Abstract
The generation of reactive oxygen species (ROS) in cells stimulated with growth factors requires the activation of phosphatidylinositol 3-kinase (PI3K) and the Rac protein. We report here that the COOH-terminal region of Nox1, a protein related to gp91phox (Nox2) of phagocytic cells, is constitutively associated with βPix, a guanine nucleotide exchange factor for Rac. Both growth factor-induced ROS production and Rac1 activation were completely blocked in cells depleted of βPix by RNA interference. Rac1 was also shown to bind to the COOH-terminal region of Nox1 in a growth factor-dependent manner. Moreover, the depletion of Nox1 by RNA interference inhibited growth factor-induced ROS generation. These results suggest that ROS production in growth factor-stimulated cells is mediated by the sequential activation of PI3K, βPix, and Rac1, which then binds to Nox1 to stimulate its NADPH oxidase activity.
Reactive oxygen species (ROS), such as superoxide anions and hydrogen peroxide (H2O2), are produced in mammalian cells in response to the activation of various cell surface receptors and contribute to intracellular signaling and to the regulation of various biological activities, including host defense and metabolic conversion (15, 23, 35). Receptor-mediated ROS production has been studied extensively in phagocytic cells. The enzyme NADPH oxidase of such cells is composed of at least five protein components, namely two transmembrane flavocytochrome b components (gp91phox and p22phox) and three cytosolic components (p47phox, p67phox, and p40phox) (2). The exposure of resting phagocytic cells to an appropriate stimulus results in extensive phosphorylation of the cytosolic components of NADPH oxidase and their association with the transmembrane flavocytochrome b components (1, 11, 33, 36). The assembled oxidase complex catalyzes the transfer of an electron to molecular oxygen to yield the superoxide anion, which is then spontaneously or enzymatically converted to H2O2. The small GTPase Rac is also required for the activation of NADPH oxidase in phagocytic leukocytes (12, 28). Hematopoietic cell-specific Rac2 and the ubiquitously expressed protein Rac1 are the major and minor Rac isoforms, respectively, in human neutrophils (19).
Nonphagocytic cells also produce superoxide anions in response to a variety of extracellular stimuli, including platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) (3, 5, 35, 38) Several homologs (Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2) of gp91phox (Nox2) have been identified in various nonphagocytic cells (8, 13, 21, 23, 37). Nox proteins contain binding sites for FAD, NADPH, and heme, and their NH2-terminal portions contain a cluster of six hydrophobic segments that are predicted to form transmembrane α helices (23). Some of the gp91phox homologs likely associate with p22phox to form functional cytochrome b in nonphagocytes, given that the latter protein is widely expressed (8) and that p22phox antisense RNA was shown to inhibit angiotensin II-induced ROS generation in smooth muscle cells (41). The expression of p40phox is restricted to hematopoietic cells, and the roles of p47phox and p67phox in ROS production in nonphagocytic cells remain unclear. Nox1, whose amino acid sequence has 56% identity to that of gp91phox, has been implicated in PDGF-induced ROS production based on the effect of Nox1 antisense RNA in smooth muscle cells. PDGF also increases the expression of Nox1 in smooth muscle cells (24).
Activated Rac is also required for the activation of ROS generation in nonphagocytic cells, as revealed by the observation that the expression of a dominant-negative form of Rac1 blocks PDGF-induced ROS generation in HepG2 cells (4, 10). Moreover, the expression of a constitutively active form of Rac increases ROS generation in several different types of cells. Activation of Rac is achieved through the exchange of bound GDP for GTP, a process that is catalyzed by a family of proteins known as guanine nucleotide exchange factors (GEFs). GEFs that target Rac include Sos; Vav1, -2, and -3; α- and βPix; Ras-GRF1 and -2; and P-Rex1 (18, 25, 29, 42). Some of these proteins also function as GEFs for other small GTPases, such as Rho and Cdc42. All of these GEF proteins contain a pleckstrin homology (PH) domain that flanks the catalytic Dbl homology (DH) domain. Various GEFs also contain additional specific motifs or homology domains. For example, hematopoietic cell-specific Vav contains a calponin homology domain, a cysteine-rich region, and both Src homology 2 (SH2) and SH3 domains, whereas Pix contains an SH3 domain, a GIT1 binding domain, a proline-rich (PXXP) region, and a leucine zipper (LZ) motif. The LZ motif, which is located in the COOH-terminal region, mediates the homodimerization of Pix (22).
We previously showed that the activation of phosphatidylinositol 3-kinase (PI3K) is required for PDGF-induced ROS production (4). Several Rac-GEFs are activated by phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] or PtdIns(3,4)P2, but not by other phosphoinositides such as PtdIns(4,5)P2. These observations, together with the essential role of Rac in PDGF-induced ROS production, suggest that the activation of a Rac-GEF through the interaction of PtdIns(3,4,5)P3 or PtdIns(3,4)P2 with its PH domain may contribute to this process. Alternatively, a phox homology (PX) domain-containing protein similar to p40phox might mediate growth factor-induced ROS generation. In the case of neutrophils, the production of ROS is triggered by the formation of PtdIns(3)P, which binds to the PX domain of p40phox (14).
We now show that βPix and Nox1 participate in the growth factor-induced generation of ROS in nonphagocytic cells. Our results demonstrate that βPix is essential for Rac1-mediated Nox1 activation and that βPix, Rac1, and Nox1 form a ternary complex in response to growth factor stimulation.
MATERIALS AND METHODS
Cell culture.
Caco-2, HEK293T, and Cos-7 cells were maintained at 37°C in an atmosphere of 5% CO2 in culture dishes containing Dulbecco's modified Eagle's medium (JBI) supplemented with 10% fetal bovine serum (JBI) and 1% antibiotic-antimycotic solution (Life Technologies).
Expression of wild-type and mutant forms of βPix.
Caco-2 cells (105) were plated in 35-mm-diameter culture dishes and transfected for 24 h with pFLAG-CMV-βPix, which encodes FLAG-tagged wild-type βPix, or with pFLAG-CMV plasmids containing various βPix mutants by use of Effectine (Qiagen). The cells were then deprived of serum for 16 h, incubated for 10 min at 37°C in the absence or presence of EGF (100 ng/ml; Upstate Biotechnology), and assayed for the production of H2O2. Cell lysates were also subjected to an immunoblot analysis of recombinant βPix expression, using monoclonal antibodies to FLAG (Sigma); the filter was then reprobed with monoclonal antibodies to β-actin (Sigma), which served as a loading control.
Electroporation of Caco-2 cells.
Electroporation (Amaxa Biosystems, Cologne, Germany) was performed as described previously (26). Caco-2 cells (2 × 105) were subjected to centrifugation, and then the pellet was resuspended in the specified Amaxa Nucleofector solution. Plasmid DNAs (1 to 5 μg) were mixed with 100 μl of cell suspension, transferred into Amaxa certified cuvettes (2.0 mm wide), and electroporated with an Amaxa Nucleofector apparatus, using an appropriate program supplied by the manufacturer's protocol. After electroporation, the cells were immediately transferred to complete medium and cultured in 35-mm-diameter culture dishes at 37°C until analysis.
Preparation of GST fusion proteins and GST pull-down assays.
The plasmid pGEX4T1-Nox1-C, which encodes a glutathione S-transferase (GST) fusion protein containing amino acids 217 to 550 of human Nox1, was introduced into Escherichia coli, and the cells were cultured at 22°C for 7 h. Expression of the GST fusion proteins was induced by 0.05 mM isopropyl-β-d-thiogalactopyranoside (ICN), and the cells were subsequently collected by centrifugation at 2,000 × g for 15 min and sonicated in 10× (wt/vol) phosphate-buffered saline (PBS) containing 1% Triton X-100 and protease inhibitors (aprotinin [1 μg/ml], leupeptin [1 μg/ml], and 0.5 mM phenylmethylsulfonyl fluoride). After centrifugation for 30 min at 19,000 × g, the resulting supernatant was incubated with glutathione-Sepharose 4B (Amersham Pharmacia Biotech) for 4 h at 4°C. The mixture was centrifuged for 2 min at 5,000 × g, and the resulting pellet was washed with 10 bed volumes of PBS containing 1% Triton X-100. For GST pull-down assays, the bead-conjugated GST-Nox1-C fusion proteins were incubated with lysates of Caco-2 or HEK293T cells transfected with pFLAG-βPix or with purified Rac1 from E. coli for 3 h at 4°C in PBS containing 1% Triton X-100. The beads were then separated by centrifugation, washed three times, and subjected to immunoblot analysis with antibodies to FLAG or with monoclonal antibodies to Rac1 (Upstate Biotechnology).
Phosphoinositide binding assay.
Pull-down assays with phosphoinositide analogue beads (Echelon Research Laboratories Inc.) were performed as previously described (17). Briefly, the PH domains (amino acids 295 to 400) of βPix were expressed in E. coli as fusion proteins with GST. Fusion proteins were purified by using glutathione. Purified GST-βPix-PH domain (∼200 ng) was incubated with phosphoinositide analogue beads (Echelon Research Laboratories Inc.) in PBS containing 1% Triton X-100 overnight at 4°C. Bead-bound proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting with antibodies against GST.
Assay of intracellular H2O2 production.
The intracellular production of H2O2 was assayed after the stimulation of cells with EGF (100 ng/ml) in serum-free Dulbecco's modified Eagle's medium. Dishes of confluent cells were washed with Hanks' balanced salt solution and incubated for 5 min in the dark at 37°C with the same solution containing 5 μM 2′,7′-dichlorofluorescein diacetate (DCF-DA; Molecular Probes). DCF-DA is oxidized by H2O2 to the highly fluorescent 2′,7′-dichlorofluorescein (DCF). The cells were then examined with a laser scanning confocal microscope (model LSM 510; Carl Zeiss) equipped with an argon laser tuned to an excitation wavelength of 488 nm, an LP505 emission filter (515 to 540 nm), and a Zeiss Axiovert ×100 objective lens. Images were digitized and stored at a resolution of 512 by 512 pixels. Five groups of cells were randomly selected from each sample, and the mean relative fluorescence intensity for each group of cells was measured with a Zeiss vision system (LSM510, version 2.3) and then averaged for all groups. All experiments were repeated at least five times.
Measurement of H2O2 by FACSCalibur.
HEK293T cells were stimulated with EGF for 10 min, stained with DCF-DA to detect ROS, and subjected to measurement in a FACSCalibur instrument from Becton Dickinson (excitation wavelength, 488 nm; emission wavelength, 515 to 545 nm). ROS are expressed as a histogram of the fluorescence generated by 10,000 cells.
Construction of siRNA for βPix and Nox1.
Specific sequences of 19 nucleotides of human βPix cDNA (GAGCTCGAGAGACACATGG, residues 721 to 739), human Nox1 cDNA (CCAGGATTGAAGTGGATGG, residues 1130 to 1148), and humanVav2 cDNA (GATGACGTCTACCGCAGCC, residues 415 to 433) were selected for the synthesis of small interfering RNAs (siRNAs) (7). The pSUPER vector for siRNAs was purchased from Oligoengine. The phosphorylated oligonucleotides were annealed and cloned into the pSUPER vector by the use of BglII (5′ end) and HindIII (3′ end). Cells were transfected with the resulting construct and cultured for 48 h in complete medium. The transfected cells were deprived of serum for 12 h, incubated for 10 min in the absence or presence of growth factor, and then analyzed for the production of H2O2. The depletion of endogenous βPix, Vav2, or Nox1 by the relevant siRNA was confirmed by immunoblot analysis (βPix or Vav2) or reverse transcription-PCR (RT-PCR) (Nox1).
RT-PCR.
The total RNA was prepared from Caco-2 cells by the use of Trisol (Invitrogen). RT was performed, with the total RNA as the template, by use of an RT-for-PCR kit (Promega). PCR amplification of Nox1 mRNA was carried out with the primers 5′-GTACAAATTCCAGTGTGCAGACCAC-3′ and 5′-CAGACTGGAATATCGGTGACAGCA-3′. Glyceraldehyde-3-phosphate dehydrogenase served as the loading control.
RESULTS
Role of βPix in growth factor-induced ROS generation.
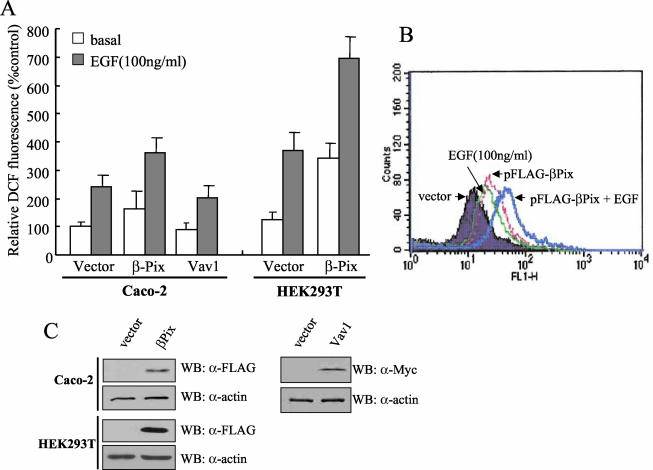
The activation of both PI3K and Rac1 is required for PDGF-induced H2O2 generation (4). We hypothesized that a Rac-GEF such as βPix might link the activation of these two proteins. PtdIns(3,4,5)P3 or PtdIns(3,4)P2 produced by PI3K might thus bind to the PH domain of βPix and thereby result in the activation of Rac1, and consequently, Nox1. To investigate this possibility, we first examined the effect of βPix overexpression on EGF-induced ROS generation in Caco-2 cells. The generation of ROS by Caco-2 or HEK293T cells was determined by measurement of the oxidation of DCF-DA to DCF with a laser-based confocal microscope or by fluorescence-activated cell sorting analysis. The exposure of control Caco-2 or HEK293T cells to EGF (100 ng/ml) resulted in an increased generation of ROS, as revealed by an increase in DCF fluorescence (Fig. 1A and B). The presence of ectopically expressed FLAG-tagged βPix resulted in a marked increase in the level of ROS in response to EGF in both cell lines compared with the level measured in control cells. An immunoblot analysis with antibodies to βPix revealed that the level of βPix-FLAG in the transfected cells was about three times that of endogenous βPix (data not shown). HepG2 cells expressing βPix showed a similar pattern of ROS generation in response to PDGF (data not shown). Recent evidence suggested that Vav1 is the most effective Rac-GEF for the activation of Nox2 (gp91phox) reconstituted with p22phox, p47phox, p67phox, and Rac1 in COS cells (34). The overexpression of Vav1 in Caco-2 cells, however, affected neither basal nor EGF-induced ROS generation (Fig. 1A). Although these overexpression experiments clearly suggest that βPix, but not Vav1, is involved in ROS production in nonphagocytic cells, the requirement of βPix for EGF-induced ROS formation is not clear since the ROS production of unstimulated cells was also increased by βPix overexpression. Activated Rac is known to regulate a variety of cellular functions, including membrane ruffling and lamellipodium formation, and the overexpression of βPix and another Rac-GEF, P-Rex protein, was shown to cause membrane ruffling and exaggerated lamellipodium formation, respectively, in the absence of PDGF stimulation (22, 42).
FIG. 1.
Effect of βPix overexpression on EGF-induced ROS generation in Caco-2 or HEK293T cells. (A) Caco-2 or HEK293T cells were transfected with an empty vector (pFLAG-CMV) or a vector encoding FLAG-tagged βPix or Myc-tagged Vav1. After incubation with EGF for 10 min, the generation of H2O2 was monitored by confocal microscopic analysis of DCF fluorescence (see Materials and Methods). The mean fluorescence intensity of DCF in experiments was measured and expressed relative to that of unstimulated cells transfected with empty vector. Data are means ± standard errors (SE) of values from five independent experiments. (B) HEK293T cells were transfected with empty vector (pFLAG-CMV) or with a vector encoding FLAG-tagged βPix. Cells were stimulated with EGF for 10 min and then stained with DCF-DA. The fluorescence was analyzed in 10,000 cells each with FACSCalibur (Becton Dickinson), with excitation at 488 nm and emission at 530 nm. (C) Cell lysates from each sample from panels A and B were prepared and subjected to immunoblot analysis (WB) with antibodies to FLAG (for recombinant βPix) or Myc (for recombinant Vav1); the filter was reprobed with antibodies to β-actin.
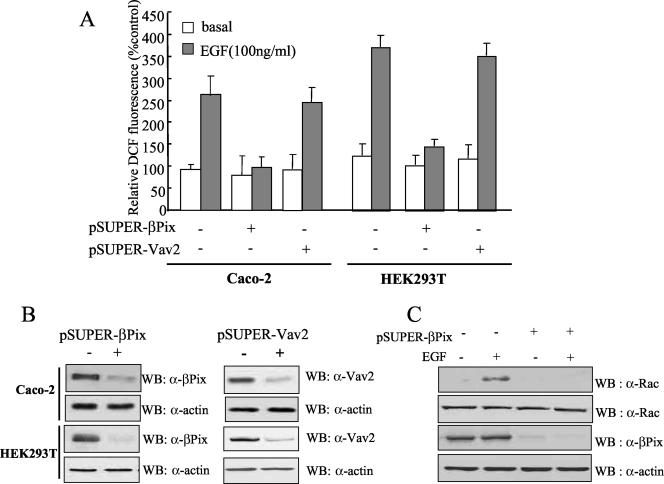
To confirm the role of βPix in EGF-induced ROS generation in Caco-2 and HEK293T cells, we subjected Caco-2 and HEK293T cells to transient transfection with pSUPER-βPix, encoding a siRNA specific for the βPix gene. The cells transfected with the siRNA vector exhibited a marked reduction in the abundance of endogenous βPix protein compared with that in cells transfected with the empty pSUPER vector (Fig. 2B). Either Caco-2 cells or HEK293T cells transfected with the pSUPER-βPix vector failed to generate ROS in response to EGF, whereas both cells transfected with pSUPER alone exhibited a marked increase in the ROS response to growth factor stimulation, suggesting that βPix is essential for EGF-induced ROS production (Fig. 2A). In order to determine whether the effect of βPix on ROS generation is restricted to a type of cell line (Caco-2 and HEK293T cells) or to EGF stimulation, we examined the effect of βPix depletion on ROS generation in HepG2 cells with PDGF stimulation. Knockout of the βPix protein by transfection of pSUPER-βPix into HepG2 cells led to a failure in PDGF-dependent ROS generation (data not shown). This result indicates that βPix is a key molecule in growth factor-mediated ROS generation in various cell lines. A recent report indicated that Vav2 is important for Rac activation in response to EGF stimulation (40). To verify the effect of Vav2 on EGF-induced ROS generation, we also subjected Caco-2 and HEK293T cells to transient transfection with pSUPER-Vav2. A transient knockout of Vav2 in both cell lines resulted in no inhibitory effect on EGF-induced ROS generation. It is likely that Vav2 is not involved in EGF-mediated ROS generation in Caco-2 and HEK293T cells.
FIG. 2.
Effect of βPix depletion by siRNA expression on growth factor-induced ROS generation. (A) Two different cell lines (Caco-2 cells and HEK293T cells) were transfected with either pSUPER-βPix, encoding a βPix-specific siRNA, or pSUPER-Vav2, encoding a Vav2-specific siRNA. Control cells were transfected with only the vector control (pSUPER). After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF treatment for 10 min, the generation of H2O2 was then monitored by confocal microscopic analysis of DCF fluorescence. Data are means ± SE of values from five independent experiments. (B) Cell lysates were then prepared and subjected to immunoblot analysis with antibodies to βPix and Vav2 (Babraham Co., Cambridge, United Kingdom); the filter was reprobed with antibodies to β-actin. (C) HEK293T cells were transfected with pSUPER (control) or pSUPER-βPix. After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF treatment for 10 min, cell lysates were then prepared with PBS containing 1% Triton X-100 and were incubated for 3 h with a GST fusion protein of PAK-RBD conjugated to glutathione-Sepharose 4B beads. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to Rac1 (upper panel). Cell lysates were also directly subjected to immunoblot analysis with antibodies to Rac (second panel), βPix (third panel), and β-actin (bottom panel).
We next examined whether endogenous βPix regulates Rac1 activity in HEK293T cells as was previously reported (25). The amount of GTP-bound (activated) Rac1 was assayed by the use of the Rac binding domain of p21-activated protein kinase (PAK-RBD), which binds Rac1-GTP but not Rac1-GDP (25, 27). Whereas EGF induced a marked increase in the abundance of Rac1-GTP in HEK293T cells transfected with pSUPER alone, Rac1 activation by EGF was completely eliminate in cells transfected with pSUPER-βPix (Fig. 2C). This result demonstrates that βPix is an essential GEF for Rac1.
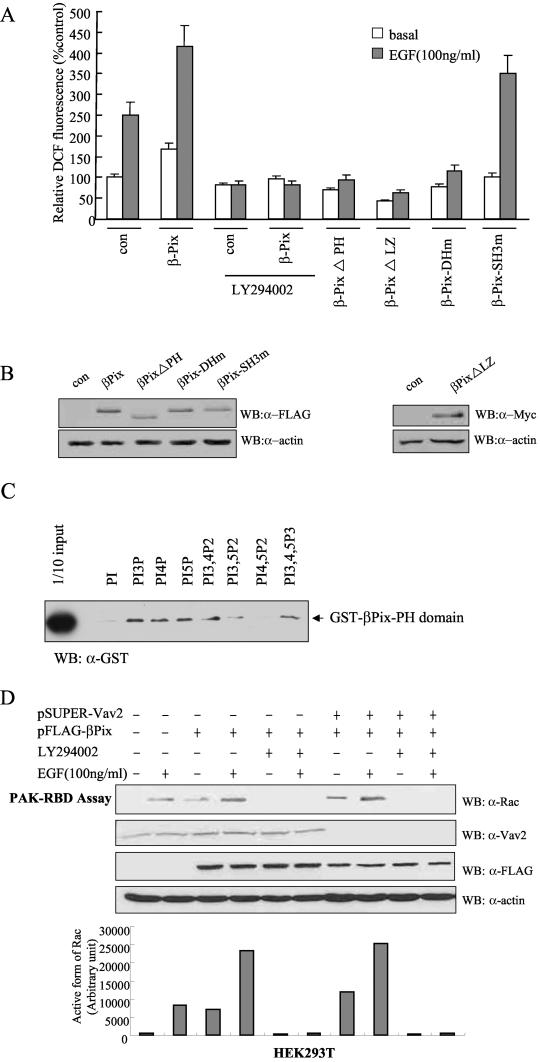
We examined whether the effect of βPix on ROS generation originates from PI3K activity. Treatment of the cells with LY294002 as a PI3K inhibitor blocked βPix-induced ROS generation as well as ROS generation in control cells upon the addition of EGF (Fig. 3A). To investigate the role of the various functional domains of βPix in ROS generation, we examined EGF-induced ROS production in Caco-2 cells expressing various βPix mutants. The expression of a βPix protein containing a point mutation in the SH3 domain (βPix-SH3m, in which Trp43 is replaced with Lys) affected neither basal nor EGF-induced ROS production, whereas βPix mutants lacking either the PH domain (βPix-ΔPH, lacking amino acid residues 295 to 400) or the LZ motif (βPix-ΔLZ, lacking amino acid residues 587 to 634) and the DH domain mutant of βPix (βPix-DHm, in which Leu238 and Leu239 are replaced with Ser and Arg, respectively) completely blocked ROS generation in response to EGF (Fig. 3A). These results thus suggest that the GEF activity of βPix through the DH domain and the abilities of βPix to bind a product of PI3K through its PH domain and to dimerize through its LZ motif are essential for EGF-induced ROS generation and that βPix mutants lacking either the PH or LZ domain exert a dominant-negative effect.
FIG.3.
Roles of the PH domain and LZ motif of βPix in EGF-induced ROS generation. (A) Caco-2 cells were transfected with empty vector (pFLAG-CMV, or pcDNA3.0 for Myc epitope-tagged βPix-ΔLZ) or with vectors encoding either wild-type βPix or βPix mutants. They were then deprived of serum for 16 h and incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cells were pretreated with LY294002 (10 μM) for 30 min before the treatment with EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are means ± SE of values from five independent experiments. (B) Lysates of the transfected cells were subjected to immunoblot analysis with antibodies to the FLAG or Myc epitope, as indicated. (C) Phosphoinositide binding assay. Purified GST-βPix-PH domain (∼200 ng) was incubated with phosphoinositide analogue beads (Echelon Research Laboratories Inc.) overnight at 4°C and then washed with PBS containing 1% Triton X-100. Bead-bound proteins were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against GST. (D) HEK293T cells were transfected with FLAG-βPix only or together with pSUPER-Vav2. After being cultured for 48 h, the cells were deprived of serum for 12 h. The cells were pretreated with LY294002 (10 μM) for 30 min before a treatment with EGF (100 ng/ml). After EGF treatment for 10 min, cell lysates were then prepared in PBS containing 1% Triton X-100 and were incubated for 3 h with a GST fusion protein of PAK-RBD conjugated to glutathione-Sepharose 4B beads. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to Rac1 (top). Cell lysates were also directly subjected to immunoblot analysis with antibodies to Vav2 (Babraham Co.), FLAG, and β-actin. The quantitative results for an active form of Rac from the PAK-RBD assay are shown in graph form (bottom).
To verify the direct activation of βPix by PI3K, we measured the binding affinity of the PH domain of βPix to various phosphoinositides. The GST-conjugated PH domain was incubated with phosphoinositide-coupled beads and washed with PBS three times. Bound complexes of the GST-PH domain of βPix and phosphoinositide were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against GST. The PH domain of βPix bound to PtdIns3P, PtdIns(3,4)P2, PtdIns(3,5)P2, and PtdIns(3,4,5)P3 as well as PtdIns4P and PtdIns5P, but not PtdIns and PtdIns(4,5)P2, indicating that PtdIns 3-kinase products directly interact with the PH domain of βPix (Fig. 3C). We next investigated the effect of binding of PtdIns 3-kinase products with the PH domain of βPix on Rac1 activity. The expression of βPix in HEK293T cells resulted in Rac1 activation as a downstream effect of βPix, whereas pretreatment with LY294002 abolished Rac1 activation in response to EGF stimulation (Fig. 3D). Moreover, the overexpression of βPix in HEK293T cells depleted of Vav2 (βPix+ Vav2−) resulted in Rac1 activation in response to EGF stimulation. This result suggests that PI3K activation regulates Rac1 activation through βPix.
Effect of mitochondrial ROS on EGF-mediated ROS generation.
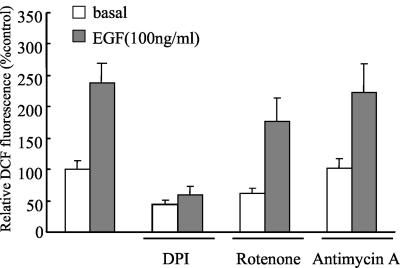
It is well known that the electron transport chain in mitochondria is a major source of ROS production for many cell types (6, 28, 31). Approximately 1 to 3% of the electrons are leaked from mitochondria and then reduce free oxygen to make superoxide anions. We first asked whether ROS from mitochondria contribute to EGF-mediated ROS production. To examine this possibility, we used diphenyliodonium (DPI) as an NADPH oxidase inhibitor and rotenone and antimycin A as electron transport chain blockers. Treatment of Caco-2 cells with DPI resulted in a complete inhibition of ROS production in response to EGF stimulation, whereas treatment with rotenone or antimycin A had a marginal effect on the inhibition of EGF-mediated ROS production (Fig. 4). This result suggests that EGF-induced ROS originates not from mitochondria, but from NADPH oxidase activity.
FIG. 4.
Effect of pharmacological inhibitors on EGF-induced ROS generation. Caco-2 cells were pretreated with DPI (10 μM), rotenone (1 μM), and antimycin A (25 mg/ml) for 30 min before treatment with EGF. After EGF (100 ng/ml) treatment for 10 min, the generation of H2O2 was measured on the basis of DCF fluorescence. Data are means ± SE of values from three independent experiments.
Association of βPix with Nox1.
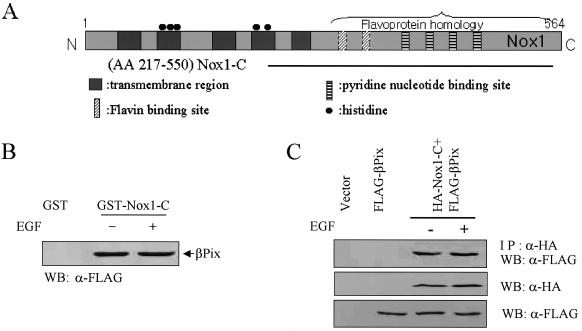
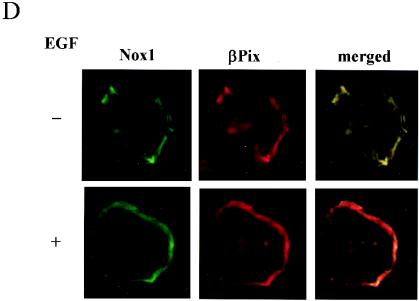
βPix contains a PtdIns(3,4,5)P3-responsive PH domain and is able to catalyze the GDP-GTP exchange of Rac1. The results shown in Fig. 2 suggest that βPix contributes to EGF-induced ROS generation. We hypothesized that this specificity is attributable to the interaction of βPix with a Nox isoform that is responsible for EGF- or PDGF-induced ROS formation. We have confirmed that Nox1 is the most abundant isozyme in Caco-2 cells by a TaqMan real-time quantitative PCR (data not shown). To test our hypothesis, we examined whether βPix interacts with Nox1. Nox1 contains five putative transmembrane domains in its NH2-terminal region and both FAD and NADPH binding domains in its cytosolic COOH-terminal region (23) (Fig. 5A). We prepared GST fusion proteins containing the transmembrane segment and COOH-terminal residues 217 to 550 (Nox1-C) and conjugated them to glutathione-Sepharose 4B beads. The incubation of bead-conjugated GST-Nox1-C with lysates of Caco-2 cells expressing FLAG-tagged βPix revealed a specific association of FLAG-tagged βPix with Nox1-C in an EGF-independent manner (Fig. 5B). To investigate whether Nox1 associates with βPix in cells, we cotransfected HEK293T cells with vectors for hemagglutinin (HA)-tagged Nox1-C and for FLAG-tagged βPix. Cells were incubated in the absence or presence of EGF and then cell lysates were subjected to immunoprecipitation with antibodies to HA. Immunoblot analyses of the resulting precipitates with antibodies to FLAG revealed that FLAG-tagged βPix interacted with Nox1-C and that the extent of these interactions was not affected by EGF stimulation (Fig. 5C). For verification of the colocalization between βPix and Nox1 in vivo, pFLAG-βPix was transfected into COS-7 cells with pEGFP-N1-full-length Nox1. Figure 5D shows that TRITC-FLAG-βPix is colocalized with GFP-Nox1 at the plasma membrane region independent of growth factor treatment.
FIG. 5.
Growth factor-independent interaction of βPix with Nox1. (A) Schematic representation of the structure of Nox1. The horizontal line represents Nox1-C. (B) Caco-2 cells were transfected with FLAG-tagged βPix. After serum deprivation, they were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then prepared, incubated for 2 h with bead-conjugated GST or GST-Nox1-C, and then subjected to immunoblot analysis with antibodies to FLAG. (C) pFLAG-CMV-βPix was transiently expressed in HEK293T cells either alone or together with pcDNA3.0-HA-Nox1-C, as indicated. After serum starvation for 16 h, cells were incubated in the absence or presence of EGF (100 ng/ml) for 10 min. Cell lysates were then subjected to immunoprecipitation (IP) with antibodies to HA, and the resulting precipitates were subjected to immunoblot analysis with antibodies to FLAG (upper panel). Lysates were also directly subjected to immunoblot analysis with antibodies to HA (middle panel) or FLAG (lower panel). (D) Cos-7 cells cotransfected with pEGFP-N1-Nox1 and pFLAG-βPix were starved of serum for 16 h and then fixed before (top panels) or after (bottom panels) stimulation with EGF (100 ng/ml) for 10 min. βPix distribution was visualized by using an anti-FLAG antibody and a tetramethyl rhodamine isocyanate-conjugated secondary antibody. These results are representative of three independent experiments.
Direct interaction of Rac1 with Nox1.
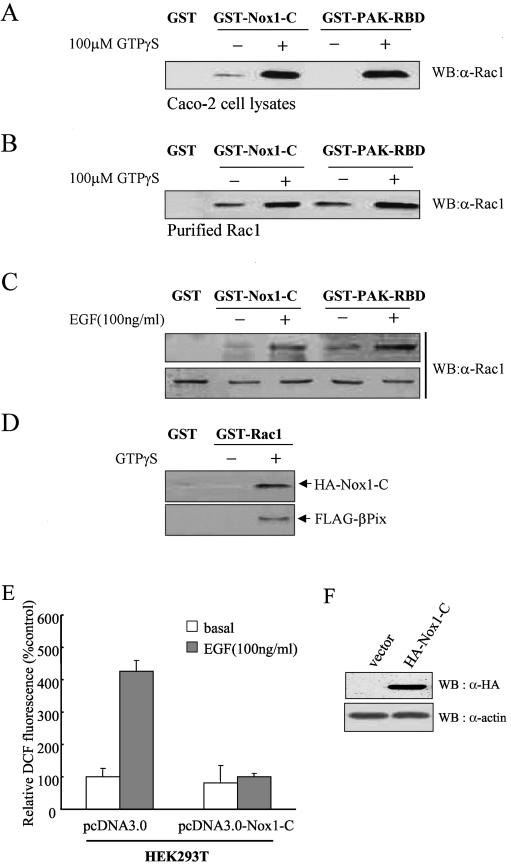
In activated phagocytic cells, Rac2 translocates from the cytoplasm to the plasma membrane and associates with the NADPH oxidase by interacting with both p67phox and gp91phox (2). We therefore examined whether the active form of Rac1 binds to Nox1. GST-Nox1-C associated with Rac1 present in Caco-2 cell lysates in a manner that was dependent on the presence of guanosine 5′-O-(3′-thiotriphosphate) (GTP-γ-S) (Fig. 6A). As a positive control, GST-PAK-RBD was examined for binding to Rac1 in the presence of GTP-γ-S (Fig. 6A and B). Given that Rac1 interacts with βPix and that βPix interacts with Nox1 (Fig. 5), the observed physical association of Rac1 with Nox1 might have occurred indirectly through βPix. However, we found that GST-Nox1-C binds to purified Rac1 in a GTP-γ-S-dependent manner (Fig. 6B), suggesting that Rac1 interacts directly with Nox1. We investigated whether the stimulation of Caco-2 cells by EGF results in the production of activated Rac1 molecules that are able to associate with GST-Nox1-C. GST-Nox1-C fusion proteins were found to interact with Rac1 present in the lysates of EGF-treated cells to a much larger extent than with that in the lysates of untreated cells (Fig. 6C). These results thus indicate that EGF induces the formation of a complex containing Nox1 and the active form of Rac1.
FIG. 6.
Direct interaction of Rac1 with Nox1. A Caco-2 cell lysate (A) or purified Rac1 (1 μg) (B) was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then for 3 h at 4°C with bead-conjugated GST, GST-Nox1-C, or GST-PAK-RBD (10 μg) in a final volume of 500 μl of PBS containing 1% Triton X-100 in the continued absence or presence of 100 μM GTP-γ-S. Proteins that bound specifically to the beads as well as to the cell lysate or purified Rac1 were then subjected to immunoblot analysis with antibodies to Rac1. (C) Caco-2 cells were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). Cell lysates were then incubated for 3 h with bead-bound GST, GST-Nox1-C, or GST-PAK-RBD. Proteins retained specifically by the beads were subjected to immunoblot analysis with antibodies to Rac1 (upper panel). Cell lysates were also subjected directly to immunoblot analysis with the same antibodies (lower panel). (D) GST-Rac was incubated first for 10 min at room temperature in the absence or presence of 100 μM GTP-γ-S and then with lysates of HEK293T cells transfected with HA-Nox1-C together with FLAG-βPix for 3 h at 4°C. The beads were then washed with ice-cold cell lysis buffer, and bound proteins were subjected to immunoblot analysis with antibodies to HA or FLAG. (E) HEK293T cells transfected with a vector encoding Nox1-C (pcDNA3.0-HA-Nox1-C) or the corresponding empty vector (pcDNA3.0) were deprived of serum for 16 h and then incubated for 10 min in the absence or presence of EGF (100 ng/ml). The generation of H2O2 was assayed on the basis of DCF fluorescence. Data are expressed as mean fluorescence intensities relative to that for the corresponding unstimulated cells transfected with empty vector and are means ± SE of values from five independent experiments. (F) Cells transfected as for panel E were lysed and subjected to immunoblot analysis with antibodies to HA; filters were reprobed with antibodies to β-actin.
We next determined whether the complex formation of βPix-Rac1-Nox1 occurs in cells by performing GST pull-down assays using GST-Rac1 with or without GTP-γ-S. GST-Rac1-GTP-γ-S interacted simultaneously with Nox1 and βPix (Fig. 6D). These results indicate that the COOH-terminal region of Nox1 contributes to the complex formation of βPix-Rac1-Nox1. We therefore examined the effect of Nox1-C expression on growth factor-induced ROS generation. The stimulation of HEK293T cells with EGF that had been transfected with an empty vector resulted in a 4.5-fold increase in DCF fluorescence (Fig. 6E). In contrast, growth factor stimulation of HEK293T cells expressing Nox1-C failed to induce ROS generation. These results thus suggest that Nox1-C blocks the formation of an active NADPH oxidase complex in growth factor-stimulated cells by acting in a dominant-negative manner to sequester βPix, activated Rac1, and other components of NADPH oxidase.
Inhibition of EGF-induced ROS generation by knockout of Nox1.
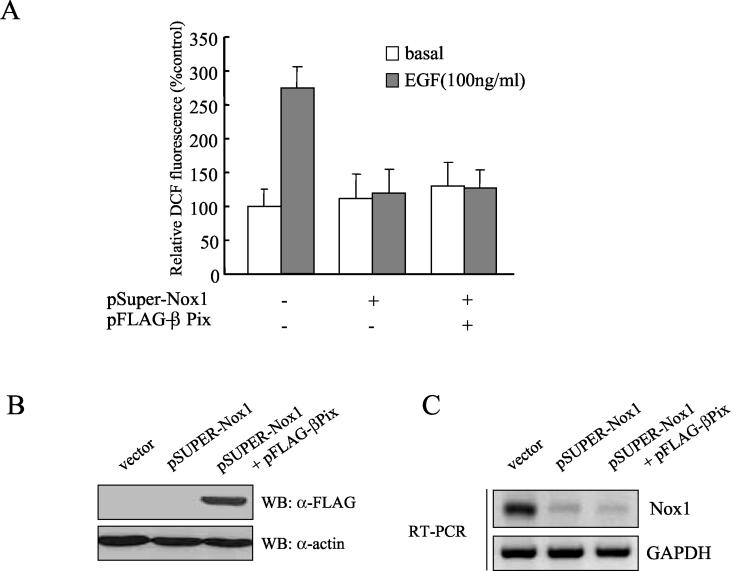
To determine the role of Nox1 in EGF-induced ROS generation, we tested the effect of a knockout of Nox1 on ROS generation in Caco-2 cells electroporated with pSUPER-Nox1 encoding a siRNA specific for the Nox1 gene. As shown in Fig. 7A, the silencing of Nox1 in Caco-2 cells resulted in a significant decrease in ROS generation in response to EGF stimulation. Moreover, the overexpression of βPix had no effect on ROS generation in Caco-2 cells depleted of Nox1 protein (Fig. 7). This result demonstrates that Nox1, as a downstream molecule of βPix and the EGF receptor, is essential for the formation of an active NADPH oxidase complex in EGF-stimulated Caco-2 cells.
FIG. 7.
Role of Nox1 in EGF-induced ROS generation. (A) Caco-2 cells were electroporated with either pFLAG-βPix or the empty vector together with pSUPER-Nox1. After being cultured for 48 h, the cells were deprived of serum for 12 h. After EGF (100 ng/ml) treatment, the generation of H2O2 was measured on the basis of DCF fluorescence. Data are means ± SE of values from three independent experiments. (B) Total cell lysates of each sample from panel A were prepared and subjected to immunoblot analysis with antibodies to FLAG; the filter was then reprobed with antibodies to β-actin. (C) Total RNA was prepared from each sample from panel A. An RT-PCR demonstrates Nox1 expression. GAPDH served as a loading control.
DISCUSSION
Previous observations have indicated that the activation of PI3K is essential for growth factor-induced ROS production in nonphagocytic cells (4). Our present data are consistent with this notion and further suggest that PtdIns(3,4,5)P3 and PtdIns(3,4)P2, which are products of PI3K action, are required for the activation of Rac1 through interaction with the PH domain of the Rac-GEF βPix. Our observation that growth factor-induced ROS formation or Rac1 activation was completely blocked by a siRNA-based depletion of βPix in two different human cell lines (Caco-2 and HEK293T) suggests that βPix is the predominant Rac-GEF in the activation of Nox1 by growth factors (Fig. 2). In addition to its role in the PI3K-βPix-Rac1-Nox1 pathway, PI3K activation is linked to ROS production by another pathway, as indicated by the observation that PtdIns(3)P, a potential breakdown product of PtdIns(3,4,5)P3, binds to the PX domains of p40phox and p47phox and thereby results in the activation of the NADPH oxidase complex in human neutrophils (14, 20, 44). However, given that the expression of p40phox is restricted to cells of hematopoietic lineages (43, 44), this pathway is likely operative only in phagocytic cells.
In addition to the PH domain, which is common to all Rac-GEF proteins, βPix contains an SH3 domain as a PAK binding site, a DH domain for GEF activity, and an LZ motif for dimerization (22). Our mutational analysis demonstrated that the PH domain, the DH domain, and the LZ motif are essential for the function of βPix in ROS production, whereas the SH3 domain is dispensable. It is well known that an interaction between Pix and PAK enhances the GEF activity (32). The expression of a PAK kinase mutant failed to induce growth factor-induced ROS generation (data not shown). Our data show that PAK has no effect on βPix-related ROS generation. These results suggest that both the interaction of βPix with PI3K products via its PH domain and its homodimerization via the LZ domain are essential for growth factor-induced ROS generation but that the association of βPix with PAK via its SH3 domain is not required for this process.
Studies with the NADPH oxidase of phagocytic cells have indicated that GTP-bound, but not GDP-bound, Rac2 directly interacts with gp91phox and that this physical interaction is required for the electron flow from NADPH to the heme group of cytochrome b (12, 30). We have now shown that Rac1 binds to the COOH-terminal flavoprotein domain (Nox1-C) of Nox1 in an activation-dependent manner. To investigate what region of Nox1 is responsible for binding to Rac1, we prepared GST fusion proteins containing the COOH-terminal residues 217 to 550 (Nox1-LC) or 336 to 550 (Nox1-SC) of Nox1 and conjugated them to glutathione-Sepharose 4B beads. Both GST-Nox1-SC and GST-Nox1-LC associated with Rac1 present in Caco-2 cell lysates in a manner that was dependent on the presence of GTP-γ-S. The amount of Rac1 associated with Nox1-LC was larger than that bound to Nox1-SC (data not shown). Unlike that of Nox1-SC, the NH2-terminal region of Nox1-LC contains a transmembrane domain. The region of Nox1 that interacts with Rac1 might thus include the membrane-proximal domain.
We also found that βPix associates with this domain of Nox1. This appears to be the first demonstration of a direct interaction between any Rac-GEF protein and a member of the Nox family. There is a large family of Rac-GEFs (though some can also act as GEFs for other monomeric GTPases), including Sos, Vav, Tiam, Ras-GRF1, and Pix (18, 25, 29, 40, 45). We have determined that Vav1 does not associate with the flavoprotein domain of Nox1 (data now shown). These results suggest that the large numbers of identified Rac-GEFs are not likely functionally redundant and that they may mediate the activation of specific Nox isoforms through physical interactions. This notion is consistent with the previous observation that Vav1 is the most effective Rac-GEF for the promotion of ROS production by the NADPH oxidase of phagocytic cells despite the fact that other Rac-GEF proteins (Vav2 and Tiam1) are more efficient at catalyzing GDP-GTP exchange on Rac (34).
Although several homologs (Nox1, Nox3, Nox4, Nox5, Duox1, and Duox2) of gp91phox from phagocytic cells have been identified in various nonphagocytic cells, no physical coupling between growth factor receptors and Nox isozymes has been proposed. Lassègue et al. suggested that the Nox1 isoform acts downstream of the PDGF receptor on the basis of the observation that PDGF-induced ROS formation was completely blocked by the expression of Nox1 antisense RNA in smooth muscle cells (24). This conclusion is consistent with our observation that expression of the siRNA of Nox1 resulted in an inhibition of growth factor-induced ROS formation in Caco-2 cells.
Several papers recently suggested that novel human homologs of p47phox (NOXO) and p67phox (NOXA) are capable of supporting the activation of Nox1 (9, 16, 39). To verify the function of NOXO and NOXA in the regulation of ROS generation through βPix-Rac-Nox1, we measured ROS generation in response to EGF in either HEK293T cells overexpressing Nox1, NOXO, and NOXA (HEK293T/Nox1/NOXO/NOXA) or Caco-2 cells overexpressing NOXO and NOXA (Caco2/NOXO/NOXA). We found that the stimulation of either HEK293T/Nox1/NOXO/NOXA or Caco2/NOXO/NOXA cellswith EGF caused a marked increase in ROS generation compared with EGF-stimulated control HEK293T or Caco-2 cells. The knockout of endogenous βPix protein in HEK293T/Nox1/NOXO/NOXA or Caco2/NOXO/NOXA cells resulted in an inhibition of ROS generation in response to EGF, indicating that βPix is essential for EGF-induced ROS production via Nox1 and for the supporting activities of NOXO and NOXA (data not shown).
In conclusion, we have established a sequential mechanism by which growth factor stimulation induces the production of ROS. The binding of growth factors to their receptors results in the activation of PI3K, the products of which, namely PtdIns(3,4,5)P3 and PtdIns(3,4)P2, then bind to the PH domain of Nox1-associated βPix and stimulate the GDP-GTP exchange activity of βPix. The activated βPix converts Rac1-GDP to Rac1-GTP, which then also associates with Nox1 to promote the electron transfer from NADPH to molecular oxygen.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the Center for Cell Signaling Research at Ewha Womans University, by a grant of Critical Technology 21 (M1001602000102B160200210), by the 21C Frontier Functional Proteomics Project (FPR02A7-32-110), by a grant from CFGC (CG 133), by an NRL program (M1-0203-00-0072) from the Korea Ministry of Science and Technology, and by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (HMP-00-GN-01-0001). H.S.P. was the recipient of a BK21 scholarship.
REFERENCES
- 1.Ago, T., H. Nunoi, T. Ito, and H. Sumimoto. 1999. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox. J. Biol. Chem. 274:33644-33653. [DOI] [PubMed] [Google Scholar]
- 2.Babior, B. M. 1999. NADPH oxidase. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 3.Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. [PubMed] [Google Scholar]
- 4.Bae, Y. S., J. Y. Sung, O. S. Kim, Y. J. Kim, K. C. Hur, A. Kazlauskas, and S. G. Rhee. 2000. Platelet-derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J. Biol. Chem. 275:10527-10531. [DOI] [PubMed] [Google Scholar]
- 5.Bokoch, G. M., and U. G. Knaus. 2003. NADPH oxidases: not just for leukocytes anymore. Trends Biochem. Sci. 9:502-508. [DOI] [PubMed] [Google Scholar]
- 6.Boveris, A., and B. Chance. 1973. The mitochondrial generation of hydrogen peroxide: general properties and effect of hyperbaric oxygen. Biochem. J. 134:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, G., Z. Cao, X. Xu, E. G. van Meir, and J. D. Lambeth. 2001. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131-140. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, G., and J. D. Lambeth. 2003. NOXO1: regulation of lipid binding, localization and activation of Nox1 by the PX domain. J. Biol. Chem. 279:4737-4742. [DOI] [PubMed] [Google Scholar]
- 10.Cool, R. H., E. Merten, C. Theiss, and H. Acker. 1998. Rac1, and not Rac2, is involved in the regulation of the intracellular hydrogen peroxide level in HepG2 cells. Biochem. J. 332:5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross, A. R., R. W. Erickson, and J. T. Curnutte. 1999. The mechanism of activation of NADPH oxidase in the cell-free system: the activation process is primarily catalytic and not through the formation of a stoichiometric complex. Biochem. J. 341:251-255. [PMC free article] [PubMed] [Google Scholar]
- 12.Diebold, B. A., and G. M. Bokoch. 2001. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2:211-215. [DOI] [PubMed] [Google Scholar]
- 13.Edens, W. A., L. Sharling, G. Cheng, R. Shapira, J. M. Kinkade, T. Lee, H. A. Edens, X. Tang, C. Sullards, D. B. Flaherty, G. M. Benian, and J. D. Lambeth. 2001. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154:879-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellson, C. D., S. Gobert-Gosse, K. E. Anderson, K. Davidson, H. Erdjument-Bromage, P. Tempst, J. W. Thuring, M. A. Cooper, Z. Y. Lim, A. B. Holmes, P. R. Gaffney, J. Coadwell, E. R. Chilvers, P. T. Hawkins, and L. R. Stephens. 2001. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox). Nat. Cell Biol. 3:679-682. [DOI] [PubMed] [Google Scholar]
- 15.Finkel, T., and T. H. Holbrook. 2000. Oxidant, oxidative stress, and the biology of aging. Nature 408:239-247. [DOI] [PubMed] [Google Scholar]
- 16.Geiszt, M., K. Lekstrom, J. Witta, and T. L. Leto. 2003. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J. Biol. Chem. 278:20006-20012. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, S., J. C. Fanzo, C. Hu, D. Cox, S. Y. Jang, A. E. Lee, S. Greenberg, and A. B. Pernis. 2003. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J. Biol. Chem. 278:43541-43549. [DOI] [PubMed] [Google Scholar]
- 18.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 19.Heyworth, P. G., B. P. Bohl, G. M. Bokoch, and J. T. Curnutte. 1994. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. J. Biol. Chem. 269:30749-30752. [PubMed] [Google Scholar]
- 20.Kanai, F., H. Liu, S. J. Field, H. Akbary, T. Matsuo, G. E. Brown, L. C. Cantley, and M. B. Yaffe. 2001. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nature 3:675-679. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi, H., M. Hikage, H. Miyashita, and M. Fukumoto. 2000. NADPH oxidase subunit, gp91phox homologue, preferentially expressed in human colon epithelial cells. Gene 254:237-243. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S., S.-H. Lee, and D. Park. 2001. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, βPix. J. Biol. Chem. 276:10581-10584. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth, J. D. 2000. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidase. Curr. Opin. Hematol. 9:11-17. [DOI] [PubMed] [Google Scholar]
- 24.Lassègue, B., D. Sorescu, K. Szocs, Q. Yin, M. Akers, Y. Zhang, S. L. Grant, J. D. Lambeth, and K. K. Griendling. 2000. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circulation 88:888-894. [DOI] [PubMed] [Google Scholar]
- 25.Lee, S. H., M. Eom, S. J. Lee, S. Kim, H. J. Park, and D. Park. 2001. βPIX-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J. Biol. Chem. 276:25066-25072. [DOI] [PubMed] [Google Scholar]
- 26.Lenz, P., S. M. Bacot, M. R. Frazier-Jessen, and G. M. Feldman. 2003. Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett. 538:149-154. [DOI] [PubMed] [Google Scholar]
- 27.Manser, E., T. Leung, H. Salihuddin, Z.-S. Zhao, and L. Lim. 1994. A brain serine/threonine kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 28.Nemoto, S., K. Takeda, Z. X. Yu, V. J. Ferrans, and T. Finkel. 2000. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell. Biol. 19:7311-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimnual, A. S., B. A. Yatsula, and D. Bar-Sagi. 1998. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 279:560-563. [DOI] [PubMed] [Google Scholar]
- 30.Nisimoto, Y., J. L. Freeman, S. A. Motalebi, M. Hirshberg, and J. D. Lambeth. 1997. Rac Binding to p67phox. Structural basis for interaction of the Rac1 effector region and insert region with compositions of respirator burst oxidase. J. Biol. Chem. 272:18834-18841. [DOI] [PubMed] [Google Scholar]
- 31.Nohl, H., and D. Hegner. 1978. Do mitochondria produce oxygen radicals in vivo? Eur. J. Biochem. 82:563-567. [DOI] [PubMed] [Google Scholar]
- 32.Obermeier, A., S. Ahmed, E. Manser, S. C. Yen, C. Hall, and L. Lim. 1998. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 17:4328-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J. W., C. R. Hoyal, J. E. Benna, and B. M. Babior. 1997. Kinase-dependent activation of the leukocyte NADPH oxidase in a cell-free system: phosphorylation of membranes and p47PHOX during oxidase activation. J. Biol. Chem. 272:11035-11043. [DOI] [PubMed] [Google Scholar]
- 34.Price, M. O., S. J. Atkinson, U. Knaus, and M. Dinauer. 2002. Rac activation induces NADPH oxidase activity in transgenic Cosphox cells, and the level of superoxide production is exchange factor-dependent. J. Biol. Chem. 277:19220-19228. [DOI] [PubMed] [Google Scholar]
- 35.Rhee, S. G., Y. S. Bae, S. R. Lee, and J. Kwon. 2000. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 53:1-6. [DOI] [PubMed] [Google Scholar]
- 36.Someya, A., H. Nunoi, T. Hasebe, and I. Nagaoka. 1999. Phosphorylation of p40-phox during activation of neutrophil NADPH oxidase. J. Leukoc. Biol. 66:851-857. [DOI] [PubMed] [Google Scholar]
- 37.Suh, Y. A., R. S. Arnold, B. Lassegue, J. Shi, X. Xu, D. Sorescu, A. B. Chung, K. K. Griendling, and J. D. Lambeth. 1999. Cell transformation by the superoxide-generating oxidase Nox1. Nature 401:79-82. [DOI] [PubMed] [Google Scholar]
- 38.Sundaressan, M., Z. X. Yu, V. J. Ferrans, K. Irani, and T. Finkel. 1995. Requirement of generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296-299. [DOI] [PubMed] [Google Scholar]
- 39.Takeya, R., N. Ueno, K. Kami, M. Taura, M. Kohjima, T. Izaki, H. Nunoi, and H. Sumimoto. 2003. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 278:25234-25246. [DOI] [PubMed] [Google Scholar]
- 40.Tamas, P., Z. Solti, P. Bauer, S. Illies, A. Bauer, A. Farago, J. Downward, and L. Buday. 2003. Mechanism of EGF regulation of Vav2, a GEF for Rac. J. Biol. Chem. 278:5163-5171. [DOI] [PubMed] [Google Scholar]
- 41.Ushio-Fukai, M., A. M. Zafari, T. Fukui, N. Ishizaka, and K. K. Griendling. 1996. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 271:23317-23321. [DOI] [PubMed] [Google Scholar]
- 42.Welch, H. C., W. J. Coadwell, C. D. Ellson, G. J. Ferguson, S. R. Andrews, H. Erdjument-Bromage, P. Tempst, P. T. Hawkins, and L. R. Stephens. 2002. P-Rex1, a Ptdlns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108:809-821. [DOI] [PubMed] [Google Scholar]
- 43.Zhan, S., N. Vazquez, S. Zhan, F. B. Wientjes, M. L. Budarf, E. Schrock, T. Ried, E. D. Green, and S. J. Chanock. 1996. Genomic structure, chromosomal localization, start of transcription, and tissue expression of the human p40-phox, a new component of the nicotinamide adenine dinucleotide phosphate-oxidase complex. Blood 88:2714-2721. [PubMed] [Google Scholar]
- 44.Zhan Y., J. Virbasius, X. Song, D. P. Pomerleau, and G. W. Zhou. 2002. The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J. Biol. Chem. 277:4512-4518. [DOI] [PubMed] [Google Scholar]
- 45.Zheng, Y. 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26:724-732. [DOI] [PubMed] [Google Scholar]