Infants and children are unique patient populations compared with adults as they not only differ physiologically and anatomically but also experience rapid changes in growth and development over the course of their childhood. These dynamic and variable developmental changes can have major impact on the pharmacokinetic and pharmacodynamic profile of a drug from its absorption, distribution, metabolism and elimination (ADME) properties to its effects [1].
Since oral administration is one of the primary routes for giving a drug to children, many studies have been implemented to provide evidence for developmental differences in the absorption of drugs [1, 2]. As is well known, the bioavailability of orally administered drugs is regulated by various metabolic enzymes and transporters expressed in the small intestine. Therefore, the developmental changes in activity of metabolic enzymes and transporters could explain developmental differences in absorption. To date, several studies have characterized the ontogeny of metabolic enzymes in tissues including the small intestine [1–3]. However, there is little information on the ontogeny of drug transporters in the human intestine.
P-glycoprotein (MDR1/ABCB1), an ATP-binding cassette transporter, located in the small intestine mediates the efflux of substrates from the epithelial cells to the intestinal lumen [4]. Thus, the inter-individual variability in the expression of intestinal ABCB1 is an important factor which determines the bioavailability of ABCB1 substrates, as has been shown for tacrolimus [5]. Since many drugs used in neonates, infants and children are substrates of ABCB1, it is important to characterize the effects of developmental changes of intestinal ABCB1 on drug disposition in children [1, 2].
Here, we evaluate ABCB1 mRNA expression patterns in human small intestine tissues collected from children to provide fundamental information on the age-dependent expression. mRNA expression data were obtained as part of a previous study at Kyoto University Hospital, Japan [5]. In short, 206 paediatric patients undergoing living donor liver transplantation were enrolled in the study. All mucosal specimens of the upper jejunum were obtained from a part of the Roux-en-Y limb for biliary reconstruction or around the bile drainage tube during surgery. Samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis. ABCB1 mRNA expression was determined by quantitative RT-PCR as previously described [5]. The amount was determined with a standard curve generated using known amounts of standard plasmid DNA. To verify the quality of the total cellular RNA extracted, GAPDH mRNA expression was also quantified as an internal standard. Demographics of patients were as follows: median age (range) 1.27 years (62 days–18.9 years), median body weight (range) 8.5 kg (3.7–70 kg), 85 male and 121 female patients. The study was approved by the Institutional Review Board and all participants, patients and/or care givers, gave written informed consent or assent.
Large inter-individual variability in intestinal ABCB1 expression was observed across all ages (Figure 1). Over the studied age range from 62 days to 18.9 years, no clear maturation trajectory of ABCB1 expression was observed. This may indicate that the expression of intestinal ABCB1 has reached at the certain level by at least 2 months after the birth. A most recent report showed no significant difference in intestinal ABCB1 mRNA expression between neonates and adults [6]. Although our data are not completely comparable due to the lack of samples in neonates, the report indirectly supports our observation. On the other hand, there is an earlier study demonstrating a very weak or no expression of ABCB1 in the fetal intestine [7]. Furthermore, a study using enterocyte cell lines demonstrated that breast milk induces ABCB1 expression in a dose-and time-dependent manner [8]. An animal study also demonstrated that the ABCB1 expression in the small intestine of neonatal rodents dramatically increased shortly after initiation of breastfeeding [8]. Since lactation is generally started right after the birth, the expression of intestinal ABCB1 is anticipated to initiate at the same time and rapidly reach levels comparable with those seen in adults.
Figure 1.
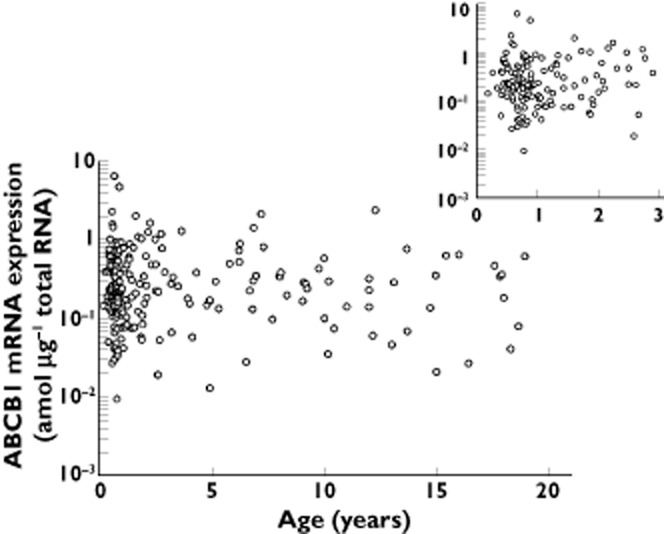
Age related changes of intestinal ABCB1 mRNA expression. Data represent individual ABCB1 mRNA expression
As with the small intestine, developmental changes in hepatic ABCB1 expression has been of great interest as an important consideration in the development of age-appropriate dosing strategies. In a recent preliminary report, it was noted that hepatic ABCB1 mRNA expression in fetuses, neonates, infants and children was 25–60-fold lower compared with adult values [9]. In addition, in a recent review article, Klaassen & Aleksunes also reported that mRNA expression of ABCB1 in perinatal human liver samples was very low and gradually increased over time until the age of 7 years [10]. The maturation pattern of ABCB1 expression is most likely organ-specific and needs to be studied in each organ.
In conclusion, this analysis suggests that the maturation of intestinal ABCB1 mRNA expression is completed relatively soon after birth. This finding contributes to an improved understanding of the age dependent maturation of ABCB1 transporter mRNA expression and its potential effect on the disposition of drug substrates.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We thank the members of Department of Pharmacy, Kyoto University Hospital and surgeons of Department of Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan for providing the clinical samples and information. This work was supported in part by a Funding Program for Next Generation World-Leading Researchers (NEXT Program: LS073) initiated by the Council for Science and Technology Policy of the Japan Society for the Promotion of Science of Japan. TM was supported by the Japan Research Foundation for Clinical Pharmacology.
References
- 1.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 2.Mooij MG, Koning de BA, Huijsman ML, Wildt de SN. Ontogeny of oral drug absorption processes in children. Expert Opin Drug Metab Toxicol. 2012;8:1293–1303. doi: 10.1517/17425255.2012.698261. [DOI] [PubMed] [Google Scholar]
- 3.Johnson TN, Tanner MS, Taylor CJ, Tucker GT. Enterocytic CYP3A4 in a paediatric population: developmental changes and the effect of coeliac disease and cystic fibrosis. Br J Clin Pharmacol. 2001;51:451–460. doi: 10.1046/j.1365-2125.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet. 2003;42:59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Masuda S, Goto M, Fukatsu S, Uesugi M, Ogura Y, Oike F, Kiuchi T, Takada Y, Tanaka K, Inui K. Intestinal MDR1/ABCB1 level at surgery as a risk factor of acute cellular rejection in living-donor liver transplant patients. Clin Pharmacol Ther. 2006;79:90–102. doi: 10.1016/j.clpt.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Mooij MG, de Wildt SN, Koning de BA, Schwarz UI, Samson JN, Spaans E, Tibboel D, Kim RB. 2013. Developmental change in human intestinal and hepatic drug transporter expression: [Late breaking abstract for the 114th American Society for Clinical Pharmacology and Therapeutics Annual Meeting]
- 7.Kalken van CK, Giaccone G, Valk van der P, Kuiper CM, Hadisaputro MM, Bosma SA, Scheper RJ, Meijer CJ, Pinedo HM. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol. 1992;141:1063–1072. [PMC free article] [PubMed] [Google Scholar]
- 8.Guner YS, Franklin AL, Chokshi NK, Castle SL, Pontarelli E, Wang J, Wang L, Prasadarao NV, Upperman JS, Grishin AV, Ford HR. P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis. Lab Invest. 2011;91:1668–1679. doi: 10.1038/labinvest.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooij MG, Wildt de SN, de Koning BA, Schwarz UI, Tibboel D, Kim RB. Lower MDR1 mRNA expression in hepatic fetal and pediatric tissue compared to adults. Clin Pharmacol Ther. 2013;93:S63. [Abstract for the 114th American Society for Clinical Pharmacology and Therapeutics Annual Meeting] [Google Scholar]
- 10.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]


