control antigen) eliminated the signal of P2X7R, demonstrating the validity of this antibody (Figure 1, middle panel). P2X7R and GAPDH, as a negative control, did not show significant co-localization in vascular cells of the mouse aorta (Figure 1, bottom panel).
Figure 1. P2X7R and TLR4 co-localize in thoracic aortic rings from C57BL/6 mice.
Confocal images showed the localization of P2X7R (P2X7; red), TLR4 (green), and GAPDH (green) proteins in endothelial and smooth muscle cells of the thoracic aortas of C57BL/6 mice. Pre-treatment of P2X7R antibody with control antigen peptide (control antigen; red) almost completely removed the signals. Merged images showed co-localization of P2X7R and TLR4 (top panel), but not with the negative control GAPDH (bottom panel).
LPS-induced decrease in mean arterial blood pressure is attenuated in P2X7KO mice
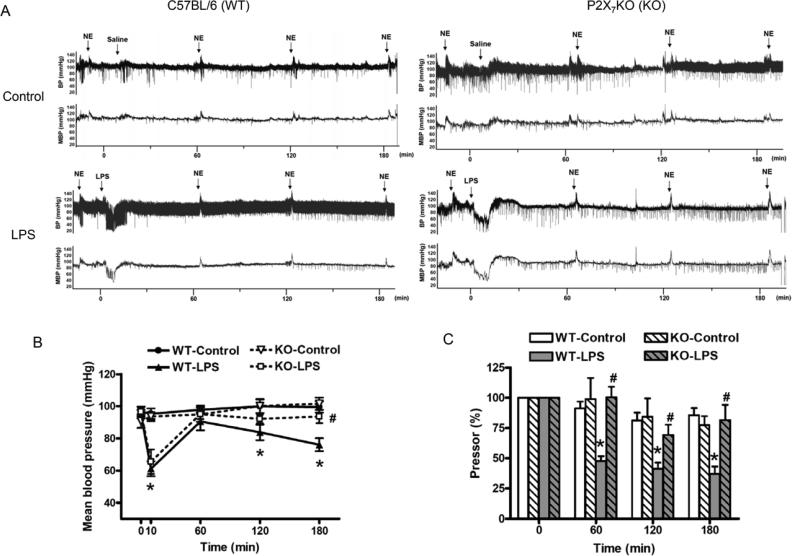
Representative trace recordings of arterial blood pressure in C57BL/6 and P2X7KO mice during 180 min after saline or LPS injection are shown at Figure 2A. Baseline values for mean arterial pressure were between 91±4 and 97±3 mmHg in C57BL/6 and P2X7KO mice, with no significant differences between the groups (Figure 2B). The injection of LPS (time 0) to C57BL/6 mice (WT-LPS) resulted in a rapid decrease in mean arterial pressure to 61±5 mmHg within 10 min, followed by an increase to 91±6 mmHg at 60 min and a progressive decrease to 76±4 mmHg at 180 min. Although the early transient hypotension (66±8 mmHg) was observed after LPS injection in P2X7KO mice (KO-LPS), LPS-induced decrease in arterial mean blood pressure was significantly attenuated at 180 min (94±4 mmHg) comparing to WT-LPS.
Figure 2. Effects of LPS on arterial blood pressure and pressor response to NE in conscious C57BL/6 and P2X7KO mice.
The original tracings illustrate LPS effects on arterial blood pressure and pressor responses to NE in C57BL/6 and P2X7KO mice (A). The statistical analysis presents the changes in mean arterial blood pressure (B) and pressor responses to NE (C) in C57BL/6 and P2X7KO mice, which were cannulated and treated with saline [C57BL/6 mice (WT-Control, n=6); P2X7KO mice (KO-Control, n=5)] or LPS [C57BL/6 mice (WT-LPS, n=6-10); P2X7KO mice (KO-LPS, n=6)]. Data are expressed as mean±S.E.M of n animals. *P<0.05, WT-LPS vs. WT-Control, #P<0.05, KO-LPS vs. WT-LPS.
LPS-induced decrease of pressor responses to NE is attenuated in P2X7KO mice
Pressor responses to intravenous injection of NE (2 μg/kg) were determined in C57BL/6 and P2X7KO mice. The area under curve was analyzed and baseline values for the pressor responses to NE were normalized in the groups studied (Figure 2A and 2C). Saline injection in C57BL/6 mice (WT-Control) or P2X7KO mice (KO-Control) had no significant effects on NE-induced pressor responses during the experimental period. In contrast, LPS injection in C57BL/6 mice (WT-LPS) resulted in a substantial, time-dependent attenuation of NE-elicited pressor responses (100% at 0 min, 47.66±4.03% at 60 min, 41.31±5.01% at 120 min and 37.18±6.02% at 180 min) (Figure 2C). However, LPS-induced attenuation of pressor responses to NE was decreased in P2X7KO mice (KO-LPS; 100% at 0 min, 100.41±8.74% at 60 min, 69.30±8.60% at 120 min and 81.66±12.57% at 180 min) (Figure 2C).
LPS-induced decrease of reactivity to PE in isolated mesenteric arteries is not observed in P2X7KO mice
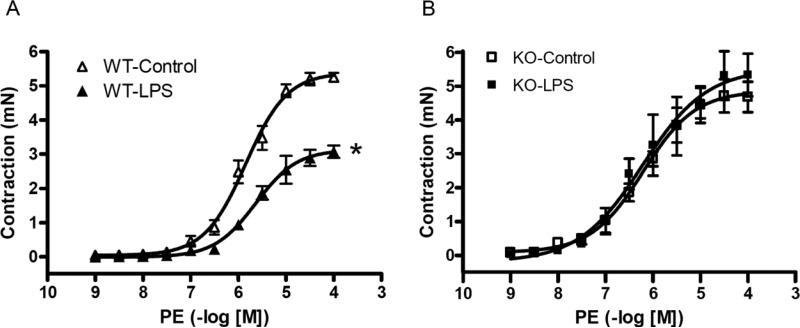
In addition to directly observing the vascular response to NE in vivo, we also measured the isolated mesenteric arterial reactivity. After 180 minutes injection of LPS (50 mg/kg. i.v.) contractile responses to PE were determined in isolated mesenteric arteries. LPS treatment significantly attenuated the maximal contractile response (Emax) to PE in isolated mesenteric arteries from C57BL/6 mice (Emax to PE: WT-Control; 5.39±0.13 mN, and WT-LPS; 3.13±0.12 mN) (Figure 3A), but not in arteries from P2X7KO mice (Emax to PE: KO-Control; 4.86±0.30 mN, and KO-LPS; 5.52±0.61 mN) (Figure 3B).
Figure 3. Effects of LPS on vascular reactivity to PE in isolated mesenteric arteries from C57BL/6 (A) and P2X7KO (B) mice.
Endothelium–intact mesenteric arterial rings were isolated from saline-treated C57BL/6 mice (WT-Control, n=10), LPS-treated C57BL/6 mice (LPS 50 mg/kg, i.v.; WT-LPS, n=9), saline-treated P2X7KO mice (KO-Control, n=5), and LPS-treated P2X7KO mice (LPS 50 mg/kg i.v.; KO-LPS, n=5). Data are expressed as mean±S.E.M of n animals. *P<0.05, vs. WT-Control.
LPS-induced decrease of pressor responses to NE is attenuated by IL1ra
Since plasma IL-1β levels increase after LPS injection, we pre-treated with IL1ra 30 min before LPS injection in C57BL/6 mice (WT-IL1ra+LPS) to evaluate the involvement of IL-1β in the process. IL1ra showed a tendency to attenuate the decreasing effect of LPS on arterial blood pressure at 180 min (86±3 mmHg), although it was not statistically significant (Figure 4A). Treatment with IL1ra prevented LPS-induced attenuation of pressor responses to NE at 120 min and 180 min in C57BL/6 mice (WT-IL1ra+LPS; 100% at 0 min, 68.46±11.78% at 60 min, 73.19±10.47% at 120 min and 69.30±10.11% at 180 min) (Figure 4B)
Figure 4. Pre-treatment with IL1ra attenuates LPS-induced decreases in pressor responses to NE.
The statistical analysis presents the changes in mean arterial blood pressure (A) and pressor responses to NE (B) in C57BL/6 mice, which were cannulated and treated with saline (WT-Control; n=6), LPS (WT-LPS; n=6-10), IL1ra plus LPS [IL1ra (80 μg/kg) for 30 min i.v. before treatment with LPS; WT-IL1ra+LPS; n=6], or IL1ra only (80 μg/kg, i.v.; WT-IL1ra; n=4). Data are expressed as mean±S.E.M of n animals. *P<0.05, WT-LPS vs. WT-Control, #P<0.05, WT-IL1ra+LPS vs. WT-LPS.
IL1ra, L-NAME, and indomethacin, but not 1400W or TFA abrogate LPS-induced decrease of vascular reactivity to PE
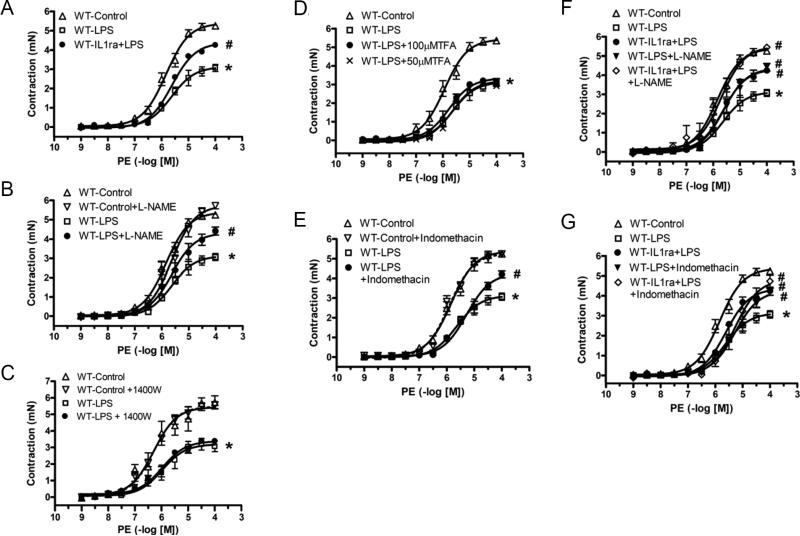
Treatment with IL1ra (80 μg/kg, i.v. 30 min before LPS injection) also significantly attenuated LPS-induced decrease of vascular reactivity to PE in C57BL/6 mice (Figure 5A). To determine the role of nitric oxide and prostaglandin I2 in LPS-induced mesenteric arterial hypo-reactivity, we tested the effects of a nonselective NOS inhibitor (L-NAME, 100 μM), a selective iNOS inhibitor (1400W, 10 μM), a selective nNOS inhibitor (TFA, 50 and 100 μM) and a COX inhibitor (indomethacin, 10 μM). Incubation for 40 min with L-NAME (Figure 5B; WT-LPS+L-NAME) and indomethacin (Figure 5E; WT-LPS+indomethacin), but not with 1400W (Figure 5C; WT-LPS+1400W) or TFA (Figure 5D; WT-LPS+TFA), reversed the decreased response to PE in mesenteric arteries isolated from LPS-injected C57BL/6 mice (WT-LPS). Incubation with L-NAME, 1400W, TFA or indomethacin did not change the Emax to PE in arteries from saline-injected C57BL/6 mice (WT-Control). Incubation of isolated mesenteric arteries from IL1ra plus LPS-injected C57BL/6 mice with L-NAME (WT-IL1ra+LPS+L-NAME) amplified IL1ra effects on vascular reactivity to PE (Figure 5F). However, incubation of isolated mesenteric arteries from IL1ra plus LPS-injected mice with indomethacin (WT-IL1ra+LPS+Indomethacin) did not have additional effects (Figure 5G).
Figure 5. Effects of IL1ra (A), L-NAME (B), 1400W (C), TFA (D), indomethacin (E), IL1ra plus L-NAME (F) and IL1ra plus indomethacin (G) on LPS-induced hypo-reactivity to PE in isolated mesenteric arteries.
Endothelium–intact mesenteric arterial rings were isolated from C57BL/6 mice, which were cannulated and treated with saline (WT-Control; n=10) or LPS (50 mg/kg, i.v. for 3 h; WT-LPS; n=9) for three hours. Isolated arteries were incubated with L-NAME (100 μM; WT-Control+L-NAME; n=5 and WT-LPS+L-NAME; n=6), 1400W (10 μM; WT-Control+1400W; n=6 and WT-LPS+1400W; n=7), or indomethacin (10 μM; WT-Control+Indomethacin; n=3 and WT-LPS+Indomethacin; n=5) for 40 min. Arteries from LPS-injected C57BL/6 mice were also treated with TFA (WT-LPS+50μMTFA; n=6, WT-LPS+100μMTFA; n=6). Mesenteric arteries from C57BL/6 mice treated with LPS (50 mg/kg) and IL1ra (80 μg/kg, i.v., 30 min before LPS treatment; WT-IL1ra+LPS; n=6) were also incubated with L-NAME (WT-IL1ra+LPS+L-NAME; n=6), or indomethacin (WT-IL1ra+LPS+indomethacin; n=5) Data are expressed as mean±S.E.M of n animals. *P<0.05, WT-LPS vs. WT-Control, #P<0.05, vs. WT-LPS.
LPS-induced IL-1β, TNF-α and IL-10 release is attenuated in P2X7KO mice
LPS induced a significant increase in plasma levels of IL-1β, TNF-α and IL-10 in C57BL/6 (WT-LPS), and in P2X7KO (KO-LPS) mice except the level of TNF-α. No significant changes in IL-1β, TNF-α or IL-10 levels were observed in the control groups (WT-Control and KO-Control), indicating that the surgical procedure alone did not influence levels of these cytokines. LPS-induced increases of IL-1β, TNF-α and IL-10 plasma levels were significantly attenuated in P2X7KO mice (KO-LPS) (Figure 6A-C). In addition, pre-treatment of C57BL/6 mice with 80 μg/kg IL1ra significantly attenuated LPS-induced increases in TNF-α and IL-10 plasma levels (WT-IL1ra+LPS) (Figure 6B and 6C). However, the injection of IL1ra alone to C57BL/6 mice had no significant effects on plasma levels of TNF-α or IL-10 (Figure 6B and 6C).
Figure 6. Effects of LPS on plasma levels of IL-1β (A), TNF-α (B) and IL-10 (C) in C57BL/6 and P2X7KO mice.
Depicted are changes in plasma levels of cytokines after the experimental procedures in C57BL/6 mice that received injections of saline (WT-Control; n=9), LPS (50 mg/kg, i.v.; WT-LPS; n=12), or IL1ra plus LPS [IL1ra (80 μg/kg) for 30 min i.v. before treatment with LPS; WT-IL1ra+LPS; n=6], or IL1ra only (80 μg/kg, i.v.; WT-IL1ra; n=4) and in P2X7KO mice injected with saline (KO-Control; n=10) or LPS (50 mg/kg, i.v.; KO-LPS; n=10). Data are expressed as mean±S.E.M. of n animals. *P<0.05, WT-LPS vs. WT-Control; #P<0.05, KO-LPS vs. KO-Control; +P<0.05, vs. WT-LPS.
LPS-induced increase in vascular expression of eNOS and COX2 is attenuated in P2X7KO mice
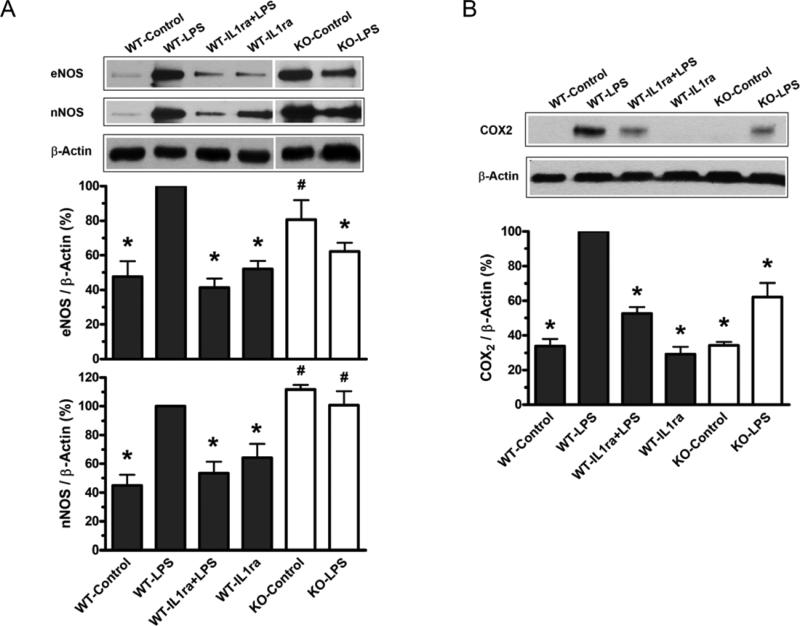
iNOS protein expression was undetectable in mesenteric arteries from LPS-treated C57BL/6 mice at 3 hours after injection (data not shown). For eNOS, nNOS and COX2 protein, the levels of LPS-treated C57BL/6 mice (WT-LPS) were set to 100% and the other groups were normalized to it, accordingly. Significant increases in eNOS, nNOS, and COX2 protein expressions were observed in arteries from LPS-treated C57BL/6 mice (WT-LPS). Pre-treatment of C57BL/6 mice with IL1ra significantly attenuated this effect of LPS (WT-IL1ra+LPS) (Figure 7). In P2X7KO mice (KO-Control), the basal levels of eNOS and nNOS protein were significantly higher than that in C57BL/6 mice (WT-Control). LPS-induced expressions of eNOS (Figure 7A) and COX2 (Figure 7B), but not nNOS, were significantly attenuated in P2X7KO mice (KO-LPS) comparing to wild type animals (WT-LPS).
Figure 7. Effects of LPS on vascular protein expressions of NOS isoforms (A) and COX2 (B) in C57BL/6 and P2X7KO mice.
The representative graph depicts a typical western blot image of protein expressions in mesenteric arteries from C57BL/6 and P2X7KO mice (top of A and B). Bar graphs show quantitative results of NOSs (A) and COX2 (B) protein expressions from three repeated experiments. C57BL/6 mice were injected with saline (WT-Control), LPS (50 mg/kg, i.v.; WT-LPS), IL1ra plus LPS [IL1ra (80 μg/kg) for 30 min i.v. before treatment with LPS; WT-IL1ra+LPS], or IL1ra only [IL1ra (80 μg/kg) for 30 min i.v. before treatment with saline; WT-IL1ra]. P2X7KO mice were injected with saline (KO-Control) or LPS (50 mg/kg, i.v.; KO-LPS). Data are expressed as mean±S.E.M. of n animals. *P<0.05, vs. WT-LPS; #P<0.05, vs. WT-Control.
DISCUSSION
The present study indicates that P2X7 receptor contributes an initial essential role to mediate vascular hypo-reactivity by E. Coli LPS in vivo, and elucidates the possible down-stream signaling pathway. A mouse model is used to demonstrate that P2X7 receptor plays an integral upstream role in LPS-induced vascular dysfunction. We measured acute cardiovascular-related responses following LPS treatment since we were interested in the initial events that might lead to circulatory collapse.
Our study showed that toll like receptor subtype 4 (TLR4) co-expressesd with P2X7 receptor in mouse aorta (Figure 1). This is of importance, since inhibition of early/upstream signaling may abrogate LPS-induced severe inflammatory responses. Intravenous administration of LPS decreased mean arterial pressure (Figure 2A and 2B) as well as pressor responses to NE in C57BL/6 mice (Figure 2A and 2C). However, in P2X7KO mice, LPS did not decrease mean arterial pressure (Figure 2A and 2B) or pressor response to NE (Figure 2A and 2C). In addition, LPS-induced vascular hypo-reactivity to PE was not observed in arteries from P2X7KO mice (Figure 3). Treatment of isolated rat or mouse aorta with LPS for 24 hours incubation did not change vascular contractile response to PE [12,21]. However, LPS-induced decrease of mouse vascular reactivity was observed in the presence of a P2X7 agonist in our previous study [12]. It has been demonstrated that ATP, the natural P2X7 agonist, could be released from platelets within 10 minutes after LPS treatment [22]. Thus, the mesenteric arterial hypo-reactivity to PE induced by LPS injection to mice might be due to transient endogenous ATP released from platelets in the blood, and subsequent activation of P2X7 receptors.
Treatment with P2X7 agonist amplifies LPS-induced IL-1β release in macrophages [18], monocytes [23], and further augments LPS-induced vascular hypo-reactivity to PE [12]. Since IL-1β levels increase after LPS injection [24], we pre-treated LPS-injected conscious mice with IL-1 receptor antagonist (IL1ra). IL1ra is a competitive inhibitor of IL-1α and IL-1β binding to the IL-1 receptor, and it blocks IL-1-dependent signal transduction, thus functioning as an endogenous IL-1-selective inhibitor of inflammation [25]. Pre-treatment of C57BL/6 mice with IL1ra for short-term attenuated LPS-caused decrease of pressor responses to NE (Figure 4B). However, in addition to vessel resistance, sympathetic nervous system, baroreceptor reflex, peptides, and hormones are the other factors known to regulate the changes of blood pressure. Thus, this acute treatment of IL1ra only partially reversed the change of blood pressure in LPS-induced hypotension (Figure 4A). Whereas IL1ra significantly decreased LPS-induced hypo-reactivity to PE in isolated mouse mesenteric arteries (Figure 5A). Considering the fact that LPS-induced IL-1β release was attenuated in P2X7KO mice (Figure 6A) and IL1ra did not completely reverse LPS-induced vascular changes, these results also imply that P2X7 receptor may also transduce signals through other IL-1β-independent pathways.
IL-1β was able to induce iNOS protein expression in endothelium-intact vessels [12]. Thus, we additionally explored the role of nitric oxide in this study. Mesenteric arteries from LPS-treated mice were incubated with L-NAME (non-selective NOS inhibitor) or 1400W (iNOS inhibitor). Forty minutes incubation with L-NAME (Figure 5B), but not 1400W (Figure 5C) reversed LPS-induced mesenteric arterial hypo-reactivity to PE. Based on these functional studies, we speculate that constitutive NOS (eNOS and nNOS) participates in early LPS-induced vascular hypo-reactivity. Systemic treatment with LPS induced eNOS and nNOS protein expression in mesenteric arteries, which were inhibited by IL1ra (Figure 7A), however iNOS protein expression was not detected after 3 hours of LPS treatment (data not shown). It should be mentioned that LPS-induced mesenteric arterial hypo-reactivity to PE was not reversed by the nNOS inhibitor-TFA (Figure 5D). Thus, the functional and molecular experimental results demonstrated that acute LPS treatment induced IL-1β release and produced vascular hypo-reactivity via eNOS (Figure 5B-D, 7A). Whereas IL1ra or L-NAME produced a partial recovery of LPS-induced mesenteric vascular hypo-reactivity to PE (Figure 5A and 5B), IL1ra plus L-NAME completely reversed LPS-induced vascular hypo-reactivity to PE (Figure 5F). These results indicate that LPS-induced vascular hypo-reactivity is not only due to indirect eNOS activation via IL-1β, but also results from direct activation of eNOS. In addition, it is common to see eNOS and nNOS protein induction in the cardiovascular system under physiological stress conditions [26,27]. We, indeed observed eNOS and nNOS protein accumulation in P2X7KO mice (KO-Control) comparing to C57BL/6 mice (WT-Control) (Figure 7A). LPS-induced eNOS protein expression was significantly inhibited in P2X7KO mice (KO-LPS) comparing to LPS-induced wild type animals (WT-LPS). Although LPS-induced nNOS protein expression was not inhibited in P2X7KO mice, LPS could not induce higher nNOS protein expression in P2X7KO mice (KO-LPS) comparing to the control group (KO-Control) (Figure 7A), either. Accordingly, we suspect that nNOS is still involved in the downstream of P2X7 receptor-mediated TLR4 signaling.
Along with nitric oxide, prostacyclin is another endothelial cell-derived relaxing factor [28]. Incubation with indomethacin (COX inhibitor) reversed LPS-induced hypo-reactivity to PE (Figure 5E). IL1ra plus indomethacin did not show additive effects greater than IL1ra or indomethacin alone (Figure 5G), indicating that COX2 was downstream to IL-1β. In addition, the current study showed that LPS-induced COX2 protein expression in C57BL/6 mice was inhibited by IL1ra pre-treatment (WT-IL1ra+LPS), as well as in P2X7KO mice (KO-LPS) (Figure 7B). Thus, we speculate that LPS-induced mesenteric arterial hypo-reactivity is mediated by IL-1β to COX2 upon P2X7 receptor activation.
The cytosolic C-terminal domain of P2X7 receptor presents a putative LPS-binding region [8] and a TNF receptor I homology domain [7]. Tumor necrosis factor (TNF)-α seems to be of particular importance for endotoxic effects [29]. Antisera or antibody against TNF-α attenuated lethality and improved hemodynamic functions provoked by sepsis or endotoxin [30,31]. In addition, Guerra et al observed that pre-treatment of the Raw 264.7 cells with P2X7 antagonist blocked the capacity of LPS to induce the production of TNF-α [18]. Application of the P2X7 receptor blocker Brilliant Blue G completely blocked LPS-induced febrile response, IL-1β and TNF-α release [32]. Thus, besides IL-1β, we also measured plasma TNF-α after LPS treatment. LPS-induced release of TNF-α was attenuated in C57BL/6 mice pretreated with IL1ra (Figure 6B). Furthermore, LPS-induced release of IL-1β and TNF-α was attenuated in P2X7KO mice (Figure 6A and 6B). These results illustrated that the action of LPS involved the release of TNF-α, which was mediated by IL-1β via P2X7 receptor and induces vasorelaxation [33,34]. It is noteworthy that IL-1β increases protein kinase C activity, which is required for the subsequent induction of TNF-α mRNA and protein [35]. Also, protein kinase C-γ interacts with P2X7 receptor complex and positively regulates the receptor-mediated Ca2+ signaling [36]. Thus, we speculate that in P2X7KO mice, Ca2+ signaling is affected, which abolish protein kinase C activation and subsequent TNF-α release. In addition, anti-inflammatory cytokine IL-10 is released to down-regulate production of TNF-α and other pro-inflammatory cytokines in an autocrine-like feedback loop [37,38]. Our data presented that IL-10 release was elevated following TNF-α release due to LPS challenge and abolished following the decrease of TNF-α in response to IL1ra treatment (Figure 6B and 6C), indicating a balance between both cytokines. LPS activates TLR4, inducing immature IL-1β accumulation in the cytoplasm. Endogenous ATP release then activates P2X7, promoting IL-1β maturation, which mediates vascular hypo-reactivity. Our results demonstrate for the first time that P2X7 receptor activation contributes to an initial upstream mechanism in LPS-induced vascular dysfunction in endotoxemia, which is involved in mediating the downstream activation of eNOS, COX2 and TNF-α via IL-1β.
We pre-treated mice with P2X7 antagonists or utilized P2X7KO mice to prevent LPS-induced vascular hypo-reactivity in endotoxemia, however the progression of sepsis always happens very fast to be caught unawares. Thus, to evaluate the therapeutic effect of post-treatment with P2X7 antagonist after sepsis occurrence, which possesses more representative clinical meanings, may be the next step to study. In fact, we did try to apply P2X7 antagonist oxidized ATP in LPS-induced mice. Unfortunately, injection of oxidized ATP in mice dominantly decreased blood pressure, induced tahcypnoea, and seizure (data not shown). These effects indicate that this kind of P2X7 antagonists is unsuitable for systemic injection in endotoxemia or the structure of this P2X7 antagonist need to be remodeled. It also emphasizes that not only the efficacy, but also the safety issues for new P2X7 antagonist development. In addition, the P2X7 gene is reported to have high polymorphisms, raising the concerns for general applications of P2X7 antagonists in inflammatory diseases [39].
P2X7 antagonists are currently under clinical trials for the treatments of several inflammatory diseases, such as inflammatory bowel disease and rheumatoid arthritis. However, a more efficacious and selective P2X7 antagonist for sepsis treatment remains to be developed. Thus, understanding the early effects triggered by P2X7 receptor activation after LPS injection in vivo may contribute to the development of novel clinical therapeutic strategies for sepsis/septic shock.
CLINICAL PERSPECTIVES.
There are no efficacious strategies to prevent or treat sepsis/septic shock, since it is very difficult to block multiple downstream pro-inflammatory factor activations at the same time during sepsis. P2X7 receptor includes a LPS binding region and amplifies LPS-induced IL-1β release. Thus, we attempt to understand the contribution of P2X7 receptor in upstream signaling of LPS-induced endotoxemia.
These results show that LPS-induced vascular dysfunction in endotoxemia are alleviated in P2X7 receptor knockout mice, which is correlated with the changes of downstream eNOS and COX2 activation, and TNF-α release via IL-1β.
Understanding the initial signaling mechanism mediated by P2X7 receptor activation after LPS-induced endotoxemia may contribute to the development of novel clinical strategies for the management of sepsis/septic shock.
Acknowledgments
FUNDING
This study was supported by grants from the National Institutes of Health (HL071138 and DK083685).
Footnotes
AUTHOR CONTRIBUTION
Chin-Wei Chiao was involved in conception and design, acquisition, analysis and interpretation of data, and drafting and editing the paper; J. Eduardo da Silva-Santos and Fernanda Giachini performed some of the vascular reactivity studies; Rita Tostes and Ming-Jai Su contributed to the discussion and critical revision of the manuscript for important intellectual content. Clinton Webb supervised the entire study and critical revision of the manuscript. All of the authors had full access to the data and take responsibility for its integrity and the accuracy of the analysis. All authors had read and agree to the paper as written.
REFERENCES
- 1.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat. Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 3.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 4.Di VF. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 5.Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di VF. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J. Clin. Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buell GN, Talabot F, Gos A, Lorenz J, Lai E, Morris MA, Antonarakis SE. Gene structure and chromosomal localization of the human P2X7 receptor. Receptors Channels. 1998;5:347–354. [PubMed] [Google Scholar]
- 7.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 8.Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, Proctor RA, Bertics PJ. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J. Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 10.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 11.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 12.Chiao CW, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release. J. Pharmacol. Exp. Ther. 2008;326:864–870. doi: 10.1124/jpet.107.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 14.Sanz JM, Di VF. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J. Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- 15.Beasley D, Cohen RA, Levinsky NG. Interleukin 1 inhibits contraction of vascular smooth muscle. J. Clin. Invest. 1989;83:331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N. Engl. J. Med. 1993;328:106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 17.Takizawa S, Ozaki H, Karaki H. Possible involvement of K+ channel opening to the interleukin-1 beta-induced inhibition of vascular smooth muscle contraction. J. Vet. Med. Sci. 1999;61:357–360. doi: 10.1292/jvms.61.357. [DOI] [PubMed] [Google Scholar]
- 18.Guerra AN, Fisette PL, Pfeiffer ZA, Quinchia-Rios BH, Prabhu U, Aga M, Denlinger LC, Guadarrama AG, Abozeid S, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J. Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- 19.Pernerstorfer T, Hollenstein U, Hansen J, Knechtelsdorfer M, Stohlawetz P, Graninger W, Eichler HG, Speiser W, Jilma B. Heparin blunts endotoxin-induced coagulation activation. Circulation. 1999;100:2485–2490. doi: 10.1161/01.cir.100.25.2485. [DOI] [PubMed] [Google Scholar]
- 20.Oury C, Toth-Zsamboki E, Vermylen J, Hoylaerts MF. The platelet ATP and ADP receptors. Curr. Pharm. Des. 2006;12:859–875. doi: 10.2174/138161206776056029. [DOI] [PubMed] [Google Scholar]
- 21.Wylam ME, Metkus AP, Umans JG. Nitric oxide dependent and independent effects of in vitro incubation or endotoxin on vascular reactivity in rat aorta. Life Sci. 2001;69:455–467. doi: 10.1016/s0024-3205(01)01137-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Han J, Welch EJ, Ye RD, Voyno-Yasenetskaya TA, Malik AB, Du X, Li Z. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J. Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward JR, West PW, Ariaans MP, Parker LC, Francis SE, Crossman DC, Sabroe I, Wilson HL. Temporal interleukin-1beta secretion from primary human peripheral blood monocytes by P2X7-independent and P2X7-dependent mechanisms. J. Biol. Chem. 2010;285:23147–23158. doi: 10.1074/jbc.M109.072793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izeboud CA, Hoebe KH, Grootendorst AF, Nijmeijer SM, van Miert AS, Witkamp RR, Rodenburg RJ. Endotoxin-induced liver damage in rats is minimized by beta 2-adrenoceptor stimulation. Inflamm. Res. 2004;53:93–99. doi: 10.1007/s00011-003-1228-y. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DL, Jr., Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J. Appl. Physiol. 2009;107:1438–1444. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte PM. Vascular biology. Old-timer makes a comeback. Nature. 1998;396:213, 215–213, 216. doi: 10.1038/24261. [DOI] [PubMed] [Google Scholar]
- 29.Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 30.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu. Rev. Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 31.Thiemermann C, Wu CC, Szabo C, Perretti M, Vane JR. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br. J. Pharmacol. 1993;110:177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, Gourine VN. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br. J. Pharmacol. 2005;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns DG, Webb RC. TNF-alpha-induced endothelium-independent vasodilation: a role for phospholipase A2-dependent ceramide signaling. Am. J. Physiol. 1998;275:H1592–H1598. doi: 10.1152/ajpheart.1998.275.5.H1592. [DOI] [PubMed] [Google Scholar]
- 34.Zemse SM, Chiao CW, Hilgers RH, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1160–H1167. doi: 10.1152/ajpheart.00763.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bethea JR, Gillespie GY, Benveniste EN. Interleukin-1 beta induction of TNF-alpha gene expression: involvement of protein kinase C. J. Cell Physiol. 1992;152:264–273. doi: 10.1002/jcp.1041520207. [DOI] [PubMed] [Google Scholar]
- 36.Hung AC, Chu YJ, Lin YH, Weng JY, Chen HB, Au YC, Sun SH. Roles of protein kinase C in regulation of P2X7 receptor-mediated calcium signalling of cultured type-2 astrocyte cell line, RBA-2. Cell Signal. 2005;17:1384–1396. doi: 10.1016/j.cellsig.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 37.de Waal MR, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit. Care Med. 2002;30:S58–S63. [PubMed] [Google Scholar]
- 39.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert. Opin. Investig. Drugs. 2011;20:897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]