Abstract
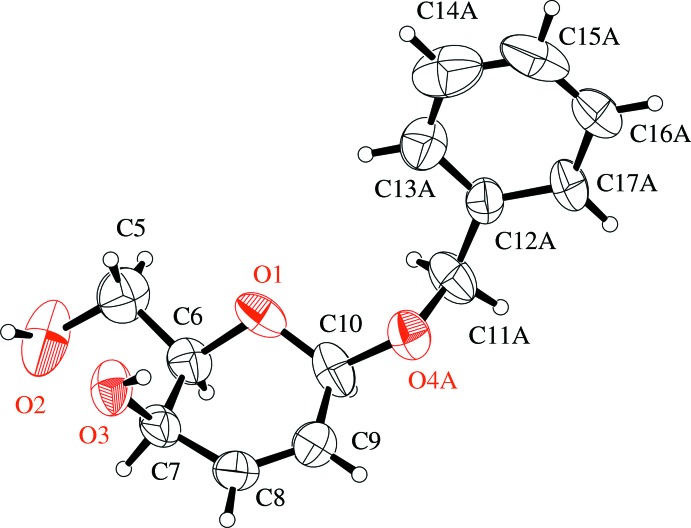
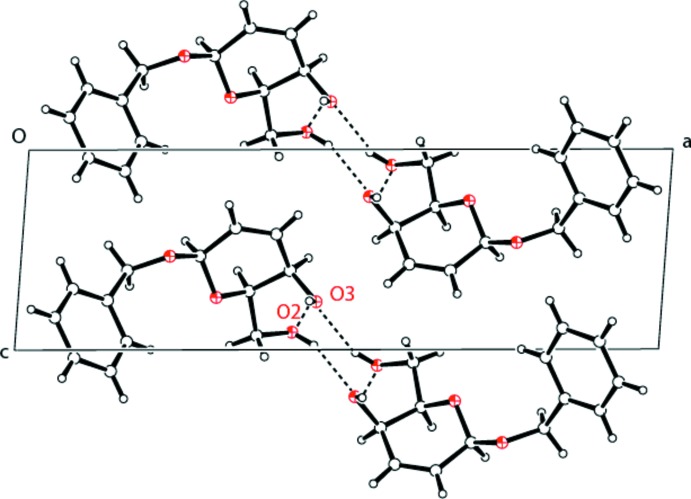
In the title compound, C13H16O4, the six-membered ring of the sugar moiety shows a half-chair conformation. In the crystal, molecules are connected via O—H⋯O hydrogen bonds, forming columns around twofold screw axes along the b-axis direction. There is a disorder of the benzyloxy group, which has two possible orientations with the phenyl group lying on a common plane [site-occupancy factors = 0.589 (9) and 0.411 (9)].
Related literature
For the phenolic Ferrier reaction, see: Ferrier & Prasad (1969 ▶); Noshita et al. (1995 ▶). For the structure of the related compound α-glycoside, see: Wingert et al. (1984 ▶). For the synthesis of β-glycoside, see: Di Bussolo et al. (2002 ▶, 2004 ▶). For the enzymatic regioselective acylation of d-glucal, see: Calveras et al. (2010 ▶).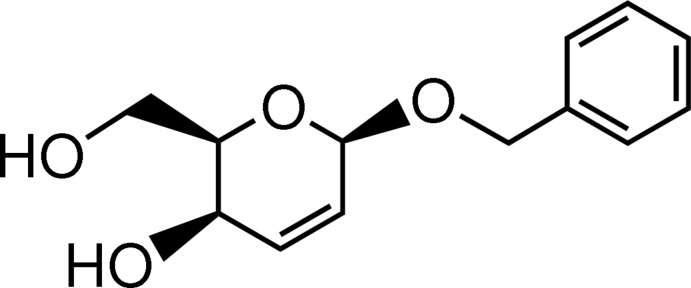
Experimental
Crystal data
C13H16O4
M r = 236.26
Monoclinic,
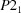
a = 19.500 (9) Å
b = 5.291 (2) Å
c = 6.0809 (15) Å
β = 94.27 (3)°
V = 625.7 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.09 mm−1
T = 292 K
0.60 × 0.40 × 0.20 mm
Data collection
Rigaku AFC-7R diffractometer
1713 measured reflections
1585 independent reflections
786 reflections with F 2 > 2σ(F 2)
R int = 0.034
3 standard reflections every 150 reflections intensity decay: 0.8%
Refinement
R[F 2 > 2σ(F 2)] = 0.056
wR(F 2) = 0.255
S = 1.04
1579 reflections
193 parameters
33 restraints
H-atom parameters constrained
Δρmax = 0.16 e Å−3
Δρmin = −0.17 e Å−3
Data collection: WinAFC Diffractometer Control Software (Rigaku, 1999 ▶); cell refinement: WinAFC Diffractometer Control Software (Rigaku, 1999 ▶); data reduction: CrystalStructure (Rigaku, 2010 ▶); program(s) used to solve structure: SIR92 (Altomare et al., 1993 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEPII (Johnson, 1976 ▶); software used to prepare material for publication: CrystalStructure (Rigaku, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) General, I. DOI: 10.1107/S1600536813031140/is5321sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813031140/is5321Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813031140/is5321Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O3i | 0.82 | 1.95 | 2.692 (8) | 151 |
| O3—H3⋯O2ii | 0.82 | 1.89 | 2.614 (8) | 147 |
Symmetry codes: (i) 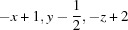 ; (ii)
; (ii) 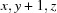 .
.
Acknowledgments
This work was supported by the Keio Gijuku Academic Development Funds.
supplementary crystallographic information
1. Experimental
1.1. Synthesis and crystallization
To a solution of 1c (669 mg, 2.17 mmol) in CH3CN (22 ml) were added Cs2CO3 (1.41 g, 4.33 mmol) and BnOH (4.53 ml, 43.5 mmol) and the mixture was stirred for 8 h at room temperature. The mixture was diluted H2O and organic materials were extracted with CHCl3. The combined extract was washed with brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (20 g). Elution with hexane-EtOAc (2:1) afforded the title compound (I) as a colorless solid (414 mg, 81%). The plate-like crystals of (I) were grown by slow cooling of a t-butylmethylether/hexane solution from ca 340 K to the room temperature. The specific rotation [α]D of (I) at 295 K is -107° (c 1.33, EtOH).
1.2. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1. The absolute structure was assigned based on the known absolute configuration around the C6 atom, which originated from (+)-D-glucose. In the absence of significant anomalous scattering effects, Friedel pairs were averaged before the final refinement. There is an orientational disorder of the benzyloxy group, where the split phenyl group moieties lie on a common plane. The site occupation factor of the major part (O4A, C11A—C17A) was refined to 58.9 (9)%. All the H atoms were positioned geometrically, and refined as riding, with C—H = 0.93–0.98 Å and O—H = 0.82 Å, and with Uiso(H) = 1.2Ueq(C,O). The hydroxy groups were allowed to rotate but not to tip.
2. Comment
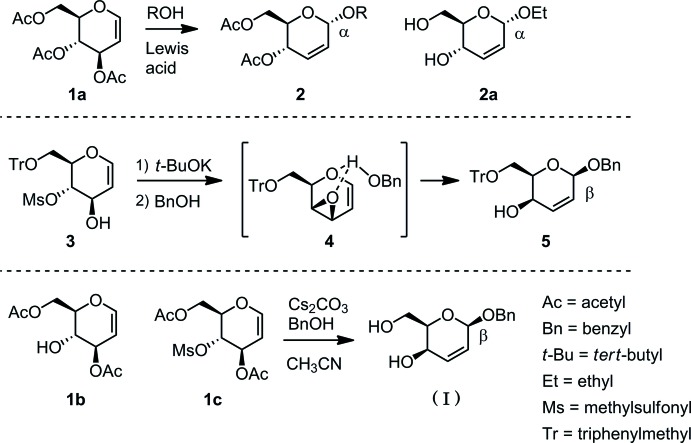
Glycosidic bond formation on glycals such as (1a in Fig. 3) accompanied with the migration of double bond from C1—C2 to C2—C3 had been recognized as Ferrier reaction to give 2-enopyranosides (2) (Ferrier & Prasad, 1969; Noshita et al., 1995). Generally, α-glycosides predominate by the well known oxygen anomeric stabilizing effect, and the anomeric stereochemistry was elucidated by X-ray structure analysis of 2a (Wingert et al., 1984). In contrast, Di Bussolo et al.(2002, 2004) have demonstrated unique β-selective approach, starting from glycals such as 3 with leaving group at C4—OH. By neighboring group participation with free C3—OH, via an epoxide intermediate (4), Ferrier-like rearrangement occurred to give 5. Their proposed mechanism is shown in Fig. 3. In this case, a hydrogen bonding between epoxy ring in 4 and nucleophile dominates the stereochemistry to β rather than α. We submitted 3,6-di-O-acetyl-D-glucal (1b), which is very easily available by an enzymatic regioselective acylation of D-glucal (Calveras et al., 2010), to Crotti's protocol. Introduction of methylsulfonyl group on free C4—OH (1c) and subsequent nuleophilic attack with benzyl alcohol provided the title compound, (I)(81%).
In the present study, the regio- and stereochemistry of (I) has been determined, although there is a complicated disorder. The benzyloxy group has two possible orientations, O4A/C11A—C17A and O4B/C11B—C17B, and their site occupation factors are 58.9 (9) and 41.1 (9)%, respectively. The C10—O4A and C10—O4B bond directions make an angle of 25.4 (7)°.
Figures
Fig. 1.
Molecular structure of the title compound with anisotropic displacement parameters drawn at the 30% probability level. The minor part of the disordered benzyloxy group was omitted for clarity.
Fig. 2.
Crystal packing viewed along the b axis with intermolecular O—H···O hydrogen bonds as dashed lines. The minor part of the disordered benzyloxy group was omitted for clarity.
Fig. 3.
Ferrier reaction and synthesis of the title compound.
Crystal data
| C13H16O4 | F(000) = 252.00 |
| Mr = 236.26 | Dx = 1.254 Mg m−3 |
| Monoclinic, P21 | Melting point = 353–354 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71069 Å |
| a = 19.500 (9) Å | Cell parameters from 25 reflections |
| b = 5.291 (2) Å | θ = 10.1–12.5° |
| c = 6.0809 (15) Å | µ = 0.09 mm−1 |
| β = 94.27 (3)° | T = 292 K |
| V = 625.7 (4) Å3 | Plate, colorless |
| Z = 2 | 0.60 × 0.40 × 0.20 mm |
Data collection
| Rigaku AFC-7R diffractometer | Rint = 0.034 |
| Radiation source: Rigaku rotating Mo anode | θmax = 27.5° |
| Graphite plate monochromator | h = −9→25 |
| ω–2θ scans | k = 0→6 |
| 1713 measured reflections | l = −7→7 |
| 1585 independent reflections | 3 standard reflections every 150 reflections |
| 786 reflections with F2 > 2σ(F2) | intensity decay: 0.8% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.056 | H-atom parameters constrained |
| wR(F2) = 0.255 | w = 1/[σ2(Fo2) + (0.1473P)2 + 0.0601P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.001 |
| 1579 reflections | Δρmax = 0.16 e Å−3 |
| 193 parameters | Δρmin = −0.17 e Å−3 |
| 33 restraints | Absolute structure: see text |
| Primary atom site location: structure-invariant direct methods |
Special details
| Experimental. Spectroscopic data: IR ν max: 3292, 2937, 2872, 1377, 1325, 1176, 1144, 1122, 1051, 970, 951, 876, 787, 744, 696 cm-1; 1H NMR (CDCl3): δ = 7.26–7.40 (m, 5H), 6.14 (ddd, J = 1.3, 4.6, 10.0 Hz, 1H), 5.89 (ddd, J = 1.0, 1.1, 10.0 Hz, 1H), 5.18 (ddd, J = 1.1, 1.3, 1.5 Hz, 1H), 4.92 (d, J = 11.8 Hz, 1H), 4.70 (d, J = 11.8 Hz, 1H), 4.02–4.09 (m, 1H), 3.97 (ddd, J = 5.9, 6.7, 12.0 Hz, 1H), 3.88 (ddd, J = 4.3, 7.3, 12.0 Hz, 1H), 3.79 (ddd, J = 3.1, 4.3, 6.7 Hz, 1H), 2.21 (dd, J = 5.9, 7.3 Hz, 1H), 1.97 (d, J = 10.6 Hz, 1H); 13C NMR (CDCl3): δ = 137.2, 130.9, 130.2, 128.4, 128.0, 127.9, 96.5, 75.1, 70.3, 62.9, 62.5. |
| Refinement. Refinement was performed using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.3098 (2) | 0.1914 (7) | 0.7411 (7) | 0.0920 (13) | |
| O2 | 0.4326 (4) | −0.2905 (9) | 0.9178 (11) | 0.152 (3) | |
| O3 | 0.4652 (2) | 0.2679 (10) | 0.7637 (8) | 0.0988 (14) | |
| O4A | 0.2323 (7) | 0.485 (3) | 0.5340 (18) | 0.090 (4) | 0.589 (9) |
| O4B | 0.2402 (8) | 0.476 (4) | 0.638 (2) | 0.068 (4) | 0.411 (9) |
| C5 | 0.3777 (5) | −0.1093 (15) | 0.9332 (13) | 0.116 (3) | |
| C6 | 0.3606 (3) | 0.0062 (11) | 0.7108 (10) | 0.0822 (15) | |
| C7 | 0.4216 (3) | 0.1300 (14) | 0.6082 (9) | 0.0837 (16) | |
| C8 | 0.3960 (3) | 0.2985 (17) | 0.4253 (8) | 0.0933 (19) | |
| C9 | 0.3317 (3) | 0.3656 (15) | 0.3939 (10) | 0.096 (2) | |
| C10 | 0.2796 (3) | 0.2768 (14) | 0.5335 (12) | 0.0946 (17) | |
| C11A | 0.1683 (5) | 0.409 (3) | 0.605 (3) | 0.097 (4) | 0.589 (9) |
| C11B | 0.1941 (7) | 0.406 (4) | 0.797 (4) | 0.107 (6) | 0.411 (9) |
| C12A | 0.1400 (7) | 0.603 (3) | 0.754 (2) | 0.078 (4) | 0.589 (9) |
| C12B | 0.1432 (11) | 0.611 (4) | 0.826 (4) | 0.078 (4) | 0.411 (9) |
| C13A | 0.1743 (7) | 0.635 (3) | 0.960 (2) | 0.108 (4) | 0.589 (9) |
| C13B | 0.1432 (12) | 0.747 (5) | 1.019 (4) | 0.108 (4) | 0.411 (9) |
| C14A | 0.1520 (9) | 0.811 (4) | 1.107 (3) | 0.153 (8) | 0.589 (9) |
| C14B | 0.0960 (16) | 0.938 (7) | 1.038 (5) | 0.153 (8) | 0.411 (9) |
| C15A | 0.0936 (10) | 0.947 (4) | 1.041 (3) | 0.143 (8) | 0.589 (9) |
| C15B | 0.0485 (15) | 0.990 (5) | 0.867 (4) | 0.143 (8) | 0.411 (9) |
| C16A | 0.0592 (8) | 0.927 (3) | 0.837 (3) | 0.109 (4) | 0.589 (9) |
| C16B | 0.0481 (10) | 0.852 (4) | 0.674 (3) | 0.109 (4) | 0.411 (9) |
| C17A | 0.0842 (9) | 0.750 (3) | 0.696 (3) | 0.095 (4) | 0.589 (9) |
| C17B | 0.0940 (15) | 0.661 (5) | 0.659 (5) | 0.095 (4) | 0.411 (9) |
| H2 | 0.4679 | −0.2355 | 0.9822 | 0.1820* | |
| H3 | 0.4543 | 0.4176 | 0.7592 | 0.1185* | |
| H51 | 0.3919 | 0.0215 | 1.0387 | 0.1391* | |
| H52 | 0.3374 | −0.1926 | 0.9836 | 0.1391* | |
| H6 | 0.3411 | −0.1243 | 0.6103 | 0.0986* | |
| H7 | 0.4491 | −0.0040 | 0.5463 | 0.1005* | |
| H8 | 0.4271 | 0.3589 | 0.3291 | 0.1120* | |
| H9 | 0.3188 | 0.4743 | 0.2779 | 0.1150* | |
| H10A | 0.2558 | 0.1336 | 0.4596 | 0.1135* | 0.589 (9) |
| H10B | 0.2496 | 0.1490 | 0.4601 | 0.1135* | 0.411 (9) |
| H11A | 0.1737 | 0.2499 | 0.6832 | 0.1165* | 0.589 (9) |
| H11B | 0.1361 | 0.3836 | 0.4779 | 0.1165* | 0.589 (9) |
| H11C | 0.2197 | 0.3725 | 0.9365 | 0.1280* | 0.411 (9) |
| H11D | 0.1701 | 0.2528 | 0.7494 | 0.1280* | 0.411 (9) |
| H13A | 0.2127 | 0.5360 | 1.0006 | 0.1298* | 0.589 (9) |
| H13B | 0.1750 | 0.7105 | 1.1365 | 0.1298* | 0.411 (9) |
| H14A | 0.1753 | 0.8367 | 1.2442 | 0.1836* | 0.589 (9) |
| H14B | 0.0965 | 1.0323 | 1.1672 | 0.1836* | 0.411 (9) |
| H15A | 0.0767 | 1.0598 | 1.1409 | 0.1720* | 0.589 (9) |
| H15B | 0.0164 | 1.1182 | 0.8812 | 0.1720* | 0.411 (9) |
| H16A | 0.0211 | 1.0264 | 0.7962 | 0.1307* | 0.589 (9) |
| H16B | 0.0168 | 0.8893 | 0.5555 | 0.1307* | 0.411 (9) |
| H17A | 0.0621 | 0.7311 | 0.5564 | 0.1141* | 0.589 (9) |
| H17B | 0.0920 | 0.5625 | 0.5324 | 0.1141* | 0.411 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.089 (3) | 0.071 (3) | 0.121 (3) | 0.007 (2) | 0.040 (3) | −0.013 (3) |
| O2 | 0.198 (6) | 0.072 (3) | 0.169 (5) | 0.023 (4) | −0.094 (5) | −0.033 (3) |
| O3 | 0.083 (3) | 0.091 (3) | 0.117 (3) | 0.003 (3) | −0.029 (2) | −0.023 (3) |
| O4A | 0.079 (6) | 0.079 (5) | 0.111 (8) | 0.007 (5) | 0.009 (7) | 0.007 (8) |
| O4B | 0.051 (6) | 0.067 (6) | 0.087 (9) | 0.012 (5) | 0.010 (7) | 0.010 (9) |
| C5 | 0.132 (6) | 0.088 (5) | 0.128 (5) | 0.001 (5) | 0.010 (5) | 0.019 (5) |
| C6 | 0.089 (4) | 0.060 (3) | 0.097 (4) | 0.007 (3) | 0.003 (3) | −0.022 (3) |
| C7 | 0.076 (3) | 0.093 (4) | 0.082 (3) | 0.020 (3) | −0.000 (3) | −0.032 (3) |
| C8 | 0.080 (4) | 0.128 (6) | 0.073 (3) | 0.004 (4) | 0.012 (3) | −0.012 (4) |
| C9 | 0.086 (4) | 0.118 (6) | 0.083 (3) | 0.005 (4) | −0.001 (3) | −0.008 (4) |
| C10 | 0.067 (3) | 0.075 (4) | 0.141 (5) | 0.010 (4) | 0.005 (4) | −0.015 (4) |
| C11A | 0.057 (5) | 0.094 (8) | 0.141 (9) | −0.013 (6) | 0.016 (6) | −0.026 (8) |
| C11B | 0.060 (8) | 0.116 (13) | 0.146 (15) | 0.024 (9) | 0.023 (9) | 0.042 (13) |
| C12A | 0.062 (4) | 0.084 (4) | 0.088 (10) | 0.003 (4) | 0.002 (6) | 0.018 (6) |
| C12B | 0.062 (4) | 0.084 (4) | 0.088 (10) | 0.003 (4) | 0.002 (6) | 0.018 (6) |
| C13A | 0.107 (9) | 0.109 (10) | 0.106 (8) | 0.002 (7) | −0.009 (7) | −0.000 (8) |
| C13B | 0.107 (9) | 0.109 (10) | 0.106 (8) | 0.002 (7) | −0.009 (7) | −0.000 (8) |
| C14A | 0.159 (14) | 0.19 (2) | 0.107 (10) | −0.077 (16) | 0.007 (9) | −0.040 (11) |
| C14B | 0.159 (14) | 0.19 (2) | 0.107 (10) | −0.077 (16) | 0.007 (9) | −0.040 (11) |
| C15A | 0.169 (16) | 0.104 (10) | 0.171 (17) | 0.018 (11) | 0.109 (13) | 0.011 (11) |
| C15B | 0.169 (16) | 0.104 (10) | 0.171 (17) | 0.018 (11) | 0.109 (13) | 0.011 (11) |
| C16A | 0.096 (7) | 0.100 (9) | 0.134 (11) | 0.022 (7) | 0.031 (8) | −0.012 (7) |
| C16B | 0.096 (7) | 0.100 (9) | 0.134 (11) | 0.022 (7) | 0.031 (8) | −0.012 (7) |
| C17A | 0.076 (7) | 0.087 (13) | 0.121 (7) | 0.032 (8) | 0.004 (6) | 0.004 (8) |
| C17B | 0.076 (7) | 0.087 (13) | 0.121 (7) | 0.032 (8) | 0.004 (6) | 0.004 (8) |
Geometric parameters (Å, º)
| O1—C6 | 1.416 (8) | C16B—C17B | 1.36 (4) |
| O1—C10 | 1.426 (8) | O2—H2 | 0.820 |
| O2—C5 | 1.445 (11) | O3—H3 | 0.820 |
| O3—C7 | 1.424 (8) | C5—H51 | 0.970 |
| O4A—C10 | 1.438 (17) | C5—H52 | 0.970 |
| O4A—C11A | 1.409 (18) | C6—H6 | 0.980 |
| O4B—C10 | 1.477 (19) | C7—H7 | 0.980 |
| O4B—C11B | 1.42 (3) | C8—H8 | 0.930 |
| C5—C6 | 1.499 (10) | C9—H9 | 0.930 |
| C6—C7 | 1.531 (9) | C10—H10A | 0.980 |
| C7—C8 | 1.483 (9) | C10—H10B | 0.980 |
| C8—C9 | 1.304 (9) | C11A—H11A | 0.970 |
| C9—C10 | 1.449 (9) | C11A—H11B | 0.970 |
| C11A—C12A | 1.499 (19) | C11B—H11C | 0.970 |
| C11B—C12B | 1.49 (3) | C11B—H11D | 0.970 |
| C12A—C13A | 1.388 (18) | C13A—H13A | 0.930 |
| C12A—C17A | 1.36 (3) | C13B—H13B | 0.930 |
| C12B—C13B | 1.38 (3) | C14A—H14A | 0.930 |
| C12B—C17B | 1.37 (4) | C14B—H14B | 0.930 |
| C13A—C14A | 1.38 (3) | C15A—H15A | 0.930 |
| C13B—C14B | 1.38 (4) | C15B—H15B | 0.930 |
| C14A—C15A | 1.38 (3) | C16A—H16A | 0.930 |
| C14B—C15B | 1.37 (4) | C16B—H16B | 0.930 |
| C15A—C16A | 1.37 (3) | C17A—H17A | 0.930 |
| C15B—C16B | 1.39 (3) | C17B—H17B | 0.930 |
| C16A—C17A | 1.38 (3) | ||
| C6—O1—C10 | 110.5 (5) | C7—C6—H6 | 108.989 |
| C10—O4A—C11A | 111.4 (12) | O3—C7—H7 | 108.061 |
| C10—O4B—C11B | 118.8 (16) | C6—C7—H7 | 108.071 |
| O2—C5—C6 | 109.1 (7) | C8—C7—H7 | 108.069 |
| O1—C6—C5 | 106.0 (6) | C7—C8—H8 | 118.624 |
| O1—C6—C7 | 109.3 (5) | C9—C8—H8 | 118.631 |
| C5—C6—C7 | 114.5 (6) | C8—C9—H9 | 118.863 |
| O3—C7—C6 | 113.1 (5) | C10—C9—H9 | 118.877 |
| O3—C7—C8 | 109.9 (6) | O1—C10—H10A | 108.127 |
| C6—C7—C8 | 109.5 (5) | O1—C10—H10B | 112.184 |
| C7—C8—C9 | 122.7 (6) | O4A—C10—H10A | 108.127 |
| C8—C9—C10 | 122.3 (6) | O4B—C10—H10B | 112.183 |
| O1—C10—O4A | 117.6 (7) | C9—C10—H10A | 108.128 |
| O1—C10—O4B | 92.3 (7) | C9—C10—H10B | 112.184 |
| O1—C10—C9 | 111.1 (5) | O4A—C11A—H11A | 109.342 |
| O4A—C10—C9 | 103.3 (8) | O4A—C11A—H11B | 109.349 |
| O4B—C10—C9 | 115.4 (9) | C12A—C11A—H11A | 109.342 |
| O4A—C11A—C12A | 111.4 (11) | C12A—C11A—H11B | 109.335 |
| O4B—C11B—C12B | 110.7 (18) | H11A—C11A—H11B | 107.980 |
| C11A—C12A—C13A | 117.0 (12) | O4B—C11B—H11C | 109.503 |
| C11A—C12A—C17A | 123.9 (12) | O4B—C11B—H11D | 109.498 |
| C13A—C12A—C17A | 119.1 (14) | C12B—C11B—H11C | 109.496 |
| C11B—C12B—C13B | 121.7 (19) | C12B—C11B—H11D | 109.495 |
| C11B—C12B—C17B | 119 (2) | H11C—C11B—H11D | 108.069 |
| C13B—C12B—C17B | 119 (2) | C12A—C13A—H13A | 119.650 |
| C12A—C13A—C14A | 120.7 (13) | C14A—C13A—H13A | 119.655 |
| C12B—C13B—C14B | 120 (2) | C12B—C13B—H13B | 120.088 |
| C13A—C14A—C15A | 117.1 (15) | C14B—C13B—H13B | 120.082 |
| C13B—C14B—C15B | 120 (3) | C13A—C14A—H14A | 121.432 |
| C14A—C15A—C16A | 124.1 (18) | C15A—C14A—H14A | 121.427 |
| C14B—C15B—C16B | 120 (3) | C13B—C14B—H14B | 119.853 |
| C15A—C16A—C17A | 116.3 (15) | C15B—C14B—H14B | 119.850 |
| C15B—C16B—C17B | 119 (2) | C14A—C15A—H15A | 117.945 |
| C12A—C17A—C16A | 122.6 (15) | C16A—C15A—H15A | 117.953 |
| C12B—C17B—C16B | 122 (3) | C14B—C15B—H15B | 120.031 |
| C5—O2—H2 | 109.474 | C16B—C15B—H15B | 120.027 |
| C7—O3—H3 | 109.475 | C15A—C16A—H16A | 121.839 |
| O2—C5—H51 | 109.847 | C17A—C16A—H16A | 121.842 |
| O2—C5—H52 | 109.853 | C15B—C16B—H16B | 120.433 |
| C6—C5—H51 | 109.854 | C17B—C16B—H16B | 120.424 |
| C6—C5—H52 | 109.864 | C12A—C17A—H17A | 118.712 |
| H51—C5—H52 | 108.286 | C16A—C17A—H17A | 118.718 |
| O1—C6—H6 | 108.978 | C12B—C17B—H17B | 119.115 |
| C5—C6—H6 | 108.981 | C16B—C17B—H17B | 119.099 |
| C6—O1—C10—O4A | −173.3 (5) | C8—C9—C10—O4A | 147.8 (7) |
| C6—O1—C10—O4B | −173.0 (4) | C8—C9—C10—O4B | 124.1 (7) |
| C6—O1—C10—C9 | −54.6 (6) | O4A—C11A—C12A—C13A | −68.8 (15) |
| C10—O1—C6—C5 | −168.0 (4) | O4A—C11A—C12A—C17A | 109.9 (13) |
| C10—O1—C6—C7 | 68.1 (5) | O4B—C11B—C12B—C13B | −111.9 (19) |
| C10—O4A—C11A—C12A | 139.3 (9) | O4B—C11B—C12B—C17B | 70 (2) |
| C11A—O4A—C10—O1 | −74.9 (11) | C11A—C12A—C13A—C14A | 179.4 (11) |
| C11A—O4A—C10—O4B | −75.6 (14) | C11A—C12A—C17A—C16A | 179.8 (12) |
| C11A—O4A—C10—C9 | 162.3 (9) | C13A—C12A—C17A—C16A | −2 (3) |
| C10—O4B—C11B—C12B | −160.8 (10) | C17A—C12A—C13A—C14A | 1 (2) |
| C11B—O4B—C10—O1 | −58.0 (13) | C11B—C12B—C13B—C14B | 178.8 (17) |
| C11B—O4B—C10—O4A | 121 (3) | C11B—C12B—C17B—C16B | −177.4 (19) |
| C11B—O4B—C10—C9 | −172.7 (11) | C13B—C12B—C17B—C16B | 4 (4) |
| O2—C5—C6—O1 | −176.5 (5) | C17B—C12B—C13B—C14B | −3 (4) |
| O2—C5—C6—C7 | −56.0 (7) | C12A—C13A—C14A—C15A | 2 (3) |
| O1—C6—C7—O3 | 77.9 (6) | C12B—C13B—C14B—C15B | 1 (4) |
| O1—C6—C7—C8 | −45.0 (6) | C13A—C14A—C15A—C16A | −3 (3) |
| C5—C6—C7—O3 | −40.7 (8) | C13B—C14B—C15B—C16B | −1 (5) |
| C5—C6—C7—C8 | −163.7 (5) | C14A—C15A—C16A—C17A | 3 (3) |
| O3—C7—C8—C9 | −111.7 (7) | C14B—C15B—C16B—C17B | 2 (4) |
| C6—C7—C8—C9 | 13.0 (9) | C15A—C16A—C17A—C12A | −0 (3) |
| C7—C8—C9—C10 | −1.1 (11) | C15B—C16B—C17B—C12B | −4 (4) |
| C8—C9—C10—O1 | 20.8 (10) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O3i | 0.82 | 1.95 | 2.692 (8) | 151 |
| O3—H3···O2ii | 0.82 | 1.89 | 2.614 (8) | 147 |
Symmetry codes: (i) −x+1, y−1/2, −z+2; (ii) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS5321).
References
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst. 26, 343–350.
- Calveras, J., Nagai, Y., Sultana, I., Ueda, Y., Higashi, T., Shoji, M. & Sugai, T. (2010). Tetrahedron, 66, 4284–4291.
- Di Bussolo, V., Caselli, M., Pineschi, M. & Crotti, P. (2002). Org. Lett. 4, 3695–3698. [DOI] [PubMed]
- Di Bussolo, V., Caselli, M., Romano, M. R., Pineschi, M. & Crotti, P. (2004). J. Org. Chem. 69, 8702–8708. [DOI] [PubMed]
- Ferrier, R. J. & Prasad, N. (1969). J. Chem. Soc. C, pp. 570–575.
- Johnson, C. K. (1976). ORTEPII Report ORNL-5138. Oak Ridge National Laboratory, Tennessee, USA.
- Noshita, T., Sugiyama, T., Kitazumi, Y. & Oritani, T. (1995). Biosci. Biotechnol. Biochem. 59, 2052–2055.
- Rigaku (1999). WinAFC Diffractometer Control Software Rigaku Corporation, Tokyo, Japan.
- Rigaku (2010). CrystalStructure Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wingert, L. M., Ruble, J. R. & Jeffrey, G. A. (1984). Carbohydr. Res. 128, 1–10. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) General, I. DOI: 10.1107/S1600536813031140/is5321sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813031140/is5321Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813031140/is5321Isup3.cdx
Additional supplementary materials: crystallographic information; 3D view; checkCIF report