Abstract
AIMS
To assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of intranasal SB-705498, a selective TRPV1 antagonist.
METHODS
Two randomized, double-blind, placebo-controlled, clinical studies were performed: (i) an intranasal SB-705498 first time in human study to examine the safety and PK of five single escalating doses from 0.5 to 12 mg and of repeat dosing with 6 mg and 12 mg twice daily for 14 days and (ii) a PD efficacy study in subjects with non-allergic rhinitis (NAR) to evaluate the effect of 12 mg intranasal SB-705498 against nasal capsaicin challenge.
RESULTS
Single and repeat dosing with intranasal SB-705498 was safe and well tolerated. The overall frequency of adverse events was similar for SB-705498 and placebo and no dose-dependent increase was observed. Administration of SB-705498 resulted in less than dose proportional AUC(0,12 h) and Cmax, while repeat dosing from day 1 to day 14 led to its accumulation. SB-705498 receptor occupancy in nasal tissue was estimated to be high (>80%). Administration of 12 mg SB-705498 to patients with NAR induced a marked reduction in total symptom scores triggered by nasal capsaicin challenge. Inhibition of rhinorrhoea, nasal congestion and burning sensation was associated with 2-to 4-fold shift in capsaicin potency.
CONCLUSIONS
Intranasal SB-705498 has an appropriate safety and PK profile for development in humans and achieves clinically relevant attenuation of capsaicin-provoked rhinitis symptoms in patients with NAR. The potential impact intranasal SB-705498 may have in rhinitis treatment deserves further evaluation.
Keywords: capsaicin challenge, intranasal SB-705498, nasal hyper-responsiveness, rhinitis, TRPV1
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Transient receptor potential vanilloid 1 (TRPV1) is an ion channel expressed on peripheral nerves, activated by several physical, chemical and biological factors.
In the nose, overstimulation of TRPV1-expressing sensory nerves may lead to nasal hyper-reactivity and development of rhinitis symptoms in sensitive individuals.
Targeting TRPV1 may offer the potential to control medical conditions characterized by sensory neuronal hyper-responsiveness, including the nasal hyper-reactivity that underlies non-allergic rhinitis (NAR).
WHAT THIS STUDY ADDS
This study provides an insight on the early clinical evaluation of SB-705498, a selective TRPV1 antagonist, as a potential intranasal therapeutic modality for NAR.
The safety and pharmacokinetic (PK) profile of intranasal SB-705498 was established in humans and an agonist-antagonist intranasal dose–response capsaicin challenge was initiated to provide evidence of pharmacology in the target patient population.
Intranasal SB-705498 could be developed as a potential new blocker of nasal hyper-reactivity in rhinitis patients.
Introduction
Rhinitis is a common condition that affects up to 30% of adults and 40% of children and poses a significant economic burden to the world population [1–3]. A regular pathophysiological feature of rhinitis is nasal hyper-responsiveness. Nasal hyper-responsiveness is characterized by exaggerated nasal sensory and reflexogenic responses to environmental factors, such as weather changes, household chemicals, pollution or strong odours, that result in generation of symptoms of rhinorrhoea, nasal congestion, sneezing and itch [4,5]. Nasal hyper-responsiveness is often a consequence of allergic inflammation, as allergic mediators affect directly the sensory nasal nerves and reduce their threshold potential for activation [4]. Nasal hyper-responsiveness is also the cardinal feature of non-allergic rhinitis (NAR), a disease entity thought to be driven by over-reactivity of nasal sensory nerves or over-interpretation of normally transmitted signals by the CNS response centres [6]. Blocking the sensory neuronal pathways involved in nasal hyper-responsiveness has been proposed as a novel form of treatment for difficult to treat rhinitis patients. This concept is supported by clinical data demonstrating that cold dry air or hypertonic saline triggered nasal hyper-responsiveness is inhibited after anaesthetization or desensitization of the nasal sensory fibres [4,7].
TRPV1 (also known as VR1, vanilloid/capsaicin receptor, OTRPC1) [8] is a sensory nerve receptor, a member of a super-family of structurally related transmembrane ion channels, known to serve a multitude of cellular roles, including many facets of sensory transduction [9,10]. TRPV1 is activated by several physiological stimuli including capsaicin, heat, low pH, osmotic stress and by endogenous inflammatory mediators, such as histamine, prostaglandins and lipoxygenases [9,11]. TRPV1 is expressed on afferent sensory nerves, particularly on non-myelinated C-fibre nociceptors, and is present in the airways. In the upper airways expression of TRPV1 has been confirmed on the trigeminal sensory neurones that innervate the epithelium and subepithelium of nasal mucosa [12]. The expression of TRPV1 has been found enhanced in chronic inflammation [13]. Several lines of experimental evidence indicate that TRPV1 is a key player in the excitability of airway sensory neurons and TRPV1 sensitive nerves have been shown to contribute in the development of lower and upper airway hyper-responsiveness, bronchoconstriction and cough [14,15]. This suggests that TRPV1 may be a primary target for pharmacological intervention for a range of respiratory disorders.
To assess if TRPV1 antagonism can prevent nasal hyper-responsiveness and therefore become a promising therapeutic modality for NAR, SB-705498, a potent and selective TRPV1 antagonist [16,17], has been developed for intranasal administration. SB-705498 is a known inhibitor of the multiple modes of TRPV1 activation. Treatment with oral SB-705498 has been applied successfully in models of neuropathic and inflammatory pain [18]. In a pre-clinical rhinitis model, intranasal as well as oral administration of SB-705498 has been shown to block the capsaicin-evoked nasal secretions [19]. In this rhinitis model 10-fold lower doses of intranasal SB-705498 were required to achieve the same efficacy as the orally dosed compound. Furthermore, intranasal SB-705498 has a good safety and pre-clinical toxicology profile that allowed initiation of clinical studies.
In this article we describe a first time in human (FTIH) study to characterize the safety and PK profiles of intranasal SB-705498 in healthy volunteers and a pharmacodynamics (PD) study to evaluate its effects against capsaicin-provoked nasal reactivity in subjects with NAR.
Methods
The FTIH study to determine the safety, tolerability and PK of single and repeat dosing with intranasal SB-705498 in healthy volunteers was conducted at Hammersmith Medicines Research, London, UK (GlaxoSmithKline protocol: VR1111610; clintrials.gov: NCT00907933). The PD study to evaluate the effect of intranasal SB-705498 on capsaicin-evoked nasal reactivity in patients with NAR was conducted at Academisch Medisch Centrum, Amsterdam, the Netherlands (GlaxoSmithKline protocol: VR1111925; clintrials.gov: NCT01439308).
Clinical study populations
FTIH study
Non-smoking male and female healthy volunteers aged 18–60 years with no previous history of nasal disorders were included in the study. A total of 14 and 30 healthy volunteers participated in the single and repeat dose arm of the study respectively.
PD study
Forty-one male and female, non-smoking NAR patients aged 18–55 years with no other concomitant disorders participated in the study. All patients had a diagnosis of NAR >1 year, as determined by the presence of perennial rhinitis symptoms triggered by environmental provocateurs (i.e. weather changes, irritants, air pollution etc.) for at least 9 months of the year. All NAR patients were required to have normal levels of total plasma IgE and negative allergen-specific skin or serum IgE tests.
All study participants provided written, informed consent to participate in the studies. Local Ethics Committees provided formal approval for the studies which were conducted in accordance with all known regulatory requirements and the guiding principles of the Declaration of Helsinki [20].
Clinical study designs
FTIH study
This study had two-arms, First the safety, tolerability and PK of five single ascending doses of intranasal SB-705498 (0.5, 1.5, 3, 6, and 12 mg) were evaluated in healthy volunteers following a randomized, double-blind, placebo-controlled, five period, incomplete block crossover design. The incomplete block design was intended to make the study more manageable for participants and more time efficient. Successfully screened subjects were randomized to receive a single intranasal dose of either SB-705498 or placebo in each treatment period, and over the course of the study each subject received five out of the six possible treatments, each once only. In each treatment period subjects attended the unit approximately 24 h before dosing and remained resident for 24 h post-dosing. A follow-up phone call was made 7 to 10 days later. At the end of each treatment period all available blinded safety and PK data were reviewed to allow dose escalation. Treatment periods were separated by a 7 day washout.
After the safety of single dosing was established a randomized, double-blind, placebo-controlled, parallel group study was initiated to evaluate the safety, tolerability and PK of 6 and 12 mg of intranasal SB-705498 administered twice daily for 14 days. New healthy volunteers were recruited for this arm of the study. Eligible subjects attended the study 24 h before the first dosing (day −1) and remained resident for approximately 36 h post-dosing. Subjects left the unit with a diary card and sufficient study medication to continue self-administration until day 7, when they returned to the unit for review of diary information and adherence and to get new medication supplies to continue self-administration until day 13. On day 13 subjects returned to the unit again and remained resident for approximately 48 h after their last dosing for assessments and collection of PK samples. A follow-up phone call was made 7–10 days after the final dose.
PD study
This was a randomized, double-blind, placebo-controlled, parallel group study to evaluate the effect of a single intranasal administration of 12 mg SB-705498 on capsaicin-evoked nasal reactivity in patients with NAR. PK and safety were also assessed. Subjects with NAR went through an initial screening to assess their eligibility for enrolment in the study and confirm their responsiveness to a single, unilateral, intranasal challenge with 50 μg capsaicin. Only subjects who developed a total symptom score (TSS) ≥3 in response to the capsaicin challenge entered the treatment phase of the study. On the dosing day 1 h after administration of SB-705498 or placebo, all patients underwent a baseline unilateral, intranasal vehicle control challenge and subsequently received three unilateral, intranasal challenges with incremental doses of capsaicin (2.5 μg, 12.5 μg and 50 μg). Clinical symptoms were assessed and nasal secretions were collected after each challenge. Patients were followed-up by telephone 48 h after treatment.
Justification of dose selection
The selection of doses for the FTIH and PD studies was based on the estimated SB-705498 dose–response from a guinea pig rhinitis PD model over the oral dose range 3 to 30 mg kg−1 [19]. The estimated ED50 parameter (10 mg kg−1) in the guinea pig was converted to the corresponding drug levels of 1.4 μg ml−1 (EC50) in the nasal turbinates using clearance data obtained in guinea pigs after intravenous and intra-nasal dosing and blood to nasal tissue partition (in house data). The EC50 value in the guinea pig turbinates was then scaled to corresponding equivalent drug levels (EC50) in human turbinates based on the known human clearance parameter of the drug and free drug fraction and assuming similar blood to nasal tissue partition as in the guinea pig. As described below, an estimation of a total intranasal dose of 12 mg (6 mg/nostril) would be required in humans to achieve intranasal concentrations corresponding to those found to be effective in the guinea pig. It was predicted that the intranasal dose of SB-705498 12 mg (6 mg in each nostril) would lead to high receptor occupancy (∼ >80%) at the target nasal tissues. This estimate took into account the binding affinity of SB-705498 at the human TRPV1 receptor (pKb = 7.5 in house data), an assumed volume of human nasal tissue of 20 ml [21] and an intranasal bioavailability of the drug of approximately 20% after intranasal dosing. The intranasal bioavailability in humans was approximated from preclinical data in the dog where the drug was given intranasally with and without charcoal (unpublished data on file, GlaxoSmithKline, Stevenage, UK). The charcoal block test is a standard and useful method to estimate the tissue bioavailability following topical administration (such as inhaled or intranasal route) of the drug compared with oral administration. Co-administration of charcoal with the drug prevents its oral absorption resulting in minimal systemic availability of the administered drug. The predicted nasal tissue and systemic exposure in humans with doses of intranasal SB-705498 up to 12 mg were within the safety margins derived from preclinical safety studies.
Safety assessments
Adverse events were recorded throughout the FTIH and PD studies. The investigator graded adverse event intensity (mild, moderate or severe) and relationship with study drug. Body temperature, vital signs, 12-lead electrocardiogram (ECG), nasal tolerability (nasal symptom scoring by visual analogue scale [VAS], nasal endoscopy (performed in the FTIH only) and visual nasal examination were evaluated at several time points post-dosing. Furthermore, there was repeat assessment of laboratory safety parameters, including plasma progesterone, adrenocorticotrophic hormone and cortisol concentrations (because administration of SB-705498 to rat or dog at high doses above the no adverse effect level (NOAEL) has been associated with vacuolation and hypertrophy of some hormone producing organs, most notably the adrenal cortex, but also the ovaries and testes).
PK assessment
In the FTIH, blood samples were collected for PK analysis pre-dose, 15 and 30 min and 1, 2, 4, 8, 12 and 24 h post each single dosing. In the 14 days repeat dosing arm, samples were taken at the same times relative to dosing on day 1 and day 14, and additional samples were taken 36 and 48 h after the last dose received on day 14. In the PD study blood samples were collected pre-dose, 30 min and 1, 2, 3 and 4 h post-dosing. Plasma was analyzed for parent drug by high performance liquid chromatography/tandem mass spectrometry using a TurboIonspray interface and multiple reaction monitoring [22,23]. The method had a lower limit of quantification (LLQ) of 2.5 ng ml−1 using a 50 μl aliquot of human plasma over a linear calibration range of 2.5 to 2000 ng ml−1. Quality control (QC) samples, prepared at three different analyte concentrations and stored with study samples, were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one-third of the QC results were to deviate from the nominal concentration by more than 15% and at least 50% of the results from each QC concentration were to be within 15% of nominal. All applicable analytical runs met all predefined run acceptance criteria.
SB-705498 PK parameters were derived from the initial time–concentration data by standard non-compartmental analysis using WinNonLin Pro (Version 4.1; Pharsight Products, Cary, NC, USA). The following parameters were assessed: AUC(0,t), AUC from time 0 to 4 h, from time 0 to 12 h and from time 0 to 24 h post-dose (AUC(0,4 h), AUC(0,12 h), AUC(0,24 h)), maximum observed plasma concentration (Cmax) and time to maximum observed plasma concentration (tmax).
PD assessments
Intranasal capsaicin challenge
In the PD study the nasal response to unilateral, intranasal capsaicin challenge was assessed and the effect of prior treatment with intranasal SB-705498 vs. placebo analyzed. In brief, patients blew their nose to clear any secretions and both nostrils were then washed 20 times in 1 min with 0.9% saline (10 ml). The lavage fluid was discarded and the nostrils were dried. Initially, a baseline assessment of the response to a unilateral intranasal vehicle control challenge was made by spraying saline into the right nostril using a metered pump device (25 μl or 50 μl per actuation). Subsequently the response to capsaicin challenge was evaluated by spraying a single (at screening) or incremental capsaicin doses (2.5 μg, 12.5 μg and 50 μg) into the right nostril using a metered pump device. The number of actuations was determined by the dose of capsaicin required. Challenges with saline or each dose of capsaicin were separated by an interval of 20 min during which a series of assessments were made.
At 1, 5, and 9 min after each challenge, patients were asked to grade the intensity of symptoms of burning sensation, rhinorrhoea, lacrimation and nasal congestion as follows: 0 = none; 1 = mild; 2 = moderate and 3 = severe. The individual scores were summed to produce a TSS. Patients also completed a 10 cm long VAS for nasal congestion, rhinorrhoea, lacrimation and burning sensation.
Peak nasal inspiratory flow (PNIF) was measured using an InCheck PNIF meter (Clement Clarke International Ltd, Harlow, United Kingdom) 15 min after each challenge. Three inspiratory efforts were made and the highest measure was recorded.
Statistical analysis
FTIH study
Sample sizes were based on logistic feasibility. In the single dose arm dose proportionality using Cmax and AUC was assessed using a power model and analysis of variance (anova). In the repeat dose arm, a statistical analysis was performed on AUC(0,12 h) and Cmax (after morning dosing) to evaluate the accumulation ratio. A mixed effect model was fitted with dose (categorical variable), day and dose by day interaction as fixed effects and repeated measures analysis was carried out on day using subject as a blocking effect. Day 14 was compared with day 1 in order to estimate the accumulation ratio for each treatment group.
PD study
An unblinded adaptive sample size re-estimation was planned for when 20 patients had completed the study to determine whether to terminate the study for futility or efficacy. Due to a high rate of recruitment, it was conducted after 37 patients had been dosed but the analysis used data from only 20 patients. Following the interim analysis the planned number of patients (n = 40) were subsequently recruited. Treatment differences and ratios (SB-705498 12 mg vs. placebo) of adjusted means were analyzed for TSS and nasal secretion weights using a repeated measures anova. A Bayesian analysis was conducted to derive the posterior probability distributions for total nasal secretion weights, mean TSS and average VAS measures for nasal congestion, rhinorrhoea, lacrimation and burning sensation. The probabilities were derived using a mixed effects model (fitted for the frequentist analysis). However, a Student's t cumulative distribution function was used to obtain the probabilistic statements, assuming a non-informative prior. The difference between SB-705498 12 mg and placebo for change from baseline in PNIF was analyzed using a repeated measures anova.
Dose ratio analysis
A quantitative approach was performed in the PD study to evaluate the effect of single dose SB-705498 (antagonist) in the presence of incremental challenge with capsaicin (agonist) to estimate the shift in dose–response. Clinical endpoints corrected for saline baseline were evaluated including average TSS, components of TSS (nasal congestion, lacrimation, burning sensation, and rhinorrhoea), VAS scores for individual components (nasal congestion, lacrimation, burning sensation, rhinorrhoea) and PNIF. The standard parallel line assay method [24] was applied to each of the clinical endpoints. With this method, an overall anova is carried out and tests of significance performed on the regression slope, linearity of dose–response and evidence of parallelism. For each clinical endpoint, the dose–response was compared only for the agonist and in the presence of the drug (antagonist). This comparison was done by estimation of the potency ratio (with associated 95% confidence intervals [CIs]), which corresponds to the inverse of the ratio for the doses that produce equivalent responses in the two treatment groups for each endpoint. This analysis was performed using PLA Version 2.0 software (Stegmann Systems, Rodgan, Germany) for parallel line and parallel logistics assays. This software includes a suite of transformation functions for the response variables to account for any heteroscedasticity. Individual datasets for each clinical endpoint for both studies were fitted to the appropriate model with a detailed statistical output of the overall dose ratio analysis. Dose ratio estimates for each clinical endpoint and associated 95% CIs are graphically presented.
Results
Participants
FTIH study
Fourteen healthy volunteers (HVT) with mean age 32.9 (23–52) years and thirty HVT with mean age 28.5 (21–48) years were randomized in the single and repeat dose arms of the study respectively. All subjects completed the study. The populations were predominantly Caucasian (11 subjects [79%] in the single dose arm and 24 subjects [80%] in the repeat dose arm) and male (11 subjects [79%] and 22 subjects [73%], respectively).
PD study
Forty-one patients (26 females and 15 males) were randomized (SB-705498 12 mg: 19 patients; placebo: 22 patients). All completed, except one patient who received SB-705498 12 mg and withdrew because of an adverse event (intermittent hypertension). Mean (range) ages were 40.1 (19–57) years in the SB-705498 group and 34.0 (18–55) years in the placebo group.
Safety and tolerability results
FTIH study
Single and repeat dosing with intranasal SB-705498 was well tolerated at all dose levels tested. No serious adverse events were reported in the study and no dose relationship in the incidence of adverse events was observed. Subjects who received single administration of 1.5 mg intranasal SB-705498 presented a slightly greater incidence of drug-related adverse events. However, this finding was not repeated at higher doses and therefore it was not considered clinically relevant. All adverse events were transient and of mild/moderate intensity. The most frequently reported adverse events were headache and oropharyngeal pain (Table 1). Clinically significant changes for vital signs, cardiac monitoring, body temperature, and standard haematology and biochemistry tests (including plasma progesterone, adrenocorticotrophic hormone and cortisol concentrations) were not detected. Furthermore, there were no consistent or dose dependent signs of nasal irritancy with clinical significance, as assessed by individual scoring of nasal symptoms, nasal endoscopy and visual nasal examination, after either single or repeat intranasal SB-705498 administration.
Table 1.
Summary of adverse events reported by more than one subject in the FTIH study
| Adverse event | Part 1 | Part 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo | SB-705498 | Placebo | SB-705498 | ||||||
| 0.5 mg | 1.5 mg | 3 mg | 6 mg | 12 mg | 6 mg | 12 mg | |||
| n = 11 | n = 12 | n = 12 | n = 11 | n = 12 | n = 12 | n = 10 | n = 10 | n = 10 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Any event | 2 (18) | 1 (8) | 5 (42) | 5 (45) | 4 (33) | 2 (17) | 5 (50) | 4 (40) | 3 (30) |
| Any event judged drug-related* | 1 (9) | 0 | 2 (17) | 1 (9) | 0 | 1 (8) | 5 (50) | 4 (40) | 3 (30) |
| Headache | 2 (18) | 1 (8) | 1 (8) | 2 (18) | 0 | 0 | 3 (30) | 1 (10) | 2 (20) |
| Oropharyngeal pain | 0 | 0 | 3 (25) | 0 | 0 | 1 (8) | 1 (10) | 1 (10) | 1 (10) |
| Upper respiratory tract infection | 0 | 0 | 0 | 2 (18) | 1 (8) | 0 | 0 | 1 (10) | 2 (20) |
| Abdominal pain | 0 | 1 (8) | 0 | 1 (9) | 0 | 0 | 0 | 0 | 0 |
| Nasal congestion | 0 | 0 | 0 | 1 (9) | 1 (8) | 0 | 0 | 0 | 0 |
Assesments of relationship were made prior to unblinding and, therefore, events could be judged related to placebo.
PD study
Administration of intranasal SB-705498 was well tolerated by NAR patients. Twenty-two patients reported adverse events: 11 (58%) had received SB-705498 and 11 (50%) had received placebo (Table 2). The most frequently reported event was cough, which was reported by five (26%) patients who received SB-705498 compared with two (9%) patients who received placebo. No serious adverse events were reported. One patient was withdrawn from the study because of intermittent hypertension of mild intensity that occurred 26 min after dosing with SB-705498 12 mg. The hypertension resolved approximately 2 h later. No clinically significant abnormalities in vital signs, 12-lead ECG, body temperature, nasal examination or clinical laboratory tests were observed. Nasal capsaicin challenge did not appear to cause other events than the expected nasal reactivity.
Table 2.
Adverse events reported by more than one patient in the PD study
| Adverse event | Placebo | SB-705498 12 mg |
|---|---|---|
| n = 22 | n = 19 | |
| n (%) | n (%) | |
| Any event | 11 (50) | 11 (58) |
| Cough | 2 (9) | 5 (26) |
| Headache | 3 (14) | 3 (16) |
| Fatigue | 3 (14) | 2 (11) |
| Sneezing | 3 (14) | 1 (5) |
| Throat irritation | 2 (9) | 1 (5) |
| Feeling cold | 0 | 2 (11) |
| Lacrimation increased | 1 (5) | 1 (5) |
| Nausea | 2 (9) | 0 |
| Upper airway obstruction | 2 (9) | 0 |
Pharmacokinetic results
FTIH study
Following intranasal administration, SB-705498 was fairly rapidly absorbed in HVT achieving maximum plasma concentration at 1–2 h post-dose (Table 3) and had slow distribution and elimination. This rate of absorption remained largely unaffected after repeat administration (Table 4) with plasma concentrations of SB-705498 declining slowly (Figure 1). Repeat administration led to higher plasma concentrations of SB-705498 compared with those achieved after single intranasal dosing (Figure 1). Generally, SB-705498 systemic exposure increased with dose escalation from 0.5 mg to 12 mg. However, the results of the power model suggest that the increase in systemic exposure was less than dose proportional in terms of AUC(0,12 h) (slope: 0.753 ng ml−1 h mg−1, 90% CI 0.644, 0.862) and Cmax (slope: 0.826 ng ml−1 mg−1, 90% CI 0.730, 0.921). Repeat intranasal administration of 6 and 12 mg SB-705498 for 14 days was associated with systemic drug accumulation. Values of AUC(0,24 h) and Cmax increased 2–3-fold on day 14 compared with day 1.
Table 3.
Derived PK parameters [geometric mean (95% confidence interval)] after single dosing in the FTIH study
| Part 1 | |||||
|---|---|---|---|---|---|
| SB-705498 dose | |||||
| 0.5 mg | 1.5 mg | 3 mg | 6 mg | 12 mg | |
| n = 12 | n = 12 | n = 11 | n = 12 | n = 12 | |
| AUC(0,t) (ng ml−1 h) | NC | 152.8 (121.0, 193.0) | 249.8 (100.7, 619.4) | 440.6 (346.2, 560.9) | 903.6 (617.8, 321.7) |
| Cmax (ng ml−1) | 4.7 (3.3, 6.6) | 22.2 (18.2, 27.1) | 33.1 (20.1, 54.7) | 43.0 (33.5, 55.2) | 86.4 (64.4, 115.9) |
| tmax (h)* | 1.0 [0.5, 2.0] | 1.0 [1.0, 8.0] | 2.0 (0.25, 4.02) | 2.0 (1.00, 4.00) | 2.0 (1.00, 8.00) |
Presented as median [range]. AUC(0,t), area under the plasma concentration–time curve from time zero to time t; Cmax, maximum plasma concentration; NC, Non-calculable due to non-quantifiable concentrations; tmax, time to Cmax.
Table 4.
Derived PK parameters [geometric mean (95% confidence interval)] after repeat dosing in the FTIH study
| Part 2 | ||||
|---|---|---|---|---|
| SB-705498 6 mg | SB-705498 12 mg | |||
| Day 1 a.m. | Day 14 a.m. | Day 1 a.m. | Day 14 a.m. | |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| AUC(0,24 h) (ng ml−1 h) | 634.5 (307.8, 1308.0) | 1522.1 (628.3., 3687.4) | 1601.3 (1296.7, 1977.5) | 3416.3 (2280.0, 5118.8) |
| Cmax (ng ml−1) | 38.2 (15.6, 93.4) | 90.3 (39.5, 206.4) | 102.1 (76.1, 137.0) | 196.3 (125.3, 307.7) |
| tmax (h)1 | 2.0 (NC, 4.1) | 2.0 (0.5, 4.0) | 2.0 (0.5, 4.03) | 3.0 (0.3, 12.0) |
Presented as median (range) AUC(0,24 h), area under the plasma concentration–time curve from time zero to 24 h; Cmax, maximum plasma concentration; NC, Non-calculable due to non-quantifiable concentrations; tmax, time to Cmax.
Figure 1.
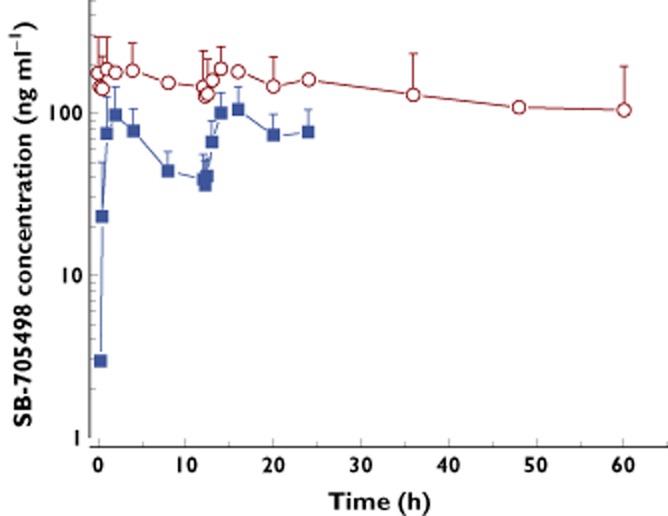
Mean (SD) plasma concentrations of SB-705498 after twice daily intranasal dosing on day 1 and day 14.  day 1 12 mg twice daily;
day 1 12 mg twice daily;  , day 14 12 mg twice daily
, day 14 12 mg twice daily
PD study
PK analysis of blood samples from NAR patients confirmed that maximum plasma concentrations were achieved at 3 h post-dose. Geometric mean Cmax was 77.7 ng ml−1 (95% CI 54.1, 111.5) and AUC(0,t) was 140.5 ng ml−1 h (95% CI 91.5, 215.9).
Pharmacodynamic results
PD study
Administration of a single dose of 12 mg intranasal SB-705498 to patients with NAR prior to intranasal challenge with capsaicin resulted in a decrease of the capsaicin-provoked symptoms, including burning sensation, rhinorrhoea, nasal congestion and lacrimation (Figure 2A). At baseline, following challenge with saline control, both SB-705498 and placebo groups reported similar symptoms. The TSS adjusted mean (95% CI) values were 1.45 (1.03, 1.86) for the placebo and 1.21 (0.75, 1.68) for the SB-705498 group. However, the TSS induced by all doses of capsaicin were markedly reduced in the SB-705498 treated group compared with placebo. Specifically, after challenge with 2.5 μg capsaicin, the adjusted mean (95% CI) values of TSS were 4.16 (3.35, 4.97) in the placebo and 2.97 (2.08, 3.87) in the SB-705498 group. After challenge with 12.5 μg capsaicin, the TSS values were 6.01 (5.10, 6.93) and 4.76 (3.74, 5.78), respectively, and after challenge with 50 μg capsaicin, the TSS values were 7.14 (6.04, 8.24) and 5.90 (4.969, 7.12) respectively. Bayesian analyses of mean TSS concluded that the probability that SB-705498 treatment led to some inhibition of challenge response (>0%) compared with placebo was P > 0.9 for all capsaicin doses.
Figure 2.
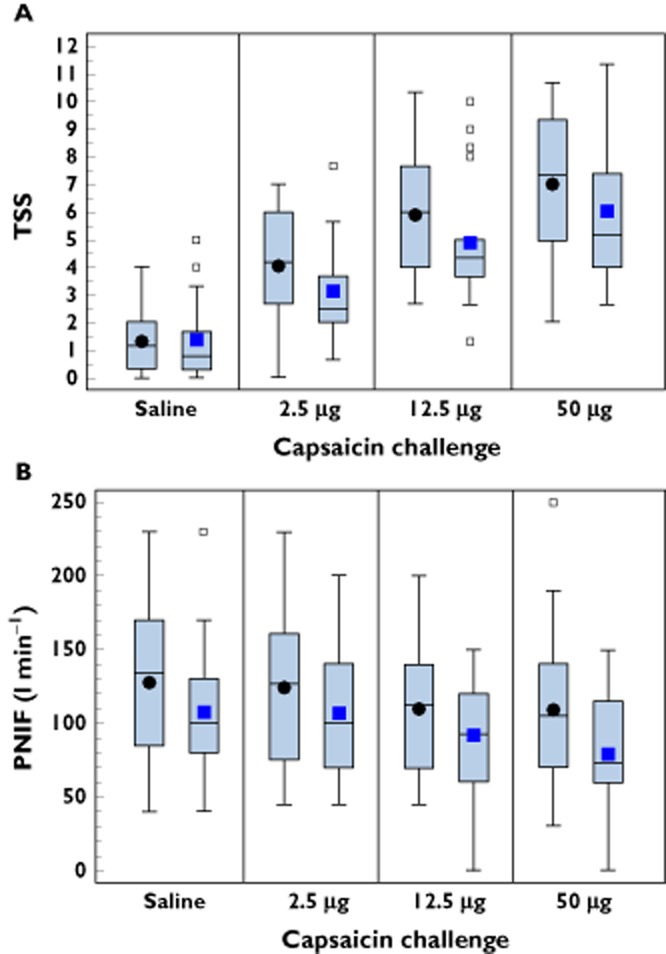
(A) Boxplots showing the effect of treatment with intranasal SB-705498 or placebo on total symptom scores (TSS) in patients with NAR. Square and circle symbols represent mean, horizontal line within the box represents median, lower and upper edges of box represent the 25th and 75th values percentiles, respectively. The 5th and 95th percentiles, not shown graphically, were, respectively,: SB-705498 12 mg: saline 0 and 5; 2.5 μg capsaicin dose 0.67 and 7.67; 12.5 μg capsaicin dose 1.33 and 10.00; 50 μg capsaicin dose 2.67 and 11.30. Placebo: saline 0 and 3.67; 2.5 μg capsaicin dose 1.33 and 6.3; 12.5 μg capsaicin dose 2.67 and 8.67; 50 μg capsaicin dose 4.0 and 10.3. (B) Boxplots showing the effect of treatment with intranasal SB-705498 or placebo and peak nasal inspiratory flow (PNIF) in patients with NAR. Square and circle symbols represent mean; horizontal line within the box represents median; lower and upper edges of box represent the 25th and 75th values percentiles respectively. The 5th and 95th percentiles, not shown graphically, were respectively: SB-705498 12 mg: saline 40 and 230; 2.5 μg capsaicin dose 45 and 200; 12.5 μg capsaicin dose 0 and 150; 50 μg capsaicin dose 0 and 150. Placebo: saline 45 and 200; 2.5 μg capsaicin dose 50 and 200; 12.5 μg capsaicin dose 50 and 190; 50 μg capsaicin dose 40 and 190. ••, placebo;  , SB-705498 12 mg
, SB-705498 12 mg
Assessment of VAS scores for individual symptoms triggered by capsaicin indicated that all recorded symptoms were affected by treatment with SB-705498. Figure 3 illustrates the effect of treatment with SB-705498 compared with placebo on burning sensation provoked by incremental capsaicin challenge. Burning sensation was of particular PD importance for the development of intranasal SB-705498, as capsaicin-induced burning sensation is mediated directly by TRPV1 engagement in sensory neurones [25] and therefore evaluation of its inhibition is a direct measure related to target TRPV1 inhibition.
Figure 3.
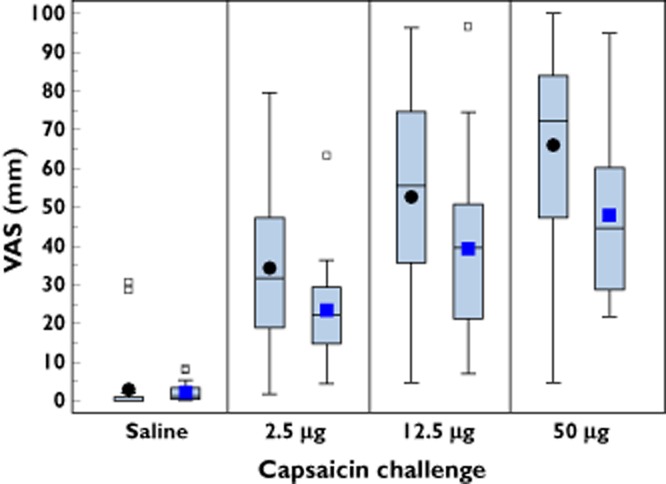
Boxplots showing the effect of treatment with intranasal SB-705498 or placebo on burning sensation, assessed by visual analogue scale (VAS). Square and circle symbols represent mean, horizontal line within the box represents median, lower and upper edges of box represent the 25th and 75th values percentiles, respectively. The 5th and 95th percentiles, not shown graphically, were, respectively,: SB-705498 12 mg: saline 0 and 8.3; 2.5 μg capsaicin dose 4.33 and 63.3; 12.5 μg capsaicin dose 7 and 96.67; 50 μg capsaicin dose 21.67 and 95. Placebo: saline 0 and 28.67; 2.5 μg capsaicin dose 3.3 and 73.0; 12.5 μg capsaicin dose 9 and 90; 50 μg capsaicin dose 9.67 and 99.3. ••, placebo;  , SB-705498 12 mg
, SB-705498 12 mg
The dose ratio analyses carried out on the TSS and VAS scores (nasal congestion, rhinorrhoea and burning sensation) confirmed that the shifts in the relative potency between placebo and SB-705498 treated subjects were parallel. The parallel shift in the dose–response is consistent with the competitive mechanism of inhibition of TRPV1 activation by the drug, which has also been demonstrated previously in in vitro cellular assays [17]. Detailed evaluation of the shift in the capsaicin-induced TSS dose–response following SB-705498 administration showed a mean change of 2.8-fold in relative potency (Figure 4). For individual VAS scores a 2-to 4-fold change in relative potency was observed on average (Figure 4).
Figure 4.
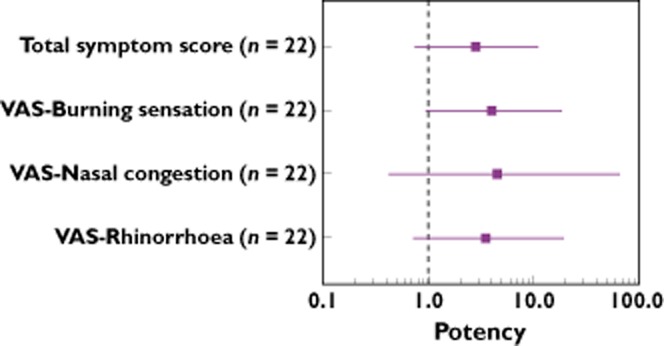
Forest plot depicting relative dose potency (mean and 95% CI) for clinical symptoms in patients with NAR. A ratio greater than 1 signifies a positive clinical endpoint response signal
Before administration of treatment, PNIF values were slightly greater in the placebo group than in the SB-705498 group; these differences were maintained after dosing with capsaicin (Figure 2B). A statistical analysis of the change from baseline indicated that challenge with increasing capsaicin dose resulted in some decrease (worsening) in PNIF values in both treatment arms. Treatment with SB-705498 compared with placebo, resulted in a minimal effect on PNIF values (mean change from baseline) which was more obvious after challenge with the lower doses of capsaicin.
Discussion
The results of this study support the concept that selective blockade of TRPV1 stimulation in the nose can reduce nasal hyper-responsiveness and development of rhinitis symptoms triggered by exogenous agents provoking sensory nerve excitability. This is the first study to explore and describe the safety, PK and PD efficacy of a novel intranasal formulation of SB-705498 in healthy volunteers and patients with NAR. Single and twice daily repeat intranasal administration of SB-705498, at doses up to 12 mg, was found to be safe overall and well tolerated. Treatment with intranasal SB-705498 showed target-specific local PD activity against nasal hyper-reactivity provoked by capsaicin challenge.
Because of the central role of TRPV1 in multi-modal activation of sensory nerves, TRPV1 antagonism has attracted significant interest as a target for the treatment of a wide range of disorders characterized by enhanced neural excitability, including neurological, gastrointestinal, urinary and respiratory conditions. However, to date the foremost application of TRPV1 antagonists has been in the treatment of pain. At least 11 TRPV1 antagonists have been identified and assessed for safety and tolerability following systemic or oral administration [17]. From these, five compounds have already progressed into ‘proof of concept’ studies to provide early efficacy readouts in patients with neuropathic or inflammatory pain. The GSK TRPV1 antagonist SB-705498 was initially developed as a novel oral analgesic. SB705498 blocked effectively in vitro the activation of TRPV1 by capsaicin, low pH and temperature [16,17]. In healthy volunteers single dosing with oral SB-705498 up to levels within the safety margin set by preclinical toxicology, was well tolerated and associated with a significant reduction of capsaicin-evoked skin flare and a lesser effect on thermal pain sensation [22]. Furthermore in the same study, oral SB-705498 produced a marked decrease on the flare and hyperalgesia elicited by UVB-irradiation of skin, implying that TRPV1 antagonism may exert an effect on neurogenic inflammation. The degree of SB-705498 PD efficacy on skin symptoms was correlated with the levels of systemic drug exposure which suggested that high oral SB-705498 doses would be necessary to achieve adequate restriction of nociceptive activity [22]. Single oral administration of SB-705498 in healthy volunteers [22] up to 400 mg, resulted in tmax of 2 h (0.75–4 h) and terminal phase elimination half-life of 54 h (35–93 h) that corresponded with a low oral clearance of approximately 9 l h−1.
The clinical development of oral TRPV1 antagonists has been recently confounded by the finding that their administration carries the risk of eliciting hyperthermia. Significant, acute increase in body temperature has been observed in preclinical species, as well as in humans and has already led to discontinuation of the development of several potent TRPV1 inhibitor compounds [26–29]. Oral SB-705498 administration to guinea pigs has been shown to result in mild body temperature increase at 30 mg kg−1 (0.6°C), and 100 mg kg−1 (0.8°C) but not at 10 mg kg−1, a dose which elicits an analgesic activity in the guinea pig (unpublished, in house data). Also, in a GSK clinical trial in subjects with dental pain single administration of oral SB-705498, at doses of 400–1000 mg (1.18–6.55 μg ml−1 (Cmax)), led to a potential trend towards a slight transient increase in body temperature 2 h after dosing (ClinicalTrials.gov Identifier: NCT00281684). The mechanisms underlying the effect of TRPV1 antagonists on body temperature are not entirely clear, but they appear to arise from inhibition of TRPV1 signalling on afferents innervating the viscera [30,31]. In most studies the induced hyperthermia seems to be dependent on the levels of systemic exposure. In the context of rhinitis treatment, it was thought that local application of a TRPV1 inhibitor in the nose could achieve effective local TRPV1 blockade with doses much lower than those needed to achieve the same effect following oral drug administration. This would reduce decisively the likelihood of treatment-related systemic adverse effects, including hyperthermia. It was therefore of interest to explore an intranasal formulation of SB-705498, as this would allow topical delivery at the site of action and thereby minimize the systemic exposure.
The challenge was to select a dose of SB-705498 that would maximize its pharmacology in the nasal tissue when administered topically. In addition, direct correlation of systemic drug concentrations with the corresponding pharmacodynamics in target nasal tissues would not be appropriate mainly due to uncertainty in the kinetics of the drug effect in the nasal turbinates. However, as described earlier in this manuscript, based on translation of findings from a PD guinea pig rhinitis model a maximum intranasal dose of 12 mg SB-705498 was selected for evaluation in the clinic. This dose was expected to achieve a high level of receptor occupancy after taking into account the human systemic exposure and the assumption of nasal bioavailability of the drug based on preclinical data. The charcoal block test in the dog has been shown to be a good predictor for assessing local drug deposition in humans [32,33]. Following intranasal administration about 20% of the SB-705498 dose is estimated to be available in the nasal tissues and absorbed across the nasal mucosa, while the remainder is expected to be swallowed and absorbed across the gastrointestinal tract. If complete drug absorption occurred in the nasal tissues, then 20% of the 12 mg of SB-705498 (e.g. approximately 2 mg) administered in this PD study could be anticipated in the nasal tissues and fluid in a maximum aqueous volume of approximately 20 ml (SB-705498 tissue concentration of 2 mg 20 ml–1) [21]. If nasal ciliary clearance (t1/2 of 15 min) is taken into consideration [34], even one tenth of the estimated nasal tissue concentration would still be associated with high receptor occupancy.
The SB-705498 PK findings in the intranasal FTIH study were consistent with the predictions based on the PK data from the FTIH study with oral SB-705498 [22] and the nasal deposition values described above. The marked increase in plasma concentrations of SB-705498 after repeat intranasal dosing compared with single dosing was predictable based on the long terminal phase elimination half-life of approximately 54 h observed in a previous study where the drug was administered orally [22]. As expected from the predicted systemic exposure, single and repeat administration of intranasal SB-705498 up to 12 mg was not associated with increase in body temperature in any of the study participants or with any other significant treatment-emergent AEs. In terms of PD efficacy, the results of our study indicated that the reduction of capsaicin-evoked rhinitis symptoms following administration of intranasal SB-705498 in patients with NAR is consistent with effective local TRPV1 antagonism. The differences in capsaicin-induced TSS between the SB-705498 12 mg and placebo group remained similar across all capsaicin doses used and were in the range of 1.19–1.25, suggesting a uniform response by the antagonist SB-705498 to all capsaicin challenges conducted in the study. Some outlier TSS points were observed in the group of patients treated with SB-705498, but the most conservative approach was taken and these data were included in the statistical analysis. From a clinical perspective, it is difficult to comment at this stage if there are patients with particular characteristics who may not respond adequately to SB-705498, because the total number of participants per arm in the study was limited. Most likely, the data reflect the normal spectrum of variability in the response to capsaicin, as well as to drug, as outliers with high TSS were noted following saline challenge, and an outlier with a much lower TSS was seen in the SB-705498 treated group after challenge with 12.5 μg capsaicin. An improvement in TSS of at least 1 unit represents a shift from one assessment grade to a lower grade, (e.g. from moderate to mild) and therefore, a change >1 unit is considered to be of clinical relevance. As shown in Figure 4, there was a consistent trend in the SB-705498 treatment effect on the clinical endpoints with their dose ratios greater than unity. It was estimated that a receptor occupancy at the TRPV1 target of about 66% was achieved based on the dose ratio of 2.8 computed for TSS endpoint using the equation for competitive antagonism (fractional occupancy = dose ratio – 1/dose ratio) [35]. Although treatment with SB-705498 reduced all individual symptoms assessed in the study, the magnitude of the treatment effect on each of them was different. Burning sensation was more profoundly affected (a 4-fold shift in dose–response relative to placebo) compared with the other rhinitis-like symptoms suggesting this endpoint is most directly coupled to TRPV1 activity. Thus, the 4-fold shift in dose–response for the burning sensation endpoint would be associated with a high receptor occupancy at the TRPV1 target of 75% and is consistent with that predicted as discussed before.
Although the effect of treatment with intranasal SB-705498 on capsaicin-evoked symptoms was marked, the results from the assessment of PNIF do not fully support the same conclusion. After challenge with the lowest dose of capsaicin (2.5 μg), patients treated with SB-705498 presented an improvement in PNIF compared with those who received placebo. However, this difference did not achieve statistical significance, while no significant effect was observed between SB-705498 and placebo after the challenges with higher doses of capsaicin. PNIF assessment is a simple objective tool to evaluate changes in nasal patency by both inflammatory and obstructive causes [36]. It is known that application of low doses of capsaicin in the nose induces changes in the nasal mucosa that lead to vasodilation, increased vascular permeability and glandular exudation that underlie the development of rhinitis-like symptoms [37]. Capsaicin, is a potent and selective activator of the TRPV1 receptor at concentrations up to 1 μm, but may engage other targets at higher concentrations [38]. Capsaicin can exert direct effects on vascular tone [39,40], smooth muscle tension [41], ion fluxes [41], nitric oxide synthesis and COX2 gene expression [42]. In the context of the nasal mucosa it was recently shown, using ex vivo functional experiments with human nasal tissue, that capsaicin induces TRPV1-independent vasodilation of the nasal vascular bed [38]. This vasodilatory effect was mediated by modulation of COX-2 enzymatic activity associated with reduced prostaglandin E2 production and could be suppressed by sulprostone, an agonist of prostaglandin E receptors [38]. Therefore, we could speculate that antagonism of the TRPV1 receptor by the administration of intranasal SB-705498 may lead to effective attenuation of the direct TRPV1-mediated effects, as reflected by the marked reduction of the burning sensation, but have a lesser effect on capsaicin-induced responses via other signalling pathways that are engaged with high local concentrations of capsaicin. This may explain why we observed variability in the degree of reduction on the PD parameters assessed in this study.
The results of a topical, low dose of SB-705498 on symptoms of allergic rhinitis in a 7 days repeat allergen challenge study were recently reported [5]. In this study 15 ml of a 30 μm solution of SB-705498 (equivalent to approximately 0.2 mg) was delivered via nasal lavage to patients with seasonal allergic rhinitis 2 min prior to allergen challenge and the effect on allergen challenge driven symptoms was measured following the allergen challenge. The selected dose of SB-705498, although it was found previously adequate to inhibit symptoms induced by a 5 μm capsaicin nasal spray, was shown to be ineffective in attenuating symptoms induced by allergen. Whilst these results may suggest that TRPV1 is not a key driver of allergen evoked symptoms, it is possible that the formulation used in this study did not have the necessary duration of action to inhibit TRPV1 beyond the 2 min explored for the capsaicin challenge (allergen symptoms were recorded at 10 min post-challenge). Hence, it is uncertain as to whether the effect of this formulation was still sufficient to block TRPV1 at the point where allergen symptoms were recorded. Duration of action studies are required to evaluate fully the effect of novel SB-705498 formulations before conclusions can be drawn about the role of TRPV1 in rhinitis symptoms. Furthermore, it is expected that TRPV1 may play a more prominent role in nasal hyper-responsiveness where the primary defect is directly linked to sensory over-sensitivity, as in many cases of NAR, than in conditions with a major immunopathology involvement, as in allergy.
In conclusion, TRPV1 antagonists offer a new mechanism of action for the potential treatment of nasal hyper-responsiveness. The results of these studies indicate that intranasal SB-705498, at a clinically safe and well-tolerated dose, has target specific PD activity in humans. The data provide the first clinical evidence that local application of a TRPV1 antagonist in the nose may alleviate symptoms triggered by stimulation of capsaicin sensitive nasal nerves. This suggests that SB-705498 could be further developed as a novel form of treatment for rhinitis patients with difficult to treat nasal hyper-responsiveness.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare CH, CvD, CS, IT and WF are employees of the Department of Otorhinolaryngology, Academic Medical Center, Amsterdam, the Netherlands and this institution received funding from GSK for the conduct of the study. CvD has received research grants from GSK, Allergopharma and ALK-Abello A/S. JD, KS, AN, MB and DT are all employees of GSK and hold GSK shares.
This study was funded by GlaxoSmithKline (GSK). The authors would like to acknowledge editorial support in the form of collating authors’ comments, editing and formatting of final draft which was provided by Kate Hollingworth of Continuous Improvement Ltd. This support was funded by GSK. Daphne Tsitoura is a fellow of the A. Onassis foundation.
References
- 1.Varjonen E, Kalimo K, Lammintausta K, Terho P. Prevalence of atopic disorders among adolescents in Turku, Finland. Allergy. 1992;47:243–248. doi: 10.1111/j.1398-9995.1992.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 2.Druce H. Allergic and nonallergic rhinitis. In: Middleton E, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger W, Busse WW, editors. Allergy Principles and Practice. 5th edn. St Louis, MO: Mosby-Year Book; 1998. pp. 1005–1016. [Google Scholar]
- 3.Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24:147–154. [PubMed] [Google Scholar]
- 4.Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Allerg Clin Immunol. 2006;118:999–1014. doi: 10.1016/j.jaci.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Alenmyr L, Greiff L, Andersson M, Sterner O, Zygmunt PM, Högestätt ED. Effect of mucosal TRPV1 inhibition in allergic rhinitis. Basic Clin Pharmacol Toxicol. 2012;110:264–268. doi: 10.1111/j.1742-7843.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Rijswijk JB, Blom HM, Fokkens WJ. Idiopathic rhinitis, the ongoing quest. Allergy. 2005;60:1471–1481. doi: 10.1111/j.1398-9995.2005.00975.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Rijswijk JB, Boeke EL, Keizer JM, Mulder PG, Blom H, Fokkens WJ. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: a double-blind randomized application regimen study. Allergy. 2003;58:754–761. doi: 10.1034/j.1398-9995.2003.00203.x. [DOI] [PubMed] [Google Scholar]
- 8.Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 10.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh Y-G, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology. 2006;44:128–134. [PubMed] [Google Scholar]
- 13.O'Hanlon S, Facer P, Simpson KD, Sandhu G, Saleh HA, Anand P. Neuronal markers in allergic rhinitis: expression and correlation with sensory testing. Laryngoscope. 2007;117:1519–1527. doi: 10.1097/MLG.0b013e3180ca7846. [DOI] [PubMed] [Google Scholar]
- 14.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 15.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Cur Opin Pharmacol. 2009;9:243–249. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rami HK, Thompson M, Stemp G, Fell S, Jerman JC, Stevens AJ, Smart D, Sargent B, Sanderson D, Randall AD, Gunthorpe MJ, Davis JB. Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett. 2006;16:3287–3291. doi: 10.1016/j.bmcl.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Gunthorpe MJ, Hannan SL, Smart D, Jerman JC, Arpino S, Smith GD, Brough S, Wright J, Egerton J, Lappin SC, Holland VA, Winborn K, Thompson M, Rami HK, Randall A, Davis JB. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and hear-mediated activation of the receptor. J Pharmacol Exp Ther. 2007;321:1183–1192. doi: 10.1124/jpet.106.116657. [DOI] [PubMed] [Google Scholar]
- 18.Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14:56–67. doi: 10.1016/j.drudis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Changani K, Hotee S, Campbell S, Pindoria K, Dinnewell L, Saklatvala P, Thompson S-A, Coe D, Biggadike K, Vitulli G, Lines M, Busza A, Denyer J. 2011. Efficacy of the TRPV1 antagonist SB-705498 in an MRI guinea pig model of rhinitis. ERS Annual Congress, Amsterdam. [DOI] [PMC free article] [PubMed]
- 20.World Medical Association. Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Available at http://www.wma.net/en/30publications/10policies/b3/ (last accessed 31 October 2011)
- 21.Tarhan E. Acoustic rhinometry in humans: accuracy of nasal passage area estimates, and ability to quantify paranasal sinus volume and ostium size. J Appl Physiol. 2005;99:616–623. doi: 10.1152/japplphysiol.00106.2005. [DOI] [PubMed] [Google Scholar]
- 22.Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM, Appleby JM. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Lambert GA, Davis JB, Appleby JM, Chizh BA, Hoskin KL, Zagami AS. The effects of the TRPV1 receptor antagonist SB-705498 on trigeminovascular sensitisation and neurotransmission. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:311–325. doi: 10.1007/s00210-009-0437-5. [DOI] [PubMed] [Google Scholar]
- 24.Finney DJ. A computer program for parallel line bioassays. J Pharmacol Exp Ther. 1976;198:497–506. [PubMed] [Google Scholar]
- 25.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 26.Gavva NR, Bannon AW, Supraneni S, Hovland DN, Jr, Lehto SG, Gore A. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, Simren M, Funch-jensen P, Drewes AM. Randomised clinical trial; the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Aliment Pharmacol Ther. 2011;33:1113–1122. doi: 10.1111/j.1365-2036.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- 29.Round P, Priestley A, Robinson J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol. 2011;72:921–931. doi: 10.1111/j.1365-2125.2011.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgstrom L, Nillsson M. A method for determination of the absolute pulmonary bioavailability of inhaled drugs: terbutaline. Pharm Res. 1990;7:1068–1070. doi: 10.1023/a:1015951402799. [DOI] [PubMed] [Google Scholar]
- 33.McDowell JE, Mackie AE, Ventresca GP, Bye A. Pharmacokinetics and bioavailability of intranasal fluticasone in humans. Clin Drug Invest. 1997;1:44–52. [Google Scholar]
- 34.Squillante E. Measurement of nasal residence time by microdialysis. Curr Sep. 2002;19:127–130. [Google Scholar]
- 35.Rang HP, Ritter JM. A new kind of drug antagonism: evidence that agonists cause a molecular change in acetylcholine receptors. Mol Pharmacol. 1969;5:394–411. [PubMed] [Google Scholar]
- 36.Teixeira RU, Zappelini CE, Alves FS, da Costa EA. Peak nasal inspiratory flow evaluation as an objective method of measuring nasal airflow. Braz J Otorhinolaryngol. 2011;77:473–480. doi: 10.1590/S1808-86942011000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanico AM, Philip G, Proud D, Naclerio RM, Togias A. Comparison of nasal mucosal responsiveness to neuronal stimulation in non-allergic and allergic rhinitis: effects of capsaicin nasal challenge. Clin Exp Allergy. 1998;28:92–100. doi: 10.1046/j.1365-2222.1998.00182.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Crombruggen K, Van Nassauw L, Derycke L, Timmermans J-P, Holtappels G, Hall D, Bachert C. Capsaicin-induced vasodilatation in human nasal vasculature is mediated by modulation of cyclooxygenase-2 activity and abrogated by sulprostone. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:613–626. doi: 10.1007/s00210-011-0638-6. [DOI] [PubMed] [Google Scholar]
- 39.Yeon D, Kwon S, Lee Y, Leem J, Nam T, Ahn D. Capsaicin-induced relaxation in rabbit coronary artery. J Vet Med Sci. 2001;63:499–503. doi: 10.1292/jvms.63.499. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Lozano-Cuenca J, Villalón CM, de Vries R, Garrelds IM, Avezaat CJ, van Kats JP, Saxena PR, MaassenVanDenBrink A. Pharmacological characterisation of capsaicin-induced relaxations in human and porcine isolated arteries. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:29–38. doi: 10.1007/s00210-007-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto S, Mori M. Characterization of capsaicin-induced, capsazepine-insensitive relaxation of ileal smooth muscle of rats. Eur J Pharmacol. 2004;487:175–182. doi: 10.1016/j.ejphar.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 2003;140:1077–1087. doi: 10.1038/sj.bjp.0705533. [DOI] [PMC free article] [PubMed] [Google Scholar]


