Abstract
The left insula or Broca’s area have been proposed as the neuroanatomical correlate for apraxia of speech (AOS) based on studies of patients with both AOS and aphasia due to stroke. Studies of neurodegenerative AOS suggest the premotor area and the supplementary motor areas as the anatomical correlates. The study objective was to determine the common infarction area in patients with pure AOS due to stroke. Patients with AOS and no or equivocal aphasia due to ischemic stroke were identified through a pre-existing database. Seven subjects were identified. Five had pure AOS, and two had equivocal aphasia. MRI lesion analysis revealed maximal overlap spanning the left premotor and motor cortices. While both neurodegenerative AOS and stroke induced pure AOS involve the premotor cortex, further studies are needed to establish whether stroke-induced AOS and neurodegenerative AOS share a common anatomic substrate.
Keywords: Apraxia of speech, stroke, aphemia, premotor cortex
1. Introduction
Apraxia of speech (AOS) is a motor speech disorder characterized by slow speech rate, segmentation of syllables, sound distortions, distorted substitutions, trial-and error articulatory movements, and increased difficulty with increased length and complexity of utterances (Duffy, 2013). Although AOS is frequently grouped under the heading of aphasia, the two disorders are clinically distinguishable even though they frequently co-occur. Numerous synonyms for AOS exist including verbal apraxia, phonetic disintegration syndrome, and aphemia, adding to the confusion (Duffy, 2013).
The neuroanatomical correlates of AOS are controversial. In neurodegenerative disease, atrophy in premotor and supplementary motor cortices correlate with AOS (Josephs et al., 2006). Case reports of AOS due to stroke implicate Broca’s area, the precentral gyrus or the insula (Schiff, Alexander, Naeser, & Galaburda, 1983; Shuren, 1993). Few larger studies have addressed the neuroanatomy of AOS in stroke. The insula was implicated in two studies with larger lesions corresponding to more severe apraxia (Dronkers, 1996; Ogar et al., 2006). The premotor region has been implicated in lesions with pure AOS, although these findings should be interpreted with caution because the study was a post-hoc analysis of prior MRI lesion studies (Robin, Jacks, & Ramage, 2007). Another study, which took into account the relative vulnerability of the insula to middle cerebral artery thrombosis, concluded that the left posterior inferior frontal gyrus (Broca’s area) was the neuroanatomical correlate of AOS (Hillis et al., 2004). The findings of Broca’s area as the anatomical correlate for AOS were replicated in a large high-resolution structural and perfusion MRI study (Richardson, Fillmore, Rorden, Lapointe, & Fridriksson, 2012). Perfusion imaging can demonstrate areas outside the diffusion weighted abnormality identifying areas which are dysfunctional but eventually survive the ischemic insult. Therefore, studies which include only traditional MRI or CT scans may miss areas that were dysfunctional but subsequently recovered. Prior work has shown that Broca’s area can be hypoperfused but not infarcted in some patients with apraxia of speech (Hillis, et al., 2004).
Subjects with pure AOS due to stroke are rare because the commonest cause of AOS, middle cerebral artery infarction, often results in aphasia. Investigators have reported different neuroanatomical localizations of AOS because of different study designs (e.g. lesion analysis, perfusion-weighted imaging), different patient populations investigated (acute AOS, chronic AOS), the common co-occurrence between aphasia and AOS, and the possibility that more than one region is responsible for motor speech programming. The present study attempts to address the third issue (i.e. co-occurrence of aphasia with AOS) by studying participants with AOS but not aphasia. The area of infarction in patients with AOS but without aphasia may be smaller than AOS with aphasia and allow for more precise identification of the area or areas of the brain crucial to (or sufficient for) the development of AOS. Our objective was to determine the common area of infarction in patients with AOS with equivocal or absent aphasia.
2. Material and methods
2.1. Patients
All stroke patients who underwent evaluation by a speech-language pathologist between January 1st, 1998 and February 1st, 2012 were identified through a database kept by the speech-language pathologists. We identified a subgroup of patients with AOS from ischemic stroke. From these, by chart review, we identified those with “pure” apraxia of speech (i.e. absent or equivocal aphasia). Patients with hemorrhagic strokes were excluded.
2.2. Speech and language testing
AOS diagnosis was based on the presence of the perceptual features of the disorder described in the Introduction and summarized in Table 1; these diagnostic features are consistent with those described in the literature (Duffy, 2013). AOS and aphasia diagnoses were determined by one of two speech-language pathologists (JRD and EAS) who examined each patient with a speech and language protocol designed to elicit features of aphasia (e.g., spoken or written language comprehension errors; semantic or phonologic errors; word retrieval delays or reduced rapid word retrieval; grammatical errors, spelling errors) and AOS, including picture description, word and sentence repetition, comprehension of language, naming and writing. Prior studies have established reliability in determining AOS between these examiners (Josephs, et al., 2006). All patients consented to the use of their clinical records for the purpose of research and the study was approved by the Mayo Clinic IRB.
Table 1.
Speech and language characteristics in each patient
| Case Number | Slow rate | Distorted substitutions | Vowel distortions | Increased sound errors with increased complexity | Segmentation of words and/or syllables | Self- correction of sound level errors | Articulatory groping | Verbal comprehension errors | Naming (semantic or phonemic paraphasias) | Reading comprehension errors | Writing errors (spelling, semantic or grammatic) | Word retrieval delays |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | yes | yes | yes | yes | yes | yes | yes | Normal | Normal | Normal | a Abnormal | Occasional |
| 2 | yes | yes | yes | yes | yes | yes | no | Normal | Normal | Normal | b Abnormal | None |
| 3 | yes | yes | yes | yes | yes | no | no | Normal | Normal | Normal | Normal | None |
| 4 | yes | yes | yes | no | yes | no | yes | Normal | Normal | Normal | Normal | None |
| 5 | yes | yes | yes | yes | yes | yes | yes | Normal | Normal | Normal | Normal | None |
| 6 | yes | yes | yes | no | no | yes | yes | Normal | Normal | Normal | Not tested | None |
| 7 | yes | yes | yes | yes | yes | yes | yes | Normal | Normal | Normal | Normal | None |
Self-generated writing was normal, but written sentences to dictation contained spelling errors (e.g., plese/please).
Normal to dictation, some mild difficulty starting self-generated sentence.
2.3. Neuroimaging Methods
The brain magnetic resonance imaging (MRI) studies performed closest to the time of the infarction were used for the lesion analysis (range: 1–10 days). The MRI scanners and acquisition parameters varied widely across the 14 years surveyed in this analysis commensurate with the clinical workflow in place at the time of the individual study’s acquisition. Field of view ranged from 20–22 cm with an in-plain matrix of 256 × 256 and slice thicknesses ranging from 4–5 mm. Field strength varied from 1.5–3T. With this protocol variability and the small number of subjects identified with pure AOS, a detailed morphometric analysis would not provide valid results. However, given the well circumscribed lesions identified in patients presenting with pure AOS, the data were well suited to be subjected to a lesion tracing analysis focusing on the frequency of involvement of large-scale functional brain areas.
There are several circumstances in which large functional regions of interest may be preferred rather than analyzing on a millimeter-scale. One of the most common rationales for such an approach is avoiding type I error by limiting the multiple comparisons problem. Another common rational is to reduce type II error by using a priori anatomic and functional information (e.g. Brodmann’s areas) to increase the sensitivity of the analysis to identify large-scale functional brain regions at the expense of millimeter level resolution. In addition, given that the information processing units in the brain span multiple spatial scales (e.g. neuron, column, circuit, and large-scale systems of brain areas), it is important to consider the scale of observation that is best suited to the available data and the scientific question under consideration. In this study, we aim to investigate the lesional disruption of the large-scale motor-language network at the spatial resolution of large-scale functional brain areas.
The T2 weighted sequences were reoriented, normalized and resampled to the resolution of the SPM8 T2 template using the unified segmentation and normalization procedure in SPM8. All resampled and normalized images were visually inspected and confirmed to have undergone high quality normalization. Each subject’s lesion was then manually traced using the MRIcron software package (Rorden & Brett, 2000). Lesion location was verified with diffusion weighted images when available. The Brodmann’s areas (BA) template available within the MRIcron software package was used to identify the location of maximal lesion overlap and regional topographic frequency of lesion occurrence. The regional topographic frequency was defined as the number of lesions located within a functional brain region (BA) relative to the number of lesions examined (n = 7).
3. Results
3.1. Patient characteristics
Seven patients met entry criteria, median age 68 (range: 49–72). Two had mild right upper extremity weakness, and two had mild right facial weakness; neurological examinations were otherwise unremarkable. Patients were evaluated at a median of 3 days after stroke (range: 1–17 days) by a speech-language pathologist. Language and AOS features for each patient are summarized (Table 1). No patients had non-verbal oral apraxia or significant dysarthria. Five had “pure” AOS, while two had AOS and equivocal evidence of aphasia. In patient 1, aphasia was considered equivocally present based on reduced rapid word retrieval and written language errors, although his writing difficulty may have reflected long-standing learning problems. In patient 2, difficulty initiating a self-generated phrase suggested equivocal aphasia. Four strokes were cardioembolic, one was from carotid artery disease, one was embolic in the setting of a hypercoagulable state (metastatic ovarian cancer), and one was cryptogenic.
3.2. MRI findings
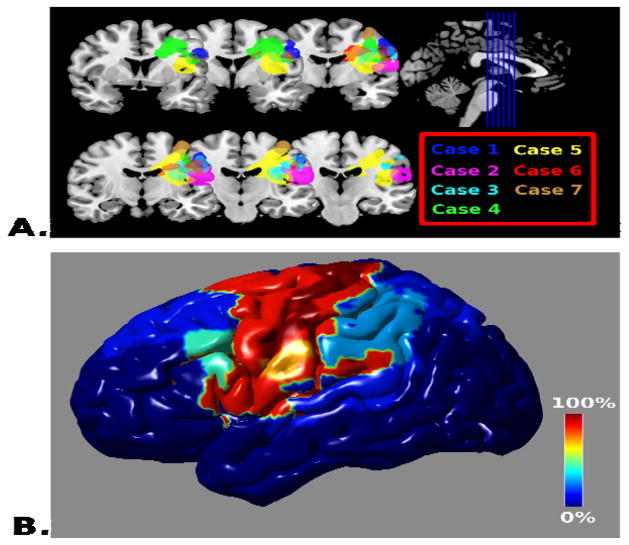
All strokes occurred in the left hemisphere with an area spanning the motor and premotor cortices being the region of maximum overlap (Figure 1a). Six of seven strokes involved premotor cortex (BA6) and 7/7 involved the primary motor cortex (BA 4). Three of seven strokes involved BA 44. Two of seven strokes involved the left insula. Table 2 reports topographic frequency for BAs. See Figure 1b for a frequency mapping of lesion occurrence for all BAs involved.
Figure 1.
Topographic Distribution of Lesion Apraxia of Speech (A) The lesions for each of the seven cases is overlaid on coronal sections of the template brain using MRIcron(Rorden & Brett, 2000) with color coding matching the case numbers in the Table. (B) The regional frequency of lesion occurrence within Brodmann’s areas (expressed as the percentage of cases located within the region) displayed on a rendering of the cortical surface using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Table 2.
Lesion topographic frequency using Brodmann’s area (BA)
| BA | Percent | Case # | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 100 | 1–7 | yes | yes | yes | yes | yes | yes | yes |
| 3 | 85.71428571 | 1–6 | yes | yes | yes | yes | yes | yes | no |
| 6 | 85.71428571 | 1,3–7 | yes | no | yes | yes | yes | yes | yes |
| 48 | 85.71428571 | 1–6 | yes | yes | yes | yes | yes | yes | no |
| 43 | 71.42857143 | 1–4,6 | yes | yes | yes | yes | no | yes | no |
| 44 | 42.85714286 | 1,4,5 | yes | no | no | yes | yes | no | no |
| 2 | 28.57142857 | 2,3 | no | yes | yes | no | no | no | no |
| 40 | 28.57142857 | 1,3 | yes | no | yes | no | no | no | no |
| 1 | 14.28571429 | 3 | no | no | yes | no | no | no | no |
| 5 | 14.28571429 | 3 | no | no | yes | no | no | no | no |
| 7 | 14.28571429 | 3 | no | no | yes | no | no | no | no |
| 8 | 14.28571429 | 4 | no | no | no | yes | no | no | no |
| 9 | 14.28571429 | 4 | no | no | no | yes | no | no | no |
| 22 | 14.28571429 | 2 | no | yes | no | no | no | no | no |
| 23 | 14.28571429 | 5 | no | no | no | no | yes | no | no |
| 42 | 14.28571429 | 2 | no | yes | no | no | no | no | no |
4. Discussion
In this study, we found that in patients with stroke induced AOS without significant coexisting aphasia, the neuroanatomical correlate was in the premotor cortex and adjacent precentral gyrus. Prior studies have included patients with AOS and aphasia caused by larger areas of infarction (Dronkers, 1996; Hillis, et al., 2004). Although few cases of AOS without aphasia have been reported, one case of severe AOS (called aphemia) without aphasia described a lesion strikingly similar to the area of overlap in our cases at the junction of BA 4 and 6 (Fox, Kasner, Chatterjee, & Chalela, 2001). Further, in the study by Hillis et al., three subjects had lesions of the precentral gyrus without involvement of Broca’s area (Hillis, et al., 2004).
The current study is unique because most other reports of AOS have included cases with coexisting aphasia. For example, in one lesional study, all 26 AOS patients had coexisting aphasia, and in another, 17/18 patients had significant coexisting aphasia (Ogar, et al., 2006; Richardson, et al., 2012). Our patients most often had small embolic lesions and were evaluated by experienced clinicians soon thereafter. By excluding cases with coexisting aphasia, the lesion size in our cases was smaller than prior studies, allowing for more precise localization.
In the study by Dronkers (1996), 44 left hemisphere stroke patients were evaluated at least one year after the stroke. Twenty five of the patients had AOS. A common area of overlap involving the left anterior insula was identified in the AOS cases (Dronkers, 1996). As subsequently pointed out (Hillis, et al., 2004), the results reported by Dronkers may have been biased because the insula is one of the most common areas injured in all left MCA strokes. In a subsequent study of chronic AOS using high resolution MRI scans and perfusion weighted imaging, predominantly Broca’s area damage but to a lesser extent precentral gyrus and premotor region damage predicted AOS (Richardson, et al., 2012). Hillis et al. analyzed the lesions of 40 left hemisphere strokes with and without insula damage in acute stroke patients, including 31 cases with AOS. They found no association between AOS and insula damage, and instead, found that AOS was associated with ischemia in Broca’s area (Hillis, et al., 2004). Perfusion imaging was also used and demonstrated hypoperfusion in Broca’s area in the absence of diffusion weighted abnormality. These studies highlight the supplemental information perfusion imaging can provide in addition to lesion analysis. However, five cases with AOS who did not have Broca’s area lesions were also noted, and they suggested that in those patients the lesions responsible for the AOS were in the precentral and postcentral gyrus area (Hillis, et al., 2004). Our findings also demonstrate lesion overlap in the precentral and postcentral gyri.
The neuroanatomical correlates of neurodegenerative AOS without aphasia have been studied with voxel based morphometry (Josephs, et al., 2006) with findings demonstrating grey matter atrophy predominantly in the superior premotor cortex spreading to the precentral gyrus, and in the supplemental motor area. In contrast, neurodegenerative patients with aphasia plus AOS had atrophy of the premotor cortex and greater involvement of Broca’s area (Josephs, et al., 2006). In a different study, the degree of AOS correlated with premotor volume whereas agrammatic aphasia severity correlated with Broca’s area (Whitwell et al., 2013). The study by Hillis et al. seems congruent with these findings as most cases in their study likely had aphasia and AOS, akin to the neurodegenerative cases with aphasia and AOS (Hillis, et al., 2004). While the cases in our study also involve the premotor and precentral gyrus similar to neurodegenerative cases, differences in methodologies between the studies limit our ability to match region of maximal atrophy in the neurodegenerative cases to the region of greatest lesion overlap reported here.
Previously, the discrepant anatomic findings from the neurodegenerative causes of AOS could not be reconciled with stroke-induced AOS. Our study provides a link between the stroke-induced lesion cases and degenerative cases. When AOS occurs with aphasia, in both degenerative and stroke cases, Broca’s area appears to be damaged consistently. In contrast, when AOS occurs without aphasia, the left premotor cortex and precentral gyrus are involved in both the stroke and neurodegenerative causes.
A motor speech network has been reliably identified through meta-analysis of functional magnetic resonance imaging (Eickhoff, Heim, Zilles, & Amunts, 2009). The network consists of the inferior frontal gyrus, face region of the motor cortex, anterior insula, and the lateral premotor cortex in addition to the cerebellum and head of the caudate. Interestingly, the inferior frontal gyrus, the anterior insula and the premotor cortex have all been implicated in stroke induced or neurodegenerative AOS. Rather than a single anatomic lesion causing AOS, it is more likely that a lesion in a key hub or the connections between hubs of the motor speech network can cause AOS. The location and size of lesion likely play critical roles in determining the presence and severity of coexisting features with AOS. For example, lesions involving the anterior insula tend to be large and cause chronic AOS and aphasia (Trupe et al., 2013). Broca’s area lesions also cause AOS and aphasia (Hillis, et al., 2004). This is in contrast to rare lesions involving the premotor cortex as it joins the primary motor cortex which can cause a pure AOS.
While the premotor cortex is consistently activated as part of the motor speech network, its role in speech is incompletely understood. Electrical stimulation of the ventral premotor cortex during surgical mapping induces anarthria (Duffau et al., 2003). A network model of the motor speech network suggests that premotor cortex serves as a final common pathway for translating intended movements after receiving information from the basal ganglia and cerebellum (Eickhoff, et al., 2009). Therefore, based on this model, a directed stroke to the premotor region could cause AOS.
Limitations of the present study include the small sample size due to the rarity of AOS with aphasia. In addition, since perfusion imaging was not performed in our cases it is possible that hypoperfusion of Broca’s area was present in the acute setting in the four cases without Broca’s area involvement. Comparison of stroke induced AOS to the anatomy of neurodegenerative AOS must be made with caution given the differences in imaging modalities used. While both stroke induced AOS and neurodegenerative AOS overlap in BA 4 and 6, subregional analysis was not possible due to difference between studies. Additionally, only acute stroke patients were studied, thus, limiting our ability evaluate what damage to these regions means for speech motor programming generally. Limited follow-up information was available on these patients. AOS may have resolved in these patients; it is possible that in order to have persistent AOS disconnection or a functional impairment of Broca’s area may be required.
5. Conclusions
In stroke-induced acute AOS with equivocal or no aphasia, an area involving the left premotor and motor cortices is the region of greatest overlap. When this clinical syndrome is seen, physicians should suspect an embolic stroke in the premotor/motor area. Our results differ from prior studies because we excluded coexisting aphasia. Further research is needed to establish if stroke-induced AOS and neurodegenerative AOS share a common anatomic substrate.
Highlights.
We assessed the neuroanatomical correlate of pure apraxia of speech after stroke.
MRI lesion analysis revealed a region of maximal overlap spanning the left premotor and motor cortices.
Our study links stroke-induced pure AOS to primary progressive apraxia of speech
Acknowledgments
Role of the funding source- The study was funded by National Institutes of Health grants R01 DC 010367
Footnotes
Disclosure statement
The authors do not have any conflicts of interest.
Disclosures:
Dr. Graff-Radford and Dr. Jones have no disclosures
Dr. Josephs receives research support from the NIH (NIDCD and NIA), and the Dana Foundation.
Dr. Rabinstein is an editor for Neurology
Dr. Duffy receives research support from the NIH (NIDCD).
Dr. Strand receives research support from the NIH (NIDCD).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, et al. The role of dominant premotor cortex in language: a study using intraoperative functional mapping in awake patients. NeuroImage. 2003;20(4):1903–1914. doi: 10.1016/s1053-8119(03)00203-9. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor Speech Disorders. 3. St. Louis: Elsevier; 2013. Apraxia of Speech; pp. 269–289. [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. A systems perspective on the effective connectivity of overt speech production. Philosophical transactions Series A, Mathematical, physical, and engineering sciences. 2009;367(1896):2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ, Kasner SE, Chatterjee A, Chalela JA. Aphemia: an isolated disorder of articulation. Clinical neurology and neurosurgery. 2001;103(2):123–126. doi: 10.1016/s0303-8467(01)00126-3. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain : a journal of neurology. 2004;127(Pt 7):1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(Pt 6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo J, Wilkins D, Ludy C, Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain and language. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Re-establishing Broca’s initial findings. Brain and language. 2012;123(2):125–130. doi: 10.1016/j.bandl.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin DA, Jacks A, Ramage AE. The Neural Substrates of Apraxia of Speech as Uncovered by Brain Imaging: A Critical Review. In: IRJ, editor. Neuroimaging in Communication Sciences and Disorders. San Diego: Plural Publishing; 2007. [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Schiff HB, Alexander MP, Naeser MA, Galaburda AM. Aphemia. Clinical-anatomic correlations. Archives of neurology. 1983;40(12):720–727. doi: 10.1001/archneur.1983.04050110038005. [DOI] [PubMed] [Google Scholar]
- Shuren J. Insula and aphasia. J Neurol. 1993;240(4):216–218. doi: 10.1007/BF00818707. [DOI] [PubMed] [Google Scholar]
- Trupe LA, Varma DD, Gomez Y, Race D, Leigh R, Hillis AE, et al. Chronic Apraxia of Speech and Broca’s Area. Stroke; a journal of cerebral circulation. 2013;44(3):740–744. doi: 10.1161/STROKEAHA.112.678508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain and language. 2013;125(3):245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]