Abstract
Objective
The role of TGF-β1 in venous ulcer healing and the signaling cascades regulating dermal fibroblast function are poorly understood. To elucidate these processes, we hypothesized that TGF-β1 facilitates wound healing by increasing chronic venous insufficiency (CVI) induced matrix contraction via intracellular cross-talk between TGF-β1 and the ERK-1/2 MAP kinase signaling cascades.
Methods
Fibroblasts isolated from calf biopsies (LC) of patients with different severity of CVI (CEAP, Clinical Etiological Anatomical Pathological classes) were seeded into 200μl collagen gels under isometric conditions. Fibroblasts from neonatal foreskins (HS68), non-CVI patients (NC), and the ipsilateral normal thigh of each CVI patient (LT) served as controls. Thirteen patients with CVI (class 2, n=5; class 4, n=5; class 6, n=3) and 2 non-CVI controls (NC, n=2) were included in the study. All experimental conditions were determined by dose-response and time-course experiments. Gels were cultured with/without 0.1 ng/ml TGF-β1 and with/without 50 μM PD98059 (MEK and downstream MAPK inhibitor). Additional patient fibroblasts were transfected with constitutively active Ras (pCMV-Ras) or an empty vector (pCMV-β) with/without 0.1 ng/ml TGF-β1 and with/without 50 μm PD98059. The collagen gels were released after 4 days and the percent contraction was determined by area measurements using image analysis. Differences in α-smooth muscle actin (α-SMA) and ERK-1/2 MAPK (phosphorylated and total) protein levels were analyzed with western blotting.
Results
Gels seeded with CVI fibroblasts contracted more than HS68, NC and LT fibroblasts. Inhibition of MAPK and/or stimulation with TGF-β1 increased the contraction of LC gels compared to un-stimulated controls. Agonist induced gel contraction correlated with CVI disease severity. α-SMA protein expression in LC fibroblasts increased with MAPK inhibition with/without TGF-β1 stimulation, and correlated with the degree of gel contraction. Transfection with pCMV-Ras (activator of ERK-1/2) inhibited gel contraction; this inhibition was not reversed by addition of TGF-β1. Transfection with the pCMV-β empty vector had no effect on gel contraction.
Conclusions
TGF-β1-stimulation of CVI patient fibroblasts grown in 3D collagen gels results in conversion to a contractile phenotype through upregulation of α-SMA, and in enhanced gel contraction. Inhibition of MAPK further increases gel contraction, while Ras activation of ERK-1/2 inhibits TGF-β1-induced gel contraction. These responses correlate with increasing CEAP severity. CVI fibroblast mediated gel contraction is therefore regulated through cross-talk between the ERK-1/2 MAPK and TGF-β1 signaling cascades. These data identify potentially cliniclly relevant therapeutic molecular targets that could enhanc matrix contraction and thereby improve venous ulcer wound healing.
Keywords: TGF-β1, matrix contraction, chronic venous insufficiency
Introduction
CVI and venous ulcer formation are the sequelae of chronic ambulatory venous hypertension. Venous hypertension causes extravasation of red blood cells (RBCs) and macromolecules that leads to inflammatory activation of the venous microcirculation and leukocyte recruitment. In our previous publications, we have demonstrated that CVI dermal fibroblasts are an important target for leukocyte-derived TGF-β1 and that CVI disease progression is associated with increased tissue levels of TGF-β1, MMP-2 activity, and decreased TGF-β1 induced mitogenic responses of fibroblasts 1,2-4. Although we have described how TGF-β1 regulates the development of CVI induced dermal fibrosis and tissue remodelling, we have not identified the mechanisms that regulate venous ulcer formation or healing. Wound formation and healing is dependent upon contractile interactions between fibroblasts and their surrounding extra-cellular matrix. TGF-β1 influences these processes by regulating the differentiation of fibroblasts into contractile myofibroblasts and stimulating the synthesis of other contractile proteins. This process is tightly regulated by the ERK (Extracellular Regulated Kinase) MAP Kinase signalling cascade (ERK-1/2, p42/44) 5-8. We therefore hypothesized that venous ulcer healing and formation would be regulated by the effects TGF-β1 and the MAP Kinases have on fibroblast mediated matrix contraction 9. To test this hypothesis we utilized three dimensional collagen gels seeded with CVI dermal fibroblasts, in order to simulate the environmental conditions observed in the lower extremities of CVI patients.
Materials & Methods
Patient selection and CVI severity classification
Thirteen patients with different CVI severity (CEAP Class 2- visible varicose veins, Class 4-varicose veins and skin hyperpigmentation, Class 6- active skin ulceration) were included in this study: class 2 (n=5), class 4 (n=5), class 6 (n=3). All patients underwent venous duplex ultrasound scanning (Quantrum 2000; Siemens, Seattle, WA) to confirm the presence of venous reflux. Six mm punch biopsies were obtained from the affected lower calf (LC) and compared to controls. Two subjects without CVI were included to serve as non-CVI normal controls (NC). Ipsilateral thigh biopsies (LT) of normal skin from CVI patients and neonatal foreskin fibroblasts (HS68) were used as additional normal controls. Informed consent was obtained from each participant. The study was approved by the IRB of the New Jersey Medical School.
Skin biopsy, fibroblast isolation from dermal explants and western blotting
Skin biopsies, fibroblast isolation from biopsy samples and western blotting were performed as previously described 1-4, 10-12. Bands were detected using an ECL-plus western blotting detection kit on ECL Hyper-film (Amersham, Piscataway, NJ). Band intensities were analyzed with IS100 Digital Imaging. Blots were normalized by comparing the ratios of samples to internal controls. All experiments were performed in triplicate on passage 3 or less cells.
Gel contraction assay
As described by Vaughn et al., we utilized in-vitro, isometric, three dimensional fibroblast-populated collagen gel assays to test our hypothesis. These gels closely mimic the environmental conditions observed in the dermis of CVI patients 11, 13. We first used a commercially available neonatal foreskin fibroblast cell line (HS68; American Tissue Type Culture Collection, Manassas, VA) to standardize the isometric contraction assay. Subconfluent fibroblasts were trypsinized, harvested, and 105 cells were seeded into 200μl collagen solution. The solution comprised of 1.5% Type I collagen (Upstate Biotech, New York, NY), 10% FBS, 3.5 mN NaOH with phenol red free 2× DMEM (DMEM powder 26.72 g/L, sodium bicarbonate 7.4 g/L, sodium pyruvate 100 mg/L). The fibroblast-collagen solution was placed on pre-scored 6-well plates and allowed to polymerize, first at room temperature for 15 min and then, with 5% CO2 at 37°C for 45 min. After solidification, 3 mL of media (1× phenol red free DMEM, 10% FBS, 1% PSN, 50 μg/mL ascorbic acid) was added per well, and incubated at 37°C with 5% CO2 for 4 days to allow for isometric tension to develop. Media was refreshed on day 2. Fibroblast type and reagents were varied according to the experiments described below. Gels were imaged on day 4 and re-imaged at timed intervals after release with an IS100 Digital Imaging System (Alpha Innotech Corp., San Leandro, CA). Gel images were digitally outlined (Image-Pro Plus, Media Cybernetics, Silver Spring, MD) and the degree of contraction quantified as the percent change in area after release (original area -final area / original area).
Matrix contraction by fibroblasts
In order to determine whether TGF-β1 promotes matrix gel contraction by fibroblasts, and to determine the time-course of this response, collagen gels seeded with HS68 cells were incubated with TGF-β1 (0, 0.1 and 1.0 ng/ml; Sigma, St. Louis, MO). On day 4, contraction was measured at 10 and 60 minutes after release compared to the pre-release area. Thereafter, fibroblasts from CVI patients (class 2, n=5; class 4, n=5; and class 6, n=3), internal controls (LT, n=13), non-CVI controls (NC, n=2) and controls (HS68) underwent gel contraction assays with/without 0.1 ng/mL TGF-β1. In separate experiments, gels seeded with fibroblasts were incubated with/without TGF-β1 and with/without PD98059 (0, 50 or 100 μM; MEK and downstream-MAPK inhibitor; Biomol, Plymouth Meeting, PA). Contraction by fibroblasts from the calves (LC) of class 2, 4 and 6 CVI patients were compared to several controls (LT, and NC). Furthermore, individual classes of CVI fibroblasts were also compared to each other to determine the relationship between CVI severity and matrix contraction.
ERK and α-SMA expression in fibroblast gel preparations
Collagen gels seeded with patient fibroblasts were incubated with/without TGF-β1 and/or PD98059. Fibroblasts were harvested at pre-release, 10, 60 and 120 minutes after release as above, and the extracted protein was immunoassayed to determine the time-course of ERK-1/2 expression. Thereafter, CVI patient fibroblast gels were similarly analyzed for expression levels of total/activated ERK-1/2 and α-SMA.
RAS transfection of CVI patient fibroblasts and gel contraction
RAS is a known upstream activator of ERK. We therefore utilized RAS transfection to test the effect of ERK activation on fibroblast gel contraction. Plasmid vectors containing a constitutively active mutant of H-RAS (pCMV-RAS) or β-galactosidase (pCMV-β; both by BD Biosciences Clontech, Palo Alto, CA) were transfected into fibroblasts. The TransIT-LT1 polyamine transfection reagent (MIRUS-Fischer Scientific, Pittsburgh, PA) was used according to the manufacturer's protocol. Briefly, TransIT-LT1 was diluted in serum-free OptiMEM (Invitrogen, Carlsbad, CA) and incubated at room temperature for 20 minutes. The plasmid vector was added, and the mixture was incubated an additional 20 minutes to allow the DNA and amphipathic polyamines to form complexes. Then, sub-confluent fibroblasts were transfected with a mixture of DMEM with 10% FBS, Transit-LT1 (10 μl/mL) and 1.5 μg/mL DNA for 48 hours at 37°C. After the transfection, cells were selected in medium containing neomysin (Life Technologies, Rockville, MD) for 7 days, and subcultured in 10% FBS containing DMEM. The transfection efficacy of this protocol was tested in fibroblasts isolated from the calf (LC) and thigh (LT) of a class 4 CVI patient using pCMV-β and X-gal staining. Furthermore, successful over-expression of Ras in cell lysates from pCMV-Ras transduced fibroblasts was tested by Western blotting as further evidence of transfection efficacy. Thereafter, CVI patient (LC), LT, and NC fibroblasts were transfected with pCMV-Ras. Transfected cells were seeded in collagen gels as described and incubated with/without 1.0 ng/mL TGF-β1 for 4 days, followed by contraction measurements.
Statistical Analysis
All experiments were performed with 6 gels/patient and repeated 3 times. Results are expressed as mean and standard deviation (SD). Statistical significance was evaluated using unpaired t-tests for comparing two means, and by ANOVA with Tukey's post-hoc test for multiple comparisons. Data were analyzed with Prism GraphPad Instat (GraphPad Software, San Diego, CA), and p<0.05 was considered statistically significant.
Results
TGF-β1 induces matrix contraction by CVI patient fibroblasts
TGF-β1 induced HS68 fibroblast gel contraction in a dose dependent fashion with maximal contraction being achieved at 0.1 ng/ml, 60 minutes after release.(p<0.01 vs. control; Data not shown). We therefore selected TGF-β1 0.1 ng/ml and 60 minutes as the optimal agonist dose and post-release measurement time respectively, for future experiments. Of note, we harvested and counted fibroblasts in collagen gels after 4 days to ensure that matrix contraction results were not due to TGF-β1 or three dimensional collagen gel stimulation of fibroblast proliferation. The analysis did not demonstrate an increase in fibroblasts higher than the number of cells seeded on day 1 (data not shown). Therefore, our observations of increased collagen gel contraction were likely secondary to altered phenotype, and not increased cell number.
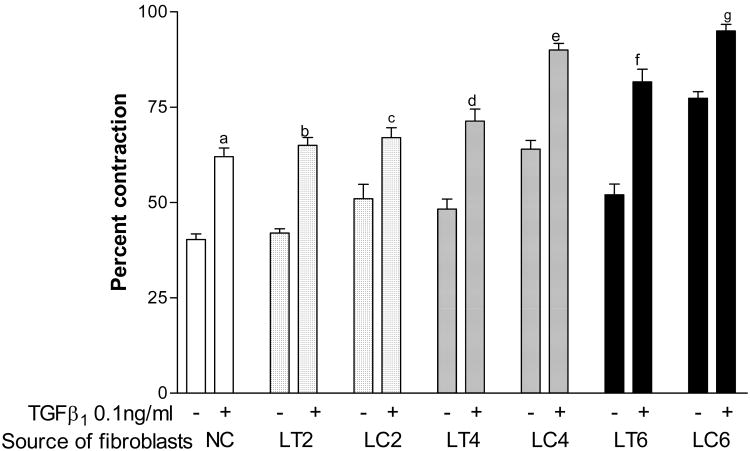
Based on these observations, we tested the effect of TGF-β1 on the contraction of gels seeded with fibroblasts derived from two non CVI patients and thirteen patients with different CVI severity (CEAP class 2 (n=5), class 4 (n=5), class 6 (n=3)). Ipsilateral thigh biopsies (LT) from normal appearing skin were used as additional internal controls. TGF-β1 at 0.1 ng/ml enhanced collagen gel contraction of normal controls (NC, p<0.001), class 2 CVI (LC2, p<0.01), class 4 (LC4, p<0.001), and class 6 (LC6, p<0.01), CVI patient fibroblasts compared to non-CVI derived fibroblasts and fibroblasts obtained from ipsilateral normal thigh biopsies (LT cells) (Fig 1). A progressive increase in fibroblast mediated gel contraction by TGF-β1 correlated with CVI disease severity (class 4 versus class 2 cells (LC4 vs. LC2, p<0.001), class 6 versus class 2 cells (LC6 vs. LC2, p<0.001), and class 6 versus class 4 fibroblasts (LC6 vs. LC4, p<0.05, Fig 1). Therefore, a graded response to TGF-β1 was observed according to increasing CVI disease severity.
Figure 1.
Patient fibroblast response to TGF-β1 stimulation. Gels were seeded with fibroblasts derived from the thighs of normal controls (NC) and CVI classes 2, 4 & 6 (LT2-6), and from the calves of CVI classes 2, 4 & 6 (LC2-6). After stimulation with 0.1 ng/ml of TGF-β1, gels were released and percent contraction was compared with untreated controls for each CVI class respectively (a, p<0.001; b, p<0.001; c, p<0.01; d, p<0.001; e, p<0.001, f, p<0.001; g, p<0.01).
Of note, untreated gels seeded with calf fibroblasts also demonstrated progressively increasing contraction with CVI severity (p<0.05 for both, LC2 vs. LC4, and LC4 vs. LC6). This was not seen when the responses of untreated thigh fibroblasts were compared (p=not significant for LT2 vs. LT4 and LT4 vs. LT6). Stimulation with TGF-β1 resulted in a further increase of gel contraction above that observed with unstimulated gels. Interestingly, TGF-β1-treated thigh cells of class 6 patients contracted more than those of class 2 patients (LT2 vs. LT6, p<0.01) indicating that molecular defects in patient fibroblasts extended well beyond the clinically affected part of the lower extremity. These data may reflect effects related to pressure as LC fibroblasts are exposed to higher levels of venous hypertension compared to LT fibroblasts in-vivo.
ERK inhibition enhances TGFβ1-induced matrix contraction by CVI patient fibroblasts
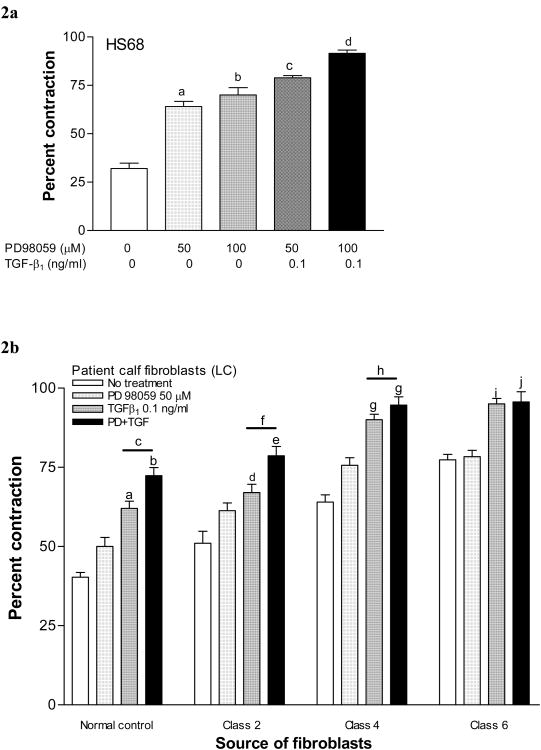
Inhibition of MAPK with 50 (p<0.01) and 100 (p<0.01) μM PD98059 enhanced gel contraction caused by HS68 fibroblasts, compared to untreated cells (Fig 2a). When gels containing HS68 cells were simultaneously incubated with 0.1 ng/mL of TGF-β1 and 50 (p<0.001) or 100 (p<0.05) μM PD98059, an even greater contraction was observed compared to PD98059 alone (Fig 2a).
Figure 2. Contraction-response of fibroblast-seeded collagen gels to MEK inhibition.
2a. Dose response to MEK inhibition (PD98059). Gels seeded with neonatal fibroblasts (HS68) were treated with increasing concentrations of PD98059 and percent contraction for each group was compared to the untreated control (a, p<0.001; b, p<0.01). Responses were not different between 50 and 100 μM concentrations. Contraction was further enhanced when 0.01 ng/ml TGF-β1 was added to PD98059 50 μM (c, p<0.001 vs. 50 μM PD alone) or to 100 mM (d, p<0.05 vs. 100 μM PD98059 alone and p<0.05 vs. combined TGF-β1+50 μM PD 98059).
2b. Patient fibroblast response to MEK inhibition (PD98059). Gels were seeded with fibroblasts derived from the thighs of normal controls (NC) and CVI classes 2, 4 & 6 (LT2-6), and from the calves of CVI classes 2, 4 & 6 (LC2-6). Gels were treated with PD98059 and/or TGF-β1, and percent contraction for each group was measured. Gels treated with PD98059 alone did not demonstrate increased contraction. Gels treated with TGF-β1 alone or in combination with PD98059 demonstrated increased contraction compared to their respective untreated controls, that varied with the severity of disease (a, p<0.001; b, p<0.001; d, p<0.01; e, p<0.001; g, p<0.001; i, p<0.001; j, p<0.001). Combined treatment with TGF-β1+PD98059 resulted in more contraction than TGF-β1 alone in normal controls, class 2 and in class 4 patients (c, p<0.05; f, p<0.001; h, p<0.05).
Based on these observations, we tested the effect of MAPK inhibition on gels constructed with CVI patient fibroblasts. In normal control (NC) patients, PD98059 alone did not alter contraction, although gel contraction was enhanced in the presence of TGF-β1 alone (p<0.001), and in conjunction with 50 μM PD98059 (p<0.001) when compared to no treatment (Fig 2b). When compared with untreated class 2 patient fibroblasts, treatment with TGF-β1 (p<0.01), and combined TGF-β1+PD98059 (p<0.001) enhanced gel contraction. Combined treatment resulted in more contraction than TGF-β1 alone (p<0.001). Similarly, class 4 fibroblasts treated with TGF-β1 (p<0.001), and combined TGF-β1+PD98059 (p<0.001) had increased contraction, while the response was maximal in the presence of the combination compared to TGF-β1 alone (p<0.05, Fig 2b). While contraction was also increased by TGF-β1 (p<0.001) or its combination with PD98059 (p<0.001) in class 6 patients, the responses were not different from each other. Therefore, a graded response to MAPK inhibition was observed according to increasing CVI disease severity.
Matrix contraction by CVI patient fibroblasts is associated with increased pERK-1/2 expression
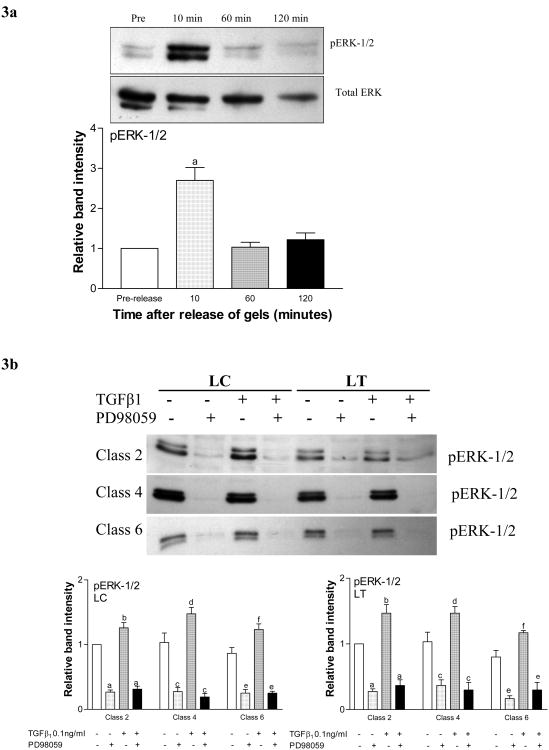
Western blotting for the phosphorylated form of ERK-1/2 demonstrated increased protein activation in un-stimulated HS68 cells 10 minutes after gel release (p<0.001). Phosphorylation returned to the pre-release baseline by 60 minutes (fig 3a). The expression of total ERK-1/2 remained unaltered (fig 3a gel image). The response by patient fibroblasts followed a similar time course (data not shown).
Figure 3. ERK-1/2 phosphorylation in response to release of fibroblast-seeded collagen gels.
3a. Time-course of ERK-1/2 response to gel-release. Representative western blot and graph demonstrating relative band intensities of protein expression for total and phosphorylated (pERK-1/2) ERK-1/2 after the release of gels seeded with HS68 fibroblasts (a, p<0.001). Total ERK-1/2 expression remained unchanged.
3b. pERK-1/2 response to TGF-β1 stimulation and MEK blockade (PD98059). Representative western blots and graphs demonstrating relative band intensities of protein expression for pERK-1/2 10 minutes after the release of gels seeded with fibroblasts derived from calves (LC) and thighs (LT) of classes 2, 4 and 6 CVI patients. Gels were stimulated with 0.01 ng/ml TGF-β1 prior to release. Percent contraction of LC gels (first graph) compared to the respective untreated controls in each group (a, p<0.001; b, p<0.05; c, p<0.001; d, p<0.001; e, p<0.01; f, p<0.05). Expression could not be restored with simultaneous stimulation with TGF-β1 (for each, compared to their respective controls). Percent contraction of LT gels (second graph). A similar response was observed when gels seeded with fibroblasts from CVI patient thighs (LT) were tested with TGF-β1 (a, p<0.01; b, p<0.05; c, p<0.01; d, p<0.05; e, p<0.001; f, p<0.05).
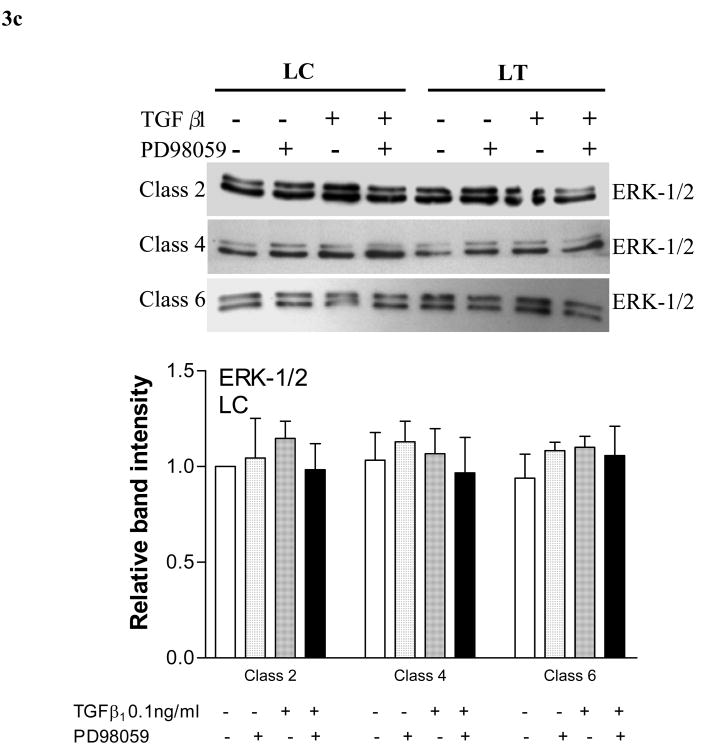
3c. Total ERK-1/2 response to TGF-β1 stimulation and MEK blockade (PD98059). Representative western blots and graphs demonstrating that no change in total ERK-1/2 expression was observed when gels seeded with CVI patient fibroblasts were treated with TGF-β1, PD98059, or a combination of TGF-β1+PD98059.
In subsequent experiments, we tested ERK activation, 10 minutes post-release, after subjecting gels seeded with patient fibroblasts to various agents. When fibroblasts derived from patient calves (LC) were exposed to PD98059 alone, or in conjunction with TGF-β1, activated ERK-1/2 was significantly reduced across all CVI classes (fig 3b). Despite being derived from visibly unaffected skin, fibroblasts from patient thighs also demonstrated a similarly reduced activation of ERK 1/2 in the presence of PD98059 alone, or in conjunction with TGF-β1 (fig 3b). The expression of total ERK-1/2 remained unchanged in both LC and LT fibroblasts across all CVI classes (fig 3c).
H-RAS transfection inhibits TGFβ1 induced matrix contraction by CVI patient fibroblasts
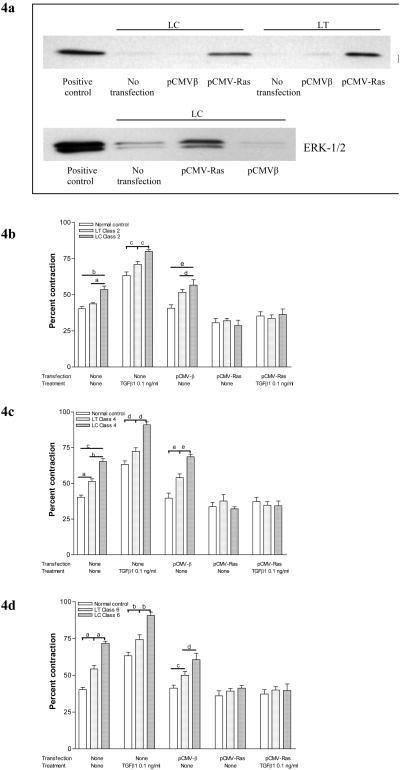
We confirmed that we were able to achieve a transfection efficacy of 56% with pCMV-β as tested with X-gal staining (data not shown) in fibroblasts derived from the calf (LC) and thigh (LT) of a class 4 CVI patient. Furthermore, pCMV-H-RAS-transfected CVI fibroblasts demonstrated enhanced Ras expression in cell lysates by western blotting (Fig 4a). Because RAS is a known upstream activator of ERK, we used it to test the significance of ERK activation on fibroblast gel contraction. Primary cultures of fibroblasts from normal subjects (NC) and patients with class 2, 4 and 6 CVI were transiently transfected with empty vector (pCMV-β) or an active mutant of Ras (pCMV-H-Ras). Cells transfected with pCMV-H-RAS demonstrated a significant reduction in matrix contraction across class 2, 4, and 6 patients, which remained attenuated even when they were stimulated with TGF-β1 (Figs 4 b, c, d). Contraction was unaltered by transfection with pCMV-β.
Figure 4. Contraction of gels seeded with Ras-transfected CVI patient fibroblasts.
4a. Transfection with Ras enhances expression of Ras and activates ERK-1/2. (Top panel) Fibroblasts derived from the lower calf (LC) and thigh (LT) of a patient with class 2 chronic venous insufficiency were transfected with an active mutant of Ras (pCMV-Ras) or empty vector (pCMV-β) and Ras expression was compared to controls by western blotting. (Lower Panel) Fibroblasts derived from the lower calf (LC) of the same patient underwent similar transfections and pERK-1/2 expression was compared with controls.
4b. ERK-1/2 activation by Ras transfection reduces TGF-β1-induced matrix contraction in class 2 CVI. Fibroblasts derived from the calves (LC) and thighs (LT) of class 2 CVI patients were transfected with (pCMV-Ras) or empty vector (pCMV-β), seeded into collagen gels and treated with TGF-β1. Percent contraction for each group was compared with the response of fibroblasts from normal patient controls (a, p<0.05; b, p<0.001; c, p<0.05; d, p<0.05; e, p<0.001).
4c. ERK-1/2 activation by Ras transfection reduces TGF-β1-induced matrix contraction in class 4 CVI Gel contraction after similar transfections of fibroblasts from the calves (LC) and thighs (LT) of class 4 CVI patients that underwent similar treatment (a, p<0.01; b, p<0.001; c, p<0.001; d, p<0.001; e, p<0.001).
4d. ERK-1/2 activation by Ras transfection reduces TGF-β1-induced matrix contraction in class 6 CVI Gel contraction after similar transfections of fibroblasts from the calves (LC) and thighs (LT) of class 6 CVI patients that underwent similar treatment (a, p<0.001; b, p<0.001; c, p<0.01; d, p<0.01).
Conversion to contractile phenotype with α-SMA expression is enhanced with TGF-β1 stimulation and/or MAPK inhibition
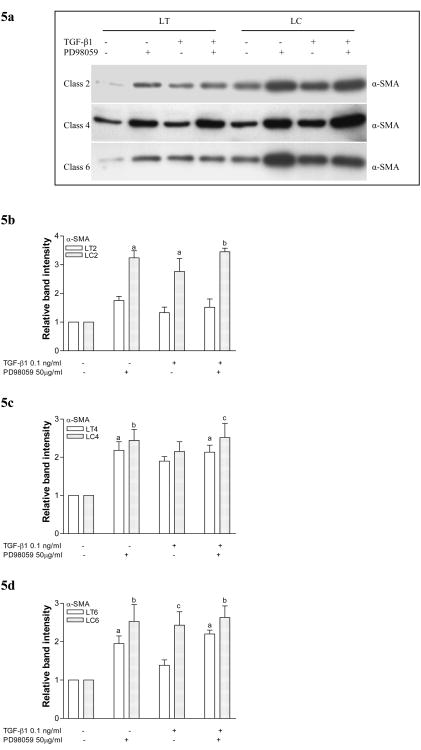
Expression of α-SMA was increased in HS68 cells grown in collagen gels in the presence of 50 μM PD98059 alone or 0.1 ng/ml TGF-β1 alone. The response was maximal when gels were stimulated with a combination of PD98059 and TGF-β1. This indicated that MAP kinases regulate TGF-β1-induced intracellular contractile proteins and the development of a myofibroblast phenotype.
Based on these observations, we tested α-SMA expression in CVI fibroblasts to examine the regulation of myofibroblast phenotype according to CVI disease severity. In class 2 fibroblasts, α-SMA expression was increased in the diseased (LC) fibroblasts in response to MAPK inhibition (p<0.001), TGF-β1 stimulation (p<0.01), and a combination of both (p<0.001) when compared to LT and to un-stimulated LC cells (fig 5a, b). In class 4 (fig 5a, c) and class 6 (fig 5a, c) CVI fibroblasts, MAPK inhibition with or without TGF-β1 stimulation increased α-SMA expression. This observation suggests that with CVI disease progression, TGF-β1 does not affect α-SMA formation and that other mechanisms are involved in the transformation of fibroblasts to myofibroblasts.
Figure 5. Expression of α-SMA in CVI patient fibroblasts in response to TGF-β1 stimulation and MAPK inhibition.
5a. Representative western blots for α-SMA expression in fibroblasts from class 2, 4 and 6 CVI patients. Cells were seeded in collagen gels, treated with TGF-β1 and/or PD98059, and protein measured prior to release.
5b. Graphs demonstrating relative band intensities of protein expression for α-SMA before release of gels seeded with fibroblasts derived from calves (LC) and thighs (LT) of classes 2 CVI patients. Gels were treated with 0.01 ng/ml TGF-β1 and/or 50 μM PD98059 (a, p<0.01; b, p<0.001 vs. untreated cells).
5c. Graph demonstrating results of similar experiments with fibroblasts derived from classes 4 CVI (a, p<0.05; b, p<0.01; c, p<0.001 vs. untreated cells).
5d. Graph demonstrating results of similar experiments with fibroblasts derived from classes 6 CVI (a, p<0.05; b, p<0.01; c, p<0.05; d, p<0.001 vs. untreated cells).
Discussion
TGF-β1 mediates tissue remodelling through regulation of collagen synthesis and degradation, matrix metalloproteinase (MMP) synthesis, and differentiation of fibroblasts into contractile myofibroblasts 2, 4. We have previously reported modest changes in MMP composition and decreased fibroblast proliferative responses in patients with CVI 14. Based on our previous publications, it appears unlikely that dysregulation of MMP synthesis or inhibition of fibroblast proliferation, plays a major role in venous ulcer formation or healing. We therefore hypothesized that decreased fibroblast mediated matrix contraction was the underlying cause for poor venous ulcer healing. Fibroblast mediated matrix contraction, in association with keratinocyte epithelialization is the primary method for normal wound healing. Our current data indicates that CVI fibroblasts placed in three dimensional collagen matrices, exhibit progressive increases in matrix remodeling and contraction that correlates with disease severity and the presence of TGF-β1 (Figure 2b). TGF-β1-induced contraction was further enhanced when MAPK signaling was blocked. MAPK blockade was also associated with a corresponding increase in α-SMA expression. Conversely, contraction was reduced when ERK-1/2 was up-regulated, thereby providing evidence that ERK-1/2 modulates TGF-β1-induced matrix contraction. The observation that the ERK MAP Kinases regulate matrix contraction indicates that these proteins may be molecular targets for drug development aimed at increasing wound contraction.
Importantly, we found that the crosstalk between ERK-1/2 and TGF-β1 was altered according to CVI disease severity. Contrary to our hypothesis, our data indicate that CVI disease progression is associated with increased fibroblast mediated contractile properties and a potential ability to accelerate venous ulcer healing. A consequence of this adaptive wound healing response is increased stored kinetic energy and tension in dermis of CVI patients. We observed gel contraction in unstimulated gels indicating that fibroblasts surrounded by an ECM, exert a baseline degree of tension on their surrounding environment. This tension is increased when stimulated with TGF-β1 and/or ERK inhibition. Injury to the CVI dermal architecture releases stored kinetic energy in the dermis and clinically manifests itself initially as wound separation. This clinical situation is analogous to a stretched rubber band. When external forces are used to stretch a rubber band, tension is exerted on the elastic fibres within the band. When the rubber band is released, the stored kinetic energy within the elastic fibres is released and the band contracts. It is clear that increased fibroblast contractility is beneficial in healing wounds. However, in patients with dermal fibrosis and increased matrix tension, an injury that causes architectural damage may be the underlying stimulus that releases kinetic energy and causes initial wound separation.
Why do venous ulcers heal poorly if CVI dermal fibroblasts demonstrate increased matrix contraction? Numerous other factors such as bacterial contamination and persistent venous hypertension play a role in poor venous ulcer healing. We believe that venous hypertension affects ulcer wound healing via mechanotransduction of pressure to CVI dermal fibroblasts. It is our contention that pressure activates cellular senescence processes and stimulates signalling cascades that inhibit TGF-β1 regulated matrix contraction, resulting in prolonged wound healing. Similar to other investigators, we have reported a loss of growth factor responsiveness in fibroblasts isolated from venous ulcer patients indicating that cellular senescence may be involved in CVI dermal pathology 15. Although senescent fibroblasts lose their proliferative capacity, they remain synthetically active. The increased synthesis and secretion of proteins by senescent fibroblasts causes an alteration in the tissue microenvironment that can effect tissue structure and function 15. Our current data strongly suggest a link between TGF-β1 signalling and CVI fibroblast senescense. We believe this link may be RAS mediated. Campisi et al have reported that over expression of Oncogenic RAS stimulates ERK MAP Kinase signalling and the induction of a senescence response in other fibroblast strains 16, 17. RAS signalling, may therefore affect CVI ulcer healing.
There is a large body of evidence that implicates integrins as “mechanosensors” and “mechanotransducers” of externally applied forces. Integrins are heterodimeric transmembrane cell surface receptors that attach the extra cellular matrix (ECM) to the intra cellular actin cytoskeleton 16-19, 19. Reports indicate that forces exerted on the ECM are converted into chemical signals through interactions with integrin receptors in bone remodelling by osteocytes, cardiac hypertrophy by myocytes, vascular smooth muscle cells during constriction of blood vessels and maintenance of skin tone by dermal fibroblasts 13, 20-22. The contraction of ECM by dermal fibroblasts isolated from scleroderma and hypertrophic scar patients is regulated by integrins that sense and transduce signals to the actin cytoskeleton via the formation of focal adhesion contacts (FAs) 13, 21, 23. In these situations, TGF-β1 increases matrix contraction, which is also associated with increased synthesis of α-SMA and the development of a contractile phenotype. Similarly, our data demonstrate that CVI disease progression is associated with TGF-β1 directed differentiation of fibroblasts into myofibroblasts and the induction of α-SMA contractile proteins (fig 5). The development of a contractile phenotype increases mechanical stress in the matrix as evidenced by enhanced contraction of fibroblast-gels according to CVI disease severity (fig 1). These data suggest that venous hypertension may affect venous ulcer healing through stimulation of fibroblast mechanotransducers. Future experiments should investigate whether pressure applied to CVI dermal fibroblasts in three dimensional collagen matrices will transmit force through integrin activation of intra-cellular ligands such as RAS.
Conclusions
Fibroblasts grown in 3-D collagen gels are useful models to study cellular responses in CVI. TGF-β1-stimulation of CVI patient fibroblasts grown in collagen gels results in differentiation of fibroblasts to a contractile phenotype through increased expression of α-SMA. This conversion is associated with enhanced matrix contraction that correlates with increasing CEAP disease severity. Simultaneous inhibition of MAPK further increases contraction, while RAS activation of ERK-1/2 inhibits TGF-β1-induced matrix contraction. These responses also correlate with disease severity and suggest an adaptive response to chronic venous hypertension exposure. Increased fibroblast mediated matrix contraction is a positive attribute when trying to heal an ulcer. However, an unintended consequence of this adaptive response is increased tension in the dermis of CVI patients. An injury that causes loss of dermal architecture releases the kinetic energy stored in the matrix resulting initially in wound separation. Based on our data, we speculate that persistent venous hypertension may stimulate RAS production via pressure mediated mechanotransduction. RAS stimulation of the ERK MAP Kinases would inhibit TGF-β1-mediated matrix contraction providing a possible cause for poor venous ulcer healing. In addition to improving our understanding of the molecular mechanisms underlying CVI induced dermal changes, these findings provide additional molecular targets that could be modulated to inhibit the development or progression of dermal sequelae associated with CVI.
Acknowledgments
Supported, in part by grants from the American College of Surgeons, American Venous Forum, and NIH.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pappas PJ, DeFouw DO, Venezio LM, et al. Morphometric assessment of the dermal microcirculation in patients with chronic venous insufficiency. J Vasc Surg. 1997;26:784–95. doi: 10.1016/s0741-5214(97)70091-0. [DOI] [PubMed] [Google Scholar]
- 2.Lal BK, Saito S, Pappas PJ, et al. Altered proliferative responses of dermal fibroblasts to TGF-β1 may contribute to chronic venous stasis ulcers. J Vasc Surg. 2003;37(6):1285–93. doi: 10.1016/s0741-5214(02)75295-6. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PJ, You R, Rameshwar P, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-β1gene expression and protein production. J Vasc Surg. 1999;30:1129–45. doi: 10.1016/s0741-5214(99)70054-6. [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Trovato MJ, You R, et al. Role of matrix metalloproteinases 1, 2, and 9 and tissue inhibitor of matrix metalloproteinase-1 in chronic venous insufficiency. J Vasc Surg. 2001 Nov;34(5):930–8. doi: 10.1067/mva.2001.119503. [DOI] [PubMed] [Google Scholar]
- 5.Massague J. How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 6.Mori Y, Hatamochi A, Arakawa M, Ueki H. Reduced expression of mRNA for transforming growth factor β (TGF-β) and TGF-β receptors I and II and decreased TGF-β binding to the receptors in in vitro-aged fibroblasts. Arch Dermatol Res. 1998;290:158–62. doi: 10.1007/s004030050282. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa L, Tabibzadeh Lefty inhibits receptor-regulated Smad phosphorylation induced by the activated transforming growth factor-beta receptor. J Biol Chem. 2001;276:21397–404. doi: 10.1074/jbc.M010783200. [DOI] [PubMed] [Google Scholar]
- 8.Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois DM. Cross-talk between the p42/44 MAP kinase and Smad pathway in transforming growth factor-beta 1 induced furin gene transaction. J Biol Chem. 2001;276:33986–94. doi: 10.1074/jbc.M100093200. [DOI] [PubMed] [Google Scholar]
- 9.Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg. 2004;40:1248–52. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Pappas PJ, Gwertzman GA, DeFouw DO, et al. Retinoblastoma protein: A molecular regulator of chronic venous insufficiency. J Surg Res. 1998;76:149–53. doi: 10.1006/jsre.1998.5310. [DOI] [PubMed] [Google Scholar]
- 11.Vaugham MB, Howard EW, Tomasek JJ. Transforming growth factor β1 promotes the morphological and functional differenciation of the myofibroblast. Exp Cell Res. 2000;257:180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 12.Lee DJ, Rosenfeldt H, Grinell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Experimental Cell Research. 2000;257:190–7. doi: 10.1006/excr.2000.4866. [DOI] [PubMed] [Google Scholar]
- 13.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002 May;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 14.Stanley AC, Park H, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be stimulated with growth factors. J Vasc Surg. 1997;26:994–1001. doi: 10.1016/s0741-5214(97)70012-0. [DOI] [PubMed] [Google Scholar]
- 15.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005 Feb 25;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins G, Redwood KL, Meadows L, Green MR. Effect of gel re-organization and tensional forces on α2β1 integrinlevels in dermal fibroblasts. Eur J Biochem. 1999;263:93–103. doi: 10.1046/j.1432-1327.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi A, Orr AW, Tzimas E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Comm. 2005;328:751–5. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 19.Kagami S, Kondo S, Loster K, et al. α1β1 Integrin-mediated collagen matrix remodeling by rat mesangial cells is differentially regulated by transforming growth factor-P and platelet-derived growth factorr-BB. J Amer Soc Nephrol. 1999;10:779–89. doi: 10.1681/ASN.V104779. [DOI] [PubMed] [Google Scholar]
- 20.Grinnell F, Ho C, Lin Y, Skuta G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J Biol Chem. 1999;274(2):918–923. doi: 10.1074/jbc.274.2.918. [DOI] [PubMed] [Google Scholar]
- 21.Tuan T, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Molec Med Today. 1998:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 22.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: In command and control of cell motility. Nature Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast. Experimental Cell Research. 2000;257:180–9. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]