Abstract
Low voltage-activated T-type calcium channels were originally cloned in the 1990s and much research has since focused on identifying the physiological roles of these channels in health and disease states. T-type calcium channels are expressed widely throughout the brain and peripheral tissues, and thus have been proposed as therapeutic targets for a variety of diseases such as epilepsy, insomnia, pain, cancer and hypertension. This review discusses the literature concerning the role of T-type calcium channels in physiological and pathological processes related to epilepsy. T-type calcium channels have been implicated in pathology of both the genetic and acquired epilepsies and several anti-epileptic drugs (AEDs) in clinical use are known to suppress seizures via inhibition of T-type calcium channels. Despite the fact that more than 15 new AEDs have become clinically available over the past 20 years at least 30% of epilepsy patients still fail to achieve seizure control, and many patients experience unwanted side effects. Furthermore there are no treatments that prevent the development of epilepsy or mitigate the epileptic state once established. Therefore there is an urgent need for the development of new AEDs that are effective in patients with drug resistant epilepsy, are anti-epileptogenic and are better tolerated. We also review the mechanisms of action of the current AEDs with known effects on T-type calcium channels and discuss novel compounds that are being investigated as new treatments for epilepsy.
Keywords: anti-epileptic drugs, epilepsy, T-type calcium channels
Basic physiology and molecular biology of T-type calcium channels
Nomenclature
Nomenclature for ion channels and receptors referred to in this review conform to the guidelines outlined by the British Journal of Pharmacology [1]. Voltage-gated calcium channels are a class of integral membrane proteins that form pores that selectively and rapidly allow calcium ion entry to cells. Calcium channels are broadly defined as either high voltage-activated (HVA) or low voltage-activated (LVA) depending on whether they first activate and allow calcium entry at more depolarized (generally >−40 mV) or hyperpolarized (∼−70 to −60 mV) membrane potentials, respectively (Figure 1) [2]. The HVA channels in mammals are described by seven classes of α1 subunit (CaV) proteins that exhibit distinct biophysical, pharmacological and second messenger dependent modulatory characteristics: L-type (CaV1.1, CaV1.2, CaV1.3, CaV1.4), P/Q-type (CaV2.1), N-type (CaV2.2) and R-type (CaV2.3). The LVA calcium channels are also known as ‘T-type’ due to their tiny and transient calcium conductance in comparison with typical HVA channels. T-type calcium channels are widely expressed throughout the body including the heart, central and peripheral nervous systems, kidney, smooth muscle, reproductive organs and endocrine organs. In mammals, T-type channels are classified into CaV3.1, CaV3.2 and CaV3.3 isoforms, each with unique biophysical, pharmacological and regulatory properties [3]. While the expression, biophysical and modulatory properties of the HVA CaV subunits can be regulated by a number of ancillary proteins (β, α2δ and γ subunits), as yet there is little evidence that functional T-type channels require ancillary subunits.
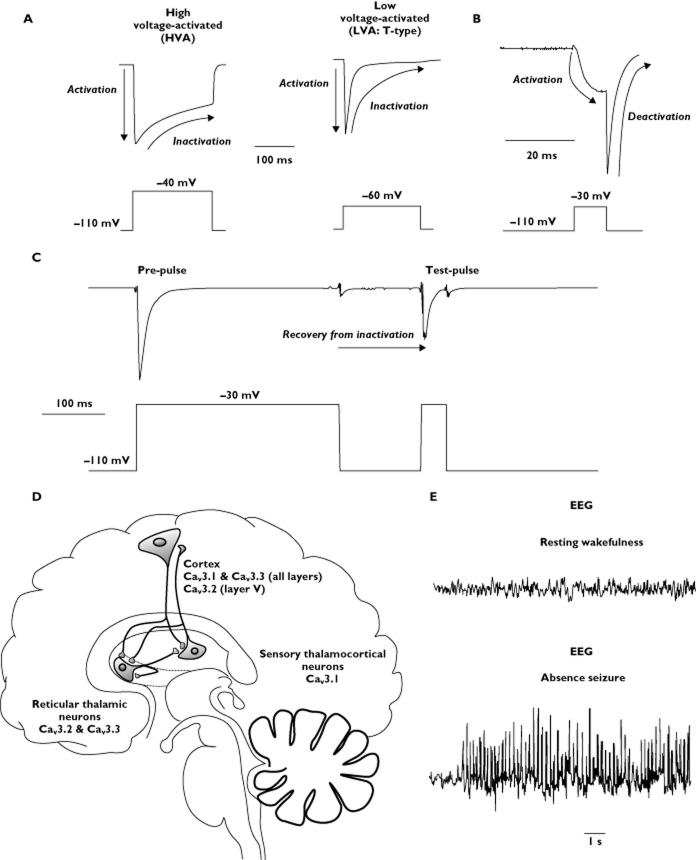
Figure 1.
Biophysical properties of T-type calcium channels. (A) Representative current traces generated by high voltage-activated (HVA; left panel, top) and low voltage-activated (LVA ‘T-type’; right panel, top) calcium channels. HVA calcium channels generally require a greater depolarization to activate than T-type calcium channels (−40 mV compared with −60 mV, respectively). Activation in response to depolarization and subsequent inactivation in response to prolonged depolarization is denoted on current traces. (B) Representative T-type calcium current trace demonstrating the deactivation (closing) process that occurs when the cell is repolarized while the T-type channels are activated (and before inactivation occurs). (C) Representative T-type calcium current trace demonstrating the time-dependent process of recovery from inactivation. A prolonged depolarizing pre-pulse is applied to induce inactivation of the T-type calcium channels. The cell is then repolarized to allow the channels to recover from inactivation before a test pulse is applied. The T-type calcium current is smaller in response to the test pulse than to the pre-pulse because the repolarization time is not sufficient to allow full recovery from inactivation in this example. (D) Schematic representation of the thalamocortical system, showing the proposed expression patterns of the three T-type calcium channel subtypes. (E) Representative electroencephalography (EEG) traces showing activity recorded over the primary somatosensory cortex during resting wakefulness (upper panel) and during an absence seizure (lower panel) in the GAERS absence epilepsy model and demonstrating 7–9 Hz spike-wave discharges
Biophysical properties
Depolarization of the cell membrane past the T-type channel activation threshold potential induces a shift from the channel closed state to an open calcium ion conducting state (Figure 1). The voltage dependence of channel activation varies between the three T-type calcium channel subtypes (hyperpolarized CaV3.1 ≥ CaV3.2 > CaV3.3 depolarized) as does the kinetic rate of channel activation (fastest CaV3.1 >> CaV3.2 > CaV3.3 slowest) [4]–[6]. Once in the open state one or more intrinsic inactivation mechanisms initiate to prevent further calcium conductance. The voltage-dependence (hyperpolarized CaV3.1 = CaV3.2 > CaV3.3 depolarized) and kinetic rate (fastest CaV3.1 >> CaV3.2 > CaV3.3 slowest) of the inactivation processes also occur in a subtype-specific manner. Once inactivated, T-type channels can only de-inactivate (revert to the closed state and once again become available for depolarization-mediated opening) by hyperpolarization of the membrane potential below that which induces inactivation (Figure 1). This process, known as recovery from inactivation, also varies between the T-type channel subtypes (fastest CaV3.1 > CaV3.3 > CaV3.2). Additionally, should the membrane potential hyperpolarize back to a point below that which induces activation before the channel inactivates (e.g. while still open), it will change confirmation to the closed state. This deactivation also occurs with subtype specificity (fastest CaV3.3 >> CaV3.1 > CaV3.2). The voltage dependencies of activation and inactivation occur over a range of membrane potentials for a population of channels from fully closed to fully open and from fully inactivated to fully de-inactivated. These ranges overlap to a degree for each of the three T-type calcium channels, which can result in a small yet measurable net inward calcium flux in the range of overlapping membrane potentials and known as a ‘window current’ [7,8].
In the nervous system, the three T-type calcium channels are often expressed in the same cell types to varying degrees. Thus a wide repertoire of different net LVA calcium currents can be generated depending on the relative contribution from each subtype [9,10]. In addition, the genes encoding the three T-type calcium channels (CaV3.1: CACNA1G, CaV3.2: CACNA1H, CaV3.3: CACNA1I) possess a number of sites that are subject to alternative splicing, adding further functional complexity to the overall spectrum of T-type currents [11]–[17]. Finally, distinct T-type channel isoforms exhibit a differential distribution across somatic, dendritic and axonal compartments, suggesting that they make unique contributions to excitability and cellular output as a result of a complex interplay between subtype specific biophysical and modulatory properties together with compartmental considerations [10,18]–[20].
Physiological roles of T-type calcium channels
T-type calcium channels in sleep
At the physiological level, extensive studies have characterized the critical role played by T-type calcium channels in the neuronal firing modalities associated with the oscillations observed on electroencephalogram (EEG) recordings during non-REM sleep, including delta waves, sleep spindles and k-complexes [21,22]. There is a well-established inter-connection between sleep and epilepsy. Sleep deprivation is a common precipitant for seizures and seizures in many patients occur preferentially during sleep or soon after awakening [23]–[26]. Conversely, patients with chronic epilepsy have a high incidence of sleep-disordered breathing and other sleep disorders such as parasomnias, insomnia and enuresis [23,27,28]. Furthermore certain anti-epileptic drugs, including lamotrigine, felbamate and levetiracetam, have been reported to adversely affect sleep quality [23]. This raises the prospect that there is a relationship between the physiological cerebral oscillations that occur during sleep and the pathological oscillations that underlie epileptic seizures.
Sleep-related cerebral oscillations arise in the thalamocortical system, comprising a loop of reciprocal glutamatergic connections between corticothalamic layer V-VI pyramidal neurons and thalamocortical relay neurons. These cortex-thalamus connecting neurons additionally innervate GABAergic neurons in the reticular thalamic nucleus, which in turn project to the thalamocortical neurons generating the hyperpolarization required to maintain T-type channel de-inactivation in thalamocortical neurons during oscillations. Burst firing is a key firing property of reticular thalamic and thalamocortical neurons during the non-REM sleep EEG oscillations, and this firing modality is dependent upon activation of highly-expressed T-type calcium channels [21]. Small membrane depolarizations induce T-type calcium channel activation, causing a cascade depolarization of the neuron and producing a low threshold calcium potential (LTCP), peaking around −40 mV, lasting between 50 to 100 ms, and allowing the generation of a burst of high frequency sodium channel-mediated action potentials upon its crest. These bursts are predicted to generate synchronization of neural networks and recruit additional neurons to the burst firing state, spreading and propagating the oscillations.
Distinct to the burst firing mode seen in reticular thalamic and thalamocortical neurons, which results from large scale T-type calcium channel activation generated LTCPs, the T-type window current is thought to contribute to slow oscillations seen in slow wave sleep [7,29]. The window current is relatively small, predicted to be rarely more than around 1% of the maximal T-type current in a cell [8]. When the window current is active, the resulting depolarization, assisted by hyperpolarization-activated and calcium-activated non-selective cation currents, produces an ‘up-state’ with action potential firing lasting up to several seconds, until leak currents hyperpolarize the neuron to the ‘down-state’ below the range of window current activation.
The transition between up-and down-states produces intrinsic slow (<1 Hz) oscillations in thalamocortical neurons and critically contributes to slow wave sleep. Mice in which the CaV3.1 channel is genetically deleted globally exhibit both increased latency to non-REM sleep and an increased number of brief waking periods during non-REM sleep [30]. Interestingly, mice with a thalamic region-specific deletion of CaV3.1 display similar sleep disturbances to globally deleted animals, whereas mice with cortical region-specific deletion of CaV3.1 display normal sleep patterns. Together, these data confirm a key role in sleep for thalamic but not cortical CaV3.1 T-type channels. Counter-intuitively, systemic administration of TTA-A2, a pan-T-type calcium channel blocker promotes slow wave sleep in mice [31,32]. Conversely, systemic administration of a different pan-T-type calcium channel blocker, Z944, does not display obvious effects on slow wave sleep, suggesting that TTA-A2 may exert some of its effects via a different mechanism from Z944 or in a distinct CNS region where Z944 has no effect [33].
T-type calcium channels and epilepsy
Classification of seizures, epilepsy and epileptogenesis
A seizure is as transient symptomatic neurological event of abnormal excessive or synchronous neuronal activity in the brain. Many people in their lifetime will experience a seizure but not necessarily go on to develop epilepsy. Epilepsy is a group of chronic neurological disorders characterized by spontaneous recurrent seizures. Epilepsy encompasses many different disorders, which can be sub-classified by aetiology, as genetic and structural/metabolic [34]. The structural/metabolic group includes epilepsy such as temporal lobe epilepsy (TLE), caused by tumours, stroke, head trauma, hypoxia, febrile seizures and metabolic disorders. The genetic group of epilepsies includes a diverse array of syndromes including childhood absence epilepsy, juvenile myoclonic epilepsy and Dravet syndrome [34]. Epilepsy affects 50 million people worldwide [35] and approximately 30% of patients do not achieve adequate seizure control with the currently available anti-epileptic drugs (AEDs) [36]. Surgery is an option for a minority of these patients with drug resistant epilepsy, but requires the origin of the seizures to be able to be localized accurately to a region of the brain that can be safely resected.
In addition to the costs associated with treating epilepsy patients, there are additional burdens on the sufferer. There is the social stigma associated with the disease as epilepsy patients may not be able to hold down a job or drive a car and epilepsy patients also have to deal with additional comorbidities such as anxiety, depression and an increased risk of sudden death. All of these factors make it all the more important to find ways to prevent or reverse the epileptic state rather than just treat the symptoms. A number of multifactorial neurobiological processes are triggered after a brain insult that progressively changes the neuronal network excitability culminating in the generation of spontaneous recurrent seizures. This period is referred to as epileptogenesis, and the neurobiological processes occurring during this time include neurodegeneration, neurogenesis, gliosis, changes in gene expression, mossy fibre sprouting and damage to the blood–brain barrier.
T-type calcium channels in seizures and epileptogenesis
T-type calcium channels are believed to play a critical role in the generation of the hypersynchronous oscillatory thalamocortical activity that underlies absence seizures [37]–[39] and of the intrinsic burst firing of hippocampal pyramidal neurons in TLE [40]–[42]. The three T-type calcium channels (CaV3.1, CaV3.2 and CaV3.3) are widely but differentially expressed in the thalamocortical circuitry implicated in absence seizures (Figure 1). Further, alterations in function and expression of T-type calcium channels have been reported in animal models of both genetic generalized epilepsy (GGE) and acquired TLE, further implicating these channels as key players in regulating neuronal excitability.
T-type calcium channels in models of acquired temporal lobe epilepsy
In the kindling model of TLE, whereby repeated electrical stimulations are applied to a brain region (such as the hippocampus or amygdala) resulting in increasing intensity and severity of seizures, T-type calcium currents were significantly larger in CA1 pyramidal cells of animals that had experienced nine class V seizures (the most severe of kindled seizures) compared with controls, and these current changes persisted up to 6 weeks after the cessation of kindling [43]. In another model of TLE (post-status epilepticus), administration of the muscarinic receptor agonist, pilocarpine, induced a period of continuous seizures (status epilepticus) which was followed by a latent seizure-free period and then the development of spontaneous recurrent seizures. The post-status epilepticus model of acquired TLE parallels human TLE closely in regards to the initial brain insult, latent period, development of spontaneous convulsive seizures, with similar AED profile and limbic histopathological changes [44]. Several studies investigating the role of T-type calcium channels during epileptogenesis after pilocarpine induced status epilepticus found that there was a selective and transient increase in CaV3.2 mRNA expression in CA1 pyramidal neurons coupled with an up-regulation of T-type calcium currents [40,41,45,46]. Additionally, hippocampal sclerosis and mossy fibre sprouting, histopathological hallmarks of TLE in humans and animal models, was absent in CaV3.2 knockout mice and these mice were resistant to the development of chronic seizures induced by pilocarpine [41].
T-type calcium channels in humans and animal models of genetic generalized epilepsy
There is also substantial evidence in the literature from human and animal studies linking T-type calcium channels to the pathogenesis of GGEs. Mutations in the gene encoding human CaV3.2, CACNA1H, have been identified in patients with a variety of GGE syndromes [47,48], in particular childhood absence epilepsy, with the mutations shown in vitro to affect channel kinetics, gating and membrane expression of CaV3.2 leading to hyperexcitability [49,50]. Additionally, a mutation in the rat CaV3.2 gene (R1584P) has been identified in the Genetic Absence Epilepsy Rats from Strasbourg (GAERS) animal model of GGE with absence seizures which was shown to have a splice variant-specific gain of function effect on the channel [51].
In Chinese Han populations no mutations in CACNA1G or CACNA1I have been identified in patients with GGE [52,53]. However several variants in CACNA1G have been identified in a Japanese population but only one variant was not observed in control samples and in vitro functional analysis of these mutations failed to show any difference in channel properties and kinetics [54]. Further evidence implicating CaV3.1 in GGE comes from studies on mutant mice. CaV3.1 knockout mice lack the burst firing mode of action potentials in thalamocortical neurons, seen during absence seizures, whereas the normal tonic mode of firing was unaffected [39]. Additionally, CaV3.1 knockout mice were resistant to the development of synchronous and bilateral spike and wave discharges (SWDs) in response to GABAB receptor activation but had the same susceptibility as wild type mice when treated with a GABAA receptor antagonist, bicuculline [39]. Conversely, transgenic mice overexpressing CaV3.1 show increased functional thalamic T-type calcium currents and frequent bilateral rhythmic SWDs that can be blocked by treating with ethosuximide [38]. However, while the role of T-type calcium channels in the pathophysiology of GGE is well established, the contribution of CaV3.1 T-type calcium currents to the generation of absence seizures is still under debate. Increased CaV3.1 T-type calcium currents have been reported to be sufficient to induce pure absence epilepsy [38] whereas in a study utilizing CaV2.1 mutant mice double crossed with CaV3.1 mutant mice it was found that increased thalamic CaV3.1 T-type calcium currents were not essential for the generation of absence seizures [55].
Changes in the expression of T-type calcium channels are also evident in animal models of GGE. CaV3.1 mRNA expression is increased in the lateral geniculate nucleus and centrolateral nucleus of the intralaminar nuclei in WAG/Rij [56] and in the ventral posterior thalamic relay nuclei of adult GAERS [57]. Increased CaV3.1 and CaV3.2 mRNA expression in the thalamus and T-type calcium currents have been reported in GAERS [37,57] and WAG/Rij rats [56]. Additionally, CaV3.3 mRNA expression is increased in the reticular nucleus of the thalamus and the centrolateral nucleus of the intralaminar nuclei in WAG/Rij [56]. Taken together, it is clear that increases in any of the T-type calcium channels are important factors in contributing to the epilepsy phenotype of GGE in these animal models.
T-type calcium channel antagonists as anti-seizure and anti-epileptogenesis agents
Current therapeutic treatment for epilepsy with AEDs is symptomatic, aimed solely at suppressing seizures, but these drugs have no disease modifying effects on epileptogenesis. There is an urgent need to develop treatments that prevent epilepsy in identified individuals at risk, whether they are at risk from genetic or acquired factors, and to mitigate the condition once established [58]. There have been recent developments investigating current AEDs as potential modifiers of epileptogenesis in GGE models. Blumenfeld and colleagues found that orally treating WAG/Rij rats with ethosuximide long term (from 3 weeks to 5 months), a drug which is believed to act in part via suppressing T-type calcium channels, suppressed seizures even up to 3 months after the cessation of treatment, indicating that the treatment had had an anti-epileptogenic effect [59]. Our group has recently demonstrated a similar anti-epileptic effect for chronic ethosuxamide treatment in the GAERS model [60]. Additionally another study found that zonisamide, a novel and new generation AED which has multiple mechanisms of action including blocking T-type calcium channels, as well as ethosuximide, administered orally from 1 month to 5 months resulted in a significant reduction in the number and duration of absence seizures in WAG/Rij rats 6 weeks after treatment had been discontinued [61,62]. Despite the fact that T-type calcium channels have been implicated in epileptogenesis in acquired models of TLE [41,45], studies into the effectiveness of AEDs as modifiers of epileptogenic processes in acquired epilepsy models have not been undertaken.
The implication of T-type calcium channels in many different disease states coupled with the need to identify truly selective T-type calcium channel antagonists has led to the emergence of drug discovery programmes and novel, potent and more selective T-type calcium channel blockers have been developed and are being tested in vitro and in animal models in the hope of identifying drugs with promising clinical use in the epilepsy field. We will review the current AEDs that are known to act on T-type calcium channels as well as discuss the effectiveness of the new T-type calcium channel antagonists in the treatment of epilepsy.
Clinically used AEDs that act on T-type calcium channels
Succinimides (ethosuximide: Zarontin®, methsuximide: Celontin®)
The two anti-convulsant succinimides currently in clinical use are ethosuximide and, to a lesser extent, methsuximide [63]. The succinimides block T-type calcium channels in a state-dependent manner, having preference for open and inactivated states [64]. Ethosuximide is the first line drug used to treat patients with GGE [65]. While ethosuximide was originally thought to act primarily via T-type calcium channels [66,67], evidence now exists which shows that ethosuximide also has effects on the persistent sodium and calcium-activated potassium currents [68]–[70].
Zonisamide (Zonegran®)
Zonisamide, a new generation AED introduced in America in 2000 and in Europe in 2005, is effective as an adjunctive therapy in adults with partial onset seizures, infantile spasms, myoclonic, absence generalized tonic-clonic seizures and tonic/atonic seizures, and has been shown to be effective in patients whose seizures are resistant to other AEDs [71,72]. Zonisamide has a unique mechanism of action, it blocks T-type calcium channels in a voltage dependent manner, having a higher affinity for the inactivated state of the channels as well as blocking voltage-gated sodium channels and in doing so raises the threshold for the generation of an action potential [73]. Through this combined effect on sodium and calcium channels, zonisamide blocks the propagation of seizures by stabilizing neuronal membranes and suppresses neuronal hypersynchronization [74,75]. Zonisamide has also been reported to enhance directly the synthesis and degradation of dopamine, serotonin [76] and acetylcholine [77] as well as inhibiting evoked glutamate release [78] and modulating GABA and glutamate transporter levels [79]. Additionally, zonisamide shows potential in alleviating central pain after stroke [80] and the symptoms of Parkinson's disease [81,82].
Valproate (Depakote®)
Valproate is a widely used broad spectrum AED and mood stabilizer that has been in clinical use for over 40 years but its mechanism of action still remains controversial [83], but given its wide spectrum of efficacy against different seizure types and other psychiatric disorders it will undoubtedly have multiple and complex modes of action. Compared with other AEDs, valproate has a very different structure with an achiral short branched fatty acid with eight carbons that does not contain a nitrogen or a cyclic ring [84]. The anti-epileptic properties of valproate have been attributed to increasing brain GABA concentrations but the mechanisms by which this occurs remain to be determined [85]–[87]. Valproate has been found to block T-type calcium currents in acutely isolated thalamocortical neurons from WAG/Rij and ACI control rats, with the degree of block being greater in WAG/Rij rats than in control rats (53% vs. 20%) [70]. Valproate also has effects on L-type calcium channels, sodium channels and voltage gated potassium channels [88] as well as attenuating NMDA-mediated excitation [89,90]. Valproate has been identified as being a histone deacetylase (HDAC) inhibitor, which has opened up avenues for research into clinical uses of valproate beyond epilepsy and psychiatric disorders [91]. Histone acetylation plays an important role in the regulation of gene expression with histone deacetylases transforming chromatin into a transcriptionally inactive form. Thus inhibition of HDAC would therefore promote gene transcription [92].
Phenytoin (Dilantin®)
Phenytoin became available as an AED in 1938 and is used to treat partial and generalized seizures. Phenytoin's primary mechanism of action is to inhibit sodium channels, however effects on T-type calcium channels have also been reported. Phenytoin voltage-dependently inhibits T-type calcium currents in HEK293 cells expressing CaV3.1 or CaV3.2 [93], native T-type calcium currents in neuroblastoma N1E-115 cells [94] and in dorsal root ganglion ND7-23 cells [95]. However it has been shown that effective inhibition of T-type calcium currents requires close to the maximal therapeutic concentrations of phenytoin [96].
Mibefradil (Posicor®)
Mibefradil was originally marketed in 1997 as a selective T-type calcium channel blocker effective as an anti-hypertensive agent [97] but subsequent studies have revealed that it also acts on sodium, potassium, chlorine and L-type calcium [98] and store-operated calcium channels [99]. Mibefradil was withdrawn from the clinical market in 1998 due to drug interactions leading to irregular heart rhythms. Despite this, mibefradil has recently been shown to block completely T-type calcium currents and low threshold calcium spikes in acutely isolated thalamocortical neurons and supress absence seizures in WAG/Rij rats when focally administered bilaterally into the ventrobasal nucleus of the thalamus [70].
Novel T-type calcium channel antagonists
Merck and Zalicus Pharmaceuticals have identified and characterized numerous potential novel selective T-type calcium channel antagonists with several of these compounds showing promise in pre-clinical epilepsy studies. Z941 and Z944 are piperazine analogues developed by Zalicus Pharmaceuticals that have nanomolar affinities for the inhibition of human CaV3.2 channels (Z941 IC50 = 120 nm; Z944 IC50 = 50 nm) in the FLIPR assay and have greater potency than ethosuximide and valproate. Z944 has similar affinity at all three human T-type calcium channels (IC50 ranges from 50–160 nm) and blocks thalamic burst firing. In vivo assessment of Z941 and Z944 revealed that both drugs suppressed absence seizure number and duration in GAERS more potently and by a mechanism distinct from that of ethosuximide [33]. Z944 is currently in phase 1 clinical trials to determine its safety, pharmacokinetics and to identify possible side effects. Merck have identified a series of piperidine-containing T-type calcium channel antagonists including TTA-P1 and TTA-P2. TTA-P1 was found to be a potent state-independent compound that inhibited human T-type calcium channels in the nanomolar range (IC50 = 32 nm) and was found to have good brain penetrance and to be effective at suppressing seizures in the WAG/Rij model of GGE [100]. Further optimization of TTA-P1s selectively and stability led to the identification of TTA-P2 which is a potent and selective antagonist at all three T-type calcium channels (IC50 = 84 nm in the depolarized FLIPR assay) and showed efficacy at suppressing seizures in WAG/Rij rats [101]. Importantly, TTA-P2 does not have any action on HVA calcium currents, sodium currents, action potentials, glutamatergic or GABAergic currents [8]. Another novel series of compounds (TTA-A) identified by Merck are structurally different from the TTA-P compounds. The amide analogue TTA-A2 selectively and potently blocks T-type calcium channels (IC50 = 9.4 nm eith depolarized FLIPR assay) by preferentially interacting and stabilizing the inactivated forms of the channels [31,102]. TTA-A2 was moderately effective at suppressing absence seizures in WAG/Rij (∼40% reduction) but the duration of efficacy was prolonged when TTA-A2 was co-administered with the quinazolinone T-type calcium antagonist TTA-Q4 [102]. Twelve hours after administration of the drugs, TTA-A2 alone no longer suppressed seizures. However TTA-A2 together with TTA-Q4 still suppressed seizures by approximately 25%. There is currently no indication that any of the Merck compounds have progressed into human clinical trials.
Conclusions
T-type calcium channels play a critical role in the generation of the pathological neuronal network oscillations and neuronal burst firing that underlies seizures. Increasing evidence from animal models and human studies indicate that T-type calcium channels play an important role in both genetic and acquired epileptogenesis, and represent a promising approach for anti-epileptogenic therapies, one of the key unmet needs for epilepsy. While a number of currently clinical used AEDs have effects on T-type calcium channels, novel compounds in the drug development pathway are more specific and potent, and represent highly promising new treatments for epilepsy which have potential to be both anti-epileptogenic as well as anti-seizure. A complicating factor in finding a truly selective T-type calcium channel blocker may be that the three T-type calcium channels (CaV3.1, CaV3.2 and CaV3.3) are widely expressed and contribute to physiological functions across the nervous, cardiovascular and endocrine systems. Additionally, multiple isoforms of each T-type channel are generated by alternative splicing and exhibit both distinct spatial distributions and biophysical properties. Thus there are likely a limited number of actual functional targets relevant to epileptogenesis. In order to maximize therapeutic ratios it will likely be necessary to develop new AEDs that exhibit both state-dependent T-type channel blockade and selectivity against the individual T-type isoforms.
Competing Interests
KLP and TJO have received research grants from Zalicus Pharmaceuticals Inc. (Cambridge, Massachusetts) in the previous 3 years. SMC received postdoctoral fellowship support from the BC Epilepsy Society and Michael Smith Foundation for Health Research. TPS is supported by an operating grant from the Canadian Institutes of Health Research (#10677) and is a Canada Research Chair in Biotechnology and Genomics-Neurobiology. TPS is a scientific consultant for Zalicus Pharmaceuticals Inc.
References
- 1.Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 4.Kozlov AS, McKenna F, Lee JH, Cribbs LL, Perez-Reyes E, Feltz A, Lambert RC. Distinct kinetics of cloned T-type Ca2 + channels lead to differential Ca2 + entry and frequency-dependence during mock action potentials. Eur J Neurosci. 1999;11:4149–4158. doi: 10.1046/j.1460-9568.1999.00841.x. [DOI] [PubMed] [Google Scholar]
- 5.Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (alpha(1G), alpha(1H) and alpha(1I)) to neuronal excitability. J Physiol. 2002;540:3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cain SM, Snutch TP. Contributions of T-type calcium channel isoforms to neuronal firing. Channels (Austin) 2010;4:475–482. doi: 10.4161/chan.4.6.14106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams SR, Toth TI, Turner JP, Hughes SW, Crunelli V. The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol. 1997;505(Pt 3):689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci. 2010;30:99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, Turner RW. Ca (V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24:2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 11.McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, Stea A, Snutch TP. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem. 2001;276:3999–4011. doi: 10.1074/jbc.M008215200. [DOI] [PubMed] [Google Scholar]
- 12.Murbartian J, Arias JM, Lee JH, Gomora JC, Perez-Reyes E. Alternative splicing of the rat Ca(v)3.3 T-type calcium channel gene produces variants with distinct functional properties(1) FEBS Lett. 2002;528:272–278. doi: 10.1016/s0014-5793(02)03341-0. [DOI] [PubMed] [Google Scholar]
- 13.Emerick MC, Stein R, Kunze R, McNulty MM, Regan MR, Hanck DA, Agnew WS. Profiling the array of Ca(v)3.1 variants from the human T-type calcium channel gene CACNA1G: alternative structures, developmental expression, and biophysical variations. Proteins. 2006;64:320–342. doi: 10.1002/prot.20877. [DOI] [PubMed] [Google Scholar]
- 14.Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet. 2006;15:1497–1512. doi: 10.1093/hmg/ddl068. [DOI] [PubMed] [Google Scholar]
- 15.Broicher T, Kanyshkova T, Landgraf P, Rankovic V, Meuth P, Meuth SG, Pape HC, Budde T. Specific expression of low-voltage-activated calcium channel isoforms and splice variants in thalamic local circuit interneurons. Mol Cell Neurosci. 2007;36:132–145. doi: 10.1016/j.mcn.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Powell KL, Kyi M, Reid CA, Paradiso L, D'Abaco GM, Kaye AH, Foote SJ, O'Brien TJ. Genetic absence epilepsy rats from Strasbourg have increased corticothalamic expression of stargazin. Neurobiol Dis. 2008;31:261–265. doi: 10.1016/j.nbd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.David LS, Garcia E, Cain SM, Thau E, Tyson JR, Snutch TP. Splice-variant changes of the Ca(V)3.2 T-type calcium channel mediate voltage-dependent facilitation and associate with cardiac hypertrophy and development. Channels (Austin) 2010;4:375–389. doi: 10.4161/chan.4.5.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, Snutch TP, Zamponi GW, Turner RW. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA. 2006;103:5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, Schneider T, Hescheler J, Kano M, Sakimura K, Watanabe M, Dieudonne S, Snutch TP. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci. 2009;29:9668–9682. doi: 10.1523/JNEUROSCI.0362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isope P, Hildebrand ME, Snutch TP. Contributions of T-type voltage-gated calcium channels to postsynaptic calcium signaling within Purkinje neurons. Cerebellum. 2012;11:651–665. doi: 10.1007/s12311-010-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- 22.Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–190. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manni R, Terzaghi M. Comorbidity between epilepsy and sleep disorders. Epilepsy Res. 2010;90:171–177. doi: 10.1016/j.eplepsyres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Parrino L, Halasz P, Tassinari CA, Terzano MG. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006;10:267–285. doi: 10.1016/j.smrv.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Parrino L, Smerieri A, Spaggiari MC, Terzano MG. Cyclic alternating pattern (CAP) and epilepsy during sleep: how a physiological rhythm modulates a pathological event. Clin Neurophysiol. 2000;111(Suppl. 2):S39–46. doi: 10.1016/s1388-2457(00)00400-4. [DOI] [PubMed] [Google Scholar]
- 26.Ferrillo F, Beelke M, Nobili L. Sleep EEG synchronization mechanisms and activation of interictal epileptic spikes. Clin Neurophysiol. 2000;111(Suppl. 2):S65–73. doi: 10.1016/s1388-2457(00)00404-1. [DOI] [PubMed] [Google Scholar]
- 27.Phillips MC, Costello CA, White EJ, Smit M, Carino J, Strawhorn A, Jackson B, Kwan P, French CR, Yerra SR, Tan KM, O'Brien TJ, Goldin J. Routine polysomnography in an epilepsy monitoring unit. Epilepsy Res. 2013;105:401–404. doi: 10.1016/j.eplepsyres.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Manni R, Terzaghi M, Arbasino C, Sartori I, Galimberti CA, Tartara A. Obstructive sleep apnea in a clinical series of adult epilepsy patients: frequency and features of the comorbidity. Epilepsia. 2003;44:836–840. doi: 10.1046/j.1528-1157.2003.55702.x. [DOI] [PubMed] [Google Scholar]
- 29.Hughes SW, Cope DW, Toth TI, Williams SR, Crunelli V. All thalamocortical neurones possess a T-type Ca2+ ‘window’ current that enables the expression of bistability-mediated activities. J Physiol. 1999;517(Pt 3):805–815. doi: 10.1111/j.1469-7793.1999.0805s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson MP, Mochizuki T, Xie J, Fischler W, Manger JP, Talley EM, Scammell TE, Tonegawa S. Thalamic Cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci U S A. 2005;102:1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus RL, Li Y, Gregan Y, Gotter AL, Uebele VN, Fox SV, Doran SM, Barrow JC, Yang ZQ, Reger TS, Koblan KS, Renger JJ. In vitro characterization of T-type calcium channel antagonist TTA-A2 and in vivo effects on arousal in mice. J Pharmacol Exp Ther. 2010;335:409–417. doi: 10.1124/jpet.110.171058. [DOI] [PubMed] [Google Scholar]
- 32.Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest. 2009;119:1659–1667. doi: 10.1172/JCI36954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, Eduljee C, Jiang X, Smith P, Morrison JL, Jones NC, Braine E, Rind G, Fee-Maki M, Parker D, Pajouhesh H, Parmar M, O'Brien TJ, Snutch TP. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med. 2012;4:121ra19. doi: 10.1126/scitranslmed.3003120. [DOI] [PubMed] [Google Scholar]
- 34.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. 2012. Fact Sheet No 999. Available at http://www.who.int/mediacentre/factsheets/fs999/en/(last accessed 29 July 2013)Epilepsy.
- 36.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 37.Tsakiridou E, Bertollini L, de Curtis M, Avanzini G, Pape HC. Selective increase in T-type calcium conductance of reticular thalamic neurons in a rat model of absence epilepsy. J Neurosci. 1995;15:3110–3117. doi: 10.1523/JNEUROSCI.15-04-03110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst WL, Zhang Y, Yoo JW, Ernst SJ, Noebels JL. Genetic enhancement of thalamocortical network activity by elevating alpha 1g-mediated low-voltage-activated calcium current induces pure absence epilepsy. J Neurosci. 2009;29:1615–1625. doi: 10.1523/JNEUROSCI.2081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 40.Su H, Sochivko D, Becker A, Chen J, Jiang Y, Yaari Y, Beck H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faas GC, Vreugdenhil M, Wadman WJ. Calcium currents in pyramidal CA1 neurons in vitro after kindling epileptogenesis in the hippocampus of the rat. Neuroscience. 1996;75:57–67. doi: 10.1016/0306-4522(96)00254-0. [DOI] [PubMed] [Google Scholar]
- 44.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Graef JD, Nordskog BK, Wiggins WF, Godwin DW. An acquired channelopathy involving thalamic T-type Ca2+ channels after status epilepticus. J Neurosci. 2009;29:4430–4441. doi: 10.1523/JNEUROSCI.0198-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaari Y, Yue C, Su H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J Physiol. 2007;580:435–450. doi: 10.1113/jphysiol.2007.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Lu J, Pan H, Zhang Y, Wu H, Xu K, Liu X, Jiang Y, Bao X, Yao Z, Ding K, Lo WH, Qiang B, Chan P, Shen Y, Wu X. Association between genetic variation of CACNA1H and childhood absence epilepsy. Ann Neurol. 2003;54:239–243. doi: 10.1002/ana.10607. [DOI] [PubMed] [Google Scholar]
- 48.Heron SE, Khosravani H, Varela D, Bladen C, Williams TC, Newman MR, Scheffer IE, Berkovic SF, Mulley JC, Zamponi GW. Extended spectrum of idiopathic generalized epilepsies associated with CACNA1H functional variants. Ann Neurol. 2007;62:560–568. doi: 10.1002/ana.21169. [DOI] [PubMed] [Google Scholar]
- 49.Vitko I, Chen Y, Arias JM, Shen Y, Wu XR, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-type calcium channel. J Neurosci. 2005;25:4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E. The I-II loop controls plasma membrane expression and gating of Ca(v)3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci. 2007;27:322–330. doi: 10.1523/JNEUROSCI.1817-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O'Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Zhang Y, Liang J, Pan H, Wu H, Xu K, Liu X, Jiang Y, Shen Y, Wu X. CACNA1I is not associated with childhood absence epilepsy in the Chinese Han population. Pediatr Neurol. 2006;35:187–190. doi: 10.1016/j.pediatrneurol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Lu J, Zhang Y, Pan H, Wu H, Xu K, Liu X, Jiang Y, Bao X, Zhou J, Liu W, Shi G, Shen Y, Wu X. T-type calcium channel gene alpha (1G) is not associated with childhood absence epilepsy in the Chinese Han population. Neurosci Lett. 2003;341:29–32. doi: 10.1016/s0304-3940(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 54.Singh B, Monteil A, Bidaud I, Sugimoto Y, Suzuki T, Hamano S, Oguni H, Osawa M, Alonso ME, Delgado-Escueta AV, Inoue Y, Yasui-Furukori N, Kaneko S, Lory P, Yamakawa K. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum Mutat. 2007;28:524–525. doi: 10.1002/humu.9491. [DOI] [PubMed] [Google Scholar]
- 55.Song I, Kim D, Choi S, Sun M, Kim Y, Shin HS. Role of the alpha1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci. 2004;24:5249–5257. doi: 10.1523/JNEUROSCI.5546-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broicher T, Kanyshkova T, Meuth P, Pape HC, Budde T. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–399. doi: 10.1016/j.mcn.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Talley EM, Solorzano G, Depaulis A, Perez-Reyes E, Bayliss DA. Low-voltage-activated calcium channel subunit expression in a genetic model of absence epilepsy in the rat. Brain Res Mol Brain Res. 2000;75:159–165. doi: 10.1016/s0169-328x(99)00307-1. [DOI] [PubMed] [Google Scholar]
- 58.Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J, Jr, Loscher W, Noebels JL, Pitkanen A, Stables J, White HS, O'Brien TJ, Simonato M. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53:571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O'Brien TJ, Jones NC. Ethosuximide reduces epileptogenesis and behavioural comorbidity in the GAERS model of genetic generalised epilepsy. Epilepsia. 2013;54:635–643. doi: 10.1111/epi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- 62.Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, Constanti A, De Sarro G. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52:1341–1350. doi: 10.1111/j.1528-1167.2011.03112.x. [DOI] [PubMed] [Google Scholar]
- 63.Bialer M. Chemical properties of antiepileptic drugs (AEDs) Adv Drug Deliv Rev. 2012;64:887–895. doi: 10.1016/j.addr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 64.McGivern JG. Pharmacology and drug discovery for T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:587–603. doi: 10.2174/187152706779025535. [DOI] [PubMed] [Google Scholar]
- 65.Goren MZ, Onat F. Ethosuximide: from bench to bedside. CNS Drug Rev. 2007;13:224–239. doi: 10.1111/j.1527-3458.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coulter DA, Huguenard JR, Prince DA. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann Neurol. 1989;25:582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 67.Coulter DA, Huguenard JR, Prince DA. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: calcium current reduction. Br J Pharmacol. 1990;100:800–806. doi: 10.1111/j.1476-5381.1990.tb14095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crunelli V, Leresche N. Block of thalamic T-type Ca(2+) channels by ethosuximide is not the whole story. Epilepsy Curr. 2002;2:53–56. doi: 10.1046/j.1535-7597.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leresche N, Parri HR, Erdemli G, Guyon A, Turner JP, Williams SR, Asprodini E, Crunelli V. On the action of the anti-absence drug ethosuximide in the rat and cat thalamus. J Neurosci. 1998;18:4842–4853. doi: 10.1523/JNEUROSCI.18-13-04842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Broicher T, Seidenbecher T, Meuth P, Munsch T, Meuth SG, Kanyshkova T, Pape HC, Budde T. T-current related effects of antiepileptic drugs and a Ca2+ channel antagonist on thalamic relay and local circuit interneurons in a rat model of absence epilepsy. Neuropharmacology. 2007;53:431–446. doi: 10.1016/j.neuropharm.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 71.Leppik IE, Willmore LJ, Homan RW, Fromm G, Oommen KJ, Penry JK, Sackellares JC, Smith DB, Lesser RP, Wallace JD. Efficacy and safety of zonisamide: results of a multicenter study. Epilepsy Res. 1993;14:165–173. doi: 10.1016/0920-1211(93)90021-x. [DOI] [PubMed] [Google Scholar]
- 72.Biton V. Clinical pharmacology and mechanism of action of zonisamide. Clin Neuropharmacol. 2007;30:230–240. doi: 10.1097/wnf.0b013e3180413d7d. [DOI] [PubMed] [Google Scholar]
- 73.Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5:3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 74.Rock DM, Macdonald RL, Taylor CP. Blockade of sustained repetitive action potentials in cultured spinal cord neurons by zonisamide (AD 810, CI 912), a novel anticonvulsant. Epilepsy Res. 1989;3:138–143. doi: 10.1016/0920-1211(89)90041-7. [DOI] [PubMed] [Google Scholar]
- 75.Kito M, Maehara M, Watanabe K. Mechanisms of T-type calcium channel blockade by zonisamide. Seizure. 1996;5:115–119. doi: 10.1016/s1059-1311(96)80104-x. [DOI] [PubMed] [Google Scholar]
- 76.Kaneko S, Okada M, Hirano T, Kondo T, Otani K, Fukushima Y. Carbamazepine and zonisamide increase extracellular dopamine and serotonin levels in vivo, and carbamazepine does not antagonize adenosine effect in vitro: mechanisms of blockade of seizure spread. Jpn J Psychiatry Neurol. 1993;47:371–373. doi: 10.1111/j.1440-1819.1993.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 77.Zhu G, Okada M, Murakami T, Kawata Y, Kamata A, Kaneko S. Interaction between carbamazepine, zonisamide and voltage-sensitive Ca2+ channel on acetylcholine release in rat frontal cortex. Epilepsy Res. 2002;49:49–60. doi: 10.1016/s0920-1211(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 78.Okada M, Kawata Y, Mizuno K, Wada K, Kondo T, Kaneko S. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br J Pharmacol. 1998;124:1277–1285. doi: 10.1038/sj.bjp.0701941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ueda Y, Doi T, Tokumaru J, Willmore LJ. Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures. Brain Res Mol Brain Res. 2003;116:1–6. doi: 10.1016/s0169-328x(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi Y, Hashimoto K, Tsuji S. Successful use of zonisamide for central poststroke pain. J Pain. 2004;5:192–194. doi: 10.1016/j.jpain.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 81.Murata M. Novel therapeutic effects of the anti-convulsant, zonisamide, on Parkinson's disease. Curr Pharm Des. 2004;10:687–693. doi: 10.2174/1381612043453180. [DOI] [PubMed] [Google Scholar]
- 82.Murata M, Horiuchi E, Kanazawa I. Zonisamide has beneficial effects on Parkinson's disease patients. Neurosci Res. 2001;41:397–399. doi: 10.1016/s0168-0102(01)00298-x. [DOI] [PubMed] [Google Scholar]
- 83.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 84.Jeavons PM, Clark JE. Sodium valproate in treatment of epilepsy. Br Med J. 1974;15:584–586. doi: 10.1136/bmj.2.5919.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21:611–615. doi: 10.1111/j.1528-1157.1980.tb04314.x. [DOI] [PubMed] [Google Scholar]
- 86.Loscher W, Siemes H. Valproic acid increases gamma-aminobutyric acid in CSF of epileptic children. Lancet. 1984;2:225. doi: 10.1016/s0140-6736(84)90509-9. [DOI] [PubMed] [Google Scholar]
- 87.Patsalos PN. Properties of antiepileptic drugs in the treatment of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl. 9):140–148. doi: 10.1111/j.1528-1167.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 88.VanDongen AM, VanErp MG, Voskuyl RA. Valproate reduces excitability by blockage of sodium and potassium conductance. Epilepsia. 1986;27:177–182. doi: 10.1111/j.1528-1157.1986.tb03525.x. [DOI] [PubMed] [Google Scholar]
- 89.Zeise ML, Kasparow S, Zieglgansberger W. Valproate suppresses N-methyl-D-aspartate-evoked, transient depolarizations in the rat neocortex in vitro. Brain Res. 1991;544:345–348. doi: 10.1016/0006-8993(91)90078-a. [DOI] [PubMed] [Google Scholar]
- 90.Gean PW, Huang CC, Hung CR, Tsai JJ. Valproic acid suppresses the synaptic response mediated by the NMDA receptors in rat amygdalar slices. Brain Res Bull. 1994;33:333–336. doi: 10.1016/0361-9230(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 91.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 92.MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 93.Todorovic SM, Perez-Reyes E, Lingle CJ. Anticonvulsants but not general anesthetics have differential blocking effects on different T-type current variants. Mol Pharmacol. 2000;58:98–108. doi: 10.1124/mol.58.1.98. [DOI] [PubMed] [Google Scholar]
- 94.Twombly DA, Yoshii M, Narahashi T. Mechanisms of calcium channel block by phenytoin. J Pharmacol Exp Ther. 1988;246:189–195. [PubMed] [Google Scholar]
- 95.Kobrinsky EM, Pearson HA, Dolphin AC. Low-and high-voltage-activated calcium channel currents and their modulation in the dorsal root ganglion cell line ND7-23. Neuroscience. 1994;58:539–552. doi: 10.1016/0306-4522(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 96.Lory P, Chemin J. Towards the discovery of novel T-type calcium channel blockers. Expert Opin Ther Targets. 2007;11:717–722. doi: 10.1517/14728222.11.5.717. [DOI] [PubMed] [Google Scholar]
- 97.Barton ME, Eberle EL, Shannon HE. The antihyperalgesic effects of the T-type calcium channel blockers ethosuximide, trimethadione and mibefradil. Eur J Pharmacol. 2005;521:79–85. doi: 10.1016/j.ejphar.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 98.Moosmang S, Haider N, Bruderl B, Welling A, Hofmann F. Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res. 2006;98:105–110. doi: 10.1161/01.RES.0000197851.11031.9c. [DOI] [PubMed] [Google Scholar]
- 99.Gackiere F, Bidaux G, Lory P, Prevarskaya N, Mariot P. A role for voltage gated T-type calcium channels in mediating ‘capacitative’ calcium entry? Cell Calcium. 2006;39:357–366. doi: 10.1016/j.ceca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Yang ZQ, Barrow JC, Shipe WD, Schlegel KA, Shu Y, Yang FV, Lindsley CW, Rittle KE, Bock MG, Hartman GD, Uebele VN, Nuss CE, Fox SV, Kraus RL, Doran SM, Connolly TM, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Marino MJ, Graufelds VK, DiLella AG, Reynolds IJ, Vargas HM, Bunting PB, Woltmann RF, Magee MM, Koblan KS, Renger JJ. Discovery of 1,4-substituted piperidines as potent and selective inhibitors of T-type calcium channels. J Med Chem. 2008;51:6471–6477. doi: 10.1021/jm800830n. [DOI] [PubMed] [Google Scholar]
- 101.Shipe WD, Barrow JC, Yang ZQ, Lindsley CW, Yang FV, Schlegel KA, Shu Y, Rittle KE, Bock MG, Hartman GD, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont T, Zrada MM, Uebele VN, Nuss CE, Connolly TM, Doran SM, Fox SV, Kraus RL, Marino MJ, Graufelds VK, Vargas HM, Bunting PB, Hasbun-Manning M, Evans RM, Koblan KS, Renger JJ. Design, synthesis, and evaluation of a novel 4-aminomethyl-4-fluoropiperidine as a T-type Ca2+ channel antagonist. J Med Chem. 2008;51:3692–3695. doi: 10.1021/jm800419w. [DOI] [PubMed] [Google Scholar]
- 102.Uebele VN, Nuss CE, Fox SV, Garson SL, Cristescu R, Doran SM, Kraus RL, Santarelli VP, Li Y, Barrow JC, Yang ZQ, Schlegel KA, Rittle KE, Reger TS, Bednar RA, Lemaire W, Mullen FA, Ballard JE, Tang C, Dai G, McManus OB, Koblan KS, Renger JJ. Positive allosteric interaction of structurally diverse T-type calcium channel antagonists. Cell Biochem Biophys. 2009;55:81–93. doi: 10.1007/s12013-009-9057-4. [DOI] [PubMed] [Google Scholar]