Abstract
Background
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a multi-system illness characterized, in part, by increased fatigue following minimal exertion, cognitive impairment, poor recovery to physical and other stressors, in addition to other symptoms. Unlike healthy subjects and other diseased populations who reproduce objective physiological measures during repeat cardiopulmonary exercise tests (CPETs), ME/CFS patients have been reported to fail to reproduce results in a second CPET performed one day after an initial CPET. If confirmed, a disparity between a first and second CPET could serve to identify individuals with ME/CFS, would be able to document their extent of disability, and could also provide a physiological basis for prescribing physical activity as well as a metric of functional impairment.
Methods
22 subjects diagnosed with ME/CFS completed two repeat CPETs separated by 24 h. Measures of oxygen consumption (VO2), heart rate (HR), minute ventilation (Ve), workload (Work), and respiratory exchange ratio (RER) were made at maximal (peak) and ventilatory threshold (VT) intensities. Data were analyzed using ANOVA and Wilcoxon’s Signed-Rank Test (for RER).
Results
ME/CFS patients showed significant decreases from CPET1 to CPET2 in VO2peak (13.8%), HRpeak (9 bpm), Ve peak (14.7%), and Work@peak (12.5%). Decreases in VT measures included VO2@VT (15.8%), Ve@VT (7.4%), and Work@VT (21.3%). Peak RER was high (≥1.1) and did not differ between tests, indicating maximum effort by participants during both CPETs. If data from only a single CPET test is used, a standard classification of functional impairment based on VO2peak or VO2@VT results in over-estimation of functional ability for 50% of ME/CFS participants in this study.
Conclusion
ME/CFS participants were unable to reproduce most physiological measures at both maximal and ventilatory threshold intensities during a CPET performed 24 hours after a prior maximal exercise test. Our work confirms that repeated CPETs warrant consideration as a clinical indicator for diagnosing ME/CFS. Furthermore, if based on only one CPET, functional impairment classification will be mis-identified in many ME/CFS participants.
Keywords: Chronic fatigue syndrome, Functional impairment, Cardiopulmonary exercise test, Exercise intolerance, Post exertional malaise
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a multi-system illness that can lead to striking debilitation. Currently, diagnosis is based on a symptom profile. A hallmark symptom is referred to as “post-exertional malaise” (PEM), and encompasses disabling and persistent fatigue following exertion, usually accompanied by increases in other symptoms, including cognitive dysfunction [1]. Other common symptoms include, but are not limited to sleep disturbance, pain, and symptoms associated with autonomic dysfunction such as orthostatic intolerance, postural orthostatic tachycardia syndrome (POTS), light and sound sensitivity, and/or gastrointestinal distress. Fatigue in ME/CFS is not alleviated by bed rest and may be exacerbated by physical or cognitive activity, or other stressors [2]. While both sexes are afflicted, the incidence of ME/CFS is about 3–4 times greater in females [3].
Prevalence estimates for ME/CFS vary from 400,000 to 800,000 [3,4] to 1 to 4 million Americans who meet a case definition criteria for ME/CFS with fewer than 20% actually diagnosed [5]. Identifying an objective indicator of ME/CFS would be useful, particularly to accelerate a normally protracted path to diagnosis. Because post-exertional fatigue associated with ME/CFS contributes to physical activity intolerance, a measurement of maximal oxygen consumption (VO2peak) would be expected to indicate low aerobic capacity compared to normal values for age, sex, and activity level. In fact, measurement of aerobic capacity or VO2peak in ME/CFS patients is not standard clinical practice, although VO2peak has been used to characterize functional capacity in adults [6-13] and adolescents [14] with ME/CFS. Typically, patients and/or physicians may not seek assessment using cardiopulmonary exercise tests (CPET) to measure VO2peak until one has been physically inactive or low active for at least six months or longer. Not surprisingly, reported VO2peak values of adults with ME/CFS range from 30-91% of healthy controls or predicted values for age and sex [7-14] and 86-90% of healthy controls in adolescents with CFS [14]. While low, these values are generally consistent with physical deconditioning and are often not considered to be clinically relevant. In other words, a low VO2peak from a single CPET reveals low functional capacity, but does not allow the conclusion that the subject responds abnormally to exercise. However, ME/CFS patients report that post-exertional fatigue is not alleviated by rest and sometimes persists for days or weeks following an exercise challenge [1]. Post-exertional malaise, or the exacerbation of symptoms following an increase in a ME/CFS patient’s typical level of activity, dramatically impacts the ability to carry out both physical and cognitive activities of daily living. This major symptom of ME/CFS is included in the most commonly used clinical [1,15] and research [16] case definitions.
As pointed out by Snell et al. [17], the predominant ME/CFS case definitions fail to operationally define, or provide guidance to assess responses to exertion. To learn more about the impact of physical activity on subsequent physical function, a two-test maximum effort CPET protocol has been used to assess the ability of ME/CFS patients to reproduce VO2peak 24 hours after an initial CPET [13,17,18]. It is well documented that VO2peak is highly reliable (test-retest difference ≤ 7%) [19-21] and reproducible (r ≥ 0.95-0.99) [20,22,23] in healthy active [21] and inactive adults [19,23], children [24] and many patient populations [25-30]. Thus, failure of ME/CFS patients to reproduce VO2peak within the well-established normative variation of ≤ 7% would indicate an underlying pathophysiology, and could provide a metric of the effects of PEM on physical activity tolerance and physical function. To date, studies of physical activity tolerance in ME/CFS using the two-CPET protocol are few [13,17,18], but indicate an impaired ability of ME/CFS patients to reproduce CPET results. For example, studies revealed an inability of ME/CFS to reproduce VO2peak [13,18], VO2 at ventilatory threshold [18] or work at peak and/or ventilatory threshold intensities [17] within normative variation. However, collectively, these studies have yet to provide consensus on a physiological indicator(s) of impaired metabolic response to exercise. While these studies reveal obvious physiological anomalies in the ME/CFS response to exercise stress, limited sample size [13,18] and contrary results [17] call for additional evidence to more clearly elucidate the abnormal exercise responses in ME/CFS. More information about the response of ME/CFS patients to exercise will help to further clarify their abnormal physiology and objectively document functional impairment. Based on the previous two-day CPET studies, we hypothesized that ME/CFS would be unable to reproduce normally physiological indices during a second CPET performed 24 hours following an initial CPET. Therefore, the purpose of this study was to assess the reproducibility of VO2peak in ME/CFS patients, and secondly, to examine if a post-exertional measure of VO2peak would change the classification of functional impairment using a standard classification scheme.
Methods
Participants
Participants were 22 patients ill with ME/CFS for greater than 6 months and were diagnosed by referring physicians based on Fukuda et al. [15]. Each completed a health/medical history form and cardiovascular screening index to ascertain health status for inclusion in the study. Patients were excluded whose cardiovascular status was determined to be high-risk based on published guidelines for cardiovascular disease risk assessment [31]. Seventeen females (44.8 ± 11.37 yrs) and five males (39.8 ± 13.92 yrs) were free from co-morbidities or orthopedic limitations that would affect ability to complete a maximum cycle test. The sample distribution was proportionate with the reported incidence of ME/CFS by sex (72% females; [3]). The institutional review boards of Ithaca College and Cornell University approved the study, and written informed consent was obtained from participants.
Procedures
Participants were instructed to abstain prior to the exercise tests from, 1) consuming food or smoking for 2 h, 2) caffeine or alcohol for 4 h, and 3) exercise for 24 h, and to use prescribed medications as usual on both test days. Next, they completed two CPETs (test 1, test 2) on a cycle ergometer separated by 23–24 hours.
Procedures for CPET began with pre-test resting measures of 12-lead ECG (Quinton Q4500 ECG, Quinton Instrument Co., Bothell, WA), blood pressure (BP) and heart rate (HR) that were made following five minutes of supine rest. The CPET began with 3 minutes of seated rest followed by cycling that began at 0 Watt and increased 25 Watts every two minutes until volitional exhaustion, a request to stop, or termination criteria were met [31]. Pedal cadence was 60–70 rpm on an electro-magnetically braked cycle ergometer (Lode Corival, Lode B.V., Groningen-Holland Medical Technology, Netherlands). Rating of perceived exertion (RPE; 6–20 scale [32]) was collected during the last 15 seconds of each workload, BP during the last minute of each workload, and ECG during each minute of the test. BP and ECG were monitored during recovery until the participant was within 20 bpm of pre-test resting HR and 10 mmHg of resting BP. Cycle seat height was positioned to approximately 175° of knee extension, and the same seat height was used for both tests. Expired gases were collected breath-by-breath through a two-way breathing valve, and analyzed using open circuit spirometry. The metabolic measurement system (PARVO Medics True Max 2400 metabolic measurement system, Salt Lake City, UT) was calibrated before each test with ambient air, standard gases of known concentrations and a 3-L calibration syringe. Ventilatory threshold (VT) is an analog of anaerobic threshold, and was identified from expired gases using the V-Slope [33] algorithm in the metabolic measurement system software. Ventilatory or anaerobic threshold is the exercise intensity at which metabolism transitions toward increased anaerobic energy production. The same trained investigator performed visual assessment and confirmation of the algorithm-derived VT. Testing took place in a controlled environment with temperature range of 20-24°C and 15-60% relative humidity.
Statistical analysis
Physiological and work variables at maximum and VT intensities were compared between CPETs using repeated measures ANOVA for VO2, heart rate, Work, and minute ventilation (Ve), as well as for variables derived from these measures. Maximal respiratory exchange ratio (RERpeak) was compared between CPETs using a non-parametric Wilcoxon’s Signed-Rank Test. Statistical significance was P < 0.05. Analyses were completed with SPSS (version 20, Armonk, NY:IBM Corp.).
Results
Participant ages ranged from 25 to 57 yrs with a mean of 43.7 ± 11.82 yrs and a body-mass index (BMI) of 27.4 ± 6.59 (Table 1), characterizing this cohort as overweight. In test 1, participants exhibited an average VO2peak of 21.9 ml.kg.min-1, which is low, and only 77.1% of the predicted VO2peak for age/sex-matched sedentary norms [23]. Physical characteristics and aerobic capacity of participants in this study were comparable to the 51 female ME/CFS participants studied by Snell et al. [17], whose age = 46.29 yrs, BMI = 25.96 and VO2peak (test 1) = 21.51 ml.kg.min-1. Likewise, Vermeulen et al. [18] studied 15 female ME/CFS patients who were younger (35.5 yrs) with normal weight status (BMI = 23.1), but had a similarly low aerobic capacity (test 1 VO2peak = 22.3 ml.kg.min-1) compared to the participants of the present study.
Table 1.
Physical characteristics and aerobic capacity of participants, N = 22 (mean ± SD)
| Age (y) |
43.7 (11.82) |
| Height (cm) |
167.3 (10.19) |
| Weight (kg) |
76.8 (20.28) |
| BMI (kg.M-2) |
27.4 (6.59) |
| VO2peak - Test 1 (ml.kg-1.min-1) |
21.9 (4.75) |
| METs@peak (1 MET = 3.5 ml.kg-1.min-1) |
6.26 (1.36) |
| %predVO2peak* - Test 1 | 77.1% (22.22) |
*%predicted VO2peak for sedentary subjects from Bruce et al. [23].
Test-retest changes in physiological and work variables appear in Table 2 and Figures 1 and 2. We detected significant differences in most parameters that were measured. Significant percent changes from test 1 to test 2 for measures at peak exercise decreased between 8.8 and 16.1%, and measures at ventilatory threshold decreased between 7.4 and 21.3%. Changes in physiological measures from test 1 to test 2 for individual subjects appear in Figures 3 and 4 for peak and ventilatory threshold intensities, respectively.
Table 2.
Physiological and work variables for Tests 1 and 2 at peak and ventilatory threshold (VT) intensities, N = 22 (mean ± SD)
| Peak exercise | Test 1 | Test 2 | %diff* | P |
|---|---|---|---|---|
| VO2peak (ml.kg-1.min-1) |
21.9 (4.75) |
18.6 (4.06) |
-13.8% |
.000¶ |
| %predVO2peak‡ |
77.1% (20.22) |
65.2% (15.74) |
--- |
.000¶ |
| HRpeak (bpm) |
159.4 (21.10) |
150.0 (23.05) |
-5.9% |
.001¶ |
| %predHRpeak† |
91.0% (10.75) |
85.2% (11.93) |
--- |
.002¶ |
| Work@peak (W) |
122.7 (28.77) |
105.7 (33.57) |
-12.5% |
.012|| |
| Ve peak (L .min-1) |
54.5 (17.56) |
44.6 (12.63) |
-14.7% |
.003¶ |
| VCO2peak (L .min-1) |
1.91 (.477) |
1.58 (.464) |
-16.1% |
.000¶ |
| O2 pulse@peak (ml.beat-1) |
10.48 (3.068) |
9.46 (2.697) |
-8.8% |
.003¶ |
| %predVO2peak‡ |
77.1% (20.22) |
65.2% (15.74) |
--- |
.000¶ |
| RERpeak |
1.17 (0.079) |
1.14 (0.081) |
-1.9% |
.157 |
|
Ventilatory threshold |
|
|
|
|
| VO2@VT (ml.kg-1.min-1) |
12.2 (3.68) |
9.9 (2.89) |
-15.8% |
.003¶ |
| HR@VT (bpm) |
113.5 (21.78) |
107.9 (20.61) |
-4.9% |
.086 |
| Work@VT (W) |
51.4 (24.97) |
41.4 (28.8) |
-21.3% |
.030|| |
| Ve@VT (L.min-1) |
21.2 (6.07) |
18.8 (4.86) |
-7.4% |
.035|| |
| VCO2@VT (L.min-1) |
0.86 (.343) |
0.72 (.265) |
-11.3% |
.014|| |
| O2 pulse@VT (ml.beat-1) | 8.15 (2.603) | 7.00 (2.323) | -12.6% | .003¶ |
*A negative %diff value indicates a decrease from Test 1 to Test 2.
†Percent of age-predicted maximum heart rate achieved.
‡% predicted VO2peak for sedentary subjects from Bruce et al. [23].
||Statistically significant difference between Test 1 and Test 2 at P < 0.05.
¶Statistically significant difference between Test 1 and Test 2 at P < 0.01.
Figure 1.
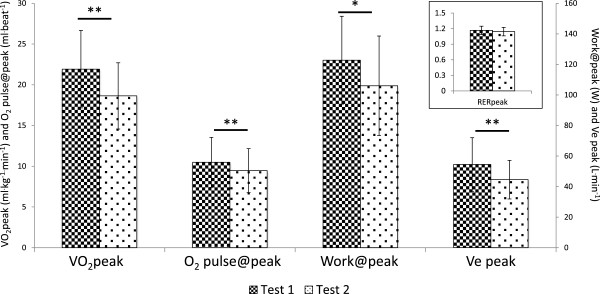
Changes in physiological and work variables from Test 1 to Test 2 at maximal intensity. Inset: Non-significant test differences for maximal respiratory exchange ratio showed that subjects achieved consistently high RER (>1.1) for Test 1 and Test 2, with maximum efforts on both tests (P = .157). Statistical significance is shown above bars with **P < 0.01 and *P < 0.05.
Figure 2.
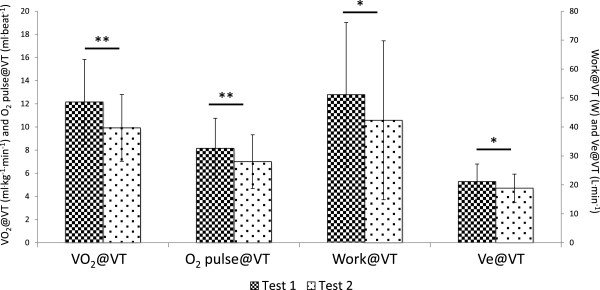
Changes in physiological and work variables from Test 1 to Test 2 at ventilatory threshold. Statistical significance is shown above bars with **P < 0.01 and *P < 0.05.
Figure 3.
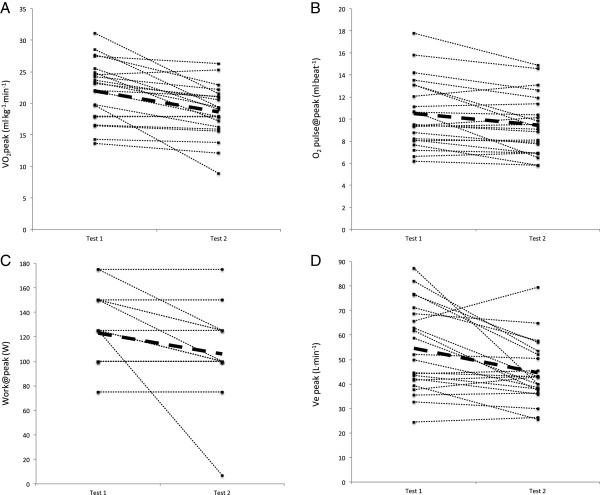
Individual changes in peak measures of VO2 (A), O2pulse (B), work (C) and Ve (D) from Test 1 to Test 2. Subjects’ whose VO2peak did not decrease during Test 2 showed a decrease in VO2@VT.
Figure 4.
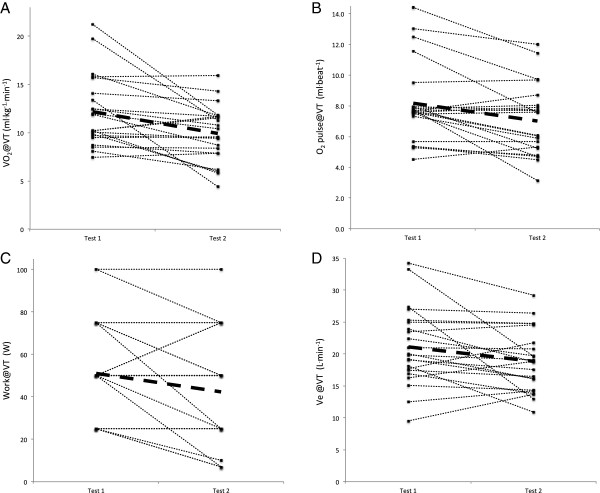
Individual changes in ventilatory threshold measures of VO2 (A), O2pulse (B), work (C) and Ve (D) from Test 1 to Test 2. Subjects’ whose VO2@VT did not decrease during Test 2 showed a decrease in VO2peak.
Participants must achieve valid indicators of maximal effort to ensure that peak exercise data reflect strong effort. Maximal effort is indicated by RER ≥1.1 [31]. The use of RER as a metric of effort during CPET is consistent with the report by the American Heart Association [22] that maximum RER during exercise is the most accurate and reliable indicator of effort. In this cohort, RER at maximal effort (RERpeak) was high (≥1.1) and did not differ between tests, indicating that ME/CFS participant effort was very strong during both CPETs.
All measures at maximal intensity decreased in test 2, including VO2, HR, Work, minute ventilation and CO2 production (VCO2). One variable, O2 pulse, a surrogate for cardiac output (O2 pulse@peak) was derived as VO2/HR. Like both VO2 and HR at peak exercise, O2 pulse also decreased indicating reduced oxygen delivery in test 2. Similarly, at ventilatory threshold intensity, all variables, except HR, decreased during test 2. A decrease in HR@VT of almost 6 bpm approached but did not achieve statistical significance (P = 0.086). The larger coefficient of variation for HR@VT (~19% for tests 1 & 2) compared to HR at maximal effort (13% for test 1, 15% for test 2) likely contributed to the lack of statistical significance. Collectively, these data indicate that ME/CFS participants were unable to reproduce most physiological measures at both maximal and ventilatory threshold intensities during test 2, despite exercising to maximal effort during both tests. Examination of individual changes from test 1 to test 2 in Figure 3 reveal that VO2peak decreased in most patients and did not change in the remaining patients. Patients whose VO2peak did not change instead demonstrated a decrease in VO2@VT shown in Figure 4. Thus, all patients demonstrated clinically significant decreases in either VO2peak and/or VO2@VT that exceed normative values for test-retest variability.
Classifying functional impairment, based on VO2peak or VO2 at ventilatory threshold, could differ for ME/CFS patients due to a decrease in CPET measures at the time of the second CPET (Table 3). Using the established classification system of Weber & Janicki [34], the functional impairment classification based on VO2peak decreased in eight participants (37%) due to the change in VO2peak from test 1 to test 2. When using VO2 at ventilatory threshold to classify functional impairment, the classification decreased in 12 participants (55%). Overall, classification of functional impairment worsened in 50% of the ME/CFS cohort due to post-exertional decrements in VO2peak and/or VO2 at VT. Using only a single CPET, 13 of 22 were classified as “A” (little to no impairment) and eight were classified as “B” (mild-moderate impairment), which would typically be attributed to physical deconditioning. Thus the actual functional impairment of ME/CFS patients is much greater than is measured by a single CPET.
Table 3.
Change from Test 1 to Test 2 in functional impairment classification using VO 2 peak and ventilatory threshold criteria[34]
| VO 2 peak(ml . kg -1. min -1 ) | Number of classes decreased (# participants) | |
|---|---|---|
|
A (>20) |
-1 class (N = 5)* |
|
|
B (16–20) |
-1 (2) |
-2 (1) |
|
C (10–15) |
|
|
|
D (<10) |
|
|
|
Ventilatory threshold(ml
.
kg
-1.
min
-1
) |
|
|
|
A (>14) |
-1 (2) |
-2 (2) |
|
B (12–14) |
-1 (3) |
-2 (1) |
|
C (9–10) |
-1 (4) |
|
|
D (6–7) |
|
|
| Number of participants whose impairment classification decreased on Test 2 based on criteria of VO2peak, VT, or both VO2peak and VT | ||
|
VO
2
peak only |
VT only |
VO
2
peak & VT |
| 4 | 7 | 5 |
*For 5 participants, impairment classification based on Test 1 VO2peak decreased from “A” to “B” using Test 2 VO2peak.
A – Little-no impairment.
B – Mild-moderate impairment.
C – Moderate-severe impairment.
D – Severe impairment.
Discussion
In this study, we sought to clarify the reproducibility of VO2 at maximal effort (VO2peak) and VO2 at ventilatory threshold (VO2@VT), an analog for anaerobic threshold, in patients ill with ME/CFS. Three studies to date [13,17,18] have demonstrated an abnormal post-exertional response to exercise in ME/CFS, but they do not agree as to which physiological measures fail to respond normally in ME/CFS. Second, we wanted to find out how a compromised test-retest response to exercise would impact a standard classification of functional impairment based on VO2peak or VO2@VT. Classification described by Weber and Janicki [34] was initially devised to categorize functional impairment/exercise intolerance in patients with chronic cardiac failure, although it is useful for other patient populations in which impaired gas exchange (oxygen consumption, carbon dioxide production, minute ventilation) contributes to exercise intolerance and limits physical function.
The test-retest changes in VO2peak that we observed are consistent with decrements reported in the three previous studies of two-day CPET response in ME/CFS [13,17,18], although the magnitude of decrease in VO2peak varied among these studies. In the first report to quantify an abnormal post-exertional response to exercise in ME/CFS, VanNess et al. [13] assessed the contribution of VO2peak measured in six females with ME/CFS and six inactive female controls to discriminate between groups. An index of maximum effort (e.g., RER) was not reported with this initial pilot study. Results indicated that a VO2peak decrement in test 2 alone correctly identified 6 of 6 ME/CFS and 5 of 6 controls, for an overall classification accuracy of 91.7%. Based on their reported mean data, VO2peak decreased during test 2 by ~22% (P = .03), in contrast to a smaller test-retest decrease observed herein of 13.8% (P < 0.001). The more robust sample size in our study may have contributed to the smaller decrease in the test-retest measures of VO2peak; however, for both studies, the test-retest decrement is considerably greater than <6-7% variability reported consistently in healthy subjects [19,20,23] and various patient populations [25-27,29,35-38].
A more recent two-day CPET assessment of ME/CFS by the same group [17] included 51 females with ME/CFS and 10 healthy, inactive controls. This study included measures at ventilatory threshold in a discriminant function analysis. Similar to their earlier study, CPET measures distinguished 95.1% of ME/CFS patients from healthy controls, with a cross-validation accuracy of 90.2%. The primary and secondary discriminating variables in this study were: 1) work at ventilatory threshold intensity (decreased ~55%) and 2) work at maximal intensity (decreased ~7%), respectively. In contrast to their first study [13], VO2peak did not contribute to the ability to distinguish ME/CFS patients in this cohort. Further, univariate analysis of VO2peak revealed no significant difference between test 1 and test 2 for ME/CFS, which was within normal variation for VO2peak.
Our results also differ from those of Vermeulen et al. [18], who measured VO2peak in 15 females with ME/CFS and 15 healthy female controls who were comparable in age and BMI. While there was a 2.2% increase (P < 0.05) in VO2peak controls, they observed a ~6.3% decrease in VO2peak (P < 0.01) in ME/CFS patients which is comparable to normal test-retest variation in healthy subjects. It is possible that methodological differences between their study and that of VanNess et al. [13] and our study contributed to the smaller decrease in VO2peak in ME/CFS patients that they detected. The cycle test protocol used by Vermeulen et al. [18] was not described in detail and appeared to vary between subjects. Reproducibility of gas exchange measures in healthy and other patient populations relies on consistent testing methodology [22]. Presumably, the protocol used for the same subject did not vary between tests, although that was not stated explicitly. Additionally, authors stated that maximum effort was assessed using RER, but the RER criterion (ie., RER ≥ 1.1) was not stated, and RER values were not reported. This is an important measure to indicate magnitude of effort, without which it is questionable whether patients gave maximal effort on both CPETs.
In addition to a 13.8% decrease in VO2peak in ME/CFS patients, we also observed decreases in maximal work (12.5%) and maximal heart rate (9 bpm). Likewise, Snell et al. [17] reported a decrease in maximal work of 7%. In repeat tests of leg extension strength and endurance, Paul et al. [39] also demonstrated a delayed recovery in ME/CFS work output with a greater decrease in quadriceps extension strength and endurance compared to controls following a 24 h repeat test. Conversely, Vermeulen et al. [18] reported no significant test-retest difference in maximal heart rate or work in ME/CFS subjects.
We observed a statistically significant test-retest decrease in maximal O2pulse of 8.8%, indicating compromised oxygen delivery in ME/CFS patients following induction of post-exertional malaise. O2pulse, a surrogate measure for stroke volume and arterio-venous oxygen content difference (a-vDO2), is a predictor of mortality in patients with cardiovascular disease [40]. It is an important index of heart function [41] and may also be associated with the onset of exercise-induced ischemia [42,43], but is also a stable and reproducible measure over time in young athletes [44] as well as adult non-athletes [45]. Vermeulen et al. [18] found a non-significant decrease of ~5% in maximal O2pulse in ME/CFS patients [18]. When this group later measured cardiac output and O2pulse during a single CPET in 178 ME/CFS patients, lower values were found in ME/CFS at VT and maximal intensities, but not at rest, compared to 11 sedentary controls. Additionally, they reported a lower arterio-venous oxygen content difference, determined non-invasively based on VO2 and cardiac output, and attributed these findings to lower O2 extraction by muscles during exercise in ME/CFS [46]. While it is not known how alteration in oxygen delivery/utilization occurs during a subsequent CPET in ME/CFS patients, these results and others [47] also suggest that the decrease in maximal O2pulse may partly explain the concomitant reduction in maximal workload in ME/CFS that we observed.
Our data showed a substantial decrease of 15.8% in test-retest VO2 at VT. Large decreases in VO2 at VT were also reported by VanNess et al. [13] (~27%) and Snell et al. [17] (~11%). Although the test-retest decrease (7%) reported by Vermeulen et al. [18] was not statistically significant, there was a significant group by test interaction (P < 0.05) due to an increase in control subjects. In contrast, gas exchange variables and work at VT are reliable and reproducible in healthy subjects and athletes [21,48], including test-retest differences of 1.5% for VO2 (r = .82-.97, Standard Error of Measurement (SE m ) = 2.64 ml.kg.min-1), and 1.5% for cycle work (SE m = 4.5 W) or treadmill velocity (SE m = 10 m.min-1) (r = .95-.99). Oxygen consumption at VT in cardiac patients (Weber class A, B, C) is also stable and reproducible in multiple measures over months, albeit with somewhat more variability (CV = 9.2%) compared to healthy subjects [38].
Work measured at VT decreased 21.3% in our subjects as well as a remarkable 55% reported by Snell et al. [17]. VanNess et al. [13] did not report work at VT, and Vermeulen et al. [18] found no significant difference in the univariate comparison of test-retest work at VT, but did find a significant group by test interaction (P < 0.05). O2 pulse at VT decreased significantly in our subjects (12.6%) and in Vermeulen et al. [18] (9%) and was not reported by VanNess et al. [13] or Snell et al. [17].
Changes in physiological measures indicate a substantial post-exertional decrement in performance at ventilatory threshold in ME/CFS 24 hours after an initial CPET. Ventilatory or anaerobic threshold intensity indicates the workload, heart rate and/or oxygen consumption at which anaerobic metabolism begins to predominate. Thus, after induction of post-exertional malaise, the threshold lowers at which anaerobic metabolism accelerates in ME/CFS. This causes premature anaerobiosis in ME/CFS patients after they have endured an earlier physical challenge, further reducing the ability to do work. It is therefore not surprising that Snell et al. [17] found that work at ventilatory threshold contributed most substantially to differentiate ME/CFS from healthy controls.
Use of a single CPET only to indicate functional impairment in ME/CFS is problematic. The results of this study, and the consensus of the three previous studies of test-retest CPETs in ME-CFS patients, provide strong evidence of impaired physiological responses to exercise. More specifically, the abnormal post-exertional responses to exercise in ME/CFS are marked by test-retest decreases in VO2 and work at both maximum and ventilatory threshold intensities. Data from a single CPET resulted in classification of 12 of 22 patients as having little or no impairment, and eight as having mild/moderate impairment. Such individuals would likely be prescribed graded exercise therapy (GET) to improve aerobic capacity. However, data from the second CPET in this and prior studies [13,17,18] indicate that aerobic energy-producing processes fail to respond normally to exercise stress in ME/CFS patients. Thus, incautiously applied GET is likely to result in exacerbation of fatigue and other symptoms of ME/CFS patients.
Little is understood about the anomalous post-exertional response to exercise in ME/CFS. We know that our data does not result from any methodological or equipment problems, because during the same time period the ME/CFS patients were being tested, we performed several repeat CPETs on healthy individuals, who demonstrated comparable or better consistency and reproducibility for VO2peak compared to published values [19-21,23,48]. The consistently high RER values during CPET 2 provide sound evidence that ME/CFS patients can, in fact, work to maximal effort in a repeat CPET. Values for maximal RER of 1.17 and 1.14 that were reported in this study would be taken as an indication of strong, maximal efforts if reported in healthy subjects and athletes [49,50]. ME/CFS patients currently represent a unique class of ill patients who do not reproduce maximal CPET measures, unlike individuals with cardiovascular disease [27,30] lung disease [28], end-stage renal disease [26], pulmonary arterial hypertension [25] and cystic fibrosis [29].
A limitation of this study should be addressed in follow-up research. Together with the three previous studies of the two-day CPET protocol [13,17,18], the collective results demonstrate consistently abnormal CPET results in ME/CFS during test 2. However, the variation in abnormal CPET responses among these studies was not clarified in the present study and requires a larger sample size with robust statistical power.
Subsequent research should strive to address the following questions regarding post-exertional fatigue in ME/CFS. Inclusion of additional males in subsequent research should allow us to ascertain whether there are sex differences in response to the two-day CPET protocol. A large sample size will be needed to determine whether we can sub-classify ME/CFS patients based on differential responses to the two-day CPET protocol at maximal and ventilatory threshold intensities. With additional participants, it would be possible to identify clinically relevant exercise measurement cutpoints and odds ratios for use by practitioners in the diagnosis and treatment of those with ME/CFS. Physical activity prior to and following the two-day CPET should be quantified to correlate changes with the decrement measured during testing.
Conclusions
The results of this study confirm previous work [13,17,18] that demonstrated an abnormal response to exercise in fatigued ME/CFS patients. The use of a two-day CPET protocol to measure the post-exertional response to exercise in ME/CFS allows us to better study the nature of this unusual, debilitating type of symptom exacerbation that follows exertion or stress, often described as post-exertional malaise or neuro-immune fatigue. Additionally, this test protocol yields information that can provide specific guidelines for exertion in ME/CFS patients in order to avoid symptom flares and that may improve daily physical function. ME/CFS patients exhibited significant post-exertional declines in VO2, work, minute ventilation and O2 pulse at both maximal and ventilatory threshold intensities. Consequently, classification of functional impairment based on VO2peak and VO2 at ventilatory threshold over-estimated the functional ability of 50% of ME/CFS in this sample when based on only one CPET.
Abbreviations
BP: Blood pressure; CDC: Centers for disease control and prevention; ME/CFS: Myalgic encephalomyelitis/chronic fatigue syndrome; CPET: Cardiopulmonary exercise test; ECG: Electrocardiogram; HRpeak: Maximal heart rate; ANOVA: Analysis of variance; PEM: Post-exertional malaise; RER: Respiratory exchange ratio; RPE: Rating of perceived exertion; Ve peak: Maximal minute ventilation; VO2peak: Maximal oxygen consumption; VT: Ventilatory threshold; Work@peak: Maximal workload; O2pulse: oxygen pulse.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. BK wrote the first draft, and LG and JP contributed to revisions until the final manuscript was achieved. JP also contributed to collection of data and preparation of the manuscript for submission. LG contributed to data analysis and preparation of the manuscript for submission. All authors read and approved the final manuscript.
Contributor Information
Betsy A Keller, Email: keller@ithaca.edu.
John Luke Pryor, Email: luke.pryor@uconn.edu.
Ludovic Giloteaux, Email: ludovicgiloteaux@gmail.com.
Acknowledgements
The authors thank Sarah Simunovich. M.S., Dept. of Exercise & Sport Sciences at Ithaca College, for assistance with the statistical analysis, and Maureen Hanson, Ph.D., Dept. of Molecular Biology & Genetics at Cornell University, for guidance and review of this manuscript. This study was supported, in part, by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) grant 1R21AI090553.
References
- Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, Bested AC, Flor-Henry P, Joshi P, Powles AP. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. 2003;11:7–115. [Google Scholar]
- Komaroff AL, Buchwald DS. Chronic fatigue syndrome: an update. Annu Rev Med. 1998;49:1–13. doi: 10.1146/annurev.med.49.1.1. [DOI] [PubMed] [Google Scholar]
- Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, McCready W, Huang CF, Plioplys S. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- Reyes M, Nisenbaum R, Hoaglin DC, Unger ER, Emmons C, Randall B, Stewart JA, Abbey S, Jones JF, Gantz N, Minden S, Reeves WC. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163:1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- The 1994 case definition. [ http://cdc.gov/cfs/diagnosis/index.html]
- Cook DB, Nagelkirk PR, Poluri A, Mores J, Natelson BH. The influence of aerobic fitness and fibromyalgia on cardiorespiratory and perceptual responses to exercise in patients with chronic fatigue syndrome. Arthritis Rheum. 2006;54:3351–3362. doi: 10.1002/art.22124. [DOI] [PubMed] [Google Scholar]
- Cook DB, Stegner AJ, Nagelkirk PR, Meyer JD, Togo F, Natelson BH. Responses to exercise differ for chronic fatigue syndrome patients with fibromyalgia. Med Sci Sports Exerc. 2012;44:1186–1193. doi: 10.1249/MSS.0b013e3182417b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Becker P, Roeykens J, Reynders M, McGregor N, De Meirleir K. Exercise capacity in chronic fatigue syndrome. Arch Intern Med. 2000;160:3270–3277. doi: 10.1001/archinte.160.21.3270. [DOI] [PubMed] [Google Scholar]
- Farquhar WB, Hunt BE, Taylor JA, Darling SE, Freeman R. Blood volume and its relation to peak O(2) consumption and physical activity in patients with chronic fatigue. Am J Physiol Heart Circ Physiol. 2002;282:H66–H71. doi: 10.1152/ajpheart.2002.282.1.H66. [DOI] [PubMed] [Google Scholar]
- Inbar O, Dlin R, Rotstein A, Whipp BJ. Physiological responses to incremental exercise in patients with chronic fatigue syndrome. Med Sci Sports Exerc. 2001;33:1463–1470. doi: 10.1097/00005768-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Sargent C, Scroop GC, Nemeth PM, Burnet RB, Buckley JD. Maximal oxygen uptake and lactate metabolism are normal in chronic fatigue syndrome. Med Sci Sports Exerc. 2002;34:51–56. doi: 10.1097/00005768-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Snell CR, VanNess JM, Strayer DR, Stevens SR. Exercise capacity and immune function in male and female patients with chronic fatigue syndrome (CFS) In Vivo. 2005;19:387–390. [PubMed] [Google Scholar]
- VanNess JM, Snell CR, Stevens SR. Diminished cardiopulmonary capacity during post-exertional malaise. J Chronic Fatigue Syndr. 2007;14:77–85. [Google Scholar]
- VanNess JM, Snell CR, Strayer DR, Dempsey L, Stevens SR. Subclassifying chronic fatigue syndrome through exercise testing. Med Sci Sports Exerc. 2003;35:908–913. doi: 10.1249/01.MSS.0000069510.58763.E8. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International chronic fatigue syndrome study group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Sharpe MC, Archard LC, Banatvala JE, Borysiewicz LK, Clare AW, David A, Edwards RH, Hawton KE, Lambert HP, Lane RJ, McDonald EM, Mowbray JF, Pearson DJ, Peto TEA, Preedy VR, Smith AP, Smith DG, Taylor DJ, Tyrrell DAJ, Wessely S, White PD, Behan PO, Rose FC, Peters TJ, Wallace PG, Warrell DA, Wright DJM. A report–chronic fatigue syndrome: guidelines for research. J R Soc Med. 1991;84:118–121. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell CR, Stevens SR, Davenport TE, Van Ness JM. Discriminative validity of metabolic and workload measurements to identify individuals with chronic fatigue syndrome. Phys Ther. 2013;93:1484–1492. doi: 10.2522/ptj.20110368. [DOI] [PubMed] [Google Scholar]
- Vermeulen RC, Kurk RM, Visser FC, Sluiter W, Scholte HR. Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity. J Transl Med. 2010;8:93. doi: 10.1186/1479-5876-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katch VL, Sady SS, Freedson P. Biological variability in maximum aerobic power. Med Sci Sports Exerc. 1982;14:21–25. doi: 10.1249/00005768-198201000-00004. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol. 1955;8:73–80. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- Weltman A, Snead D, Stein P, Seip R, Schurrer R, Rutt R, Weltman J. Reliability and validity of a continuous incremental treadmill protocol for the determination of lactate threshold, fixed blood lactate concentrations, and VO2max. Int J Sports Med. 1990;11:26–32. doi: 10.1055/s-2007-1024757. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician’s guide to cardiopulmonary exercise testing in adults a scientific statement from the American heart association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Welsman J, Bywater K, Farr C, Welford D, Armstrong N. Reliability of peak VO(2) and maximal cardiac output assessed using thoracic bioimpedance in children. Eur J Appl Physiol. 2005;94:228–234. doi: 10.1007/s00421-004-1300-5. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Sun XG, Yasunobu Y, Garafano RP, Gates G, Barst RJ, Wasserman K. Reproducibility of cardiopulmonary exercise measurements in patients with pulmonary arterial hypertension. Chest. 2004;126:816–824. doi: 10.1378/chest.126.3.816. [DOI] [PubMed] [Google Scholar]
- Koufaki P, Naish PF, Mercer TH. Reproducibility of exercise tolerance in patients with end-stage renal disease. Arch Phys Med Rehabil. 2001;82:1421–1424. doi: 10.1053/apmr.2001.26076. [DOI] [PubMed] [Google Scholar]
- Lehmann G, Kolling K. Reproducibility of cardiopulmonary exercise parameters in patients with valvular heart disease. Chest. 1996;110:685–692. doi: 10.1378/chest.110.3.685. [DOI] [PubMed] [Google Scholar]
- Marciniuk DD, Watts RE, Gallagher CG. Reproducibility of incremental maximal cycle ergometer testing in patients with restrictive lung disease. Thorax. 1993;48:894–898. doi: 10.1136/thx.48.9.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone EF, Barry SC, FitzGerald MX, Gallagher CG. Reproducibility of maximal exercise ergometer testing in patients with cystic fibrosis. Chest. 1999;116:363–368. doi: 10.1378/chest.116.2.363. [DOI] [PubMed] [Google Scholar]
- Meyer K, Westbrook S, Schwaibold M, Hajric R, Peters K, Roskamm H. Short-term reproducibility of cardiopulmonary measurements during exercise testing in patients with severe chronic heart failure. Am Heart J. 1997;134:20–26. doi: 10.1016/S0002-8703(97)70102-9. [DOI] [PubMed] [Google Scholar]
- ACSM’s Guidelines for Exercise Testing and Prescription. 9. Philadelpha: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81:II14–II30. doi: 10.1161/01.CIR.81.1.14. [DOI] [PubMed] [Google Scholar]
- Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol. 1985;55:22A–31A. doi: 10.1016/0002-9149(85)90792-1. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal A, Zannad F, Kayanakis JG, Gueret P, Aupetit JF, Kolsky H. Multicentre study of the determination of peak oxygen uptake and ventilatory threshold during bicycle exercise in chronic heart failure. Comparison of graphical methods, interobserver variability and influence of the exercise protocol. The VO2 French study group. Eur Heart J. 1991;12:1055–1063. doi: 10.1093/oxfordjournals.eurheartj.a059837. [DOI] [PubMed] [Google Scholar]
- Cox NJ, Hendriks JC, Binkhorst RA, Folgering HT, van Herwaarden CL. Reproducibility of incremental maximal cycle ergometer tests in patients with mild to moderate obstructive lung diseases. Lung. 1989;167:129–133. doi: 10.1007/BF02714939. [DOI] [PubMed] [Google Scholar]
- Dobrovolny CL, Ivey FM, Rogers MA, Sorkin JD, Macko RF. Reliability of treadmill exercise testing in older patients with chronic hemiparetic stroke. Arch Phys Med Rehabil. 2003;84:1308–1312. doi: 10.1016/S0003-9993(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Janicki JS, Gupta S, Ferris ST, McElroy PA. Long-term reproducibility of respiratory gas exchange measurements during exercise in patients with stable cardiac failure. Chest. 1990;97:12–17. doi: 10.1378/chest.97.1.12. [DOI] [PubMed] [Google Scholar]
- Paul L, Wood L, Behan WM, Maclaren WM. Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. Eur J Neurol. 1999;6:63–69. doi: 10.1046/j.1468-1331.1999.610063.x. [DOI] [PubMed] [Google Scholar]
- Oliveira RB, Myers J, Araujo CG, Abella J, Mandic S, Froelicher V. Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur J Cardiovasc Prev Rehabil. 2009;16:358–364. doi: 10.1097/HJR.0b013e3283292fe8. [DOI] [PubMed] [Google Scholar]
- Åstrand P-O, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Lacalaprice F, Carle F, Minnucci A, Cianci G, Perna G, D’Eusanio G. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J. 2003;24:1304–1313. doi: 10.1016/S0195-668X(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Munhoz EC, Hollanda R, Vargas JP, Silveira CW, Lemos AL, Hollanda RM, Ribeiro JP. Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Med Sci Sports Exerc. 2007;39:1221–1226. doi: 10.1249/mss.0b013e3180601136. [DOI] [PubMed] [Google Scholar]
- Perim RR, Signorelli GR, Araujo CG. Stability of relative oxygen pulse curve during repeated maximal cardiopulmonary testing in professional soccer players. Braz J Med Biol Res. 2011;44:700–706. doi: 10.1590/s0100-879x2011007500073. [DOI] [PubMed] [Google Scholar]
- Oliveira RB, Myers J, Araujo CG. Long-term stability of the oxygen pulse curve during maximal exercise. Clinics (Sao Paulo) 2011;66:203–209. doi: 10.1590/S1807-59322011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen RC, van Eck IW V. Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome. J Transl Med. 2014;12:20–26. doi: 10.1186/1479-5876-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.RES.58.2.281. [DOI] [PubMed] [Google Scholar]
- Aunola S, Rusko H. Reproducibility of aerobic and anaerobic thresholds in 20–50 year old men. Eur J Appl Physiol Occup Physiol. 1984;53:260–266. doi: 10.1007/BF00776600. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Wilmore KM, Jaskolska A, Jaskolski A, Daw EW, Rice T, Gagnon J, Leon AS, Wilmore JH, Rao DC, Bouchard C. Reproducibility of maximal exercise test data in the HERITAGE family study. Med Sci Sports Exerc. 1999;31:1623–1628. doi: 10.1097/00005768-199911000-00020. [DOI] [PubMed] [Google Scholar]
- Weston SB, Gabbett TJ. Reproducibility of ventilation of thresholds in trained cyclists during ramp cycle exercise. J Sci Med Sport. 2001;4:357–366. doi: 10.1016/S1440-2440(01)80044-X. [DOI] [PubMed] [Google Scholar]