Abstract
This prospective cross-sectional study attempted to determine both the usefulness of the serum intercellular adhesion molecule-1 (ICAM-1) as a biomarker for pulmonary artery hypertension secondary to congenital heart disease and the nature of this marker's association with catheter angiographic findings.
Our study included a total of 70 male and female children, comprising 30 patients with both pulmonary artery hypertension and congenital heart disease, 20 patients with congenital heart disease alone, and 20 healthy control subjects. Levels of ICAM-1 in plasma samples from all groups were measured by the enzyme-linked immunosorbent assay method. Cardiac catheterization was also performed in all patients.
The mean serum ICAM-1 levels in pediatric patients who had congenital heart disease with and without pulmonary artery hypertension were 349.6 ± 72.9 ng/mL and 312.3 ± 69.5 ng/mL, respectively (P=0.002). In healthy control subjects, the mean serum ICAM-1 level was 231.4 ± 60.4 ng/mL.
According to the results of this study, the ICAM-1 level of the pulmonary artery hypertension group was significantly higher than those of the congenital heart disease group and the healthy control group. Correlation analysis showed that ICAM-1 level was correlated with systolic and mean pulmonary artery pressures (r=0.62, P=0.001; r=0.57, P=0.001)—which are 2 important values used in diagnosis of pulmonary artery hypertension. Moreover, receiver operating characteristic analysis yielded consistent results for the prediction of pulmonary artery hypertension. Therefore, we conclude that ICAM-1 has potential use as a biomarker for the diagnosis and follow-up of pulmonary artery hypertension.
Keywords: Biological markers, blood; hypertension, pulmonary/blood/diagnosis/etiology; intercellular adhesion molecule-1/blood; prospective studies, cross-sectional
Congenital heart disease (CHD) is the most common congenital malformation: the prevalence of moderate and severe forms of CHD is about 6 in 1,000 live births, or 19 in 1,000 live births if potentially serious cases of bicuspid aortic valve are included.1 Pulmonary artery hypertension (PAH) is a frequent complication of CHD, particularly in patients who have left-to-right shunts.2 Pulmonary artery hypertension has been defined as an increase in mean pulmonary artery pressure (PAP) of ≥25 mmHg at rest, as evaluated by right-sided heart catheterization.3
Pulmonary artery hypertension has a multifactorial pathogenesis that involves various biochemical pathways and cell types. The increase in pulmonary vascular resistance is related to different mechanisms, including vasoconstriction, proliferative and obstructive remodeling of the pulmonary vessel wall, inflammation, and thrombosis.3 Persistent exposure of the pulmonary vasculature to increased blood flow and pressure can result in endothelial dysfunction and endothelial damage.2 After such damage, endothelial cells produce an array of cytokines and growth factors and promote immune cells to adhere to and migrate through the vessel wall into the extravascular space.4 Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are expressed on endothelial cells. When overexpressed on the activated endothelial layer, VCAM-1 and ICAM-1 undergo shedding, and their soluble forms (detectable in serum) are considered to be markers of endothelial cell activity or injury.5,6 In the medical literature,7–9 elevated serum concentrations of adhesion molecules have been detected in several diseases, such as diabetes mellitus, coronary heart disease, atherosclerosis, and rheumatoid arthritis. Sungprem and colleagues10 showed that serum ICAM-1 level was increased in patients with PAH.
Right-sided heart catheterization is known to be the gold-standard method for the diagnosis of PAH. However, it is an invasive test and is not always practical for repeated ongoing evaluation. Noninvasive techniques for measuring hemodynamic variables, such as echocardiography, are used increasingly in PAH patients, both before treatment and during follow-up evaluation.3 Recently, various biomarkers, such as asymmetric dimethylarginine and brain natriuretic peptide, have been proposed for the diagnosis and follow-up of PAH.11 Another possible biomarker for the development of PAH is ICAM-1, a fundamental mediator of endothelial damage and dysfunction.
In this study, we measured ICAM-1 levels in groups of patients with and without PAH and in healthy control subjects. The relationship of this particular biomarker with catheter angiographic findings was analyzed in order to learn whether ICAM-1—along with recognized diagnostic methods—has the potential to be used as a biomarker in the diagnosis and follow-up evaluation of patients who have PAH.
Patients and Methods
Study and Control Groups. A total of 74 subjects who underwent consultation in the department of pediatric cardiology at our institution were screened for diseases that might affect ICAM-1 levels, such as diabetes mellitus, systemic hypertension, hypercholesterolemia, sepsis, and renal failure; 4 patients with these diseases were excluded from the study.
The remaining 70 patients were included in this prospective, cross-sectional, single-center study. Among these 70 children, 30 had a diagnosis of both PAH (as determined by diagnostic catheter angiography) and CHD (23 patients with ventricular septal defects, 2 with truncus arteriosus type 4, 2 with patent ductus arteriosus, 2 with atrial septal defects, and 1 with double-outlet right ventricle with mitral atresia); 20 had a diagnosis of left-to-right shunt without PAH; and 20 were healthy control subjects. The group of 30 patients with PAH was further divided into 2 subgroups—cyanotic (9 patients) and acyanotic (21 patients).
All groups were matched in accordance with sex and age. In the PAH group, 15 male and 15 female patients ranged in age from 1 year to 17 years; in the CHD group, 8 male and 12 female patients ranged in age from 2 months to 16 years; and in the healthy control group, 10 male and 10 female patients ranged in age from 9 months to 16 years.
According to the World Health Organization's functional classification system, 9 of the 30 PAH patients were class 3 and the remaining 21 were class 2. The 6-minute walk-test distance of the PAH group was, on average, 312.9 m. When the control and patient groups were compared, there were no statistically significant differences in weight, height, body mass index, or body surface area.
The results of detailed history-taking, physical examination, and anthropometric measurements, together with telecardiographic, electrocardiographic, echocardiographic, and catheter angiographic findings, were recorded on previously prepared forms. We also analyzed the healthy control group, which included only patients referred to pediatric cardiology who had been found to be free of cardiac disease.
Cardiac Catheterization. Routine cardiac catheterizations and hemodynamic studies were performed with intravenous midazolam sedation. Pressure measurements were recorded with the use of fluid-filled catheters connected to pressure transducers. Oxygen consumption was estimated on the basis of age, sex, and heart rate according to the method of LaFarge and Miettinen.12 The pulmonary and systemic blood flows were calculated with the Fick equation and indexed for body surface area. Pulmonary and systemic vascular resistance, the pulmonary flow/systemic flow ratio, and the pulmonary resistance/systemic resistance ratio were calculated in accordance with the standard formulas. The patients were considered to have PAH when their mean PAP values exceeded 25 mmHg and their systolic PAP values exceeded 50 mmHg.
Blood Evaluation. After 12 hours of fasting, patients had 10-cc blood samples taken in EDTA at the beginning of cardiac catheterizations for ICAM-1 levels; plasma samples obtained by centrifugation at 3,500 rpm for 5 minutes were then stored at −80 °C for later work. These blood samples were obtained before beginning treatment for angiographic and clinical findings.
Intercellular Adhesion Molecule-1 Levels. The ICAM-1 levels were measured with use of a human soluble ICAM-1 ELISA Kit (RayBiotech, Inc.; Norcross, Ga) and an enzyme-linked immunosorbent assay method. Results were recorded in terms of ng/mL.
Statistical Analysis
The data were analyzed with use of SPSS software; the results were shown as mean ± SD. Mann-Whitney U and Kruskal-Wallis tests were used for statistical analysis, and a P value of <0.05 was accepted as significant. The cutoff level for ICAM-1 was determined by means of receiver operating curve (ROC) analysis. Optimal cutoff level is determined to be the point on the ROC curve that has the minimum Euclidean distance to the coordinate (0.1) of the ROC space (also called the perfect classification point) that represents 100% sensitivity (no false negatives) and 100% specificity (no false positives). The sensitivity, specificity, positive predictive value, and negative predictive value for each cutoff were determined. Logistic regression analysis was also conducted to verify the results found in ROC analysis. The correlation between the following variables and the serum ICAM-1 level was determined by univariate linear regression: pulmonary blood flow (Qp), systemic blood flow (Qs), pulmonary-to-systemic flow ratio (Qp/Qs), systolic PAP, mean PAP, diastolic PAP, pulmonary arteriolar resistance (Rp), systemic arteriolar resistance (Rs), pulmonary-to-systemic resistance ratio (Rp/Rs), pulmonary artery oxygen saturation, and systemic oxygen saturation. The statistically significant variables in the univariate models were entered into the multivariate backward stepwise linear regression model for further analysis.
Ethical Considerations. The study protocol was approved by our local ethics committee and performed in accordance with the Declaration of Helsinki.
Results
The values of the 2 groups of patients and the control group were compared in accordance with their statistical significance. In our study, the mean ICAM-1 levels of the control group, CHD group, and PAH group were 231.4 ± 60.4 ng/mL, 312.3 ± 69.5 ng/mL, and 349.6 ± 72.9 ng/mL, respectively (Fig. 1).
Fig. 1.
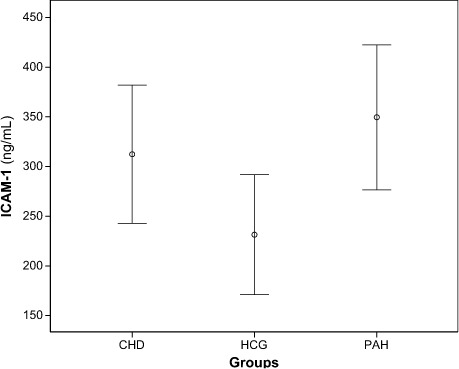
Error bar plot of mean intercellular adhesion molecule-1 (ICAM-1) levels of groups.
CHD = congenital heart disease, HCG = healthy control group; PAH = pulmonary artery hypertension
According to our results, mean ICAM-1 levels were higher in the PAH group than in the CHD group. Healthy control subjects had the lowest mean ICAM-1 level of all (Fig. 1 and Table I). The mean ICAM-1 levels in cyanotic PAH patients were higher than in acyanotic PAH patients, although the difference did not reach statistical significance (P=0.18).
TABLE I.
Comparative Intercellular Adhesion Molecule-1 Levels of the Pulmonary Artery Hypertension, Congenital Heart Disease, and Healthy Control Groups
We performed an ROC analysis to determine the most significant ICAM-1 level for PAH. The area under the ROC curve was computed as 0.87 (Fig. 2). According to our ROC analysis, a patient was predicted to have PAH (mean PAP, >25 mmHg; systolic PAP, >50 mmHg) when the ICAM-1 level reached the ICAM-1 cutoff value of 308 ng/mL. This cutoff level, which was chosen as the optimal point on the ROC curve, had a specificity of 75%, a sensitivity of 73%, a positive predictive value of 69%, and a negative predictive value of 79%. Logistic regression analysis yielded the same specificity and sensitivity values.
Fig. 2.
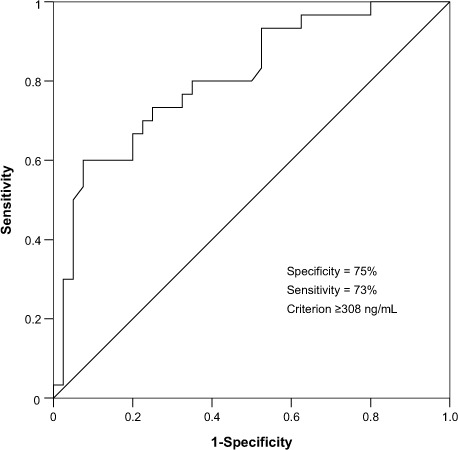
Receiver operating curve (ROC) for intercellular adhesion molecule-1.
When the patient groups were compared for hemodynamic values, we found higher systolic PAP, diastolic PAP, mean PAP, pulmonary resistance, and pulmonary-to-systemic resistance ratio values in the PAH group than in the CHD group (Table II).
TABLE II.
Hemodynamic Values of the Pulmonary Artery Hypertension and Congenital Heart Disease Groups as Detected by Catheter Angiography
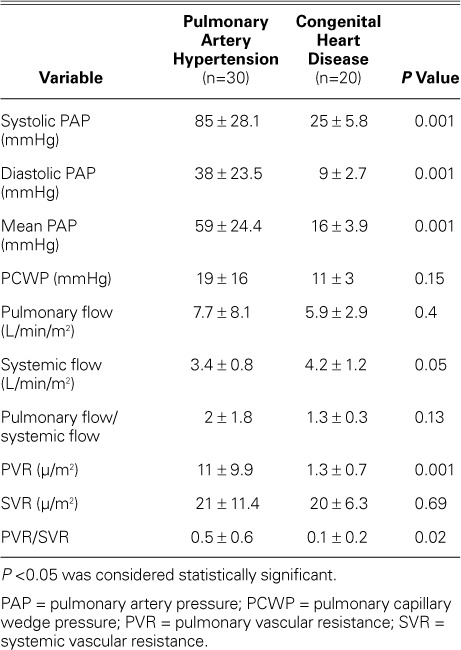
Table III shows correlations between various hemodynamic values and ICAM-1 levels. In univariate regression analysis, systolic PAP, diastolic PAP, mean PAP, pulmonary resistance, and pulmonary-to-systemic resistance ratio were significantly correlated with ICAM-1 level. In the backward multivariate stepwise linear regression models, systolic and mean PAP were left as independent predictors for ICAM-1 level (P=0.001 for both) (Fig. 3).
TABLE III.
Correlation of Catheter Angiographic Findings and Intercellular Adhesion Molecule-1 Levels in the Pulmonary Artery Hypertension Group
Fig. 3.
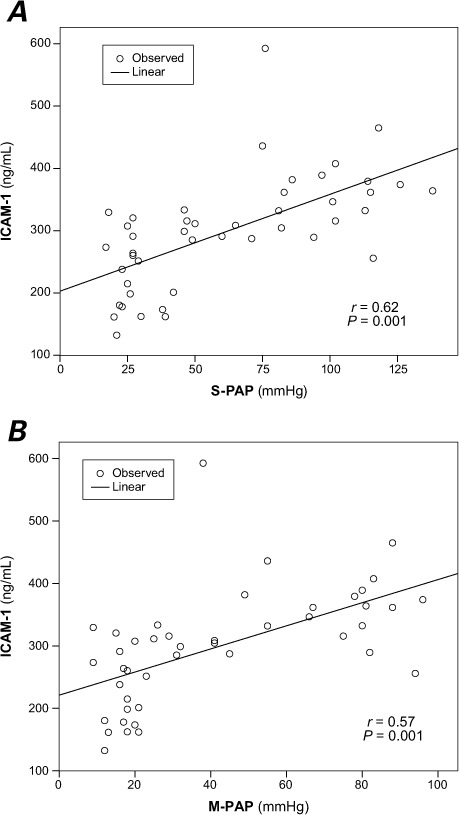
A) Scatter plot of the correlation between systolic pulmonary artery pressure (S-PAP) and serum intercellular adhesion molecule-1 (ICAM-1) level (r=0.62, P=0.001). B) Scatter plot of the correlation between mean pulmonary artery pressure (M-PAP) and serum ICAM-1 level (r=0.57, P=0.001)
P <0.05 was considered statistically significant.
Discussion
In this study, we sought to determine the usefulness of ICAM-1 as an indicator of endothelial dysfunction caused by increased high-pressure flow in association with PAP. As a result of this study, ICAM-1 levels in patients with PAH were found to be higher than levels in healthy control subjects (P=0.014) and in CHD patients (P=0.002) (Fig. 1 and Table I). The results provide evidence to suggest that elevated levels of ICAM-1 are associated with both PAH and CHD.
Increased serum levels of ICAM-1 and VCAM-1 have been used as markers for endothelial damage and activation caused by CHD and PAH.13, 14 In addition, Li and colleagues15 showed that increased pressure flow could induce messenger RNA expression, which in turn induces the synthesis of adhesion molecules and cytokine in distal pulmonary artery endothelial cells. In a study concerning PAH caused by high blood flow in rats, Jin and associates16 detected that serum and tissue ICAM-1 levels (associated with vascular inflammation) were significantly higher in rats with induced shunting than in the healthy control group. Our data were consistent with all of these studies.
In a study by Ercan and coworkers,17 the level of oxidative stress in patients with cyanotic CHD was significantly higher than in patients with acyanotic CHD and in healthy control subjects. In states of oxidative stress, endothelial cells lose their protective phenotypes and synthesize proinflammatory molecules such as ICAM-1.18 Several studies point out the essential role of oxidative stress in the induction of endothelial dysfunction.19 Endothelial dysfunction marker levels are therefore expected to be higher in a cyanotic group. In our study, the higher serum ICAM-1 levels in our cyanotic subgroup, although statistically insignificant, might have attained significance had that subgroup been larger.
In 31 patients with CHD, Sungprem and colleagues10 measured serum ICAM-1 levels and compared them with catheter angiographic findings. The ICAM-1 levels were higher in patients with a mean PAP of >25 mmHg, and mean PAP was the strongest independent marker of ICAM-1 levels, according to the multiple regression model.10 We too found that ICAM-1 level correlated rather strongly with systolic and mean PAP (Fig. 3 and Table III). We should mention that our study included a healthy control group, which was not included in Sungprem's study, and a larger patient group of 50 (30 PAH and 20 CHD).
Our ROC analysis showed that the area under the ICAM-1 ROC curve is 0.87. This implies that ICAM-1 is good at separating PAH patients from non-PAH patients. For the purpose of PAH prediction, we followed ROC criteria and chose an optimal ICAM-1 cutoff level of 308 ng/L. The choice of a cutoff value for ICAM-1 might also depend on whether clinicians aim to identify true positive cases of PAH at a higher rate, in which instance they should choose a lower cutoff value to increase sensitivity. Ideally, determining a cutoff value for clinical use would also require a meta-analysis of studies with much larger groups of subjects—of different age groups, sexes, nationalities, etc. Our study might be considered for incorporation into such a meta-analysis.
The significantly higher ICAM-1 levels in our CHD group than in our healthy control group suggests that the elevated rate of blood flow was by itself enough to increase the excretion of ICAM-1 before the emergence of PAH. Therefore, monitoring ICAM-1 levels in CHD patients might be useful in looking for the development of subsequent PAH in these patients.
Study Limitations
The main limitation of our study is its cross-sectional nature. Because of this, we could not use ICAM-1 to clearly evaluate a patient's response to therapy. Therefore, additional prospective studies are needed, specifically to compare basal ICAM-1 levels with levels after 6 to 12 months of treatment.
Conclusion
This study shows that elevation of serum ICAM-1 can be associated with PAH, the pathogenesis of which involves endothelial damage and vascular inflammation—factors known to increase ICAM-1. The study also suggests that elevation in serum ICAM-1 is a relatively good predictor of both systolic and mean PAP and might be a useful biomarker of PAH.
Footnotes
Dr. M. Oguz is now at the Department of Pediatrics, Dr. Sami Ulus Child Hospital, Ankara, Turkey. Dr. Sanli is now at the Department of Pediatric Cardiology, Kirikkale Medical School, Kirikkale, Turkey.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.D'Alto M, Mahadevan VS. Pulmonary arterial hypertension associated with congenital heart disease. Eur Respir Rev. 2012;21(126):328–37. doi: 10.1183/09059180.00004712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) [published erratum appears in Eur Heart J 2011;32(8):926] Eur Heart J. 2009;30(20):2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 4.Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26(11):587–95. doi: 10.1016/j.it.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 6.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol. 2005;24(2):111–6. doi: 10.1007/s10067-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 7.Labarrere CA, Nelson DR, Miller SJ, Nieto JM, Conner JA, Pitts DE et al. Value of serum-soluble intercellular adhesion molecule-1 for the noninvasive risk assessment of transplant coronary artery disease, posttransplant ischemic events, and cardiac graft failure. Circulation. 2000;102(13):1549–55. doi: 10.1161/01.cir.102.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Sfikakis PP, Tsokos GC. Clinical use of the measurement of soluble cell adhesion molecules in patients with autoimmune rheumatic diseases. Clin Diagn Lab Immunol. 1997;4(3):241–6. doi: 10.1128/cdli.4.3.241-246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jude EB, Douglas JT, Anderson SG, Young MJ, Boulton AJ. Circulating cellular adhesion molecules ICAM-1, VCAM-1, P- and E-selectin in the prediction of cardiovascular disease in diabetes mellitus. Eur J Intern Med. 2002;13(3):185–9. doi: 10.1016/s0953-6205(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 10.Sungprem K, Khongphatthanayothin A, Kiettisanpipop P, Chotivitayatarakorn P, Poovorawan Y, Lertsapcharoen P. Serum level of soluble intercellular adhesion molecule-1 correlates with pulmonary arterial pressure in children with congenital heart disease. Pediatr Cardiol. 2009;30(4):472–6. doi: 10.1007/s00246-008-9374-1. [DOI] [PubMed] [Google Scholar]
- 11.Warwick G, Thomas PS, Yates DH. Biomarkers in pulmonary hypertension. Eur Respir J. 2008;32(2):503–12. doi: 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 12.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4(1):23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Ongen Z, Yilmaz Y. Aterosklerozun patogenezi. In: Kultursay H, editor. Koroner Kalp Hastaligi Primer ve Sekonder Korunma [in Turkish] 1st ed. Istanbul (Turkey): Argos Iletisim Hizmetleri; 2001. pp. 31–66. In. editor. p. [Google Scholar]
- 14.Rosenthal A, Nathan DG, Marty AT, Button LN, Miettinen OS, Nadas AS. Acute hemodynamic effects of red cell volume reduction in polycythemia of cyanotic congenital heart disease. Circulation. 1970;42(2):297–308. doi: 10.1161/01.cir.42.2.297. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Scott DE, Shandas R, Stenmark KR, Tan W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng. 2009;37(6):1082–92. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin HF, Liang C, Liang JM, Tang CS, Du JB. Effects of hydrogen sulfide on vascular inflammation in pulmonary hypertension induced by high pulmonary blood flow: experiment with rats [in Chinese] Zhonghua Yi Xue Za Zhi. 2008;88(32):2235–9. [PubMed] [Google Scholar]
- 17.Ercan S, Cakmak A, Kosecik M, Erel O. The oxidative state of children with cyanotic and acyanotic congenital heart disease. Anadolu Kardiyol Derg. 2009;9(6):486–90. [PubMed] [Google Scholar]
- 18.Klings ES, Anton Bland D, Rosenman D, Princeton S, Odhiambo A, Li G et al. Pulmonary arterial hypertension and left-sided heart disease in sickle cell disease: clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol. 2008;83(7):547–53. doi: 10.1002/ajh.21187. [DOI] [PubMed] [Google Scholar]
- 19.Wever RM, Luscher TF, Cosentino F, Rabelink TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97(1):108–12. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]


