Abstract
Aims
To investigate the QT interval after high dose droperidol using continuous 12-lead Holter recordings.
Methods
This was a prospective study of patients given droperidol with a continuous Holter recording. Patients were recruited from the DORM II study which included patients with aggression presenting to the emergency department. Patients initially received 10 mg droperidol as part of a standardized sedation protocol. An additional 10 mg dose was given after 15 min if required and further doses at the clinical toxicologist's discretion. Continuous 12-lead Holter recordings were obtained for 2–24 h utilizing high resolution digital recordings with automated QT interval measurement. Electrocardiograms were extracted hourly from Holter recordings. The QT interval was plotted against heart rate (HR) on the QT nomogram to determine if it was abnormal. QTcF (Fridericia's HR correction) was calculated and >500 ms was defined as abnormal.
Results
Forty-six patients had Holter recordings after 10–40 mg droperidol and 316 QT–HR pairs were included. There were 32 abnormal QT measurements in four patients, three given 10 mg and one 20 mg. In three of the four patients QTcF >500 ms but only in one taking methadone was the timing of QTcF >500 ms consistent with droperidol dosing. Of the three other patients, one took amphetamines, one still had QT prolongation 24 h after droperidol and one took a lamotrigine overdose. No patient given >30 mg had a prolonged QT. There were no arrhythmias.
Conclusion
QT prolongation was observed with high dose droperidol. However, there was little evidence supporting droperidol being the cause and QT prolongation was more likely due to pre-existing conditions or other drugs.
Keywords: droperidol, drug safety, emergency department, Holter recording, QT prolongation, sedation
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Droperidol is a highly effective sedative and anti-emetic agent.
It has been removed or highly restricted because of concerns about QT prolongation and torsades de pointes.
Outside of spontaneous reporting there is limited published evidence that droperidol causes QT prolongation.
WHAT THIS STUDY ADDS
QT prolongation was associated with high dose droperidol for sedation in acute agitated patients.
In patients with QT prolongation, this could be attributed to another drug or pre-existing cardiac disease in all cases.
Introduction
Droperidol is a butyrophenone, antipsychotic medication that has been used extensively for decades to sedate patients with acute behavioural disturbance [1]. There have been concerns about the safety of droperidol because of its association with torsades des pointes (TdP) and QT prolongation [2]. Despite little evidence to support these claims [3], a black box warning was imposed by the United States Food and Drug Administration (FDA) in 2001 [3, 4] and other international drug regulatory bodies have removed it or restricted its use. This has led to a rapid decrease in its use and lack of availability [5].
Acute behavioural disturbance is common in the emergency department and often manifests as violence and aggression. Such behaviours put both staff and patients at risk of harm and can result in damage to property and injury [6]. Patients who cannot be settled by verbal de-escalation methods or oral sedation require mechanical restraint and parenteral sedation [7]. There is increasing evidence that droperidol is an effective drug for rapid sedation and it appears to be safer than benzodiazepines, because the latter cause over-sedation and require more additional sedation [8–11]. The increasing evidence for the benefit of droperidol [8–11] and the long safety record prior to the black box warning [12] means that there needs to be a reassessment of its safety so that a potentially beneficial drug is not restricted without good reason.
Although a number of studies have reported the association between droperidol and QT prolongation [13], they have not used standardized approaches to measuring the QT interval or have used Bazett's formula for heart rate (HR) correction, which over-corrects with heart rates greater than 70 beats min–1 [14]. There is limited information on electrocardiogram (ECG) changes following the administration of high dose droperidol for sedating agitated patients. Such studies have used a limited number of 12-lead ECGs [8, 9, 15]. A better understanding of the ECG changes following large doses of droperidol is required to provide a better assessment of the risk of QT prolongation and TdP in this setting.
The aim of this study was to investigate the cardiac effects of droperidol by accurately measuring the QT interval after the administration of droperidol using high resolution continuous 12-lead Holter recordings and assessing the risk of TdP using the QT nomogram [16].
Methods
This was a prospective study of patients given droperidol, which used high resolution Holter recordings to investigate the effect of high dose droperidol on the ECG and in particular, its effect on the QT interval. Patients were recruited as part of the DORM II study. DORM II is an observational study of patients with aggression or agitation presenting to the emergency department requiring parenteral sedation and physical restraint. Ethics approval was obtained from the local Human Research Ethics Committee. Due to the lack of decision making capacity in these patients and a duty of care to sedate them, patient consent was waived by the ethics committee.
Patients were included in this study between September 2009 and June 2011 from one hospital emergency department site involved in the DORM II study where there was access to Holter recordings. This was an urban emergency department with 30 000 annual presentations and approximately 5.5 presentations per 1000 with violence and/or agitation requiring parenteral sedation.
The DORM II study recruits adult patients (>16 years of age) presenting to the emergency department with violence and/or agitation who do not settle with verbal de-escalation or the administration of oral medication. A standardized intramuscular sedation protocol is followed for all patients, including routine observations (heart rate [HR], blood pressure [BP], respiratory rate [RR] and pulse oximetry) [6, 8] and the sedation assessment tool (SAT) to monitor agitation and sedation [17]. All patients are initially administered 10 mg intramuscular droperidol and if they do not settle within 15 min they are given a second dose of 10 mg. If patients still do not settle 30 min after their initial assessment, further sedation with droperidol is determined by the clinical toxicologist.
A purpose-designed chart was completed for all patients in the DORM II study, including observations, treatments and adverse effects. Once the patient was settled they had an ECG done and in this study were assessed by the nursing staff for suitability for a Holter recording. Patients were recruited if they were settled enough for a Holter recorder and its 12 leads to be attached, and the patient was able to tolerate this for at least 2 h. The duration of recording was for as long as the patient tolerated the Holter leads, until the patient was discharged or transferred, or 24 h had passed (maximum length of the digital Holter recording). Patients were excluded if they were not in sinus rhythm. The following data were included for the study: age, gender, drugs taken prior to droperidol and the dose and timing of droperidol.
For each admission 12-lead ECGs were extracted from the digital Holter recordings as follows. The H12+24 Hour Digital Holter Recorder (Mortara, Inc.) records a continuous 12-lead ECG onto a 24 h compact flash card. Continuous 12-lead Holter recording data were then acquired via a card reader and downloaded to a desktop computer using proprietary software (H-Scribe; Mortara, Inc.). The software allows the continuous 12-lead recordings to be stored and reviewed. The trend setting was used to determine if any arrhythmias had occurred and then high resolution digital 12-lead ECGs were extracted from the Holter recordings using H-Scribe. A 10 s 12-lead ECG was extracted every hour from the recording. The 12-lead ECGs were imported into E-scribe (Mortara, Inc.) to measure the QT interval. The E-Scribe software includes an algorithm to measure automatically the QT interval which includes averaging over multiple beats in each lead. It then displays the computer measured QT in a magnified view with the six chest and six limb leads separately overlayed, an overlay or butterfly view. On screen callipers are provided to adjust manually the QT interval if required. The measurement of the QT interval was reviewed by a clinical pharmacologist/toxicologist with expertise in the measurement and assessment of the QT. The QT interval was recorded for each ECG as well as the HR. Each QT interval measurement was plotted against the HR on the QT nomogram [16, 18]. Any QT–HR pair that was above the line on the QT nomogram was defined as abnormal. QTcF (Fridericia's HR correction of the QT interval) was also calculated and a cut-off of 500 ms was defined as abnormal.
The primary outcome for this study was the proportion of patients who had any QT–HR pairs above the ‘at risk’ line on the QT nomogram [16]. Secondary outcomes included QTcF > 500 ms and arrhythmias occurring after the administration of droperidol. Medians, ranges and interquartile ranges (IQR) are reported for continuous variables. Graphical analyses were done in GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego, California, USA, http://www.graphpad.com.
Results
There were forty-six patient admissions, 33 males and 13 females with a median age of 34 years (IQR 25 to 41 years, range 17 to 85 years) included in the study from which 3 to 23 12-lead ECGs were obtained for each admission. Three patients were excluded, two with chronic atrial fibrillation and one with left bundle branch block. Two patients had a second recording on a separate admission. Twenty-nine patients received 10 mg droperidol, 11 received 20 mg, three received 30 mg and three received 40 mg. The Holter recording was commenced a median of 60 min (IQR 38 to 111 min, range 16 to 307 min) after the first administration of droperidol. The median duration of the recordings was 6 h (range 2 to 24 h). A total of 316 QT–HR pairs were included and 284 QT–HR pairs were below the ‘at risk line on the QT nomogram (Figure 1). Thirty-two QT–HR pairs in four patients were above the ‘at risk’ line (Figure 2). The QTcF was greater than 500 ms in three of the four patients (three male patients, Table 1). Figure 3 shows the time course of QTcF after the administration of droperidol. None of six patients who received 30 or 40 mg droperidol had an abnormal QT. No patient had an arrhythmia and TdP did not occur in the four patients with prolonged QT intervals.
Figure 1.
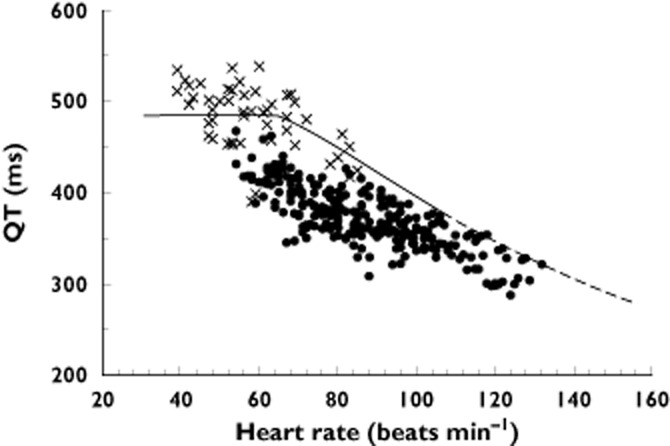
Plot of the QT interval vs. heart rate for the 42 patient admissions where the QT was normal.  , normal QT;
, normal QT;  , abnormal QT
, abnormal QT
Figure 2.
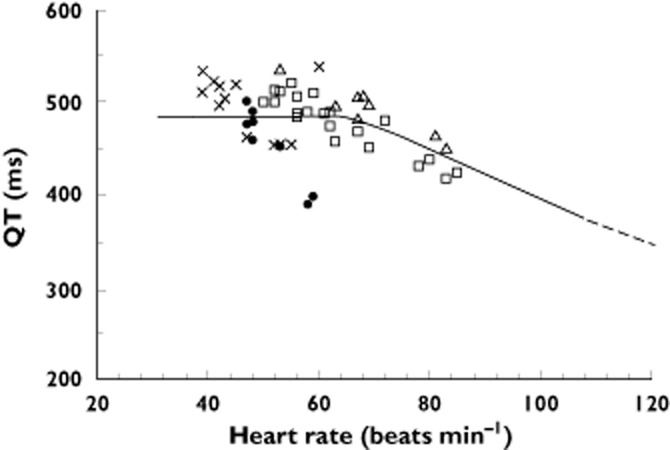
Plot of the QT interval vs. heart rate for the four patients with an abnormal QT interval (see Table 1).  , 52M patients;
, 52M patients;  , 41M patient;
, 41M patient;  , 25M patient;
, 25M patient;  , 17F patient
, 17F patient
Table 1.
Details of the four patients with QT prolongation
| Age/gender | Dose (mg) | Reason for presentation | History | Time to QT prolongation (min) | Maximum QT interval (ms) | Heart rate (beats min−1) |
|---|---|---|---|---|---|---|
| 41 M | 10 | Hallucinations due to amphetamines | Poly-substance abuse. Urine drug screen positive for amphetamines and THC | 210 | 522 | 53 |
| 25 M | 20 | Amphetamine toxicity and agitation | Taking methadone confirmed on urine drug screen. | 50 (start of recording) | 512 | 59 |
| 52 M | 10 | Attempted suicide with liquid petroleum gas | Poly-substance abuse. Urine drug screen positive for THC, amphetamines | 260 | 534 | 39 |
| 17 F | 10 | Lamotrigine overdose | Post-natal depression | 110 (start of recording) | 505 | 40 |
THC, tetrahydrocannibinol.
Figure 3.
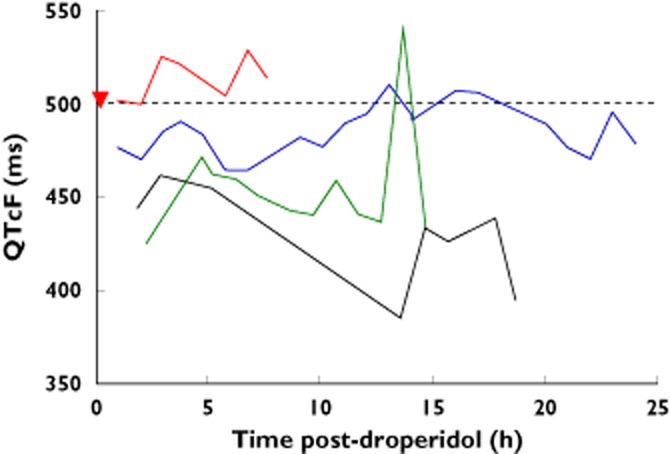
Plot of the QTcF vs. time for the four patients with an abnormal QT.  , 41M patient;
, 41M patient;  , 52M patient;
, 52M patient;  , 25M patient;
, 25M patient;  , 17F patient;
, 17F patient;  , repeat droperidol dose
, repeat droperidol dose
Details of the four patients with a prolonged QT are included in Table 1. The first patient presented to the emergency department with hallucinations after using amphetamines. He was a regular illicit intravenous drug user and a carrier of hepatitis C. His urine drug screen was positive for amphetamines and tetrahydrocannibinol. The Holter monitor was placed 1 h after droperidol 10 mg and the QT was normal until it became prolonged 11 h after droperidol (Figure 3, blue line). The second patient had been on methadone for 6 months and an ECG obtained prior to methadone commencing was normal. He was given 20 mg droperidol and the Holter placed 50 min after this. The QT interval was prolonged from the start until the end of the Holter recording 7.5 h after droperidol (Figure 3, red line). The third patient was a homeless intravenous drug user who attempted suicide using liquid petroleum gas inhalation. The Holter was placed 2.25 h after droperidol was administered. QT prolongation appeared 4.33 h after droperidol was administered and remained prolonged on discharge the next morning. However, the QTcF remained less than 500 ms except for on ECG recording 13.66 h post-droperidol (Figure 3, green line). The patient also remained bradycardic on discharge and never had a HR greater than 60 beats min–1. Despite repeated attempts the patient could not be contacted for cardiology follow-up. The fourth patient was female with post-natal depression who took an overdose of 2800 mg lamotrigine. The Holter was placed 1 h and 50 min after droperidol was administered. The QT interval was prolonged from the start of the Holter recording and resolved over several hours. She was also bradycardic and had a HR less than 60 beats min–1 for the duration. Her QTcF remained less than 500 ms for the duration.
Discussion
QT prolongation occurred in four patients after high dose droperidol administration and in these patients with an abnormal QT, there was little evidence to support droperidol being the cause. In at least one of the four patients with an abnormal QT, another drug was more likely to be the cause (methadone treatment). In addition, there was no dose dependence with droperidol and the QT prolongation, because QT prolongation occurred in three patients given 10 mg and one given 20 mg and there was no QT prolongation in patients given larger doses. The study used accurate measurement of the QT interval and a previously evaluated approach to determining abnormal QT intervals associated with TdP [18]. By employing continuous recording over many hours we were able to determine when the QT prolongation occurred in relation to the droperidol dose using QTcF (Figure 3). This intensive sampling in the first few hours after droperidol administration also meant we did not rely on one or two ECGs, or single lead ECG recordings. Importantly, no arrhythmias occurred including those who had a prolonged QT interval.
In the four patients with abnormal QT intervals, the QT prolongation could reasonably be attributed to other drugs or a pre-existing condition (e.g. undiagnosed cardiac condition). Two males with an abnormal QT were taking therapeutic drugs known to prolong the QT interval (methadone) or taking illicit drugs (e.g. amphetamines) (Table 1). The female patient presented with a large lamotrigine overdose and had a prolonged QT on the QT nomogram from the time the Holter was commenced. Lamotrigine has been shown to inhibit the human cardiac delayed rectifier potassium current in vitro and may be associated with QT prolongation [19]. This patient had a slow heart rate and the QTcF was never greater than 500 ms (Figure 3). The other male patient with poly-substance abuse had unresolved QT prolongation and bradycardia on discharge and was lost to cardiology follow-up. Such factors as undiagnosed pre-existing cardiac disease or other drugs are substantial confounders in this patient cohort who presented to the ED with agitation and violence. However, it is not possible to exclude droperidol completely as a contributing factor in these four patients.
The change in QTcF over time, shown in Figure 3, also provides some insight into whether the abnormal QT was due to droperidol. The patient on methadone (25-year-old male, Figure 3) clearly had an abnormal QTcF for the duration of the Holter recording. However, for the other two male patients, the QTcF was only abnormal between 12 and 18 h after droperidol, not consistent with the expected pharmacokinetics of droperidol.
This study used accurate measurement of the QT interval [20] and a previously evaluated approach to determining abnormal QT intervals [16, 18]. Two early studies prior to the black box warning issued by the FDA suggested that QT prolongation occurred with high dose droperidol [13, 21]. However, in both studies there were problems with QT measurement and the heart rate correction of the QT interval, and both studies were done in patients under general anaesthesia. A more recent study of patients undergoing general anaesthesia found that similar numbers of patients had QT prolongation if they were given normal saline or droperidol [22]. The first of the two earlier studies by Guy et al. [21] provided no information on the method of measuring the QT and used the mean of the QT from different leads, which provides a biased estimate of the QT interval [23]. Lischke et al. used an automatic measurement from a standard ECG machine and also used the mean of the QT from different leads [13]. Both studies used Bazett's formula to correct for HR which is known to over-correct in patients with HRs faster than 70 beats min–1 [24, 25]. This may account for the unusual finding by Lischke et al. that the mean maximal QT prolongation occurred within 1 min of drug administration, at the same time as a significant increase in HR. In addition, the rapid rise and fall of the QTc in the study by Lischke et al. is not consistent with the known slow adaptation of the QT interval to sudden or rapid changes in HR due to QT hysteresis [26]. A more recent study by Charbit et al. suggested there was a significant change in the QTcB (Bazett's) following droperidol (compared with ondansetron). However, they showed rather erratic changes in the QTcB commencing minutes after the administration of droperidol, which did not account for inter-individual variation in HR correction or QT hysteresis, making these results difficult to interpret [27].
In our study the QT nomogram was the major method used to determine if the QT was abnormal [18]. The QT nomogram provides a different approach to assessing whether a QT interval is abnormal because the QT is plotted against the HR avoiding the need for HR correction formulae. However, it is not possibly to plot easily the QT–HR pair vs. time to determine if the abnormal QT coincides with the dosing of droperidol. We therefore used QTcF to explore this relationship in the patients with an abnormal QT, despite QTcF being a population based HR correction formula which can be problematic for fast and slow HRs [16].
The use of automated measurement of the QT interval using standard ECG machines is known to be inaccurate [14, 28]. In this study we used an automated QT measurement in dedicated software for the measurement of QT which also allowed the use of on-screen magnification and callipers for manual checking of the QT measurement by a clinician experienced in reading ECGs [14]. This approach provided the most accurate method of QT measurement and the application of this in a clinical setting is unique to the study.
A limitation of this study was the variability of the commencement time of the continuous Holter recordings. This was determined by the time to sedation but in 75% of the patients the Holter was commenced within 2 h. The compliance of the patients was imperative and was difficult to predict. Another problem was that the study did not include patients where it was unsafe or not possible to put on the Holter recording device. However, this was rare and was unlikely to have biased the patient group included in the study.
The absence of baseline ECGs is also a limitation of the study but it is not possible and unsafe to attempt to record an ECG or Holter in violent and agitated patients. There is limited data on the underlying frequency of QT prolongation in this population of patients presenting with acute behavioural disturbance. Three previous studies of droperidol in this population found no significant difference between patients given another drug for sedation (midazolam or olanzapine) vs. patients given droperidol [8, 9, 15]. In the DORM study there was no difference in the number of patients with an abnormal QT with two of 31 given 10 mg droperidol, two of 29 given 10 mg midazolam and four of 29 given 5 mg midazolam and 5 mg droperidol [8]. In another study where droperidol was compared with olanzapine or control, in patients already receiving midazolam, the median QTcB (Bazett's) intervals in 211 patients having an ECG did not differ between groups and was between 440 ms and 450 ms. One patient given olanzapine and one patient in the control group (midazolam alone) had QTcB measurements of 500 ms and 512 ms respectively. These studies suggest that there is a larger proportion of patients in this population who have an abnormal QTcB, with a higher median of 440 to 450 ms [15] compared with normal populations of 410 to 420 ms [29] and a greater number of outliers [8, 15]. The number of patients in our study with an abnormal QT on the Holter is consistent with this. Studies in other populations have also found that QT prolongation is often present in a proportion of patients prior to the administration of droperidol. In a study comparing droperidol and ondansetron for postoperative nausea and vomiting, 21% of patients had a long QTcB pre-operatively before any drug was administered [30].
Although QT prolongation was observed with high dose droperidol in this study, there was little evidence to support droperidol being the cause and QT prolongation was more likely to be due to pre-existing conditions or other drugs. There was also no evidence of dose dependence in cases where QT prolongation occurred.
Competing Interests
Both authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. GKI is supported by an NHMRC Clinical Career Development Award ID 605817.
We thank Guanhao Luo and Aziz Nor for helping recruit patients to the study.
References
- 1.Richards JR, Schneir AB. Droperidol in the emergency department: is it safe? J Emerg Med. 2003;24:441–447. doi: 10.1016/s0736-4679(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 2.Wooltorton E. Droperidol: cardiovascular toxicity and deaths. CMAJ. 2002;166:932. [PMC free article] [PubMed] [Google Scholar]
- 3.Kao LW, Kirk MA, Evers SJ, Rosenfeld SH. Droperidol, QT prolongation, and sudden death: what is the evidence? Ann Emerg Med. 2003;41:546–558. doi: 10.1067/mem.2003.110. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz BZ, Bizovi K, Moreno R. Droperidol – behind the black box warning. Acad Emerg Med. 2002;9:615–618. doi: 10.1197/aemj.9.6.615. [DOI] [PubMed] [Google Scholar]
- 5.Nuttall GA, Eckerman KM, Jacob KA, Pawlaski EM, Wigersma SK, Marienau ME, Oliver WC, Narr BJ, Ackerman MJ. Does low-dose droperidol administration increase the risk of drug-induced QT prolongation and torsade de pointes in the general surgical population? Anesthesiology. 2007;107:531–536. doi: 10.1097/01.anes.0000281893.39781.64. [DOI] [PubMed] [Google Scholar]
- 6.Downes MA, Healy P, Page CB, Bryant JL, Isbister GK. Structured team approach to the agitated patient in the emergency department. Emerg Med Australas. 2009;21:196–202. doi: 10.1111/j.1742-6723.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 7.Calver LA, Downes MA, Page CB, Bryant JL, Isbister GK. The impact of a standardised intramuscular sedation protocol for acute behavioural disturbance in the emergency department. BMC Emerg Med. 2010;10:14. doi: 10.1186/1471-227X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isbister GK, Calver LA, Page CB, Stokes B, Bryant JL, Downes MA. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Ann Emerg Med. 2010;56:392–401. doi: 10.1016/j.annemergmed.2010.05.037. e1. [DOI] [PubMed] [Google Scholar]
- 9.Knott JC, Taylor DM, Castle DJ. Randomized clinical trial comparing intravenous midazolam and droperidol for sedation of the acutely agitated patient in the emergency department. Ann Emerg Med. 2006;47:61–67. doi: 10.1016/j.annemergmed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Richards JR, Derlet RW, Duncan DR. Chemical restraint for the agitated patient in the emergency department: lorazepam versus droperidol. J Emerg Med. 1998;16:567–573. doi: 10.1016/s0736-4679(98)00045-6. [DOI] [PubMed] [Google Scholar]
- 11.Martel M, Sterzinger A, Miner J, Clinton J, Biros M. Management of acute undifferentiated agitation in the emergency department: a randomized double-blind trial of droperidol, ziprasidone, and midazolam. Acad Emerg Med. 2005;12:1167–1172. doi: 10.1197/j.aem.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Chase PB, Biros MH. A retrospective review of the use and safety of droperidol in a large, high-risk, inner-city emergency department patient population. Acad Emerg Med. 2002;9:1402–1410. doi: 10.1111/j.1553-2712.2002.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 13.Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg. 1994;79:983–986. doi: 10.1213/00000539-199411000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Malik M. Errors and misconceptions in ECG measurement used for the detection of drug induced QT interval prolongation. J Electrocardiol. 2004;37(Suppl):25–33. doi: 10.1016/j.jelectrocard.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Chan EW, Taylor DM, Knott JC, Phillips GA, Castle DJ, Kong DC. Intravenous droperidol or olanzapine as an adjunct to midazolam for the acutely agitated patient: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Ann Emerg Med. 2013;61:72–81. doi: 10.1016/j.annemergmed.2012.07.118. [DOI] [PubMed] [Google Scholar]
- 16.Isbister GK, Page CB. Drug induced QT prolongation: the measurement and assessment of the QT interval in clinical practice. Br J Clin Pharmacol. 2013;76:48–57. doi: 10.1111/bcp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calver LA, Stokes B, Isbister GK. Sedation assessment tool to score acute behavioural disturbance in the emergency department. Emerg Med Australas. 2011;23:732–740. doi: 10.1111/j.1742-6723.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- 18.Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM. 2007;100:609–615. doi: 10.1093/qjmed/hcm072. [DOI] [PubMed] [Google Scholar]
- 19.Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. doi: 10.1016/j.eplepsyres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Berling I, Isbister GK, Calver L, Clunas S. Digital Holter measurement of QT prolongation in ziprasidone overdose. Clin Toxicol (Phila) 2011;49:694–696. doi: 10.3109/15563650.2011.597035. [DOI] [PubMed] [Google Scholar]
- 21.Guy JM, Andre-Fouet X, Porte J, Bertrand M, Lamaud M, Verneyre H. [Torsades de pointes and prolongation of the duration of QT interval after injection of droperidol] Ann Cardiol Angeiol (Paris) 1991;40:541–545. [PubMed] [Google Scholar]
- 22.White PF, Song D, Abrao J, Klein KW, Navarette B. Effect of low-dose droperidol on the QT interval during and after general anesthesia: a placebo-controlled study. Anesthesiology. 2005;102:1101–1105. doi: 10.1097/00000542-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Malik M, Camm AJ. Evaluation of drug-induced QT interval prolongation: implications for drug approval and labelling. Drug Saf. 2001;24:323–351. doi: 10.2165/00002018-200124050-00001. [DOI] [PubMed] [Google Scholar]
- 24.Davey P. How to correct the QT interval for the effects of heart rate in clinical studies. J Pharmacol Toxicol Methods. 2002;48:3–9. doi: 10.1016/S1056-8719(03)00008-X. [DOI] [PubMed] [Google Scholar]
- 25.Hodges M. Rate correction of the QT interval. Card Electrophysiol Rev. 1997;3:360–363. [Google Scholar]
- 26.Lau CP, Freedman AR, Fleming S, Malik M, Camm AJ, Ward DE. Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res. 1988;22:67–72. doi: 10.1093/cvr/22.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology. 109:206–212. doi: 10.1097/ALN.0b013e31817fd8c8. [DOI] [PubMed] [Google Scholar]
- 28.Isbister GK, Calver L, Gorp van F, Stokes B, Page CB. Inter-rater reliability of manual QT measurement and prediction of abnormal QT,HR pairs. Clin Toxicol (Phila) 2008;47:884–888. doi: 10.3109/15563650903333820. [DOI] [PubMed] [Google Scholar]
- 29.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72:23B–25. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 30.Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–1100. doi: 10.1097/00000542-200506000-00006. [DOI] [PubMed] [Google Scholar]


