Abstract
AIM
Twice daily dosing is often perceived as inferior to once daily dosing due to a higher likelihood of missing a dose. However, more important is the extent to which drug action is maintained when doses are delayed or missed. We compared the estimated inhibition of platelet aggregation (eIPA) for ticagrelor twice daily and clopidogrel once daily, based on their pharmacokinetic/pharmacodynamic relationships and patient dosing history data.
METHODS
Drug dosing histories of 5014 patients prescribed cardiovascular medications (primarily antihypertensive medicines) were extracted from an electronically compiled dosing history database. eIPA levels were simulated for 677 twice daily and 677 once daily dosing histories over a 30 day period, based on published onset/offset models for ticagrelor and clopidogrel IPA characteristics.
RESULTS
While many patients treated twice daily missed at least one dose in 30 days, only 25.7% missed two consecutive doses. By comparison, 46.8% of patients treated once daily missed at least one dose. Simulations based on patient adherence over time showed that the average mean eIPA for ticagrelor twice daily remained significantly higher than for clopidogrel once daily (81.1% vs. 55.0%, P < 0.001). Ticagrelor twice daily patients had an eIPA below 10% for 0.20% of the 30 day period compared with 2.05% for clopidogrel once daily (P A= 0.0001).
CONCLUSIONS
The projected level of platelet inhibition remained higher for ticagrelor twice daily than clopidogrel once daily, mainly due to the higher eIPA level achieved with ticagrelor and the relatively low likelihood of missing two consecutive twice daily doses. This modelling and simulation study suggests a therapeutic benefit of ticagrelor over clopidogrel when taking into account the most common dosing omissions.
Keywords: adherence, antiplatelet therapy, twice daily, clopidogrel, once daily, ticagrelor
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Twice daily dosing is often perceived as inferior to once daily dosing due to a higher likelihood of missing a dose.
However, analysis of the percentages of doses taken is not suitable to predict the real therapeutic consequences of non-adherence.
WHAT THIS STUDY ADDS
We found that although patients treated twice daily more frequently miss single doses compared with patients treated once daily, the calculated level of platelet inhibition remains higher for ticagrelor twice daily compared with clopidogrel once daily.
This study underlines the need to shift focus from percentages of prescribed doses taken to pharmacometrically equivalent dosing errors, which is better suited to predict therapeutic consequences of missed doses.
Introduction
Vascular damage such as rupture or erosion of an atherosclerotic plaque can trigger platelet aggregation. The resultant thrombus may lead to vascular occlusion, causing hypoxia and tissue damage [1]. Thrombus occlusion of a coronary artery, which may result in an acute myocardial infarction, is the proximate cause of acute coronary syndrome (ACS). With platelets thus playing a central role in atherothrombosis and consequent pathologies, antiplatelet therapy has emerged as a cornerstone of treatment for ACS patients [1–3].
The current standard antiplatelet therapy consists of aspirin, targeting the thromboxane-induced pathway of platelet activation, combined with a P2Y12 receptor antagonist that inhibits the ADP-induced platelet activation. The most frequently used P2Y12 receptor antagonist is clopidogrel, a thienopyridine, administered at a daily dose of 75 mg. However, clopidogrel has drawbacks such as delayed onset of action, interpatient variability in response or even resistance to clopidogrel, and irreversibility of its inhibitory effect, which increases the risk of bleeding in patients requiring surgery [4–6].
Recently, more potent antiplatelet drugs have been developed, such as ticagrelor which is the first of a new chemical class of oral cyclopentyl-triazolo-pyrimidine antiplatelet agents. Ticagrelor is a direct acting and reversibly binding P2Y12 antagonist that is able to overcome non-responsiveness to clopidogrel [7,8]. Treatment with ticagrelor, as compared with clopidogrel, has been shown to reduce significantly the rate of death from vascular causes, myocardial infarction or stroke in ACS patients. These beneficial effects were achieved without a significant increase in the rate of overall major bleeding, although an increase in the rate of non-procedure-related bleeding was noted [9]. The reversible binding of ticagrelor and its plasma half-life of 7–8.5 h mandate twice daily dosing (usually 90 mg per dose) [10–13].
Many studies have shown that patients on a once daily dosing regimen take a somewhat higher percentage of prescribed doses than patients on a twice daily dosing regimen [14–17]. However, the pertinent therapeutic question to be addressed is how these missed doses affect the clinical benefits of the drug. One intermediate endpoint that can be assessed is the extent to which drug action, i.e. platelet inhibition, is maintained in the face of occasionally delayed or missed doses during twice daily or once daily dosing. The ability of drugs to maintain therapeutic activity in patients with non-adherent dosing behaviour is referred to as ‘drug forgiveness’, whereby drugs with a duration of action far exceeding their dosing interval are considered more ‘forgiving’ [18].
Assessment of the continuity of platelet inhibition requires an integrated analysis of dosing history data together with the pharmacokinetic (PK) and pharmacodynamic (PD) properties of the drugs being compared [19,20]. Electronic methods for compiling drug dosing histories are now the recognized standard for quantifying adherence [21,22] and they provide a high temporal resolution. When combined with established pharmacometric methods, these dosing histories allow the projection of drug concentration levels and of drug action in the body [23–25].
In this study, we compared the estimated percentage inhibition of platelet aggregation (eIPA) for ticagrelor twice daily and clopidogrel once daily, based on electronically compiled dosing history data and the respective PK/PD properties of these two drugs. This hypothesis-generating analysis of patient adherence patterns and their consequences for antiplatelet therapy thus provides further insight into the consequences of non-adherence with either ticagrelor or clopidogrel. Additionally, our results shed new light on some prejudices regarding once daily and twice daily dosing. Our analyses, together with data from clinical trials assessing the clinical benefits of these drugs, may be informative for healthcare providers in determining the most effective treatments and treatment schedules for their patients.
Methods
Study design
Adherence to medication is based on three elements, initiation, implementation (execution) and discontinuation [26]. A distinction between these three elements can be made using the detailed dosing histories compiled by electronic package entries, allowing a thorough characterization of adherence [24]. In this modelling and simulation study, we focussed on implementation of the dosing regimen, i.e. the extent to which a patient's actual dosing corresponds to the prescribed dosing regimen, from initiation until the last dose taken. We selected the first 30 days of dosing history from anonymized adherence data as a proxy for implementation. These data were used to simulate the eIPA levels in the patients to investigate the effect of implementation and missed doses on drug action.
The anonymized adherence data, in the form of dosing histories of patients prescribed various cardiovascular drugs with either a once daily or twice daily regimen, were extracted from the MWV Adherence Database [22,27]. This database contains dosing history data from almost 19000 patients in 95 clinical studies that have been electronically compiled by a Medication Event Monitoring System (MEMS®, MWV, Sion, Switzerland). This monitoring system automatically registers the date and time of each opening of the medication container through micro-circuitry integrated in the closure of the container [22]. Most of the patients were receiving treatment for hypertension, while some were prescribed anticoagulants or drugs to treat angina, heart failure and hypercholesterolaemia (Supporting information Table S1). The owners of each dataset gave consent for the use of these depersonalized data for retrospective analyses. Each patient gave informed consent regarding the use of the container.
Simulation of eIPA levels
eIPA traces were simulated based on the patient dosing histories from day 1 to day 30. We simulated the final extent IPA, which is determined 6 min after the addition of 20 μm ADP and is mediated primarily by the P2Y12 receptor. For this analysis, all patients were assumed to have taken the loading dose at 09.00 h of day 0 (600 mg for clopidogrel, 180 mg for ticagrelor).
Piecewise linear functions were used to depict onset and offset IPA trajectories. Those functions were empirically derived from two different sources for the onset trajectory. For the eIPA trajectory after the first dose, the onset was based on the IPA data obtained by Gurbel and colleagues during the onset period following the loading dose (600 mg for clopidogrel, 180 mg for ticagrelor) [12]. For the maintenance trajectory, the onset was based on IPA data obtained by Husted and colleagues in patients receiving ticagrelor 100 mg or clopidogrel 75 mg [10]. In both studies, clopidogrel and ticagrelor were given in association with aspirin. The offset IPA trajectory was derived from the above mentioned study by Gurbel and colleagues [12].
Piecewise linear functions were then used to simulate deterministically hourly eIPA trajectories. Since between or within patient variability was not directly available from the published papers, no stochastic modelling approach could be performed. We ensured that the simulated eIPA trajectory did not exceed the maximum IPA described under steady-state conditions (88% for ticagrelor and 62% for clopidogrel) [12].
Statistical methods
The analysis of once daily dosing was based on a randomly selected sample of 677 patients, whereas the twice daily analysis was performed for all available 677 twice daily dosing histories extracted from the database. One-dimensional Monte Carlo simulation through re-sampling of adherence profiles was used to estimate within and between patient variability in eIPA resulting from variable drug exposure. Deterministic onset/offset models for IPA characteristics of ticagrelor twice daily and clopidogrel once daily were taken from the literature.
Descriptive statistics were used to summarize the results of the simulations. Numerical data were summarized with standard statistics (i.e. mean, SD, minimum, median, maximum and lower and upper quartile). Comparisons between the once daily and twice daily groups were tested by two-sided Wilcoxon Rank Sum test at a 5% level of significance.
We also performed a sensitivity analysis by repeating the simulation using the onset characteristics of the maintenance dose as reported by Storey and colleagues for patients on ticagrelor 90 mg [28]. In a second sensitivity analysis, once daily dosing histories were derived from the twice daily dosing histories by deleting the twice daily evening doses and using only the data from the morning doses.
Results
Effect of dosing errors on eIPA levels in twice daily and once daily regimen
By simulating the eIPA trace for ticagrelor twice daily and clopidogrel once daily based on IPA trace slopes described in literature [10,12], we investigated the consequences for the eIPA levels when one or more doses were missed. Missing a single dose of ticagrelor twice daily did not lower the eIPA below the 24 h trough concentrations of clopidogrel once daily (Figure 1). Additionally, missing two sequential ticagrelor twice daily doses resulted in comparable eIPA levels with those observed after missing one dose of clopidogrel once daily (Figure 1). Consequently, the pharmacometrically equivalent dosing error of a single missed clopidogrel once daily dose was two sequentially omitted ticagrelor twice daily doses.
Figure 1.
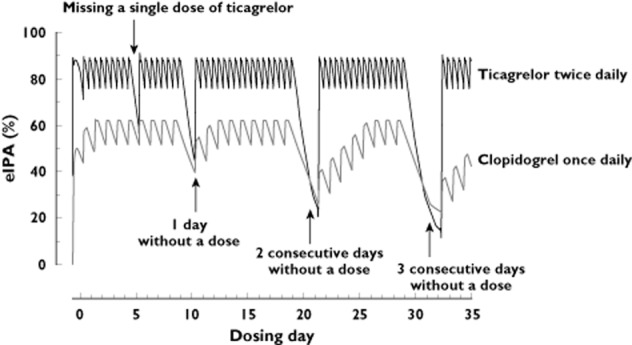
Comparison of projected eIPA levels in the presence of non-adherence.Missing one dose of ticagrelor does not lower the IPA below the 24 trough levels of clopidogrel once daily. Missing two sequential ticagrelor doses results in comparable eIPA levels as seen after missing one clopidogrel dose. eIPA = estimated inhibition of platelet aggregation
Frequency of dosing errors in twice daily and once daily regimen
A total of 5014 dosing histories from patients treated for cardiovascular disease could be retrieved from the MWV Adherence database, among which 677 were prescribed a twice daily regimen and 4337 were prescribed a once daily regimen. Most patients (90% of twice daily and 81.5% of once daily) were receiving treatment for hypertension. A random sample of 677 patients from the once daily regimen group was selected for further analysis.
The frequency of dosing errors was calculated based on the dosing intervals of the selected dosing histories (Figure 2). During month 1 of prescribed once daily dosing, 46.8% of patients missed at least one dose, compared with 75.2% of patients prescribed twice daily dosing. However, only 25.7% of patients on a twice daily dosing regimen missed two consecutive doses, which is the pharmacometrically equivalent dosing error in the ticagrelor twice daily regimen for a single missed dose in the clopidogrel once daily regimen (Figure 2).
Figure 2.
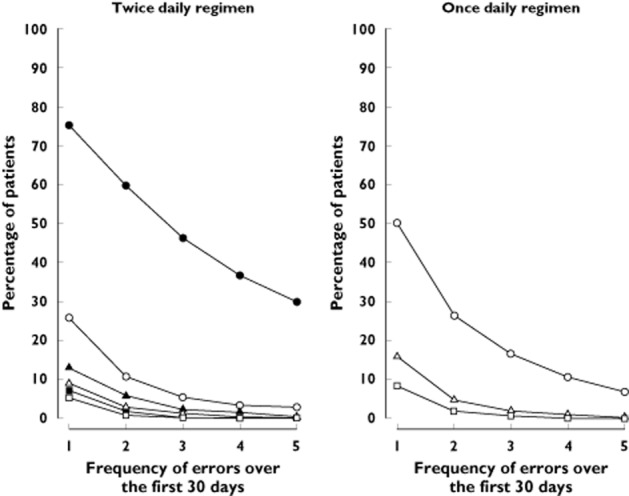
Frequency of dosing errors based on dosing intervals.  , >18 h;
, >18 h;  , >30 h;
, >30 h;  , >42 h;
, >42 h;  , >54 h;
, >54 h;  , >56 h;
, >56 h;  >66 h;
>66 h;  , >78 h. Lines show the frequency at which patients miss one or more twice daily or once daily doses, presented as specified intervals of time between doses (e.g. an interval of >18 h corresponds to missing one twice daily dose; an interval of >30 h corresponds to missing two consecutive twice daily doses or one once daily dose)
, >78 h. Lines show the frequency at which patients miss one or more twice daily or once daily doses, presented as specified intervals of time between doses (e.g. an interval of >18 h corresponds to missing one twice daily dose; an interval of >30 h corresponds to missing two consecutive twice daily doses or one once daily dose)
The random sample of 677 patients from the once daily population had similar dosing error characteristics as the entire group, indicating that this selected sample was representative for the entire population (data not shown).
Simulation of eIPA levels based on twice daily and once daily patient adherence data
The simulations based on patient adherence over time showed that the average mean eIPA for ticagrelor twice daily remained significantly higher than for clopidogrel once daily (81.1% ± 5.9% for twice daily, 55.0% ± 8.1% for once daily, P < 0.001) (Figure 3A). Also the average minimum eIPA values remained higher for ticagrelor twice daily compared with clopidogrel once daily (53.3% ± 16.1% for twice daily, 36.7% ± 11.9% for once daily, P < 0.0001) (Figure 3B).
Figure 3.
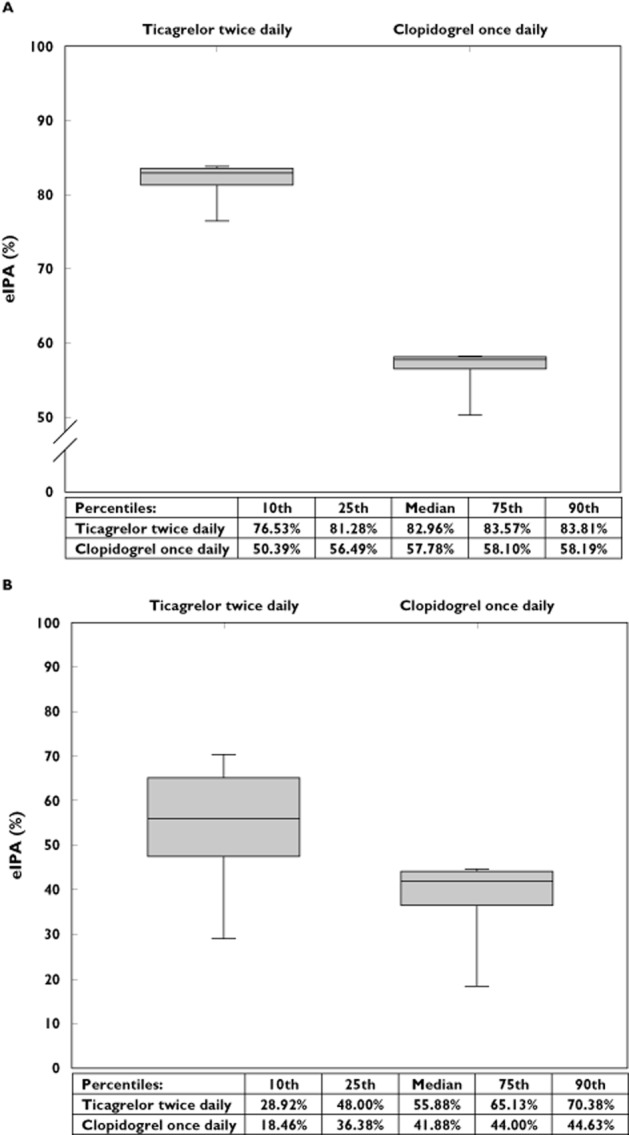
The twice dailyadvantage.(A) Box whisker plot of the mean eIPA between patients for both regimens. The central box represents the 25th to 75th percentile; the middle line represents the median. The ends of the whiskers represent the 10th and 90th percentile of the distribution. (B) The box whisker plot represents the minimum eIPA value of the eIPA trajectory projection over 30 days of each patient. The central box represents the 25th to 75th percentile; the middle line represents the median. The ends of the whiskers represent the 10th and 90th percentile of the distribution. eIPA = estimated inhibition of platelet aggregation
For each patient, we also calculated the percentage of time over the 30 day period for which the eIPA level was below a pre-defined cut-off. For ticagrelor twice daily, time spent below the cut-off was comparable for each cut-off value. For each of the IPA cut-offs, the eIPA level for ticagrelor twice daily was below the cut-off for a significantly shorter amount of time than for clopidogrel once daily. Ticagrelor twice daily patients had an eIPA below 10% for 0.20% of the 30 day period, compared with 2.05% for clopidogrel once daily (P = 0.0001). The mean percentage of time spent below the predefined cut-off increased considerably at the IPA cut-off of 60% for clopidogrel once daily (Figure 4).
Figure 4.
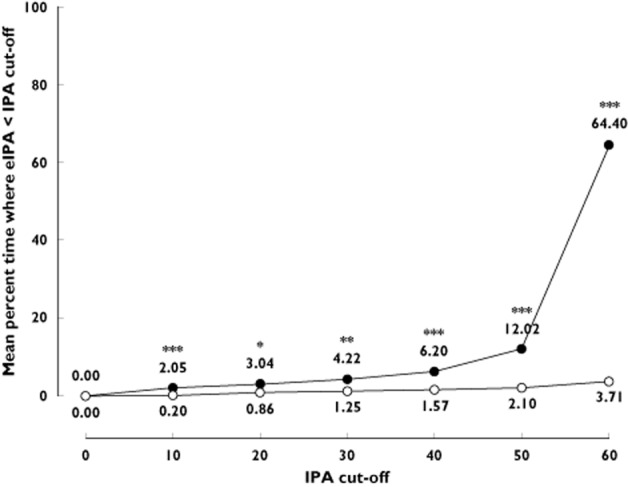
Comparison of percentage of time spent under a certain IPA cut-off.  , clopidogrelonce daily;
, clopidogrelonce daily;  , ticagrelor twice daily. The mean percentage of time below the various cut-offs was fairly stable for ticagrelor twice daily and was always lower than for clopidogrel once daily, which had a considerable increase in time spent below cut-off at 60% IPA. eIPA = estimated inhibition of platelet aggregation. *P = 0.005; **P = 0.0003; ***P ≤ 0.0001
, ticagrelor twice daily. The mean percentage of time below the various cut-offs was fairly stable for ticagrelor twice daily and was always lower than for clopidogrel once daily, which had a considerable increase in time spent below cut-off at 60% IPA. eIPA = estimated inhibition of platelet aggregation. *P = 0.005; **P = 0.0003; ***P ≤ 0.0001
In the literature, hypo-responsiveness to clopidogrel once daily has been defined by a variety of IPA thresholds, ranging from 10% to 40% IPA, with different platelet function assays used to measure these IPA levels [7,29,30]. In our simulations, 97.8% of ticagrelor twice daily patients and 93.9% of clopidogrel once daily patients never had an eIPA level below 10%. No ticagrelor twice daily patients had a simulated eIPA below 10% for more than half of the 30 day period, compared with 1.6% of clopidogrel once daily patients. In addition, 86.9% of ticagrelor twice daily patients and 74.4% of clopidogrel once daily patients never had an eIPA below 40%. The percentage of patients with an eIPA below 40% for more than 50% of the 30 day period was 0.6% for ticagrelor twice daily and 4% for clopidogrel once daily (Figure 5).
Figure 5.
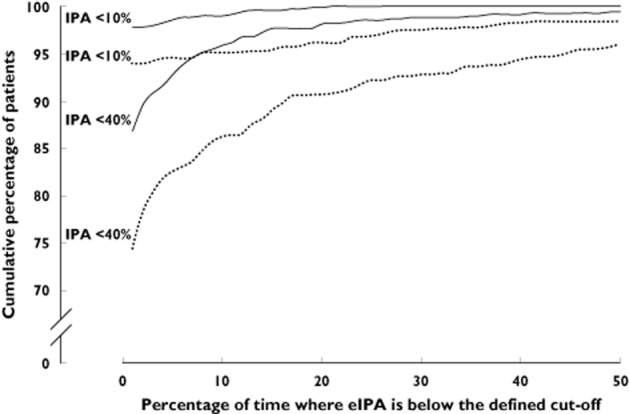
Cumulative percentage of patients with eIPA levels below 10% and below 40% for a defined percentage of time.  , ticagrelor twice daily;
, ticagrelor twice daily;  , clopidogrelonce daily. The cumulative percentages of patients with eIPA levels below 10% and 40% for a defined period of time are shown, for time periods up to 50% of the 30 day period. eIPA = estimated inhibition of platelet aggregation
, clopidogrelonce daily. The cumulative percentages of patients with eIPA levels below 10% and 40% for a defined period of time are shown, for time periods up to 50% of the 30 day period. eIPA = estimated inhibition of platelet aggregation
Sensitivity analysis
We performed two sensitivity analyses. First, we simulated the eIPA trace for ticagrelor based on Storey et al., who studied ticagrelor 90 mg in patients with ACS [28]. This simulation gave a similar trajectory as the trace based on Husted et al. who investigated ticagrelor 100 mg in patients with atherosclerotic disease [10], with only a small difference in onset after a drug holiday (i.e. 3 days or more without a dose).
Secondly, the eIPA trace of clopidogrel once daily was simulated using a twice daily dosing history in which all evening doses were omitted. This allowed us to compare the eIPA traces for ticagrelor twice daily and clopidogrel once daily in the same patient. The once daily dosing history data created by eliminating evening doses from the twice daily dosing history had similar dosing error characteristics as the sampled once daily dosing history (data not shown). Average mean and minimum eIPA values for clopidogrel based on the twice daily dosing history without the evening dose were very similar to the traces based on sampled once daily dosing history data (mean eIPA 54.7% vs. 55.0%, P = 0.06; minimum eIPA 37.3% vs. 36.7%, P = 0.04), and thus remained considerably lower than the ticagrelor twice daily traces (P < 0.0001 for mean and minimum eIPA values) (data not shown).
Discussion
It has been previously shown that patients appear to be more adherent to medication with once daily dosing than with twice daily dosing [14–16]. Consistent with this, the compiled dosing histories of patients prescribed various cardiovascular drugs, extracted from the MWV Adherence Database, showed that a higher percentage of patients treated twice daily than patients treated once daily missed at least one dose during the 30 day period. Such observations have frequently led to the conclusion that once daily dosing is superior to twice daily dosing. However, rather than considering the percentages of prescribed doses taken, these evaluations should be based on pharmacometrically equivalent dosing errors, taking into account the effect of the omitted doses on the pharmacodynamics of the drug. Our simulations of the eIPA levels showed that the pharmacometrically equivalent dosing error of a single missed clopidogrel once daily dose is two sequentially omitted ticagrelor twice daily doses. The higher drug forgiveness of ticagrelor compared with clopidogrel results from a combination of three elements (efficacy, onset and offset). While the offset in IPA is slightly faster with ticagrelor, it has a steady-state IPA of 81% which is much higher than the 55% steady-state level of clopidogrel. Moreover, the much faster onset of ticagrelor once dosing resumes, allows the IPA to normalize faster after a missed dose. This faster restoration of ticagrelor's action contributes to the greater forgiveness of ticagrelor than of clopidogrel.
Additionally, the likelihood of omitting two consecutive twice daily doses was almost half that of missing one once daily dose in the analysed dosing histories. These findings suggest that a ticagrelor twice daily regimen could be superior to a clopidogrel once daily regimen in maintaining antiplatelet activity within a therapeutically desirable range. Indeed, we found that the level of platelet inhibition calculated for patient dosing histories remained higher for ticagrelor twice daily compared with clopidogrel once daily, even though patients more frequently missed a twice daily dose. This can be explained by the higher IPA level achieved with ticagrelor and the relatively low likelihood of missing two consecutive twice daily doses. Also the percentage of time where eIPA is below a certain cut-off was lower for ticagrelor compared with clopidogrel.
The superiority of ticagrelor twice daily to maintain antiplatelet activity within a therapeutically desirable range seems to be in accordance with results from the PLATO trial, which showed a significantly decreased rate of death from vascular causes, myocardial infarction or stroke in patients treated with ticagrelor compared with clopidogrel [9]. Adherence reported for the PLATO study was relatively high (82.8%). However, this was assessed using pill counts and could thus be biased upward, in addition to mixing two elements of non-adherence (poor implementation and early discontinuation). It is thus difficult to assess if and to what extent the difference in drug forgiveness, combined with the low likelihood of missing two consecutive twice daily doses, contributed to the observed difference in clinical outcome, on top of the more intense P2Y12 inhibition that is reached with ticagrelor.
Simulation of both the ticagrelor twice daily and clopidogrel once daily trace in the same patient suggested that the observed differences were not caused by differences between the two patient groups. Additionally, these simulations indicate that ticagrelor twice daily is likely to give a more consistently high IPA level than clopidogrel once daily in patients who are not fully compliant. They also provide insight into the effect on IPA levels when a patient is switched from one drug regimen to another.
Strengths and weaknesses
Although this study was based on real-life patient adherence data, the eIPA levels were simulated and only the first 30 days of dosing history were used for simulation. Nevertheless, this is an appropriate time period to study patient implementation of the two dosing regimens unconfused by treatment discontinuation, which was the scope of this study. We should also take into account that the dosing histories were obtained from patients participating in clinical trials, which may have led to better adherence. In addition, the direct translation of IPA data into clinical outcomes should be interpreted with some caution. Some studies have suggested that a correlation with clinical outcomes exists [31,32]. However, a recent study found no predictive value in aggregation-based assays concerning recurrent ischaemic events in stable cardiovascular patients [33].
ACS is currently the only indication for use of ticagrelor. However, due to the limited number of ACS patients in the MWV database, we also included data from patients with other cardiovascular diseases. Adherence is composed of three elements, initiation, implementation (execution) and discontinuation. While it has been shown that initiation and discontinuation are very different across diseases [22], the implementation is strikingly consistent across diseases for matching dosing regimens [34–36]. Therefore, we are confident that the dosing history data used are meaningful for the ACS patient population, as we limited our analysis to the implementation phase.
Also the IPA trace slopes were based on pharmacodynamic studies in patients with other cardiovascular diseases such as stable coronary artery disease [12] and atherosclerotic disease [10], in addition to ACS patients [28]. Nevertheless, PK/PD findings in ACS seem comparable with those obtained in stable CAD and atherosclerotic patients [37].
In addition to clopidogrel and ticagrelor, the thienopyridine prodrug prasugrel is also used in the treatment of ACS, more specifically in those patients undergoing percutaneous coronary intervention. However, since head-to-head data to perform the eIPA trace simulation were not available, we were unable to include this drug in the analysis.
Our study focused on implementation without taking into account initiation and discontinuation, which are also important parts of drug adherence. In the PLATO trial, premature discontinuation of the study drug was slightly more common in the ticagrelor group than in the clopidogrel group (23.4% of patients vs. 21.5%) [9].
Finally, there are other differences between ticagrelor and clopidogrel, such as potential side effects, that should also be taken into account when determining the most effective therapy for a certain patient.
In coclusion, although patients treated twice daily more frequently miss single doses than patients treated once daily, the simulated levels of platelet inhibition remain consistently higher for ticagrelor twice daily as compared with clopidogrel once daily. The findings of this hypothesis-generating study suggest that ticagrelor twice daily is superior to clopidogrel once daily in terms of pharmacodynamics when taking into account the most common dosing omissions seen in ambulatory patients on chronic cardiovascular medication.
Funding
This study was funded by AstraZeneca.
Competing Interests
Bernard Vrijens is an employee of AARDEX Group, an MWV Healthcare Company. Marc J. Claeys received speakers/consultance fees from Eli Lilly, AstraZeneca and Medicines company. Frans Van de Werf received advisory board and lecture fees from AstraZeneca. Eef Vandendriessche is an employee of AstraZeneca.
The authors wish to thank Paulus Kristanto and John Urquhart from AARDEX Group for technical assistance and analysis support, and Joke Vandewalle and Melissa McNeely (XPE Pharma & Science, on behalf of AstraZeneca) for writing assistance and coordination of manuscript development.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Patient selection from the MWV adherence database
References
- 1.Michelson AD. Advances in antiplatelet therapy. Hematology Am Soc Hematol Educ Program. 2011;2011:62–69. doi: 10.1182/asheducation-2011.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Boden H, van der Hoeven BL, Karalis I, Schalij MJ, Jukema JW. Management of acute coronary syndrome: achievements and goals still to pursue. Novel developments in diagnosis and treatment. J Intern Med. 2012;271:521–536. doi: 10.1111/j.1365-2796.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 3.Menozzi A, Lina D, Conte G, Mantovani F, Ardissino D. Antiplatelet therapy in acute coronary syndromes. Expert Opin Pharmacother. 2012;13:27–42. doi: 10.1517/14656566.2012.642862. [DOI] [PubMed] [Google Scholar]
- 4.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationships among dose, effectiveness, and side effects: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:234S–264. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 5.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Jukema JW, Huisman MV. Clopidogrel nonresponsiveness in patients undergoing percutaneous coronary intervention with stenting: a systematic review and meta-analysis. Am Heart J. 2007;154:221–231. doi: 10.1016/j.ahj.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, Buonamici P, Gensini GF, Vergara R, Abbate R, Antoniucci D. Incidence and clinical impact of dual nonresponsiveness to aspirin and clopidogrel in patients with drug-eluting stents. J Am Coll Cardiol. 2008;52:734–739. doi: 10.1016/j.jacc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 7.Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, Rasmussen L, Storey RF, Nielsen T, Eikelboom JW, Sabe-Affaki G, Husted S, Kereiakes DJ, Henderson D, Patel DV, Tantry US. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–1199. doi: 10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 8.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 9.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 10.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 11.Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y(12) receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–496. doi: 10.1007/s00228-009-0778-5. [DOI] [PubMed] [Google Scholar]
- 12.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 13.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77. doi: 10.1111/j.1365-2125.2010.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22–e33. [PubMed] [Google Scholar]
- 15.Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–680. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- 16.Bae JP, Dobesh PP, Klepser DG, Anderson JD, Zagar AJ, McCollam PL, Tomlin ME. Adherence and dosing frequency of common medications for cardiovascular patients. Am J Manag Care. 2012;18:139–146. [PubMed] [Google Scholar]
- 17.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 18.Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88:457–459. doi: 10.1038/clpt.2010.171. [DOI] [PubMed] [Google Scholar]
- 19.Comte L, Vrijens B, Tousset E, Gerard P, Urquhart J. Estimation of the comparative therapeutic superiority of QD and BID dosing regimens, based on integrated analysis of dosing history data and pharmacokinetics. J Pharmacokinet Pharmacodyn. 2007;34:549–558. doi: 10.1007/s10928-007-9058-0. [DOI] [PubMed] [Google Scholar]
- 20.Hughes D. Less is more: medicines that require less frequent administration improve adherence, but are they better? Pharmacoeconomics. 2006;24:211–213. doi: 10.2165/00019053-200624030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21:1074–1090. doi: 10.1016/S0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 22.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 23.Rubio A, Cox C, Weintraub M. Prediction of diltiazem plasma concentration curves from limited measurements using compliance data. Clin Pharmacokinet. 1992;22:238–246. doi: 10.2165/00003088-199222030-00006. [DOI] [PubMed] [Google Scholar]
- 24.Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol. 2005;45:461–467. doi: 10.1177/0091270004274433. [DOI] [PubMed] [Google Scholar]
- 25.Savic RM, Barrail-Tran A, Duval X, Nembot G, Panhard X, Descamps D, Verstuyft C, Vrijens B, Taburet AM, Goujard C, Mentre F. Effect of adherence as measured by MEMS, ritonavir boosting, and CYP3A5 genotype on atazanavir pharmacokinetics in treatment-naive HIV-infected patients. Clin Pharmacol Ther. 2012;92:575–583. doi: 10.1038/clpt.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vrijens B, De GS, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, Dobbels F, Fargher E, Morrison V, Lewek P, Matyjaszczyk M, Mshelia C, Clyne W, Aronson JK, Urquhart J. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P, Cannon CP. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–1856. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 29.Jernberg T, Payne CD, Winters KJ, Darstein C, Brandt JT, Jakubowski JA, Naganuma H, Siegbahn A, Wallentin L. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166–1173. doi: 10.1093/eurheartj/ehi877. [DOI] [PubMed] [Google Scholar]
- 30.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Barrera-Ramirez C, Sabate M, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005;115:101–108. doi: 10.1016/j.thromres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Jennings LK. Variability in platelet response to the antiplatelet agents aspirin and clopidogrel: mechanisms, measurement, and clinical relevance. Crit Pathw Cardiol. 2009;8:20–28. doi: 10.1097/HPC.0b013e318194e45e. [DOI] [PubMed] [Google Scholar]
- 32.Sweeny JM, Gorog DA, Fuster V. Antiplatelet drug ‘resistance’. Part 1: mechanisms and clinical measurements. Nat Rev Cardiol. 2009;6:273–282. doi: 10.1038/nrcardio.2009.10. [DOI] [PubMed] [Google Scholar]
- 33.Reny JL, Berdague P, Poncet A, Barazer I, Nolli S, Fabbro-Peray P, Schved JF, Bounameaux H, Mach F, de Moerloose P, Fontana P. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: results of the Antiplatelet Drug Resistances and Ischemic Events study. Circulation. 2012;125:3201–3210. doi: 10.1161/CIRCULATIONAHA.111.085464. [DOI] [PubMed] [Google Scholar]
- 34.Parienti JJ, Barrail-Tran A, Duval X, Nembot G, Descamps D, Vigan M, Vrijens B, Panhard X, Taburet AM, Mentre F, Goujard C. Adherence profiles and therapeutic responses of treatment-naive HIV-infected patients starting boosted atazanavir-based therapy in the ANRS 134-COPHAR 3 trial. Antimicrob Agents Chemother. 2013;57:2265–2271. doi: 10.1128/AAC.02605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruse W, Weber E. Dynamics of drug regimen compliance–its assessment by microprocessor-based monitoring. Eur J Clin Pharmacol. 1990;38:561–565. doi: 10.1007/BF00278582. [DOI] [PubMed] [Google Scholar]
- 36.Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple anti-epileptic medications during a controlled clinical trial. Epilepsia. 1995;36:1111–1117. doi: 10.1111/j.1528-1157.1995.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 37.Husted SE, Storey RF, Bliden K, Tantry US, Hoimark L, Butler K, Wei C, Teng R, Gurbel PA. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET-OFFSET and RESPOND studies. Clin Pharmacokinet. 2012;51:397–409. doi: 10.2165/11599830-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient selection from the MWV adherence database


