Abstract
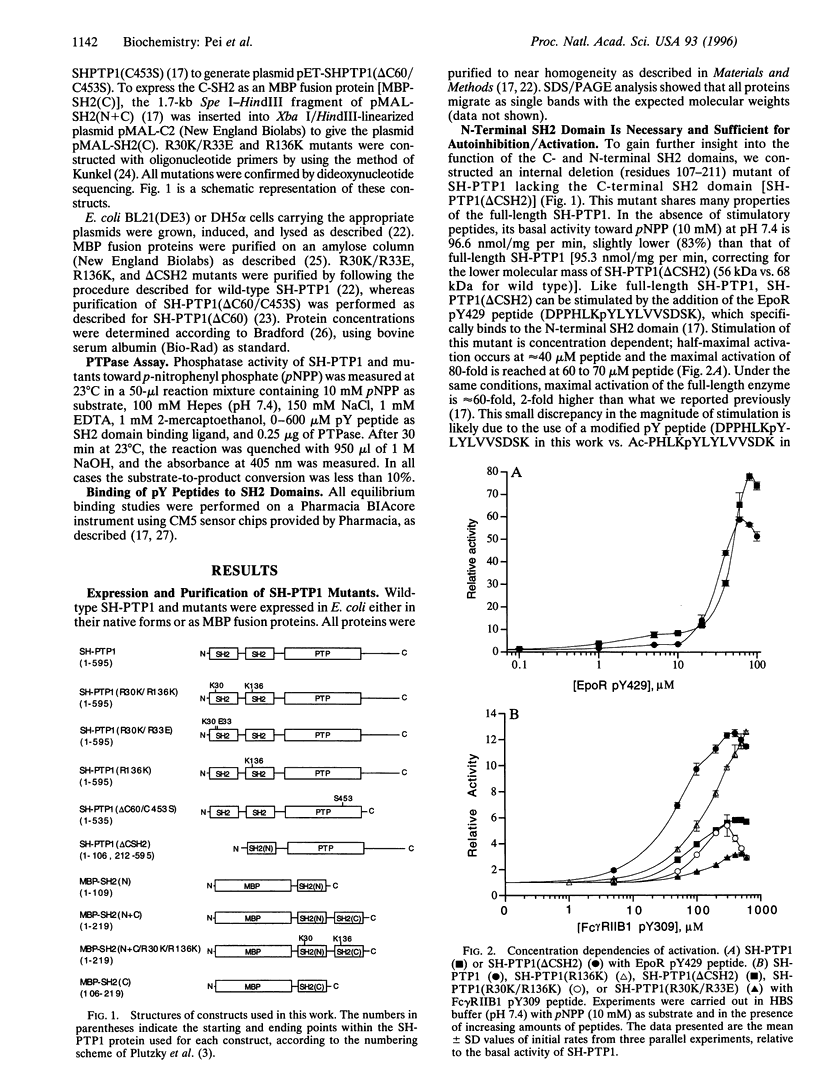
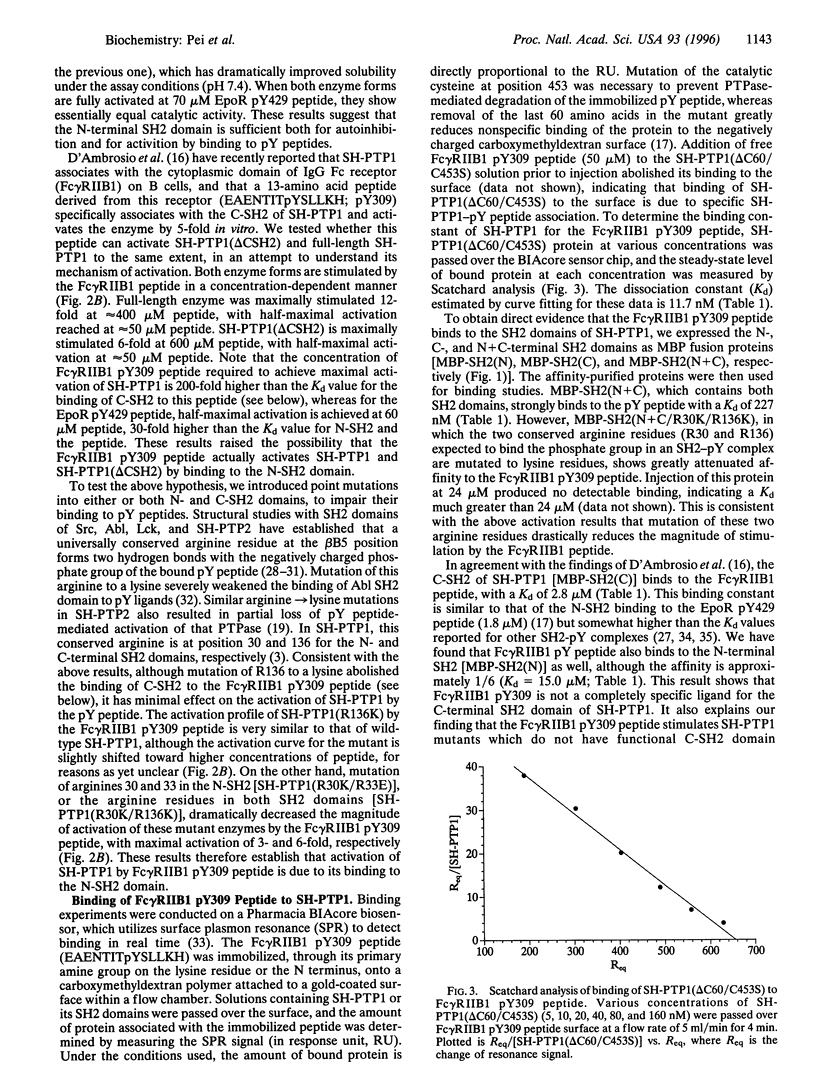
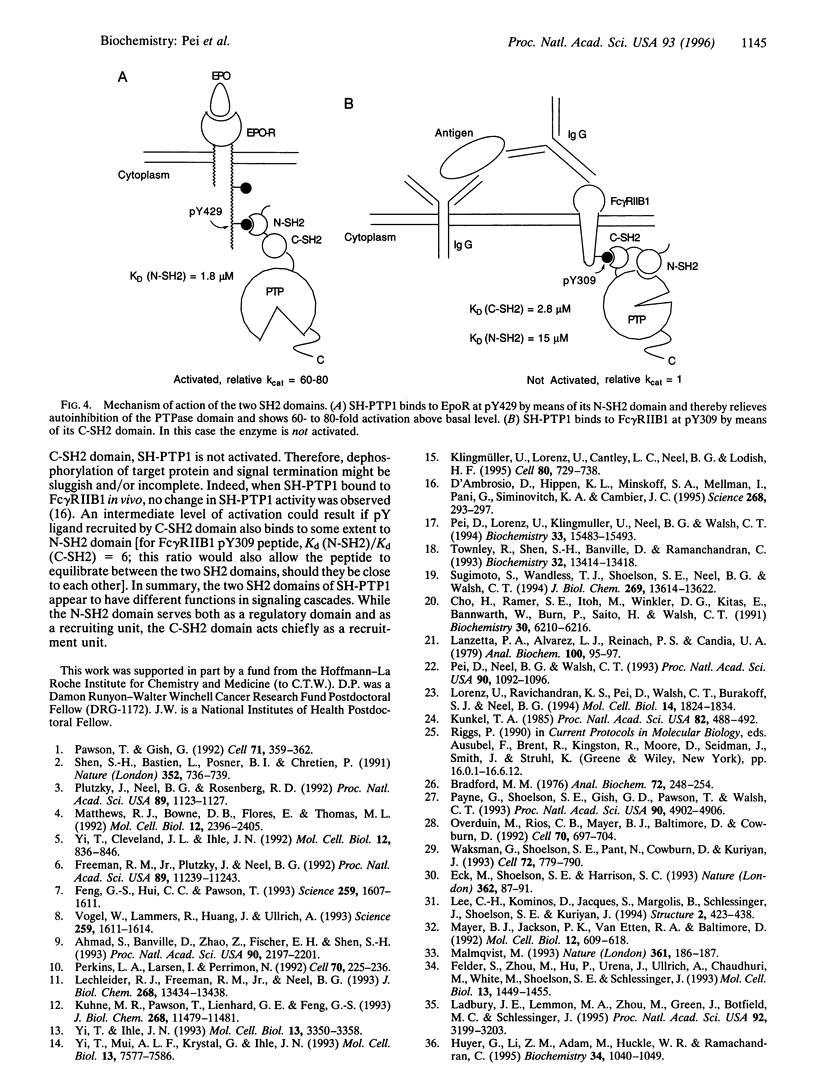
SH-PTP1 (also known as PTP1C, HCP, and SHP) is a non-transmembrane protein tyrosine phosphatase (PTPase) containing two tandem Src homology 2 (SH2) domains. We show here that the two SH2 (N-SH2 and C-SH2) domains in SH-PTP1 have different functions in regulation of the PTPase domain and thereby signal transduction. While the N-terminal SH2 domain is both necessary and sufficient for autoinhibition through an intramolecular association with the PTPase domain, truncation of the C-SH2 domain [SH-PTP1 (delta CSH2) construct] has little effect on SH-PTP1 activity. A synthetic phosphotyrosine residue (pY) peptide derived from the erythropoietin receptor (EpoR pY429) binds to the N-SH2 domain and activates both wild-type SH-PTP1 and SH-PTP1 (delta CSH2) 60- to 80-fold. Another pY peptide corresponding to a phosphorylation site on the IgG Fc receptor (Fc gamma RIIB1 pY309) associates with both the C-SH2 domain (Kd = 2.8 microM and the N-SH2 domain (Kd = 15.0 microM) and also activates SH-PTP1 12-fold. By analysis of the effect of the Fc gamma RIIB1 pY309 peptide on SH-PTP1 (delta CSH2), SH-PTP1 (R30K/R33E), SH-PTP1 (R30K/R136K), and SH-PTP1 (R136K) mutants in which the function of either the N- or C-SH2 domain has been impaired, we have determined that both synthetic pY peptides stimulate SH-PTP1 by binding to its N-SH2 domain; binding of pY ligand to the C-SH2 domain has no effect on SH-PTP1 activity. We propose that the N-terminal SH2 domain serves both as a regulatory domain and as a recruiting unit, whereas the C-terminal SH2 domain acts merely as a recruiting unit.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Banville D., Zhao Z., Fischer E. H., Shen S. H. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Ramer S. E., Itoh M., Winkler D. G., Kitas E., Bannwarth W., Burn P., Saito H., Walsh C. T. Purification and characterization of a soluble catalytic fragment of the human transmembrane leukocyte antigen related (LAR) protein tyrosine phosphatase from an Escherichia coli expression system. Biochemistry. 1991 Jun 25;30(25):6210–6216. doi: 10.1021/bi00239a019. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D., Hippen K. L., Minskoff S. A., Mellman I., Pani G., Siminovitch K. A., Cambier J. C. Recruitment and activation of PTP1C in negative regulation of antigen receptor signaling by Fc gamma RIIB1. Science. 1995 Apr 14;268(5208):293–297. doi: 10.1126/science.7716523. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Shoelson S. E., Harrison S. C. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993 Mar 4;362(6415):87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Felder S., Zhou M., Hu P., Ureña J., Ullrich A., Chaudhuri M., White M., Shoelson S. E., Schlessinger J. SH2 domains exhibit high-affinity binding to tyrosine-phosphorylated peptides yet also exhibit rapid dissociation and exchange. Mol Cell Biol. 1993 Mar;13(3):1449–1455. doi: 10.1128/mcb.13.3.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G. S., Hui C. C., Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993 Mar 12;259(5101):1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- Freeman R. M., Jr, Plutzky J., Neel B. G. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer G., Li Z. M., Adam M., Huckle W. R., Ramachandran C. Direct determination of the sequence recognition requirements of the SH2 domains of SH-PTP2. Biochemistry. 1995 Jan 24;34(3):1040–1049. doi: 10.1021/bi00003a039. [DOI] [PubMed] [Google Scholar]
- Klingmüller U., Lorenz U., Cantley L. C., Neel B. G., Lodish H. F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995 Mar 10;80(5):729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Kuhné M. R., Pawson T., Lienhard G. E., Feng G. S. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993 Jun 5;268(16):11479–11481. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbury J. E., Lemmon M. A., Zhou M., Green J., Botfield M. C., Schlessinger J. Measurement of the binding of tyrosyl phosphopeptides to SH2 domains: a reappraisal. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3199–3203. doi: 10.1073/pnas.92.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lechleider R. J., Freeman R. M., Jr, Neel B. G. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993 Jun 25;268(18):13434–13438. [PubMed] [Google Scholar]
- Lee C. H., Kominos D., Jacques S., Margolis B., Schlessinger J., Shoelson S. E., Kuriyan J. Crystal structures of peptide complexes of the amino-terminal SH2 domain of the Syp tyrosine phosphatase. Structure. 1994 May 15;2(5):423–438. doi: 10.1016/s0969-2126(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Lorenz U., Ravichandran K. S., Pei D., Walsh C. T., Burakoff S. J., Neel B. G. Lck-dependent tyrosyl phosphorylation of the phosphotyrosine phosphatase SH-PTP1 in murine T cells. Mol Cell Biol. 1994 Mar;14(3):1824–1834. doi: 10.1128/mcb.14.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist M. Biospecific interaction analysis using biosensor technology. Nature. 1993 Jan 14;361(6408):186–187. doi: 10.1038/361186a0. [DOI] [PubMed] [Google Scholar]
- Matthews R. J., Bowne D. B., Flores E., Thomas M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992 May;12(5):2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Van Etten R. A., Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992 Feb;12(2):609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin M., Rios C. B., Mayer B. J., Baltimore D., Cowburn D. Three-dimensional solution structure of the src homology 2 domain of c-abl. Cell. 1992 Aug 21;70(4):697–704. doi: 10.1016/0092-8674(92)90437-h. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Payne G., Shoelson S. E., Gish G. D., Pawson T., Walsh C. T. Kinetics of p56lck and p60src Src homology 2 domain binding to tyrosine-phosphorylated peptides determined by a competition assay or surface plasmon resonance. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4902–4906. doi: 10.1073/pnas.90.11.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D., Lorenz U., Klingmüller U., Neel B. G., Walsh C. T. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry. 1994 Dec 27;33(51):15483–15493. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- Pei D., Neel B. G., Walsh C. T. Overexpression, purification, and characterization of SHPTP1, a Src homology 2-containing protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1092–1096. doi: 10.1073/pnas.90.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Larsen I., Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992 Jul 24;70(2):225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Plutzky J., Neel B. G., Rosenberg R. D. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Bastien L., Posner B. I., Chrétien P. A protein-tyrosine phosphatase with sequence similarity to the SH2 domain of the protein-tyrosine kinases. Nature. 1991 Aug 22;352(6337):736–739. doi: 10.1038/352736a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto S., Wandless T. J., Shoelson S. E., Neel B. G., Walsh C. T. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptides derived from insulin receptor substrate-1. J Biol Chem. 1994 May 6;269(18):13614–13622. [PubMed] [Google Scholar]
- Townley R., Shen S. H., Banville D., Ramachandran C. Inhibition of the activity of protein tyrosine phosphate 1C by its SH2 domains. Biochemistry. 1993 Dec 14;32(49):13414–13418. doi: 10.1021/bi00212a006. [DOI] [PubMed] [Google Scholar]
- Vogel W., Lammers R., Huang J., Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993 Mar 12;259(5101):1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Yi T. L., Cleveland J. L., Ihle J. N. Protein tyrosine phosphatase containing SH2 domains: characterization, preferential expression in hematopoietic cells, and localization to human chromosome 12p12-p13. Mol Cell Biol. 1992 Feb;12(2):836–846. doi: 10.1128/mcb.12.2.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T., Ihle J. N. Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Mol Cell Biol. 1993 Jun;13(6):3350–3358. doi: 10.1128/mcb.13.6.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T., Mui A. L., Krystal G., Ihle J. N. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993 Dec;13(12):7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]



