Abstract
Aim
Little attention has been paid to the effects of compliance and prescription practice on treatment outcome in HIV-infected children. In this context, an evaluation of the role of covariates on pharmacokinetics is required to establish the impact of differences in dosing regimens. Here we investigate whether a once daily dosing regimen of lamivudine provides comparable exposure to the currently approved paediatric regimen.
Methods
A hypothetical group of 180 patients between 3 months and 12 years old was used to evaluate the impact of body weight on systemic exposure to lamivudine. Simulation scenarios were evaluated using AUC and Cmax as parameters of interest. The analysis was performed using a population pharmacokinetic model previously implemented in nonmem v.6.2.
Results
The simulations show that once daily dosing of lamivudine yields comparable exposure to historical values observed in children and adults, both for liquid and solid dosage forms. Simulated steady-state AUC(0–24 h) and Cmax values after once daily doses ranged respectively from 9.95 mg l−1 h and 1.9 mg l−1 for children lighter than 14 kg to 13.75 mg l−1 h and 3.0 mg l−1 for children heavier than 30 kg. These values are comparable or higher than historical values observed after once daily dosing in children and adults.
Conclusions
Our findings illustrate how dosing regimens can be evaluated taking into account the effects of developmental growth on drug disposition. Most importantly, they suggest that the reduction in dosing frequency to once daily leads to comparable lamivudine exposure, as observed after administration of a twice daily dosing regimen.
Keywords: clinical trial simulations, compliance, dose rationale, lamivudine, model-based drug development, paediatrics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Lamivudine is currently administered as a once daily dosing regimen in adults and twice daily in children.
Recent studies in a subgroup of HIV-infected children have shown similar pharmacokinetics after once and twice daily dosing with either of the available dosage forms of lamivudine (i.e. tablet and solution).
Empirical evidence of drug exposure, efficacy and safety in controlled clinical trials has been required for the evaluation and approval of novel dosing regimens for a licensed product, thereby exposing children to potentially unnecessary trials.
WHAT THIS STUDY ADDS
Although simulated exposures varied across the paediatric weight range, children in all weight bands showed predicted lamivudine AUC(0–24 h) values after once daily administration comparable with or higher than the reference values previously observed in children on twice daily regimen or adults on once daily or twice daily dosing regimens.
The predicted increase in Cmax after once daily administration is unlikely to result in a higher risk of adverse events.
The use of model-based extrapolations allows the assessment of novel dosing regimens based on the underlying pharmacokinetic and pharmacokinetic–pharmacodynamic properties of the drug(s) under investigation.
Introduction
Historically prescription practice and patient compliance have not been considered as factors determining the successful use of antiretroviral drugs in HIV-infected children. However, it is well known that current combination antiretroviral therapy (cART) regimens require large numbers of pills or capsules to be taken several times a day and the overall pill burden may thus be too large to permit adherence for periods of many years. Differences in dosing regimen and/or dosing frequency may clearly constitute a burden for patients and in particular for younger children. Based on previous studies, not only the availability of suitable paediatric dosage forms, but also dosing frequency can be an important determinant of compliance and consequently of treatment outcome [1]. In fact, a significant correlation between lower pill burden and better virological outcome has been observed for antiretroviral drugs [2–4].
The availability of fixed dose combinations and the possibility to administer all the drugs as a once daily regimen may be very advantageous, with direct implications for adherence to therapy and for the overall treatment outcome [5–7]. In addition, reducing administration frequency may significantly reduce medication error. The use of a simple once daily cART regimen may therefore be a powerful solution to optimize treatment adherence and the patient's quality of life [8–10]. Furthermore, one specific concern with older children is the stigma of taking medications during the day or having friends finding out that they have an illness, so limiting the number of times a child has to take a medication can significantly improve a patient's well-being. Once daily dosing may also provide the flexibility to maximize adherence according to individual circumstances, particularly in resource limited settings where most HIV-infected children live [11]. For example, caregivers who are sellers in the market may find it hard to give drugs in the morning if they leave before the child is awake. Caregivers who work evenings may have to rely on others to administer evening doses.
Whilst empirical evidence of drug exposure, efficacy and safety in controlled clinical trials has been required for the evaluation and approval of novel dosing regimens for a licensed product, increasing evidence is emerging regarding the relevance of model-based extrapolations, which allow the assessment of novel dosing regimens based on the underlying pharmacokinetic and pharmacokinetic–pharmacodynamic properties of the drug(s) under investigation. These principles have been recently highlighted in the concept paper of the EMA [12]. The document emphasizes how extrapolations enable one to make inferences for another condition or product, thus reducing the need to generate additional information to reach conclusions for the condition or medicinal product. However, despite such a regulatory initiative, the requirements for the design of paediatric trials or the opportunities for extrapolation remain poorly understood. The current study illustrates the concepts on extrapolation and evidence synthesis with a real-life example. Here we show how to assess the performance of a once daily dosing regimen of lamivudine in children using a model-based approach. We demonstrate how inferences can be made about the new regimen when the appropriate data are already available, thereby reducing the need to expose children to unnecessary trials. Further details on the pharmacokinetics, efficacy and safety of lamivudine properties are summarized in the next paragraphs. These data provide the basis for the simulation of pharmacokinetic profiles in a large hypothetical paediatric cohort, which in turn can be used to confirm the dose rationale without the requirement for further enrolment of children into a new clinical trial [12, 13].
Lamivudine (3TC), a nucleoside reverse transcriptase inhibitor commonly used in combination antiretroviral therapy to HIV-infected children [14], was initially administered twice daily in both adults and children. Lamivudine enters infected lymphocytes and is progressively phosphorylated by intracellular enzymes to the active moiety, which has a long half-life (16–19 h) relative to the half-life of parent lamivudine in plasma (7–9 h) [15]. The evidence of such a long half-life and intracellular pooling of the precursor lamivudine diphosphate supported the investigation of once daily dosing in adults. In fact, a once daily dose regimen was subsequently approved for adults based on clinical studies which showed equivalent antiviral activity [16] and equivalent area under the curve [AUC(0–24 h)] of plasma lamivudine and intracellular lamivudine triphosphate [17] after once and twice daily regimens. Even though a formal concentration–antiviral effect relationship is lacking due to the difficulties in routinely measuring the intracellular lamivudine triphosphate concentrations, the plasma AUC(0–24 h) of lamivudine can be considered the best plasma predictor of antiviral activity based on the mechanism of action and long half-life of the active moiety. Moreover, deviation from these levels has been correlated with the development of resistance to lamivudine [18], in particular when drug exposure does not produce suppression of viral load below the limit of detection.
Currently lamivudine is commercially available as a tablet and oral solution for children between 3 months and 12 years old. It is labelled for twice daily administration in children based on clinical trials which demonstrated antiviral activity at doses yielding similar exposure to those observed in adults. Given the mechanism of action of lamivudine [19], a once daily dose regimen in children that can match the AUC(0–24 h) of the approved twice daily regimen in children or the once or twice daily regimen in adults should yield equivalent antiviral activity in children. To that purpose, several studies have been conducted to explore the PK and feasibility of once daily dosing in children. Comparable AUC(0–24 h) values were observed for once and twice daily dosing in HIV-infected children aged 3 months to 12 years. As expected, Cmax was approximately two-fold higher on the once daily regimen [20, 21]. In spite of these data, the once daily regimen has not yet been approved. The aim of this study was therefore to assess whether administration of lamivudine doses according to a once daily regimen yielded exposure profiles comparable with what is observed after a twice daily dosing regimen to HIV-infected children between 3 months and 12 years old. The use of simulation scenarios was proposed as the basis for establishing the suitability of this new regimen in children.
Methods
Simulations were performed to compare the systemic exposure of lamivudine after once daily dosing to historical values in children and adults and to explore how differences in demographic covariates affect steady-state exposure. The hypothetical population was represented by children between 3 months and 12 years old. For the purposes of our analysis, children were split into various age groups, each with five patients with different body weight (n = 180). The correlation between age and body weight was based on the WHO weight for age tables [22]. Lamivudine total daily doses were determined according to the currently recommended dose and method of administration, as indicated in the latest Summary of Product Characteristics (Table 1) [23].
Table 1.
Currently recommended doses of lamivudine in children [23]
| Weight band | Lamivudine dose regimen | Lamivudine total daily dose |
|---|---|---|
| <14 kg | Oral solution (4 mg kg−1) twice daily | 8 mg kg−1 |
| 14 to 21 kg | One-half tablet (75 mg) twice daily | 150 mg |
| >21 to <30 kg | One-half tablet (75 mg) in the morning; one whole tablet (150 mg) in the evening | 225 mg |
| ≥30 kg | One whole tablet (150 mg) twice daily | 300 mg |
A one-compartment model with first order absorption and elimination processes previously developed and validated by our group was used to simulate the pharmacokinetic profiles [24]. Data from 77 HIV-infected children receiving lamivudine both as twice and once daily dosing regimens were pooled and used for model building. Body weight was found to be exponentially correlated with clearance and volume of distribution. Given that formulation was not found to influence the pharmacokinetic parameters, the same model was used to predict lamivudine pharmacokinetics in children receiving tablets or solution. The model was carefully scrutinized for its predictive performance during simulations by statistical and graphical methods [24].
The frequency and times for pharmacokinetic sampling were based on a serial sampling scheme (0, 1, 2, 3, 4, 6, 8, 12 and 24 h after drug administration) to mimic current practice with regard to estimating AUC over the dosing interval. Concentration vs. time data were then integrated using the trapezoidal rule to ensure realistic estimates of variability, as observed in a typical non-compartmental analysis. The hypothetical experimental protocol is depicted in Figure 1. Given that a significant concentration–effect relationship for lamivudine could not be found in the past, the adequacy of the simulated dosing regimens was assessed graphically by determining the fraction of the paediatric population reaching systemic exposure comparable with AUC(0–24 h) values previously observed in studies of adults on approved once and twice daily regimens and children on approved twice daily regimens. Cmax values of the paediatric population were also compared with historical values of Cmax from previous clinical trials in adults [25, 26]. Simulations were performed using nonmem version 6.2. Results were graphically summarized using R 2.8.2.
Figure 1.

Diagram depictingthe hypothetical experimental protocol
Results
Simulations were performed using a population pharmacokinetic model previously developed and validated by our group (see companion paper). Based on the original parameter estimates, the distribution of the area under the curve (AUC(0–24 h)) and peak concentration (Cmax) values associated with a once daily dosing regimen for lamivudine were evaluated in a hypothetical group of paediatric patients. In total, the simulated population consisted of 180 patients between 3 months and 12 years old, who represent a population with comparable demographic characteristics of HIV-infected children in a typical clinical setting. The demographic characteristics of the simulated population are summarized in Table 2.
Table 2.
Demographic characteristics of the simulated paediatric population
| Overall | <14 kg | 14–21 kg | 21–30 kg | >30 kg | |
|---|---|---|---|---|---|
| Subjects | 180 | 85 | 34 | 31 | 30 |
| Median age (years) | 3.5 | 0.91 | 4.5 | 8 | 10.5 |
| Minimum (years) | 0.25 | 0.25 | 2 | 5 | 7.5 |
| Maximum (years) | 12 | 3.5 | 7.5 | 11 | 12 |
| Median weight (kg) | 14.9 | 9.7 | 17.2 | 24.9 | 35.9 |
| Minimum (kg) | 5.4 | 5.4 | 14.1 | 21.1 | 30.2 |
| Maximum (kg) | 53.9 | 13.6 | 20.7 | 29.1 | 53.9 |
The simulation results are presented graphically in Figures 2 and 3, which show the comparison between the simulated distributions of the secondary pharmacokinetic parameters [AUC(0–24 h) and Cmax] and historical data from previous clinical trials with lamivudine in children and adults. For completeness, the pharmacokinetics parameters of lamivudine are presented in Table 3. Box plots show that the predicted lamivudine exposure reached after once daily dosing was comparable or higher in every weight range than the exposure reached in historical trials where lamivudine was administered at approved once or twice daily doses to adults and twice daily doses to children. The predicted Cmax values on the once daily regimen exceeded those of the twice daily regimen in children. However, there was considerably overlap of the predicted Cmax values with those observed in adult subjects on the once daily regimen.
Figure 2.
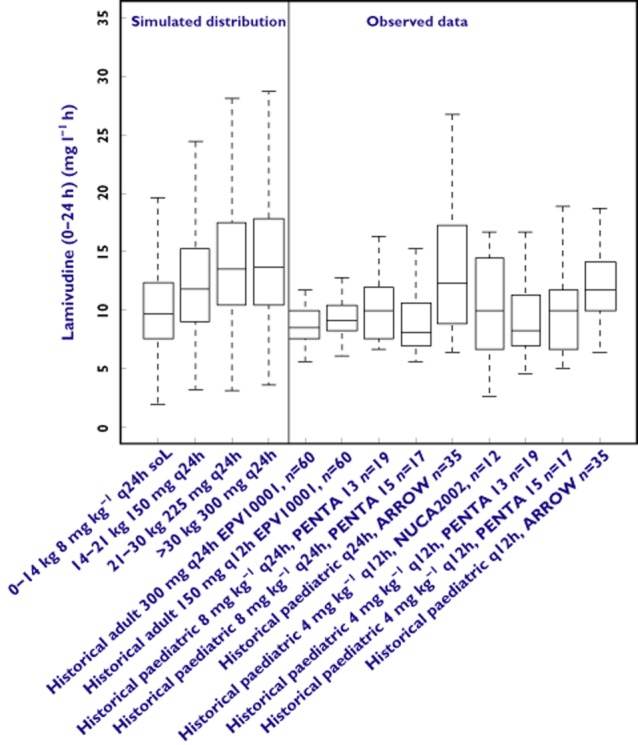
Box plots showing the comparison between simulated distributions of lamivudine AUC(0–24 h) after once daily dosing and historical data from clinical trials. Box represents median, 25th and 75th percentiles, bars represent 10th and 90th percentiles, open circles represent outliers. Simulated distributions (n = 500 replicate trials) are comparable or higher than historical data in each weight range
Figure 3.
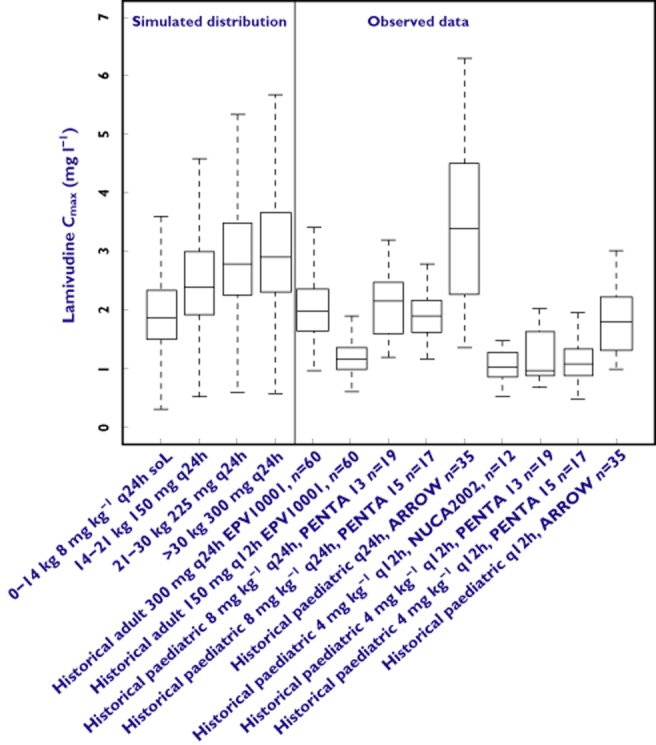
Box plots showing the comparison between simulated distributions of lamivudine Cmax after once daily dosing and historical data from clinical trials. Box represents median, 25th and 75th percentiles, bars represent 10th and 90th percentiles, open circles represent outliers
Table 3.
Summary of the pharmacokinetic parameters of the simulated population. Values are presented as geometric mean (95% CI) except for dose which is presented as median (range)
| Overall | <14 kg | 14–21 kg | 21–30 kg | >30 kg | |
|---|---|---|---|---|---|
| Dose (mg kg−1) | 8.51 | 8.45 | 8.72 | 9.01 | 8.34 |
| (5.55–10.66) | (7.84–9.25) | (7.21–10.61) | (7.75, 10.66) | (5.55, 9.91) | |
| AUC(0–24 h) (mg l−1 h) | 11.26 | 9.65 | 11.83 | 13.47 | 13.68 |
| (10.70, 11.86) | (9.01, 10.36) | (10.51, 13.26) | (11.84, 15.37) | (12.01, 15.52) | |
| Cmax(mg l−1) | 2.25 | 1.87 | 2.38 | 2.79 | 2.87 |
| (2.14, 2.36) | (1.75, 2.01) | (2.13, 2.66) | (2.50, 3.14) | (2.53, 3.22) | |
| CL/F (l h−1) | 15.19 | 10.65 | 16.14 | 21.07 | 27.68 |
| (14.42, 16.04) | (9.89, 11.53) | (14.39, 18.28) | (18.58, 23.51) | (24.26, 31.69) | |
| CL/F/kg (l h−1 kg−1) | 0.96 | 1.12 | 0.93 | 0.84 | 0.74 |
| (0.91, 1.02) | (1.04, 1.21) | (0.83, 1.06) | (0.74, 0.95) | (0.65, 0.85) |
Discussion
Evidence synthesis by modelling and simulation
Undoubtedly, the use of once daily dosing of antiretroviral drugs in HIV-infected children may offer significant clinical advantages, especially in resource-limited countries. There can be several benefits for both children and caregivers and adherence may be maximized, with consequent improvements in treatment outcome. These benefits also appear to be relevant from an economic perspective. An evaluation of the effect of antiretroviral adherence on health care costs demonstrated that high adherence to antiretroviral regimens was associated with lower mean monthly costs of direct health care, with the greatest savings in hospitalization costs, particularly important in countries with limited resources [27].
Given that previous studies have shown similar pharmacokinetics between once and twice daily dosing and evidence on the preference of caregivers for a once daily regimen, a model-based approach (i.e. evidence synthesis) should be fully considered in support of once daily dosing of lamivudine in HIV-infected children before insisting on the need for new data. We have used a population pharmacokinetic model to evaluate whether differences exist in the overall exposure to lamivudine after once daily dosing as compared with the observed profiles after twice daily administration. Our results clearly show that simulation scenarios offer the possibility to evaluate the implications of changes in dosing regimen based on existing evidence in the adult population and limited experience in children. It is unfortunate that historically population pharmacokinetic models have been used primarily as an alternative estimation method, with simulations being performed primarily as a diagnostic procedure during model validation, rather than as a tool for subsequent decision making. Yet, extrapolations are often used implicitly in many situations involving clinical or regulatory decisions, e.g. when extending conclusions from trial populations to the general population [12].
Once daily dosing regimen: systemic exposure
Simulation scenarios show that the lamivudine AUC(0–24 h) reached after once daily dosing is comparable with historical values in children on a twice daily regimen of lamivudine and adults receiving the approved once or twice daily lamivudine regimens. Figure 1 shows that the youngest group of children (between 0 and 14 kg) had a lower exposure compared with the older, heavier children. This fact, previously shown by Burger et al. [28], could be partly explained by the slightly lower dose that the small children receive (as shown in Table 3). Effectively, higher mg kg−1 doses are administered with the scored tablets because of the pre-defined tablets strengths (either 75 mg half tablet or 150 mg whole tablet) as well as the weight band cut-offs selected to minimize under-dosing in heavier children. Therefore, lighter children in the same weight band receive doses that are substantially higher than the 8 mg kg−1 day−1 when the solution is administered. There may also be some effect of the formulation, since these children receive solution and the heavy ones receive tablets, as shown in a recent study from Kasirye et al. [29]. However, such an effect is probably not large enough, as it could not be identified as a significant covariate [24]. Lastly, it is worth mentioning that distributional mechanisms may also be implicated in low exposure in younger children [30, 31]. Although simulated exposures varied across the paediatric weight range, children in all weight bands showed predicted lamivudine AUC(0–24 h) values after once daily administration comparable with or higher than the reference values previously observed in children on the twice daily regimen or adults on the once daily or twice daily dosing regimen. Given the assumption of comparable exposure–antiviral response relationship for HIV infection in adults and children, a once daily regimen that matches the AUC(0–24 h) of the approved twice daily regimen in children and the once daily regimen in adults should demonstrate equivalent antiviral activity in children.
Clearly one of the major concerns about once daily administration of antiretroviral drugs is the higher risk of viral failure. In adults it has been shown that once daily lamivudine in combination with zidovudine and efavirenz provides a comparable response as with twice daily lamivudine [16]. It has also been shown that didanosine, another NRTI with similar pharmacokinetic properties to lamivudine, despite a different threshold for the development of resistance, allows for once daily administration without increased risk of viral failure [32–34]. Regarding the increased risk of drug resistance, previous studies have demonstrated that once daily dosing of antiretrovirals is strongly correlated with increased patient adherence to therapy. Given that improved adherence may avoid development of resistance, it can be anticipated that the use of a once daily dosing regimen is unlikely to increase the probability of viral mutations and drug resistance. In fact, our findings with once daily dosing of lamivudine suggest no change in the risk of under-exposure to lamivudine, one of the main factors determining the development of resistance.
Once daily dosing regimen: peak concentrations
As expected for drugs showing linear pharmacokinetics, the reduction in dosing frequency resulted in an increase in median Cmax by approximately two-fold. Figure 2 shows that the maximum peak concentration reached after once daily dosing is much higher compared with twice daily administration. Once daily administration of lamivudine to HIV-infected children also results in higher peak concentrations (Cmax) than the historical values observed in adults and children during previous clinical trials in which twice daily dosing has been used. Given that the once-daily regimen was approved for use in adults based on good safety and efficacy and taking into account the evidence of positive tolerability and the safety profile of once daily lamivudine in small studies of children, no significant differences may be expected with regard to the safety of the once-daily regimen [35]. The predicted increase in Cmax after once daily administration is unlikely to result in a higher risk of adverse events. Again Cmax values appear to be slightly lower for children lighter than 14 kg. However, the simulated maximum peak concentration in this group of children is comparable with the reference Cmax values in adults receiving once daily dosing and higher than those observed in children and adults receiving lamivudine twice daily.
Limitations
One on the main limitations in our study was that lamivudine plasma pharmacokinetics can only be considered as a limited marker of drug exposure. As indicated previously, it is the intracellular lamivudine triphosphate metabolite that becomes pharmacologically active. However, no alternative is available due to the requirements for adequate sampling of intracellular concentrations of nucleoside transcriptase inhibitor triphosphate, which is logistically and technically difficult. This is further complicated by the volume of blood required for the bioanalysis of intracellular lamivudine triphosphate concentrations, which makes serial sampling impractical for paediatric patients. Instead, we have made explicit assumptions about the use of plasma concentrations, namely that equilibrium between plasma and intracellular concentrations is rapidly reached and drug distribution into cells is driven by a first order process, without the risk of saturation occurring within the range of concentrations observed after once or twice daily dosing.
In conclusion, the possibility of evaluating the implication of different dosing regimens using a model-based approach shows one of the various applications of virtual clinical trials in paediatric clinical pharmacology research. Our findings indicate that when the same total daily lamivudine dose is administered, the reduction in dosing frequency to once daily does not represent a potential risk of under-or over-dosing in the paediatric population. Taking into account the historical data regarding acceptability and adherence in previous paediatric and adult HIV trials, the current results suggest that further consideration should be given to an alternative, once daily dosing regimen, particularly in resource-limited settings.
Conflict of Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare CP had support from PENTA-LABNET and GlaxoSmithKline for the submitted work. ODP and KA are employed by GlaxoSmithKline, as indicated in their affiliations. WZ, DB, EJA,MD declare no conflict of interest. There are no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We thank all the children, families and staff from the centres participating in the PENTA 13, PENTA15 and ARROW studies. We acknowledge the MRC Clinical Trials Unit, London, UK and INSERM SC10, Paris, France for conducting the studies.
PENTA is a Co-ordinated Action of the European Commission (EC), supported by the Sixth Framework contract LSHP-CT-2006–018865 and Fifth Framework Program contract QLK2-2000-00150. PENTA activities are also supported by the PENTA Foundation and PENTA LABNET (EC Seventh Framework contract 201057). Financial support for PENTA 13 and 15 was also received from GlaxoSmithKline, UK.
References
- 1.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2009;6:194–200. doi: 10.1007/s11904-009-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS (London, England) 2001;15:1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 3.Moyle G. Once-daily therapy: less is more. Int J STD AIDS. 2003;14(Suppl 1):1–5. doi: 10.1258/095646203322491815. [DOI] [PubMed] [Google Scholar]
- 4.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 5.Manfredi R, Calza L. Recent availability of two novel, fixed formulations of antiretroviral nucleoside analogues: a 12-month prospective, open-label survey of their practical use and therapeutic perspectives in antiretroviral-naive and-experienced patients. AIDS Patient Care STDS. 2008;22:279–290. doi: 10.1089/apc.2007.0141. [DOI] [PubMed] [Google Scholar]
- 6.Chokephaibulkit K, Cressey T, Capparelli E, Sirisanthana V, Muresan P, Hongsiriwon S, Ngampiyaskul C, Limwongse C, Wittawatmongko O, Aurpibul L, Kabat B, Toye M, Smith M, Eksaengsri A, McIntosh K, Yogev R. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antivir Ther. 2011;16:1287–1295. doi: 10.3851/IMP1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosso R, Di Biagio A, Maggiolo F, Nulvesu L, Callegaro AP, Taramasso L, Bruzzone B, Viscoli C. Patient-reported outcomes and low-level residual HIV-RNA in adolescents perinatally infected with HIV-1 after switching to one-pill fixed-dose regimen. AIDS care. 2012;24:54–58. doi: 10.1080/09540121.2011.596511. [DOI] [PubMed] [Google Scholar]
- 8.Taburet A-M, Paci-Bonaventure S, Peytavin G, Molina J-M. Once-daily administration of antiretrovirals: pharmacokinetics of emerging therapies. Clin Pharmacokinet. 2003;42:1179–1191. doi: 10.2165/00003088-200342140-00001. [DOI] [PubMed] [Google Scholar]
- 9.Raboud J, Li M, Walmsley S, Cooper C, Blitz S, Bayoumi AM, Rourke S, Rueda S, Rachlis A, Mittmann N, Smieja M, Collins E, Loutfy MR. Once daily dosing improves adherence to antiretroviral therapy. AIDS behav. 2011;15:1397–1409. doi: 10.1007/s10461-010-9818-5. [DOI] [PubMed] [Google Scholar]
- 10.Maitland D, Jackson A, Osorio J, Mandalia S, Gazzard BG, Moyle GJ. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 2008;9:667–672. doi: 10.1111/j.1468-1293.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 11.Portsmouth SD, Osorio J, McCormick K, Gazzard BG, Moyle GJ. Better maintained adherence on switching from twice-daily to once-daily therapy for HIV: a 24-week randomized trial of treatment simplification using stavudine prolonged-release capsules. HIV Med. 2005;6:185–190. doi: 10.1111/j.1468-1293.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellanti F, Della Pasqua O. Modelling and simulation as research tools in paediatric drug development. Eur J Clin Pharmacol. 2011;67(Suppl 1):75–86. doi: 10.1007/s00228-010-0974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cock RFW, Piana C, Krekels EHJ, Danhof M, Allegaert K, Knibbe CAJ. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67(Suppl 1):5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achenbach CJ, Scarsi KK, Murphy RL. Abacavir/lamivudine fixed-dose combination antiretroviral therapy for the treatment of HIV. Adv ther. 2010;27:1–16. doi: 10.1007/s12325-010-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KH, Barrett JE, Shaw S, Pakes GE, Churchus R, Kapoor A, Lloyd J, Barry MG, Back D. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS (London, England) 1999;13:2239–2250. doi: 10.1097/00002030-199911120-00006. [DOI] [PubMed] [Google Scholar]
- 16.DeJesus E, McCarty D, Farthing CF, Shortino DD, Grinsztejn B, Thomas DA, Schrader SR, Sension MG, Gough K, Madison SJ. Once-daily versus twice-daily lamivudine, in combination with zidovudine and efavirenz, for the treatment of antiretroviral-naive adults with HIV infection: a randomized equivalence trial. Clin Infect Dis. 2004;39:411–418. doi: 10.1086/422143. [DOI] [PubMed] [Google Scholar]
- 17.Yuen GJ, Lou Y, Bumgarner NF, Bishop JP, Smith GA, Otto VR, Hoelscher DD. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48:176–182. doi: 10.1128/AAC.48.1.176-182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boucher CA, Cammack N, Schipper P, Schuurman R, Rouse P, Wainberg MA, Cameron JM. High-level resistance to (-) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37:2231–2234. doi: 10.1128/aac.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry CM, Faulds D. Lamivudine a review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs. 1997;53:657–680. doi: 10.2165/00003495-199753040-00008. [DOI] [PubMed] [Google Scholar]
- 20.Bergshoeff A, Burger D, Verweij C, Farrelly L, Flynn J, Le Prevost M, Walker S, Novelli V, Lyall H, Khoo S, Gibb D. Plasma pharmacokinetics of once-versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13) Antivir ther. 2005;10:239–246. [PubMed] [Google Scholar]
- 21.Paediatric European Network for Treatment of AIDS (PENTA) Pharmacokinetic study of once-daily versus twice-daily abacavir and lamivudine in HIV type-1-infected children aged 3–<36 months. Antivir ther. 2010;15:297–305. doi: 10.3851/IMP1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 2013. The WHO Child Growth Standards. Available at http://www.who.int/childgrowth/en/ (last accessed 20 October 2013) [Google Scholar]
- 23.Summary of Product Characteristics of lamivudine. 2011. World Health Organisation. Prequalification Programme. Available at http://apps.who.int/prequal/whopar/whoparproducts/HA414Part4v1.pdf (last accessed 20 October 2013) [Google Scholar]
- 24.Piana C, Zhao W. Adkison K, Burger D, Jacqz-Aigrain E, Danhof M, Della Pasqua O. Covariate effects and population pharmacokinetics of lamivudine in HIV-infected children. Br J Clin Pharmacol. 2014;77:861–872. doi: 10.1111/bcp.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen GJ, Esinhart JD, Rubin M. 1995. Clinical Pharmacology Study Report. GlaxoSmithKline Document Number UCR/95/004. Study Protocol ID NUCA2002. A phase I/II study of 3TC (GR109714X) in children with HIV infection (Protocol No: NUCA2002). Available at http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=NUCA2002&studyId=1DCBC204-B242-451B-A77D-6F586F8FF319&compound=lamivudine (last accessed 20 October 2013) [Google Scholar]
- 26.Yuen GJ, Dawson D, Lou Y, Martinson M, Gillum BE, Stein DS. GlaxoSmithKline Document Number RM2000/00258/00 Study Protocol ID EPV10001. An open-label, randomized, 2-way cross-over study to compare the steady-state pharmacokinetics of lamivudine and lamivudine triphosphate following lamivudine 300mg once daily versus EPIVIR 150 mg twice a day in healthy volunteers. Available at http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=EPV10001&studyId=FA1B4634-22F2-4EE6-8C22-DF6951C705E9&compound=EPV10001&type=GSK+Study+ID&letterrange=All (last accessed 20 October 2013) [Google Scholar]
- 27.Bishai D, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS (London, England) 2007;21:1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 28.Burger DM, Verweel G, Rakhmanina N, Wissen CPWGMV-V, La Porte CJL, Bergshoeff AS, Lyall H, Hartwig NG, Green H, Soldin S, Gibb DM, Groot de R. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin Pharmacol Ther. 2007;81:517–520. doi: 10.1038/sj.clpt.6100118. [DOI] [PubMed] [Google Scholar]
- 29.Kasirye P, Kendall L, Adkison KK, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, Nahirya-Ntege P, Mhute T, Kekitiinwa A, Snowden W, Burger DM, Gibb DM, Walker AS ARROW Trial Team. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin Pharmacol Ther. 2012;91:272–280. doi: 10.1038/clpt.2011.225. [DOI] [PubMed] [Google Scholar]
- 30.Minuesa G, Volk C, Molina-Arcas M, Gorboulev V, Erkizia I, Arndt P, Clotet B, Pastor-Anglada M, Koepsell H, Martinez-Picado J. Transport of lamivudine [(-)-beta-L-2′,3′-dideoxy-3′-thiacytidine] and high-affinity interaction of nucleoside reverse transcriptase inhibitors with human organic cation transporters 1, 2, and 3. J Pharmacol Exp Ther. 2009;329:252–261. doi: 10.1124/jpet.108.146225. [DOI] [PubMed] [Google Scholar]
- 31.Anderson PL, Lamba J, Aquilante CL, Schuetz E, Fletcher CV. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–449. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 32.Roca B, Lapuebla C, Vidal-Tegedor B. HAART with didanosine once versus twice daily: adherence and efficacy. Int J Infect Dis. 2005;9:195–200. doi: 10.1016/j.ijid.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Kazatchkine MD, Van PN, Costagliola D, Mohammed AS, Ledeine JM, Troccaz M, Belec L. Didanosine dosed once daily is equivalent to twice daily dosing for patients on double or triple combination antiretroviral therapy. The AI454-147 Team. J Acquir Immune Defic Syndr. 2000;24:418–424. doi: 10.1097/00126334-200008150-00003. [DOI] [PubMed] [Google Scholar]
- 34.Monno L, Cargnel A, Soranzo ML, Chirianni A, Ferraro T, Di Stefano M, Angarano G. Comparison of once and twice daily dosing of didanosine in combination with stavudine for the treatment of HIV-1 infection. AI 454-146 Team. Antivir ther. 1999;4:195–202. [PubMed] [Google Scholar]
- 35.LePrevost M, Green H, Flynn J, Head S, Clapson M, Lyall H, Novelli V, Farrelly L, Walker AS, Burger DM, Gibb DM. Adherence and acceptability of once daily Lamivudine and abacavir in human immunodeficiency virus type-1 infected children. Pediatr Infect Dis J. 2006;25:533–537. doi: 10.1097/01.inf.0000222415.40563.d4. [DOI] [PubMed] [Google Scholar]


