Abstract
Acute myocardial infarction and acute myeloid leukemia are rarely reported as concomitant conditions. The management of ST-elevation myocardial infarction (STEMI) in patients who have acute myeloid leukemia is challenging: the leukemia-related thrombocytopenia, platelet dysfunction, and systemic coagulopathy increase the risk of bleeding, and the administration of thrombolytic agents can be fatal. We report the case of a 76-year-old man who presented emergently with STEMI, myelodysplastic syndrome, and newly recognized acute myeloid leukemia transformation. Standard antiplatelet and anticoagulation therapy were contraindicated by the patient's thrombocytopenia and by his reported ecchymosis and gingival bleeding upon admission. He declined cardiac catheterization, was provided palliative care, and died 2 hours after hospital admission.
We searched the English-language medical literature, found 8 relevant reports, and determined that the prognosis for patients with concomitant STEMI and acute myeloid leukemia is clearly worse than that for either individual condition. No guidelines exist to direct the management of STEMI and concomitant acute myeloid leukemia. In 2 reports, dual antiplatelet therapy, anticoagulation, and drug-eluting stent implantation were used without an increased risk of bleeding in the short term, even in the presence of thrombocytopenia. However, we think that a more conservative approach—balloon angioplasty with the provisional use of bare-metal stents—might be safer. Simultaneous chemotherapy for the acute myeloid leukemia is crucial. Older age seems to be a major risk factor: patients too frail for emergent treatment can die within hours or days.
Keywords: Angina, unstable/diagnosis/pathology; chest pain/etiology; combined modality therapy; coronary disease/blood; fatal outcome; leukemia, myeloid, acute/complications/therapy; myocardial infarction/complications/etiology; risk factors
Reports of concomitant acute myocardial infarction (AMI) and acute myeloid leukemia (AML) are sparse. The management of ST-elevation myocardial infarction (STEMI) in patients with AML is challenging, because of the increased risk of bleeding from the thrombocytopenia, platelet dysfunction, and systemic coagulopathy in AML. No guidelines exist for the appropriate management of this combined disease phenomenon. We report a case of STEMI in a patient with coronary artery disease, myelodysplastic syndrome, and newly recognized AML transformation. In addition, we review the relevant medical literature and discuss the appropriateness of applying evidence-based acute coronary syndrome (ACS) management principles to patients with simultaneous STEMI and AML.
Case Report
A 76-year-old man with known coronary artery disease and myelodysplastic syndrome emergently presented with a 3-week history of intermittent exertional angina.
He had undergone 3-vessel coronary artery bypass grafting 10 years earlier. Cardiac catheterization performed a year before the current presentation yielded severe native-vessel disease: 90% stenosis of the distal left main coronary artery (LMCA), 50% ostial stenosis of the proximal left anterior descending coronary artery (LAD) with Rentrop grade 2 collateral vessels, ostial bifurcation lesions in the left circumflex coronary artery, and a chronically occluded right coronary artery (RCA). The graft from the left internal mammary artery to the mid-LAD and the sequential graft from the free radial artery to the distal diagonal artery were patent; however, the saphenous vein-to-RCA graft was occluded. The patient's estimated left ventricular ejection fraction (LVEF) was 0.55 by ventriculography. An electrocardiogram (ECG) then showed sinus rhythm with first-degree atrioventricular block and frequent premature ventricular complexes (Fig. 1). The patient underwent no functional testing at that time.
Fig. 1.
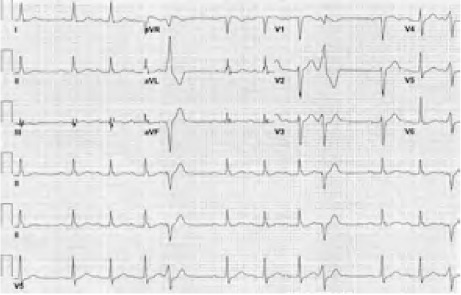
Baseline electrocardiogram from a year before the current presentation shows sinus rhythm, first-degree atrioventricular block, and frequent premature ventricular complexes.
For 4 weeks, the patient had been undergoing intermittent erythropoietin infusion and packed red blood cell transfusions to treat the symptomatic anemia from his myelodysplastic syndrome. During this time, his white blood cell (WBC) count had risen from 14 × 109/L to 63 × 109/L, with 13% blasts; his platelet count fell to 21,000 ×109/μL; and his hemoglobin was stable. His prothrombin time and activated partial thromboplastin time were normal. Results of flow cytometry were consistent with AML, French-American-British class M4.
Two days before the current presentation, the patient had begun experiencing nonradiating, nonpositional, mid-substernal chest pain at rest and upon minimal exertion. The angina, initially relieved by sublingual nitroglycerin, had become unstable—occurring more frequently, lasting longer, and responding less well to the nitroglycerin. The patient reported no nausea, vomiting, daytime diaphoresis, paroxysmal nocturnal dyspnea, orthopnea, leg edema, palpitations, or syncope. He reported a poor energy level, decreased appetite, nonvolitional weight loss, and night sweats. He had noticed ecchymosis at venipuncture sites and gingival bleeding while brushing his teeth during the preceding 2 weeks.
At the current emergent presentation, the patient was in shock, with a systolic blood pressure ranging from 80 to 89 mmHg and sinus tachycardia of approximately 120 beats/min. An ECG showed sinus tachycardia, left anterior fascicular block, ST-segment elevation in leads III, aVF, aVR, and V1, and diffuse ST-segment depression in the lateral leads (Fig. 2). These ECG changes were consistent with inferior STEMI or possibly with severe 3-vessel disease with or without LMCA involvement.1 The patient's cardiac troponin level was 17 ng/mL. Chest radiographs showed mild pulmonary edema and cardiomegaly. An urgent bedside echocardiogram revealed global hypokinesia with an LVEF <0.20. Cardiac catheterization was offered; however, the patient opted for conservative medical management. Palliative care was provided, and he died 2 hours after admission.
Fig. 2.
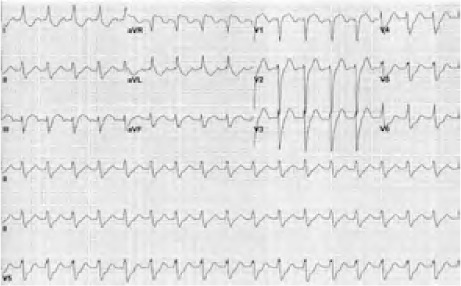
Admission electrocardiogram shows sinus tachycardia, left anterior fascicular block, ST-segment elevation in the inferior leads, and a possible global ischemic pattern.
Discussion
We sought to determine the effect of various therapeutic regimens on clinical outcomes and evaluate the prognostic indicators of in-hospital death in cases of STEMI with AML. We found only 8 relevant reports in the English-language medical literature (Table I).2–9
TABLE I.
Published Cases of Patients with Simultaneous STEMI and AML
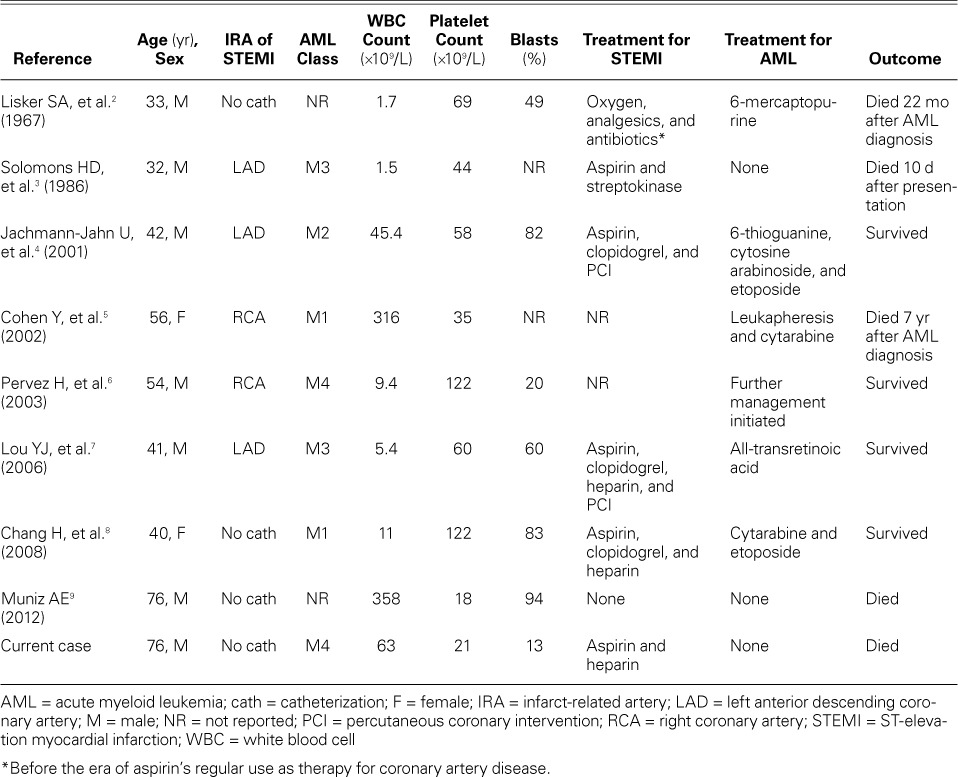
It is unclear whether AML independently contributes to AMI pathogenesis. Several pathogenic mechanisms have been proposed. Severe elevation of the WBC count to levels above 100 ×109/L can lead to leukostasis, a medical emergency most often seen in patients who have AML or chronic myeloid leukemia and are in blast crisis.10 Leukostasis is characterized by extremely high blast cell count and by symptoms of tissue hypo-perfusion that most frequently affect the lungs and brain. Epidemiologic studies have revealed a 4-fold increase in the risk of myocardial infarction in patients who have a high-normal WBC count (>9 ×109/L) in comparison with a low-normal range (<6 ×109/L); only 50% to 65% of the excess risk of the high-count individuals is explainable by their tobacco-smoking.11 A high WBC count also predicts a greater risk of repeat infarction and in-hospital death. Moreover, the risk of AMI was higher in coronary artery disease patients whose WBC counts increased after the administration of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor administered to promote neo-revascularization.12,13 Neutrophilic infiltration of culprit lesions in patients who died after AMI suggests that platelets and neutrophils interact at the site of ruptured plaques.14 The less-deformable blast cells contribute to atherothrombosis by impeding blood flow to the microcirculation.3 In addition, blasts possess potent tissue factor activity that can activate the extrinsic system and generate thrombin. If the major coronary arteries have been narrowed by atherosclerosis, leukemic-rich thrombus formation from leukostasis might lead to myocardial ischemia or infarction.5 However, WBC and blast counts do not fully explain the development of leukostasis. For example, an AML blast count of approximately 300 ×109/L or an acute lymphocytic leukemia blast count of approximately 600 ×109/L is necessary to achieve a leukocrit of 12 to 15 mL/dL, after which blood viscosity increases rapidly.10
For ACS, dual antiplatelet therapy (DAPT) and anticoagulation are administered in accordance with the premise that coronary artery thrombus is rich in both platelets and fibrin, and that maximal inhibition of each component can prevent further clot propagation. However, the concomitant presence of STEMI and AML poses a clinical dilemma, because current evidence-based antithrombotic therapies for myocardial infarction are relatively contraindicated in view of the increased risk of bleeding from AML-related thrombocytopenia, platelet dysfunction, and systemic coagulopathy. Despite the presence of thrombocytopenia, it is possible for platelet-fibrin thrombus to form in a major epicardial coronary artery in patients with AML and AMI, as Solomons and colleagues proved histopathologically.3
The first case of concurrent STEMI and AML was reported before aspirin therapy was standard for coronary artery disease and before AML was classified. That patient lived for 22 months after the onset of his illness.2 The next case—the only one involving the use of thrombolytic agents—led to fatal hemorrhagic complications3; hence, thrombolysis cannot be recommended. Two studies of the use of DAPT, anticoagulation, and percutaneous coronary intervention (PCI) with drug-eluting stents4,7 revealed no excessive bleeding or inhospital deaths, despite patients' platelet counts in the range of 58,000 to 60,000 ×109/μL. Those patients had no major or minor bleeding before the initiation of the ACS protocol. Of note, those studies were conducted before the routine use of a 600-mg clopidogrel loading dose and before the availability of newer, more potent P2Y12 antagonists such as prasugrel and ticagrelor. Finally, no one has apparently reported the use of balloon angioplasty alone, balloon angioplasty with provisional stenting, or bare-metal stenting in the management of STEMI with concomitant AML. In a meta-analysis of randomized controlled trials comparing balloon angioplasty versus PCI with bare-metal stents in the treatment of AMI (alone), Zhu and colleagues15 found no difference in rates of death or reinfarction; however, target-vessel revascularization and major adverse cardiac events occurred more often in patients who underwent angioplasty alone. In our opinion, the more conservative approach of balloon angioplasty with the provisional use of bare-metal stents might be the safer treatment for concomitant STEMI and AML, because it should reduce major bleeding through shorter DAPT exposure and eliminate the risk of stent thrombosis (in drug-eluting stents) associated with DAPT interruption. Performing PCI via a radial approach might likewise reduce the risk of bleeding complications.16
In treating AML, the goal is complete remission. Induction chemotherapy is the only therapy that eliminates blast cells from the bone marrow with a proven survival benefit.10 Hydroxyurea administration and leukapheresis are second-line therapies.10 Especially in STEMI patients with leukostasis, simultaneous chemotherapy is necessary, because response takes up to 48 hours.10 Chemotherapy is expected to worsen thrombocytopenia, so a transfusion platelet-count target of 50 to 100 ×109/L is recommended in patients undergoing invasive procedures.17 Of note, the standard of care to prevent bleeding in AML is routine platelet transfusion at a platelet-count trigger point of 10 ×109/L.18
The prognosis in concomitant STEMI and AML is clearly worse than that of either pathologic condition individually. Older age seems to be a major risk factor. If patients are too frail to undergo emergent treatment, as our case and another18 illustrate, they can die within hours or days.
Acknowledgments
We thank Dr. Shelby Dickerson and Ms Annette Black-well for their technical assistance.
References
- 1.Nikus KC, Eskola MJ, Sclarovsky S. Electrocardiographic presentations of left main or severe triple vessel disease in acute coronary syndromes--an overview. J Electrocardiol. 2006;39(4 Suppl):S68–72. doi: 10.1016/j.jelectrocard.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Lisker SA, Finkelstein D, Brody JI, Beizer LH. Myocardial infarction in acute leukemia. Report of a case in a young man. Arch Intern Med. 1967;119(5):532–5. [PubMed] [Google Scholar]
- 3.Solomons HD, Stanley A, King PC, Pienaar N, Atkinson PM. Acute promyelocytic leukaemia associated with acute myocardial infarction. A case report. S Afr Med J. 1986;70(2):117–8. [PubMed] [Google Scholar]
- 4.Jachmann-Jahn U, Cornely OA, Laufs U, Hopp HW, Meuthen I, Krakau M, O'Brien B. Acute anterior myocardial infarction as first manifestation of acute myeloid leukemia. Ann Hematol. 2001;80(11):677–81. doi: 10.1007/s002770100353. [DOI] [PubMed] [Google Scholar]
- 5.Cohen Y, Amir G, Da'as N, Gillis S, Rund D, Polliack A. Acute myocardial infarction as the presenting symptom of acute myeloblastic leukemia with extreme hyperleukocytosis. Am J Hematol. 2002;71(1):47–9. doi: 10.1002/ajh.10155. [DOI] [PubMed] [Google Scholar]
- 6.Pervez H, Potti A, Mehdi SA. Challenging and unusual cases: Case 1. Simultaneous presentation of acute myelogenous leukemia and myocardial infarction. J Clin Oncol. 2003;21(7):1416–7. doi: 10.1200/JCO.2003.06.168. [DOI] [PubMed] [Google Scholar]
- 7.Lou Y, Mai W, Jin J. Simultaneous presentation of acute myocardial infarction and acute promyelocytic leukemia. Ann Hematol. 2006;85(6):409–10. doi: 10.1007/s00277-006-0106-4. [DOI] [PubMed] [Google Scholar]
- 8.Chang H, Lin TL, Ho WJ, Hsu LA. Acute myeloid leukemia associated with acute myocardial infarction and dural sinus thrombosis: the possible role of leukemia-related hyperhomocysteinemia. J Chin Med Assoc. 2008;71(8):416–20. doi: 10.1016/S1726-4901(08)70093-5. [DOI] [PubMed] [Google Scholar]
- 9.Muniz AE. Myocardial infarction and stroke as the presenting symptoms of acute myeloid leukemia. J Emerg Med. 2012;42(6):651–4. doi: 10.1016/j.jemermed.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer CA. Larson RA, editor. Hyperleukocytosis and leukostasis [monograph on the Internet] editor. UpToDate. Available from: http://www.uptodate.com/contents/hyperleukocytosis-and-leukostasis [2012 May 10; cited 2014 Jan 31]
- 11.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257(17):2318–24. [PubMed] [Google Scholar]
- 12.Hill JM, Syed MA, Arai AE, Powell TM, Paul JD, Zalos G et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J Am Coll Cardiol. 2005;46(9):1643–8. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46(9):1636–42. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 14.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 15.Zhu MM, Feit A, Chadow H, Alam M, Kwan T, Clark LT. Primary stent implantation compared with primary balloon angioplasty for acute myocardial infarction: a meta-analysis of randomized clinical trials. Am J Cardiol. 2001;88(3):297–301. doi: 10.1016/s0002-9149(01)01645-9. [DOI] [PubMed] [Google Scholar]
- 16.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60(24):2481–9. doi: 10.1016/j.jacc.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850):1309–16. doi: 10.1016/S0140-6736(12)60689-8. [DOI] [PubMed] [Google Scholar]
- 18.Stanworth SJ, Estcourt L, Powter G, Kahan BC, Dyer C, Bakrania L et al. The effect of a no-prophylactic versus prophylactic platelet transfusion strategy on bleeding in patients with hematological malignancies and severe thrombocytopenia (TOPPS trial) [abstract] Amer Soc Hematology abstract book. 2012;120(21):1. Available from: http://www.unimedizin-mainz.de/fileadmin/kliniken/M3/Dokumente/Post_ASH/Supportive_Therapie_1_Post-ASH2012.pdf [cited 2014 Jan 31] [Google Scholar]


