Abstract
Engagement of the T-cell receptor (TCR) results in the activation of a multitude of signaling events that regulate the function of T lymphocytes. These signaling events are in turn modulated by adapter molecules, which control the final functional output through the formation of multiprotein complexes. In this report, we identified the adapter molecule Sin as a new regulator of T-cell activation. We found that the expression of Sin in transgenic T lymphocytes and Jurkat T cells inhibited interleukin-2 expression and T-cell proliferation. This inhibitory effect was specific and was due to defective phospholipase C-γ (PLC-γ) phosphorylation and activation. In contrast to other adapters that become phosphorylated upon TCR stimulation, Sin was constitutively phosphorylated in resting cells by the Src kinase Fyn and bound to signaling intermediates, including PLC-γ. In stimulated cells, Sin was transiently dephosphorylated, which coincided with transient dissociation of Fyn and PLC-γ. Downregulation of Sin expression using Sin-specific short interfering RNA oligonucleotides inhibited transcriptional activation in response to TCR stimulation. Our results suggest that endogenous Sin influences T-lymphocyte signaling by sequestering signaling substrates and regulating their availability and/or activity in resting cells, while Sin is required for targeting these intermediates to the TCR for fast signal transmission during stimulation.
In recent years, adapter molecules have emerged as critical regulators of intracellular signaling pathways and function in T cells. While these molecules lack intrinsic enzymatic activity, they regulate and integrate signaling events through protein-protein and protein-lipid interactions. T cells express a variety of adapter molecules that act as positive or negative regulators of T-cell receptor (TCR) signaling. Positive regulators of T-cell function include the bona fide adapters Grb-2 and Gads, as well as the scaffold proteins Src homology 2 domain containing leukocyte protein 76 (SLP-76), linker of activation of T cells (LAT), and degranulation-promoting adapter protein (ADAP),whereas negative regulators consist of the Cbl family of proteins, SLAP (Src-like adapter protein), and the protein associated with glycosphingolipid-enriched microdomains PAG/Cbp (19, 26, 37).
Gene targeting experiments with Jurkat T-cell lines and mice indicate that the deletion of positive regulators in general exhibit defective thymocyte development or T-cell signaling (8, 30, 31, 40, 43), whereas mice lacking negative regulators have opposite phenotypes manifested as increased thymocyte positive selection and the development of autoimmunity (23, 25, 34). Similarly, studies describing the overexpression of adapter molecules in T lymphocytes have helped to further define these molecules and have shown that negative and positive regulators either suppress or promote excessive T-lymphocyte development and function, respectively (20, 35, 36, 38).
Our studies are concentrated on elucidating the molecular mechanisms involved in TCR signaling, with particular emphasis on the role of adapter/scaffold molecules in this process. More specifically, we are interested in examining the role of the novel adapter molecule Sin in T-cell signaling and activation. Sin belongs to a small family of related proteins, the other members being p130Cas and CasL (28). These proteins share a conserved Src homology region 3 (SH3) domain, repeated tyrosine-based and proline-rich motifs, and a conserved C terminus. All three members of the p130Cas family are substrates for Src kinases, and Src kinase-mediated phosphorylation of these proteins is important for their adapter/scaffold signaling properties (2).
We became interested in determining whether Sin regulates TCR signaling because Sin was cloned as a substrate for the key TCR signaling molecule Fyn (1, 13) and because the thymus expresses higher levels of Sin than other tissues do (2, 12). Thus, in previous experiments we examined the role of Sin in thymocyte development using transgenic mice expressing a truncated form of Sin, SinΔC. SinΔC expression in the mouse thymus resulted in reduced thymic cellularity due to increased thymocyte apoptosis as well as defective thymocyte differentiation manifested as reduced numbers of mature CD4 and CD8 single-positive (SP) cells (6). We also found that the Src kinase Fyn was important for Sin-mediated thymocyte apoptosis but not for the inhibition of thymocyte maturation (6). These results suggest that Sin is a negative regulator of thymocyte differentiation and survival.
In this report, we addressed the role of Sin in TCR signaling and T-cell activation using Sin-expressing Jurkat and transgenic T cells as well as Sin-specific short interfering RNA (siRNA). We found that Sin expression inhibited TCR-induced T-cell activation and proliferation by blocking expression of the interleukin-2 (IL-2) gene. The defect in IL-2 expression correlated with reduced phospholipase C-γ (PLC-γ) phosphorylation, intracellular calcium release, and NFAT and AP-1 activation. Downregulation of endogenous Sin expression also inhibited TCR-induced transcriptional activation, an observation seemingly paradoxical to our overexpression data. Strikingly, however, we found that Sin was constitutively phosphorylated in resting cells, which correlated with the association of phosphorylated Sin with multiple signaling molecules of which we identified Fyn and PLC-γ. This adapter function of Sin was modulated through the TCR because Sin was dephosphorylated after TCR stimulation, which coincided with the release of proteins bound to Sin in resting cells including Fyn and PLC-γ. Taken together, these data suggest that Sin is a dual positive-negative regulator which influences T-lymphocyte signaling first by sequestering signaling substrates and preventing them from acting on their substrates in resting cells and second by releasing and targeting these substrates to the TCR for signal transmission upon stimulation.
MATERIALS AND METHODS
Mice.
A cDNA fragment encoding amino acids 1 to 335 of full-length Sin was cloned into the EcoRI-SmaI site of the CD2 expression cassette (44), and SinΔC transgenic mice were generated as previously described (6). C57BL/6 animals were purchased from the Jackson Laboratory (Bar Harbor, Maine).
cDNA constructs and Sin-specific siRNA oligos.
DNA manipulations were performed by standard protocols. Full-length Sin, Efs2 (missing amino acids 4 to 99), and SinΔC (amino acids 1 to 335) were cloned into the SpeI-NotI sites of the pEBB expression vector. pEBB was derived from the pEF-BOS expression vector driven by the human elongation factor 1-α promoter (21). Four different siRNA oligonucleotide duplexes were synthesized (QIAGEN), corresponding to target sequences on human full-length Sin. The target sequences were as follows: for SRi1, 5′AACGAGCGTCAGCCTTACTCA; for SRi2, 5′AGTATGACTATGTCCACCTGA; for SRi3, 5′AAGAGATGGTGCAGTGTGTAA; and for SRi4, 5′AATTCACTACCCTGCTCACTA. A nonsilencing control siRNA duplex sequence (catalog number 1022076; QIAGEN) was used as a control for the transfections. The same fluorescently labeled siRNA duplex (catalog number 80-11320; QIAGEN) was used to monitor transfection efficiency.
Cell lines and antibodies.
Jurkat cells transfected with the simian virus 40 large T antigen (SV40 Tag) (provided by A. Weiss, University of California at San Francisco, San Francisco, Calif.), the parental Jurkat E6-1, and primary splenic T cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, penicillin, streptomycin, and glutamine. Stable cells lines were generated by cotransfecting 2 × 106 Jurkat Tag cells with 1 μg of pEBB-Sin and 0.2 μg of MSC-puro vector expressing puromycin using the FuGENE-6 transfection reagent (Roche). Transfected cells were serially diluted in 96-well plates and selected in culture medium containing 300 ng of puromycin/ml.
For mouse monoclonal antibodies, anti-Sin-specific and isotype-matched control clone MOPC-31C (MOPC) were obtained from BD Transduction Laboratories; anti-Fyn, anti-Lck, and PLC-γ (clone sc-81) were obtained from Santa Cruz; antiphosphotyrosine-specific antibody was obtained from Upstate Biotechnology. Purified mouse monoclonal anti-CD3 and anti-CD28 antibodies used for cross-linking were obtained from BD Pharmingen.
Flow cytometry.
Freshly isolated splenocytes (106) were incubated with the appropriate antibodies in staining medium (3% fetal calf serum, 0.1% sodium azide in phosphate-buffered saline) for 15 min on ice. Cells were spun down and washed three times with staining medium and analyzed by flow cytometry using FACSCalibur and CELLQUEST software. Anti-CD4-allophycocyanin-, anti-CD8-peridinin chlorophyll-a protein-, and anti-CD3-fluorescein isothiocyanate-conjugated antibodies were purchased from BD Pharmingen.
T-cell purification and proliferation assays.
Splenic CD4+ T cells were purified by using the Dynabead/DETACHaBEAD mouse CD4+ system (DYNAL Biotech). A total of 105 T cells were plated per well of a 96-well plate. Cells were left untreated or induced with plate-bound mouse anti-CD3ɛ (0.5 μg/ml) and anti-CD28 (5 μg/ml) or anti-CD3ɛ (0.05 μg/ml) with or without recombinant mouse IL-2 (20 ng/ml) (BD Pharmingen). At 72 h, cells were labeled overnight with 1 μCi of [3H]thymidine, and the proliferative response was determined by levels of [3H]thymidine incorporation on a scintillation counter. In the proliferation assay, 5 μl of supernatant from each well was collected at 24, 48, and 72 h and assayed for IL-2 concentration (mouse IL-2 enzyme-linked immunosorbent assay kit; R&D Systems).
Transfections and luciferase assays.
For luciferase reporter assays, 2 × 106 Jurkat Tag cells were transfected with the indicated amounts of plasmid DNA expressing Sin, SinΔC, Efs2, CasL, or SLP-76 proteins plus 200 ng of NFAT- or AP-1-firefly-luciferase and 5 ng of pRL-thymidine kinase (TK)-Renilla-luciferase reporter constructs using FuGENE-6 transfection reagent according to manufacturer's protocol. Expression of the Renilla luciferase is under the influence of the herpes simplex virus (HSV)-TK promoter (Promega). After overnight incubation, half of the transfected cells were stimulated either by plate-bound OKT3 antibody (5 μg/ml), or by phorbol myristate acetate (PMA) (10 ng/ml) plus ionomycin (1 μM) for 8 h. Induced and uninduced cells were lysed in 50 μl of lysis buffer, and 20 μl of cell lysates was assayed by using a Dual-Luciferase reporter assay system (Promega) according to the manufacturer's protocol.
siRNA transfection in Jurkat and 293 HEK cells.
Jurkat cells were washed with RPMI 1640 medium to remove serum and antibiotics. A total of 4 × 105 cells were seeded per well in a 24-well plate in 300 μl of RPMI 1640 medium. Four hundred nanograms of annealed double-stranded siRNA, 40 ng of NFAT-firefly-luciferase, and 1 ng of pRL-TK-Renilla-luciferase plasmid DNA were transfected into the cells by using TransMessenger transfection reagent (QIAGEN) according to the manufacturer's protocol. After overnight incubation, cells were induced with OKT3 for 8 h, and cells were harvested and processed for luciferase assays as described above. In the transfection of 293 HEK cells, 100 ng of plasmid expressing human SinII/EFS2 (Image Clone ID number 4214788) and 200 ng of siRNA were transfected with the same reagent. After 36 h, the cells were harvested, and cell extracts were processed on Western blots.
Measurement of intracellular calcium.
Jurkat Tag cells (4 × 106) were incubated with 1 μM indo-1/AM for 1 h at 37°C in RPMI 1640 medium plus 1% fetal bovine serum. Cells were washed three times in buffer A (10 mM HEPES [pH 7.4], 3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 140 mM NaCl, 0.1% glucose, 1% fetal bovine serum) and suspended at a final concentration of 106 in buffer A. Cells were stimulated with 1 μg of OKT3/ml. The fluorescence ratio (405/485 nm) with excitation at 350 nm was obtained from a spectrofluorometer (Photo Technology International, Lawrenceville, N.J.). Ionomycin was added to a final concentration of 15 μM in order to obtain maximum fluorescence under saturating calcium concentrations followed by 10 mM EGTA to obtain minimum fluorescence in the absence of calcium. Calibrations and calculation of intracellular calcium concentration were conducted as previously described (9, 32).
Analysis of IP3 release.
A total of 4 × 106 cells were induced with soluble OKT3 antibody (1 μg/ml) for 3 min, and cell extracts were generated by adding 80 μl of ice-cold 100% trichloroacetic acid. Inositol 3-phosphate (IP3) levels were assessed by using the IP3 Radioreceptor assay kit (NEN Life Science Products, Boston, Mass.) according to the manufacturer's protocol.
TCR cross-linking.
Splenocytes (107) were incubated with 2 μg of anti-CD3 and anti-CD28 antibodies on ice for 15 min, washed with cold phosphate-buffered saline, and super-cross-linked with 5 μg of goat anti-hamster immunoglobulin G (IgG) for 20 min on ice. Cells were then incubated at 37°C at different time points, spun down, and immediately lysed. Cell lysates were used for immunoprecipitation and immunoblot analysis.
Immunoprecipitations.
Immunoprecipitations were performed as previously described (1). Briefly, cells were lysed in 1 ml of ice-cold NP-40 lysis buffer (1% NP-40, 20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 10% glycerol, 10 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). Cell extracts were then incubated with the specified antibodies at concentrations suggested by the manufacturers for 2 h at 4°C, and the immune complexes were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Western blots were performed as previously described (39).
In vitro kinase assays.
Protein complexes obtained by immunoprecipitation were washed three times in kinase buffer, and reactions were carried out in 20 μl of kinase buffer containing 20 mM HEPES (pH 7.4), 5 mM MnCl, 10 μM ATP, and 1 μl of [γ-32P]ATP (5,000 Ci/mmol) at room temperature for 10 min. The pellets were resuspended in 1× Laemmli buffer and boiled for 2 min, and phosphorylated proteins were analyzed by SDS-PAGE and autoradiography.
RESULTS
Expression of Sin isoforms in T cells.
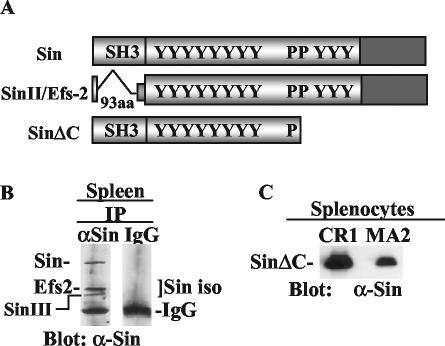
At least three isoforms of Sin can be detected on Northern and Western blots of the mouse thymus (2, 6) and with a Sin-specific monoclonal antibody in immunoprecipitates of mouse splenocyte extracts: the ∼80-kDa full-length Sin and two additional protein bands that may correspond to SinII/Efs2 and SinIII (Fig. 1B), collectively shown as Sin isoforms. SinII/Efs2 is a previously described alternative splice form of Sin in which the SH3 domain is deleted (12), whereas Sin III migrates slightly faster than SinII/Efs2 and may correspond to a different Sin isoform we isolated from the mouse thymus (2).
FIG. 1.
Expression of Sin isoforms in mature T cells. (A) Schematic structure of Sin, SinII/Efs2, and the truncated protein SinΔC. Y, tyrosine-containing sequences; P, proline-rich motifs; aa, amino acids. (B) Splenocytes (3 × 107) were immunoprecipitated (IP) with Sin-specific or isotype-matched IgG control antibodies, and Western blots of the immune complexes were probed with a Sin-specific monoclonal antibody to reveal expression of the Sin isoforms as indicated. (C) Western blot of splenocytes from two different founders expressing SinΔC probed with anti-Sin (α-Sin) antibody.
As mentioned above, transgenic mice expressing a truncated form of Sin, SinΔC (Fig. 1A), were previously used to explore the physiologic role of Sin in vivo (6). This truncated Sin mutant was chosen based on the finding that it enhanced Src kinase signaling more than the full-length protein (39). Two founder transgenic lines, CR1 and MA2, were generated expressing approximately 27- and 9-fold more SinΔC, respectively, than endogenous SinII/Efs2 (6). The expression of SinΔC was regulated by the human CD2 promoter, which allows expression of cloned genes in both thymocytes and mature T cells (44). Expression of the SinΔC transgene was demonstrated in splenocytes from two different founder mice (Fig. 1C).
SinΔC expression reduces numbers of peripheral T cells.
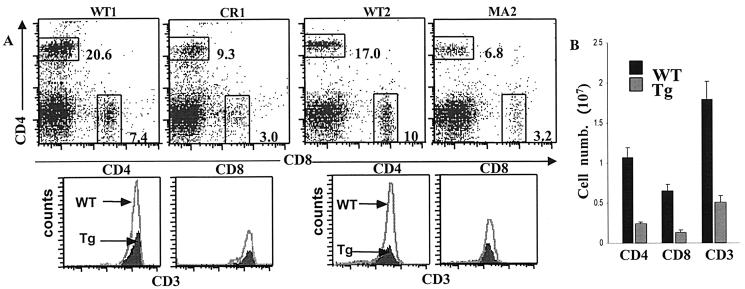
It was previously found that SinΔC expression inhibited thymocyte development, manifested as a reduced percentage of mature CD4 and CD8 SP cells in the thymus and increased thymocyte apoptosis, suggesting a negative function for SinΔC in T lymphocytes (6). Here, we used the SinΔC mice to examine the effect of SinΔC on mature T-cell activation and proliferation. First, we examined the effect of SinΔC protein expression on the production of mature T cells in the spleen. Fluorescence-activated cell sorting analysis of total splenocytes revealed that the percentages of mature splenic T cells of both the CD4+ and CD8+ lineages were substantially reduced in transgenic CR1 and MA2 animals compared to normal littermate controls (Fig. 2A, dot plots). Nevertheless, splenic T cells from transgenic animals expressed normal levels of CD3ɛ compared to wild-type controls (Fig. 2A, histograms). Consistent with this finding, it was previously found that in addition to normal TCR levels, the expression levels of maturation markers such as CD69 and CD5 in thymic T cells were also normal (6). This finding suggests that although the percentages of cells are reduced, the existing mature T cells have undergone positive selection. Analysis of several MA2 SinΔC animals further revealed a consistently dramatic decrease in total CD3+-T-cell numbers, due to a decrease of both CD4+ and CD8+ mature T-cell populations (Fig. 2B). Relative proportions of T and B cells in the spleen, determined by Thy1.2 and B220 staining, also revealed a dramatic decrease in T-cell numbers, whereas the B-cell compartment was normal (data not shown). Thus, our data show that SinΔC expression results in reduced numbers of CD4 and CD8 SP cells in the spleens of transgenic animals, consistent with T-cell-specific expression of SinΔC from the CD2 promoter.
FIG. 2.
SinΔC expression inhibits production of mature T cells. (A) Splenocytes (106) from 6- to-8-week-old wild-type (WT) and transgenic (Tg) animals were triply stained with CD4-allophycocyanin/CD8-peridinin chlorophyll-a protein and CD3-fluorescein isothiocyanate and analyzed by flow cytometry. In the dot plots, the numbers indicate the percentage of cells in each region. Histograms represent CD3 expression within the CD4 and CD8 SP populations in the dot plots. Two different transgenic founder lines, CR1 and MA2, with their respective wild-type controls, are shown. (B) Splenocytes were counted and stained, and the percentages of total CD3 T cells and CD4+ and CD8+ subpopulations were determined by fluorescence-activated cell sorting analysis and used to calculate the actual cell numbers for each population. Results from at least five wild-type and MA2 transgenic mice are represented as the mean ± the standard deviation (SD).
SinΔC expression inhibits T-cell activation.
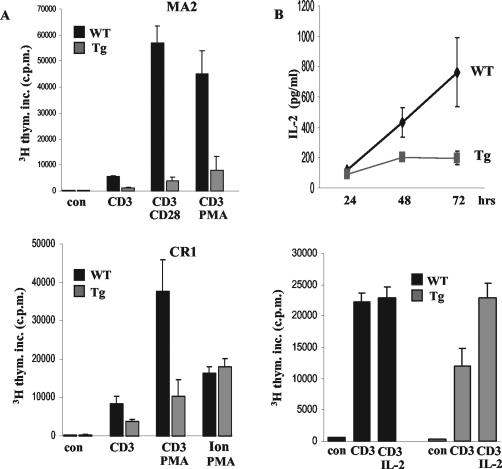
We next tested whether SinΔC-expressing T cells were able to respond to stimulation through their TCR. Equal numbers of T cells purified from the spleens of normal and SinΔC transgenic animals (CR1 and MA2) were stimulated with plate-bound CD3- and CD28-specific antibodies and PMA-ionomycin as shown in Fig. 3. As indicated above (Fig. 2A, histograms), TCR expression levels were similar in purified normal and SinΔC-expressing cells assayed by CD3 staining and flow cytometry. We found that SinΔC-expressing T cells stimulated through their TCR with CD3, CD3 and CD28, or PMA exhibited dramatically reduced proliferation compared to normal controls (Fig. 3A). In contrast, transgenic T cells stimulated with PMA and ionomycin, a nonphysiologic stimulus that bypasses the TCR, proliferated to the same extent as their wild-type counterparts, suggesting that the inhibitory effect of SinΔC on T-cell proliferation is specific to proximal TCR signaling events (Fig. 3A, bottom graph).
FIG. 3.
SinΔC expression inhibits T-cell proliferation and IL-2 production. (A) T cells (105) purified from the spleens of 6- to 8-week-old normal and SinΔC animals (CR1 and MA2) were stimulated with plate-bound CD3 and CD28 antibodies and PMA and ionomycin as indicated and as described in Materials and Methods. Proliferation was calculated by [3H]thymidine incorporation (3H thym. inc.) as the mean ± SD of radioactive counts in triplicate wells. Shown are data representative of at least three experiments with two different transgenic lines (MA2, top panel; CR1, bottom panel). (B) Five microliters of the supernatants was removed from stimulated cells at the indicated times and analyzed by enzyme-linked immunosorbent assay for the presence of secreted IL-2 (top graph). Purified T cells were stimulated with plate-bound anti-CD3 antibody (0.05 μg/ml) in the presence or absence of IL-2 (20 ng/ml). Proliferation was determined by [3H]thymidine incorporation (bottom graph). Tg, transgenic; WT, wild type.
Upon stimulation, T cells secrete the critical cytokine IL-2, which acts in an autocrine fashion to stimulate T-cell proliferation. We found that the reduced proliferation of SinΔC-expressing T cells was accompanied by a significant reduction in the level of secreted IL-2, suggesting defective TCR signaling leading to cytokine production (Fig. 3B, top graph). The addition of exogenous IL-2, however, restored the proliferative response of the transgenic T cells (Fig. 3B, bottom graph), indicating that signaling events downstream of the cytokine receptor remained intact. Consistent with this finding, the upregulation of the IL-2 receptor (CD25) was normal in transgenic cells stimulated with anti-CD3/CD28 compared to littermate controls (data not shown). These results suggest that the inability of SinΔC-expressing T cells to proliferate is specific to the TCR and is due to defective TCR-mediated IL-2 production and not to an inherent inability of these cells to respond to stimuli.
Full-length Sin expression inhibits TCR-mediated transcriptional activation in human Jurkat T cells.
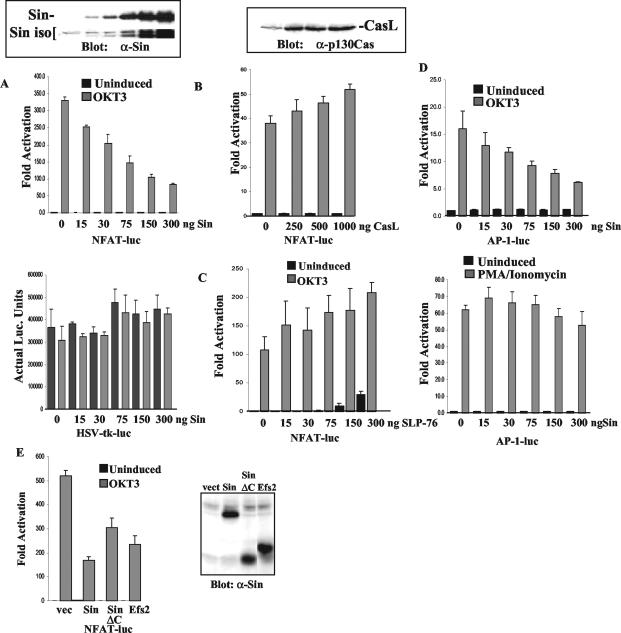
The results presented above suggest that SinΔC inhibits T-cell activation by rendering cells incapable of IL-2 production. To further analyze the inhibitory effect of Sin on TCR signaling and to examine whether SinΔC mimics the function of Sin, we used transiently or stably transfected human Jurkat T cells. Jurkat Tag cells were transfected with increasing concentrations of a mammalian vector expressing Sin along with two reporter plasmids expressing two different luciferase proteins: one from firefly under three copies of the distal NFAT/AP-1 or multimerized AP-1 binding sites from the IL-2 promoter (10, 33) and the other from the sea pansy Renilla under the HSV-TK promoter, which was included as an internal control for transfection efficiency. We found that the expression of Sin in Jurkat cells efficiently inhibited NFAT promoter activation in response to TCR stimulation in a concentration-dependent manner (Fig. 4A, top graph). In contrast, in the same experiments, activation of the Renilla-luciferase reporter (HSV promoter) remained constant, suggesting that the inhibition we observed is not because of cell toxicity due to Sin overexpression (Fig. 4A, bottom graph). Importantly, the inhibitory effect of Sin was evident even when it was expressed at levels similar to those of the endogenous Sin isoforms, arguing against a negative effect of Sin due to gross overexpression (Fig. 4A, inset). As the Sin-related protein HEF1/CasL has been shown to dimerize through its C-terminal conserved region (17), the increased amounts of endogenous Sin isoforms observed at high concentrations of overexpressed Sin (Fig. 4A, inset) may be due to dimerization and stabilization of these isoforms by overexpressed Sin.
FIG. 4.
Sin expression inhibits TCR-induced transcriptional activation. Jurkat Tag cells were transiently transfected with increasing amounts of plasmid DNA expressing Sin (A and D), CasL (B), or SLP-76 (C) in the presence of NFAT- or AP-1-firefly-luciferase (luc) as shown along with the HSV-TK-Renilla-luciferase reporter (A to E). The fold activation for NFAT-and AP-1-firefly-luciferase and actual luciferase units for HSV-TK-Renilla-luciferase are shown (A, bottom graph). (E) Jurkat Tag cells were transfected with a pEBB vector (vec) expressing Sin, SinII/Efs2, or SinΔC (300 ng each) in the presence of the NFAT- and HSV-TK-luciferase reporters as shown. The results represent one of at least three experiments each performed in triplicate, and fold activation is relative to the value obtained with pEBB vector backbone used to express Sin, CasL, or SLP-76 in unstimulated cells, which was given a value of 1. The results shown represent the mean ± SD. Whole-cell lysates of the transfected cells were separated on SDS-PAGE and Western blotted with anti-Sin (α-Sin) or anti-p130Cas (α-p130Cas) antibodies to reveal levels of Sin and CasL protein expression, respectively (A, B, and E, insets).
To further address whether the inhibitory effect on NFAT activation is specific to Sin, we also examined the effect of other adapter molecules on T-cell activation. To this end, we used SLP-76, an established positive regulator of T-cell function (24, 31), as well as CasL, a Sin-related protein also shown to stimulate T-cell signaling (15, 27). In contrast to Sin, we found that the expression of the Sin-related molecule CasL (Fig. 4B), or SLP-76 (Fig. 4C), stimulated NFAT activation in response to TCR cross-linking. These results suggest that the effects of Sin are specific and that Sin expression represses TCR-induced transcriptional activity that regulates IL-2 expression.
In addition to NFAT, we also examined the effect of Sin on AP-1 activation, which is a complex of Fos and Jun transcription factors required for IL-2 gene expression. Similarly to NFAT, we found that Sin expression also inhibited AP-1-dependent transcriptional activation in response to TCR stimulation (Fig. 4D, top graph) but not in response to stimulation with PMA-ionomycin (Fig. 4D, bottom graph). These results suggest that the inhibitory effect of Sin is specific to TCR signaling and that this effect can be bypassed by nonphysiologic stimuli.
These and previous data suggest that the expression of Sin, as well as the truncated SinΔC form, inhibits TCR signaling and transcriptional activation from the IL-2 promoter. To address whether SinΔC mimics the function of Sin, we directly compared the ability of these proteins as well as that of SinII/Efs2 to inhibit T-cell signaling in Jurkat T cells (Fig. 4E). We found that all proteins share similar inhibitory function, although SinΔC was somewhat less potent in inhibiting transcription than Sin or SinII/Efs2 (Fig. 4E) when the proteins were expressed at similar levels (Fig. 4E, inset). These results suggest that the C terminus of Sin contributes to the maximal inhibition of signaling observed with the full-length protein but is not by itself sufficient for complete inhibition of signaling since a significant block of NFAT activation is observed with SinΔC, which lacks this region. Thus, SinΔC behaves similarly to Sin and acts as a negative regulator of TCR signaling.
Sin expression inhibits PLC-γ phosphorylation and activation.
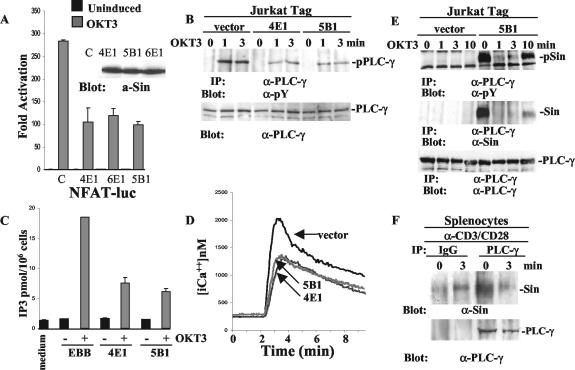
We next examined the mechanism of Sin-mediated inhibition of transcriptional activation by examining upstream signaling events that control the activation of the NFAT and AP-1 transcription factors. It has been established that TCR-induced PLC-γ phosphorylation and activation lead to the release of the second messengers IP3 and diacyl-glycerol (DAG). The production of IP3 induces intracellular calcium release and NFAT activation, whereas DAG controls AP-1 activation through the stimulation of protein kinase C-θ (PKC-θ) and Ras-guanyl-releasing protein (11, 19, 37). We thus examined whether these signaling events were compromised by Sin expression using Jurkat T-cell lines stably overexpressing Sin. Consistent with the results obtained from transient assays, we found that the expression of Sin blocked NFAT-luciferase activation in response to TCR stimulation in three different cell lines (Fig. 5A).
FIG. 5.
Defective PLC-γ phosphorylation and activation in Jurkat cells stably overexpressing Sin. (A) Sin overexpressing stable Jurkat Tag cells from three different lines were transiently transfected with NFAT-firefly- and HSV-TK-Renilla-luciferase reporters and stimulated with 5 μg of OKT3/ml as described in Materials and Methods. A representative of at least three experiments performed in triplicate is shown. The fold activation was determined as described in the legend to Fig. 4. Sin expression levels in these cell lines are shown in the inset. a-Sin, anti-Sin. (B) Jurkat cells (107) from control (vector alone) and two different Sin-expressing cell lines were stimulated with OKT3 (5 μg/ml) for the indicated times, and cell lysates were immunoprecipitated with anti-PLC-γ-specific antibody. Immune complexes were separated by SDS-PAGE, and the Western blot was probed with an antiphosphotyrosine antibody. Total lysates of the same samples normalized for protein content were processed in parallel to reveal levels of endogenous PLC-γ. (C) IP3 levels were determined as described in Materials and Methods. One of three representative experiments is shown. (D) Intracellular calcium concentration in the presence or absence of Sin from one control and two stable cell lines was measured as described in Materials and Methods. Shown is a representative of at least four experiments. (E) Control or Sin-expressing Jurkat Tag cell lines (5 × 107) were stimulated with OKT3 (5 μg/ml) for the indicated times, and cell lysates were immunoprecipitated with PLC-γ-specific antibody. The separated immune complexes were transferred on nitrocellulose membrane, and the upper half of the membrane was probed with anti-PLC-γ (bottom panel), whereas the lower half was probed first with anti-Sin (α-Sin) antibody (middle panel) and then stripped and reprobed with antiphosphotyrosine-specific antibody (top panel). (F) Extracts of 9 × 107 total splenocytes from wild-type mice stimulated with anti-CD3/CD28 (α-CD3/CD28) antibodies (15 μg/ml each) were immunoprecipitated with anti-PLC-γ or control IgG. The upper and lower halves of the membrane containing the immune complexes were probed with anti-PLC-γ and anti-Sin (α-Sin) antibodies, respectively. Protein bands were visualized by enhanced chemiluminescence (ECL). α-PLC-γ, anti-PLC-γ; pPLC-γ, phosphorylated PLC-γ, α-pY, antiphosphotyrosine; pSin, phosphorylated Sin.
Using these cell lines, we then examined the effect of Sin expression on PLC-γ phosphorylation and activation. We found that TCR-mediated PLC-γ phosphorylation was reduced in Sin-expressing Jurkat T cells compared to control cells transfected with vector alone (Fig. 5B). Consistent with decreased phosphorylation, we found that the enzymatic activity of PLC-γ was also reduced in T cells expressing Sin, shown by reduced levels of IP3 production (Fig. 5C). As a result, TCR-stimulated intracellular calcium release was also compromised in these Sin-expressing cell lines compared to cells stably transfected with vector alone (Fig. 5D). These results suggest that the defective activation of NFAT/AP1-mediated transcription in Sin-expressing T cells is the result of defective PLC-γ phosphorylation and activation and calcium release. These results also show that Sin inhibits T-cell signaling by exerting its effects on signaling events proximal to the TCR, which regulate the phosphorylation and activation of PLC-γ.
To further analyze the inhibitory effects of Sin expression on PLC-γ activation, we examined whether Sin directly interacts with PLC-γ in the Sin-expressing cell lines. Surprisingly, we found that Sin was constitutively associated with endogenous PLC-γ in resting T cells and that this association was disrupted in stimulated cells (Fig. 5E). Thus, PLC-γ activation correlated with its disassociation from Sin after TCR stimulation. Ten minutes after stimulation, the association of Sin with PLC-γ was once again evident, coinciding with the downregulation of TCR signaling (Fig. 5E). To address the physiological significance of the Sin-PLC-γ interaction, we also tested the association of endogenous Sin and PLC-γ in splenocytes. Importantly, and similar to our results with Jurkat T cells, we found that endogenous Sin was constitutively associated with endogenous PLC-γ in resting splenocytes, but less so in cells stimulated with anti-CD3/CD28 for 3 min (Fig. 5F). These results suggest that Sin preferentially binds to the nonactivated form of PLC-γ and that it may limit its availability and/or activation in unstimulated as well as activated cells under physiologic conditions. The overexpression of Sin reveals this function and results in the inhibition of PLC-γ phosphorylation and activation.
The adapter function of Sin is regulated by constitutive phosphorylation and TCR-induced dephosphorylation.
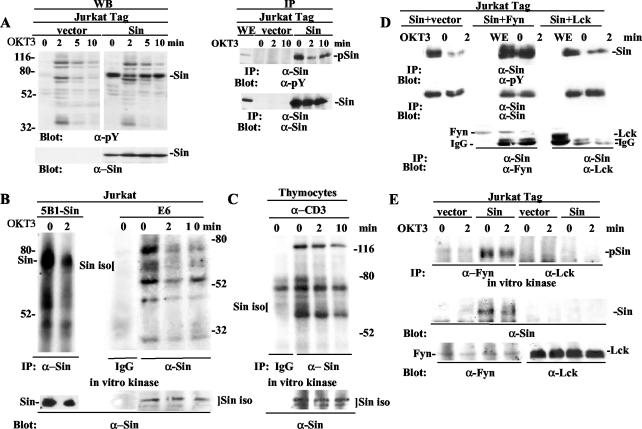
Given that TCR signaling molecules typically experience an increase in their tyrosine phosphorylation content after TCR induction, we sought to determine whether Sin also underwent such a modification. The majority of adapter proteins that regulate TCR signaling, such as LAT, SLP-76, and Cbl, are rapidly phosphorylated after T-cell receptor cross-linking under endogenous or overexpressing conditions (7, 22, 42). In stark contrast, we found that Sin was constitutively phosphorylated in resting cells and was rapidly dephosphorylated after TCR stimulation in total cell extracts and in Sin immunoprecipitates (Fig. 6A, left and right panels, respectively). The basal constitutive phosphorylation of Sin returned after 10 min of TCR stimulation, which coincided with a reduction of total cellular phosphotyrosine levels (Fig. 6A, left panels). The dephosphorylation of Sin after TCR stimulation is likely due to phosphatase activity, considering the rapid rate of dephosphorylation (2 min). Thus, Sin is regulated differently from other signaling phosphoproteins, as Sin becomes dephosphorylated rather than phosphorylated upon TCR stimulation.
FIG. 6.
Sin associates with and is constitutively phosphorylated by Fyn in resting cells. Sin is transiently dephosphorylated and dissociates from Fyn in response to TCR stimulation. (A) Jurkat cells (107) stably transfected with vector alone or Sin-expressing plasmid were stimulated with OKT3 (5 μg/ml) at the indicated time points, and whole-cell extracts normalized for protein content were separated by SDS-PAGE and blotted with antiphosphotyrosine antibody. The blot was stripped and reprobed with anti-Sin to reveal total Sin levels (lower right panel). Similarly prepared extracts from vector or Sin-expressing cell lines were immunoprecipitated with Sin-specific antibody and first blotted with antiphosphotyrosine and then stripped and reprobed with Sin antibody (right panels). (B and C) Sin-expressing (5B1) or control Jurkat E6 cells (B) and 3 × 107 thymocytes from normal mice (C) were left unstimulated or were stimulated with anti-TCR antibodies as shown. Cell extracts were immunoprecipitated with Sin-specific antibody or isotype-matched control IgG, and immune complexes were incubated in vitro in the presence of [32P]ATP. Immune complexes were separated, proteins were transferred to nitrocellulose membrane, and membranes were exposed on film overnight. Subsequently, the filters were probed with Sin antibody to reveal total Sin levels. (D) Jurkat Tag cells (5 × 106) transiently transfected with Sin alone and Sin plus Fyn or Lck were immunoprecipitated with anti-Sin antibody and processed as described above for panel A. The blots were sequentially probed with antiphosphotyrosine, Sin, and Fyn or Lck antibodies as shown. (E) Control or Sin-expressing Jurkat Tag cells were stimulated for the indicated times with OKT3 antibody, and extracts were immunoprecipitated with Fyn- or Lck-specific antibodies. Immune complexes were subjected to in vitro kinase assays in the presence of radioactive ATP and subsequently separated on SDS-PAGE. Blots were exposed on film and then probed sequentially with Sin and Fyn or Lck antibodies as shown. α-pY, antiphosphotyrosine; α-Sin, anti-Sin; pSin, phosphorylated Sin; WE, whole-cell extracts; iso, isoforms; α-Fyn, anti-Fyn; α-Lck, anti-Lck.
To further characterize the regulation of Sin phosphorylation and to determine if the pattern of phosphorylation for overexpressed Sin reflects that of endogenous Sin, we performed in vitro kinase assays using immune complexes precipitated with Sin-specific antibody. In these assays, we sought to compare the levels of kinase activity associated with Sin under resting and TCR-stimulated conditions. In Sin immunoprecipitates from Jurkat T cells, we found that both overexpressed full-length Sin (Fig. 6B, left panels) and the endogenous Sin isoforms (Fig. 6B, right panels) were phosphorylated in vitro in unstimulated Jurkat T cells by an associated kinase(s). In addition, we observed that Sin associated with several proteins that became phosphorylated in vitro in unstimulated cells (Fig. 6B, left and right panels). TCR stimulation disrupted the in vitro phosphorylation of Sin as well as that of associated substrates, suggesting either decreased kinase activity or dissociation of a kinase(s). The effects we observed were specific, as no phosphoproteins were detected from precipitates of isotype-matched control IgG (Fig. 6B, right panel). Similar results were obtained with endogenous Sin when primary mouse thymocyte cell extracts were used for the kinase assays, although with delayed kinetics for rephosphorylation (Fig. 6C). This result may be due to differences between primary cells versus cell lines or thymocytes versus T cells. These results suggest that a similar mode of regulation exists for the phosphorylation of overexpressed Sin in T-cell lines and endogenous Sin in primary T lymphocytes, such that Sin is phosphorylated in resting cells and TCR stimulation results in a loss of associated kinase activity.
The constitutive phosphorylation of Sin on tyrosine residues in resting Jurkat cells suggested a constitutive interaction with a protein tyrosine kinase. Sin was identified as a ligand for Src kinase SH3 domains, and more recently, it was found that in thymocytes the truncated form of Sin, SinΔC, also binds to and is constitutively phosphorylated by Fyn but not the related kinase Lck (6). Thus, we tested the association of Sin with Fyn in transiently transfected Jurkat T cells. Consistent with the above data, we found that transiently transfected Sin was constitutively phosphorylated in resting cells and became dephosphorylated after 2 min of stimulation (Fig. 6D, left panels). The coexpression of Sin with Fyn resulted in a Sin-Fyn association and a substantial increase in Sin phosphorylation in resting cells (Fig. 6D, middle panels). The Sin-Fyn association and Sin phosphorylation were reduced after 2 min of stimulation (Fig. 6D, middle panels). Similarly, Fyn immunoprecipitates from Jurkat cells stably expressing Sin revealed that more Sin was associated with Fyn under resting conditions than after TCR stimulation (Fig. 6E, middle panel) and that Fyn phosphorylates Sin in vitro (Fig. 6E, top panel). Consistent with previous results, we found no association of Sin with the Src kinase Lck in these experiments (Fig. 6D, right panels, and E). Taken together, these data suggest that Fyn associates with and phosphorylates Sin under basal conditions, while TCR engagement disrupts this interaction. Our results also suggest that Fyn-mediated phosphorylation of Sin regulates the adapter function of Sin and its ability to form an inactive multiprotein complex in resting cells.
Endogenous Sin is required for TCR-induced transcriptional activation.
Our experiments presented above show that constitutive, Fyn-mediated Sin phosphorylation correlates with the formation of a multiprotein complex in resting cells which includes Fyn and PLC-γ, among others. TCR stimulation leads to Sin dephosphorylation and the release of bound intermediates including Fyn and PLC-γ. These results, together with the observation that overexpressed Sin blocks TCR signaling, suggest that phosphorylated Sin acts to sequester signaling intermediates under steady-state conditions, thus limiting their availability and/or activity, while the release of these substrates upon stimulation mediates TCR-induced signal transduction. To further examine the function of Sin in T-cell activation, we sought to determine whether Sin regulates TCR signaling by acting solely as a tethering device for cytoplasmic intermediates in resting cells or whether Sin facilitates TCR signaling by targeting intermediates to the TCR signaling complex upon stimulation. To this end, we used inhibitory RNA oligonucleotides against endogenous Sin to assess the effect of Sin downregulation on TCR signaling. If the function of Sin is solely to sequester signaling intermediates in resting cells, we should observe increased signaling through the TCR in its absence. If, on the other hand, Sin actively participates in TCR signaling by targeting proteins to the greater TCR signaling complex, the downregulation of Sin expression should lead to an inhibition of TCR signaling.
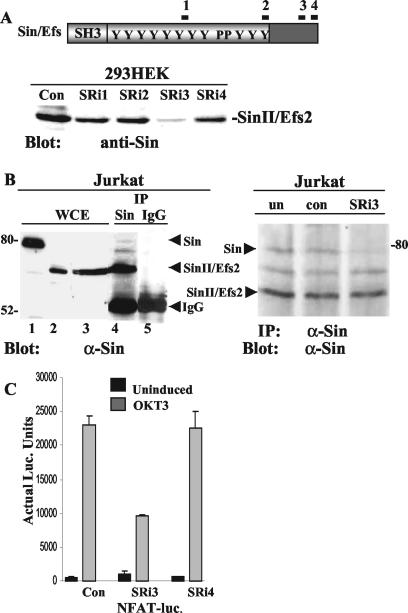
Four different Sin-specific RNA oligonucleotides were tested for their ability to block the expression of overexpressed Sin in 293 HEK cells (Fig. 7A). Cotransfection of one of these oligonucleotides, SRi3, along with a plasmid expressing human SinII/Efs2, dramatically inhibited Sin expression, while the other oligonucleotides were less effective in downregulating Sin expression (Fig. 7A). SRi3 was subsequently tested for its ability to downregulate endogenous Sin expression in Jurkat T cells. Three protein bands are detected in anti-Sin but not in control immunoprecipitates of Jurkat T-cell lysates, which comigrate with overexpressed full-length Sin and SinII/Efs2. A third band is also detected with the Sin monoclonal antibody that is not present in the mouse spleen and thymus and may represent a human-specific isoform of Sin (Fig. 7B, left panel). Transfection of SRi3 oligonucleotides inhibited full-length Sin expression (Fig. 7B, right panel), which correlated with reduced TCR-stimulated transcriptional activation of the NFAT-luciferase reporter (Fig. 7C). Although the SRi3 oligonucleotide targets human Sin on its extreme C terminus, which is common to the full-length and SinII/Efs2 isoform, expression of the SinII/Efs2 isoform was not detectably affected under these conditions. This may be due to the higher levels of expression of this isoform in Jurkat T cells in conjunction with the modest transfection efficiency (∼30%) as determined by the transfection of a fluorescently labeled control siRNA (see Materials and Methods). In contrast, transfection of nontargeting control siRNA or SRi4, which did not significantly inhibit Sin expression in 293 HEK cells, had no effect on Sin expression or NFAT-mediated transcriptional activation. These results suggest that Sin is required for T-cell activation and that it influences TCR signaling by regulating the activity and targeting of substrates in both resting and stimulated cells.
FIG. 7.
Downregulation of Sin expression inhibits TCR-induced transcriptional regulation. (A) A total of 4 × 105 293 HEK cells were transfected with 100 ng of a plasmid expressing human SinII/Efs2 along with 200 ng of Sin-specific or control (Con) siRNA. Thirty-six hours after transfection, cells were harvested and lysed. Whole-cell extracts were normalized for protein content, and proteins were separated by SDS-PAGE. Western blots were probed with Sin-specific antibody, and protein bands were visualized by ECL. (B) Jurkat Tag-cell extracts were immunoprecipitated with Sin-specific or control antibody, and Western blots of immune complexes separated by SDS-PAGE were probed with anti-Sin (α-Sin) antibody (left panel). Whole-cell extracts (WCE) from Jurkat cells overexpressing Sin (lane 1), SinII/Efs2 (lane 2), or untransfected cells (lane 3) were included as controls. Nine times more protein was loaded onto lane 3 compared to lane 2 to reveal endogenous protein levels of Sin isoforms. For downregulation of endogenous Sin expression, 4 × 105 Jurkat Tag cells/well in multiple wells were either left untransfected (un) or transfected with 400 ng of control (con) or Sin-specific siRNA. After 24 to 36 h, cells were lysed and extracts were immunoprecipitated with anti-Sin antibody. Western blots of immune complexes were probed with the same antibody and protein bands visualized by ECL (right panel). One of two independent experiments is shown. IP, immunoprecipitation. (C) Jurkat Tag cells (4 × 105) transfected with 400 ng of control or Sin-specific siRNA, 40 ng of NFAT-firefly-luciferase, and 1 ng of pRL-TK-Renilla-luciferase plasmid DNA. Twenty-four hours after transfection, cells were induced with 1 μg of OKT3/well for 8 h, cells were harvested and lysed, and luciferase (luc.) assays were performed as described in the legend to Fig. 4 and Materials and Methods. Results from one of at least four independent experiments is shown.
DISCUSSION
In this report, we identified Sin as a novel regulator of TCR signaling using overexpression as well as knockdown-of-protein-expression strategies. We found that the overexpression of two different forms of Sin inhibited TCR-induced IL-2 expression in two different cell systems. Specifically, in transgenic T cells, SinΔC expression resulted in defective proliferation and IL-2 secretion in response to TCR stimulation. Correspondingly, Sin expression in Jurkat T cells conferred defective TCR-induced transcriptional activation of IL-2-derived promoter constructs. The proliferative defect in the transgenic T cells resulted from disrupted proximal TCR signaling, as the cells proliferated normally when signals downstream of the TCR were engaged. Additionally, the impaired proliferative response in SinΔC T cells was a direct consequence of inadequate IL-2 secretion, as exogenous IL-2 treatment rescued the defect.
In Sin-expressing Jurkat T cells, the reduced activation of IL-2-promoter-derived constructs containing NFAT and AP-1 binding sites correlated with defective PLC-γ phosphorylation as well as deficient IP3 and intracellular calcium release. Activated PLC-γ cleaves the phosphoinositide PIP2 into the second messengers IP3 and DAG. IP3 production results in intracellular calcium release, which is required for NFAT activation and nuclear localization, while DAG activates PKC-θ and Ras-guanyl-releasing protein, resulting in increased AP-1 transcriptional activity. In T cells, NFAT and AP-1 then bind to the IL-2 promoter with other transcriptional activators to induce IL-2 expression (19, 26, 37). Thus, the inhibition of IL-2 transcription in Sin cell lines could be traced back to a block in PLC-γ activation and the calcium and transcriptional signaling pathways regulated by PLC-γ.
The exact mechanism Sin employs to block PLC-γ activation is still unclear. In Sin-expressing stable cell lines, we observed a Sin-PLC-γ interaction in resting cells and a reduction in PLC-γ phosphorylation in activated cells. The negative effect Sin has on PLC-γ phosphorylation may be attributed entirely to binding and sequestering PLC-γ away from the transmembrane adapter LAT due to inefficient Sin dephosphorylation because of overexpression. Consistent with this, residual Sin phosphorylation was evident during 1 to 3 min of stimulation (Fig. 5E). However, since Sin/PLC-γ association was undetectable immediately after TCR stimulation, we cannot rule out that Sin may also inhibit upstream signaling events that regulate PLC-γ phosphorylation. In addition to binding LAT, PLC-γ activation requires phosphorylation by both ZAP-70 and the Itk kinases (14, 29, 42). It is conceivable that Sin binds to and interferes with Itk and/or ZAP-70 kinase activity, as Sin contains both Itk and ZAP-70 consensus binding motifs (2; unpublished observations). Thus, Sin may regulate PLC-γ activity by direct association and sequestration as well as unidentified indirect mechanisms.
Alternatively, since Sin also binds Fyn, Sin may block Fyn-mediated phosphorylation of the TCR subunits and subsequent downstream events like PLC-γ phosphorylation. Given that Jurkat T cells express approximately 30-fold more Lck than Fyn (Fig. 6D) (5), a reasonable assumption is that blocking some Fyn activity while leaving Lck activity intact would not significantly affect proximal TCR phosphorylation events. In support of this assumption, the total TCR-induced tyrosine phosphorylation pattern in Sin-overexpressing versus control Jurkat T-cell lines does not appear notably different (Fig. 6A, left panels). Furthermore, we could not detect alterations of CD3ζ chain phosphorylation in Sin-expressing Jurkat T cells compared to control cells (unpublished observations). Therefore, it is unlikely that the defect in PLC-γ phosphorylation is due to a disruption in Fyn activity.
Evidence presented above reveals that the Src kinase Fyn constitutively phosphorylates Sin. This finding contradicts the general belief that Src kinases are inactive in resting T cells. We have previously shown that Sin can associate with Src kinases through an Src-SH3/Sin-proline interaction inducing Src kinase activity in the absence of any extracellular stimuli. The activated Src kinase can then phosphorylate Sin Y motifs and subsequently bind these motifs through the Src-SH2 domain (1, 39). Thus, Sin can activate Fyn under basal conditions, but considering that both the Fyn SH2 and SH3 domains can bind Sin, the affinity between the two proteins may prevent Fyn from phosphorylating other molecules. In support of this concept, Sin expression does not trigger Fyn-mediated TCR signaling events, such as CD3ζ phosphorylation and activation of TCR transcriptional targets, in resting cells (Fig. 4 and unpublished observations).
In addition to overexpressing Sin, we used siRNA oligonucleotides to downregulate the expression of endogenous Sin in Jurkat cells and assess its effect on TCR signaling. As with Sin overexpression, we found that the expression of Sin-specific siRNA also inhibited TCR-induced transcriptional activation, a seemingly contradictory finding compared to results obtained with Sin overexpression. However, given that Sin is found in a complex with other proteins which dissociate upon TCR stimulation, the data presented here support a dual regulatory role for Sin in T-lymphocyte signaling that involves the modulation of molecular availability-activity and targeting of signaling substrates. We propose that the means by which Sin regulates TCR signaling is by competing with and sequestering signaling molecules away from the greater TCR complex, thus acting as a negative regulator in resting cells, while it positively regulates TCR signaling by releasing these substrates for fast signal transmission upon stimulation.
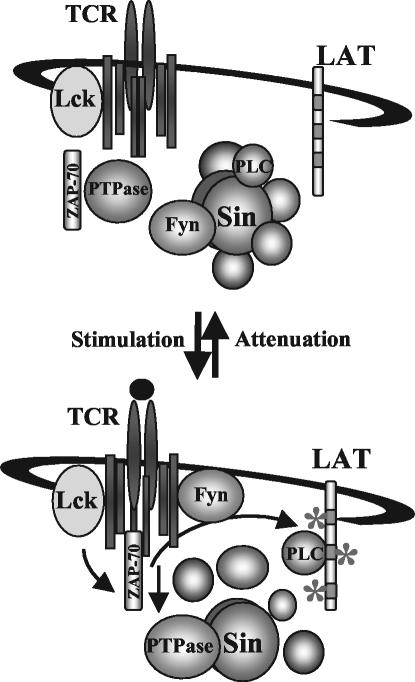
Consistent with our model, the pattern of Sin phosphorylation, as well as its association with Fyn and PLC-γ, is precisely the opposite of that of the TCR complex. In uninduced T cells, Sin is hyperphosphorylated and binds to Fyn, PLC-γ, and other proteins. After TCR engagement, Sin becomes dephosphorylated and disassociates from Fyn and PLC-γ, which then bind to the newly phosphorylated TCR complex and LAT, respectively. The rapid rate of Sin dephosphorylation is likely due to the enzymatic activity of a phosphatase and not simply to a lack of associated Fyn kinase activity. Attenuation of TCR signaling after 10 min, evidenced as a reduction of total phosphotyrosine, coincides with Sin rephosphorylation and formation of an inactive complex of TCR signaling molecules, including Fyn and PLC-γ, with Sin (Fig. 8).
FIG. 8.
Model for Sin-mediated regulation of TCR signaling. In resting T cells, Sin is constitutively phosphorylated by Fyn and bound to PLC-γ and other proteins. TCR stimulation induces the dephosphorylation of Sin, likely through the action of a tyrosine phosphatase, which correlates with the rapid and transient release of associated substrates including Fyn and PLC-γ. Fyn and PLC-γ bind to their respective targets to mediate TCR signaling. Attenuation of TCR signaling 10 min after stimulation results in the reassociation of Fyn and PLC-γ with Sin and Sin phosphorylation, reformation of the inactive complex, and return to resting conditions.
The TCR-induced dephosphorylation of Sin is intriguing given that most adapters, including positive and negative regulators, are phosphorylated after TCR stimulation (15, 27). This includes the Sin-related molecule CasL, which becomes phosphorylated upon TCR stimulation under conditions where Sin becomes dephosphorylated, i.e., overexpression in Jurkat cells. A notable exception, however, is the transmembrane protein PAG/Cbp (3, 16). In resting cells, PAG/Cbp regulates Src kinases by linking them to the Src kinase inhibitor Csk kinase. PAG/Cbp, like Sin, binds to and is phosphorylated by Fyn in resting cells (4, 41). Upon TCR stimulation, PAG/Cbp also becomes rapidly and transiently dephosphorylated, releasing Csk to the cytoplasm and allowing for Src kinase-mediated phosphorylation of the TCR subunits. The similarities between Sin and PAG/Cbp are intriguing and suggest that constitutive phosphorylation of adapter molecules by Fyn or other kinases may be a generalized mechanism for negatively regulating TCR signaling in resting cells, while TCR-stimulated dephosphorylation of the adapters attenuates their opposing influence.
It has previously been proposed that adapter molecules may be capable of generating both positive and negative signals depending on their phosphorylation state, tissue-specific expression, or subcellular localization (18). The finding that Sin also plays an active role in signal transmission during stimulation supports this idea, and to our knowledge, Sin represents the first example of such a positive-negative regulator whose dual function is regulated by changes in its phosphorylation state. Although we have identified Fyn as one of the possible kinases that phosphorylates Sin, the phosphatase responsible for Sin dephosphorylation has not yet been characterized. However, it will be important to learn whether the same phosphatase is responsible for subsequent TCR complex dephosphorylation and signal attenuation. Future experiments will address these questions as well as the identity of the putative phosphatase.
Acknowledgments
We thank C. Roman, A. Pernis, and S. Greenberg for critically reading the manuscript. We also thank A. Weiss for providing the Jurkat Tag cells and the SLP-76 expression construct.
This work was supported in part by the American Cancer Society grant RPG99-09-01MGO, by the Department of Defense grants DAMD17-99-1-9151 and DAMD17-99-1-915, by the National Institute of Allergy and Infectious Diseases (NIAID) grant RO1 AI49387-01, and by the American Cancer Society's Institutional Research grant number 177F.
Research related to the use of animals has complied with all relevant federal guidelines and institutional policies.
REFERENCES
- 1.Alexandropoulos, K., and D. Baltimore. 1996. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 10:1341-1355. [DOI] [PubMed] [Google Scholar]
- 2.Alexandropoulos, K., L. T. Donlin, L. Xing, and A. G. Regelmann. 2003. Sin: good or bad? A T lymphocyte perspective. Immunol. Rev. 192:181-195. [DOI] [PubMed] [Google Scholar]
- 3.Brdicka, T., D. Pavlistova, A. Leo, E. Bruyns, V. Korinek, P. Angelisova, J. Scherer, A. Shevchenko, I. Hilgert, J. Cerny, K. Drbal, Y. Kuramitsu, B. Kornacker, V. Horejsi, and B. Schraven. 2000. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 191:1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson, D., M. Bakinowski, M. L. Thomas, V. Horejsi, and A. Veillette. 2003. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol. Cell. Biol. 23:2017-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny, M. F., B. Patai, and D. B. Straus. 2000. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol. Cell. Biol. 20:1426-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlin, L. T., C. A. Roman, M. Adlam, A. G. Regelmann, and K. Alexandropoulos. 2002. Defective thymocyte maturation by transgenic expression of a truncated form of the T lymphocyte adapter molecule and Fyn substrate, Sin. J. Immunol. 169:6900-6909. [DOI] [PubMed] [Google Scholar]
- 7.Donovan, J. A., R. L. Wange, W. Y. Langdon, and L. E. Samelson. 1994. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J. Biol. Chem. 269:22921-22924. [PubMed] [Google Scholar]
- 8.Griffiths, E. K., C. Krawczyk, Y. Y. Kong, M. Raab, S. J. Hyduk, D. Bouchard, V. S. Chan, I. Kozieradzki, A. J. Oliveira-Dos-Santos, A. Wakeham, P. S. Ohashi, M. I. Cybulsky, C. E. Rudd, and J. M. Penninger. 2001. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science 293:2260-2263. [DOI] [PubMed] [Google Scholar]
- 9.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3450. [PubMed] [Google Scholar]
- 10.Hughes, C. C., and J. S. Pober. 1996. Transcriptional regulation of the interleukin-2 gene in normal human peripheral blood T cells. Convergence of costimulatory signals and differences from transformed T cells. J. Biol. Chem. 271:5369-5377. [DOI] [PubMed] [Google Scholar]
- 11.Isakov, N., and A. Altman. 2002. Protein kinase Cθ in T cell activation. Annu. Rev. Immunol. 20:761-794. [DOI] [PubMed] [Google Scholar]
- 12.Ishino, M., T. Ohba, J. Inazawa, H. Sasaki, Y. Ariyama, and T. Sasaki. 1997. Identification of an Efs isoform that lacks the SH3 domain and chromosomal mapping of human Efs. Oncogene 15:1741-1745. [DOI] [PubMed] [Google Scholar]
- 13.Ishino, M., T. Ohba, H. Sasaki, and T. Sasaki. 1995. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene 11:2331-2338. [PubMed] [Google Scholar]
- 14.Iwashima, M., B. A. Irving, N. S. van Oers, A. C. Chan, and A. Weiss. 1994. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science 263:1136-1139. [DOI] [PubMed] [Google Scholar]
- 15.Kanda, H., T. Mimura, N. Morino, K. Hamasaki, T. Nakamoto, H. Hirai, C. Morimoto, Y. Yazaki, and Y. Nojima. 1997. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. Eur. J. Immunol. 27:2113-2117. [DOI] [PubMed] [Google Scholar]
- 16.Kawabuchi, M., Y. Satomi, T. Takao, Y. Shimonishi, S. Nada, K. Nagai, A. Tarakhovsky, and M. Okada. 2000. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 404:999-1003. [DOI] [PubMed] [Google Scholar]
- 17.Law, S. F., Y. Z. Zhang, S. J. Fashena, G. Toby, J. Estojak, and E. A. Golemis. 1999. Dimerization of the docking/adaptor protein HEF1 via a carboxy-terminal helix-loop-helix domain. Exp. Cell Res. 252:224-235. [DOI] [PubMed] [Google Scholar]
- 18.Leo, A., and B. Schraven. 2001. Adapters in lymphocyte signalling. Curr. Opin. Immunol. 13:307-316. [DOI] [PubMed] [Google Scholar]
- 19.Leo, A., J. Wienands, G. Baier, V. Horejsi, and B. Schraven. 2002. Adapters in lymphocyte signaling. J. Clin. Investig. 109:301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67-75. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima, S., and S. Nagata. 1990. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 18:5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motto, D. G., M. A. Musci, S. E. Ross, and G. A. Koretzky. 1996. Tyrosine phosphorylation of Grb2-associated proteins correlates with phospholipase C gamma 1 activation in T cells. Mol. Cell. Biol. 16:2823-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy, M. A., R. G. Schnall, D. J. Venter, L. Barnett, I. Bertoncello, C. B. Thien, W. Y. Langdon, and D. D. Bowtell. 1998. Tissue hyperplasia and enhanced T-cell signalling via ZAP-70 in c-Cbl-deficient mice. Mol. Cell. Biol. 18:4872-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myung, P. S., G. S. Derimanov, M. S. Jordan, J. A. Punt, Q. H. Liu, B. A. Judd, E. E. Meyers, C. D. Sigmund, B. D. Freedman, and G. A. Koretzky. 2001. Differential requirement for SLP-76 domains in T cell development and function. Immunity 15:1011-1026. [DOI] [PubMed] [Google Scholar]
- 25.Naramura, M., H. K. Kole, R. J. Hu, and H. Gu. 1998. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl. Acad. Sci. USA 95:15547-15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norian, L. A., and G. A. Koretzky. 2000. Intracellular adapter molecules. Semin. Immunol. 12:43-54. [DOI] [PubMed] [Google Scholar]
- 27.Ohashi, Y., K. Tachibana, K. Kamiguchi, H. Fujita, and C. Morimoto. 1998. T cell receptor-mediated tyrosine phosphorylation of CasL, a 105-kDa Crk-associated substrate-related protein, and its association of Crk and C3G. J. Biol. Chem. 273:6446-6451. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, G. M., S. J. Fashena, and E. A. Golemis. 2000. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 10:111-119. [DOI] [PubMed] [Google Scholar]
- 29.Paz, P. E., S. Wang, H. Clarke, X. Lu, D. Stokoe, and A. Abo. 2001. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 356:461-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson, E. J., M. L. Woods, S. A. Dmowski, G. Derimanov, M. S. Jordan, J. N. Wu, P. S. Myung, Q.-H. Liu, J. T. Pribila, B. D. Freedman, Y. Shimizu, and G. A. Koretzky. 2001. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293:2263-2265. [DOI] [PubMed] [Google Scholar]
- 31.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94:229-238. [DOI] [PubMed] [Google Scholar]
- 32.Razani-Boroujerdi, S., L. D. Partridge, and M. L. Sopori. 1994. Intracellular calcium signaling induced by thapsigargin in excitable and inexcitable cells. Cell Calcium 16:467-474. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro, V. S., M. N. Mollenauer, and A. Weiss. 1998. Nuclear factor of activated T cells and AP-1 are insufficient for IL-2 promoter activation: requirement for CD28 up-regulation of RE/AP. J. Immunol. 161:6455-6458. [PubMed] [Google Scholar]
- 34.Sosinowski, T., N. Killeen, and A. Weiss. 2001. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity 15:457-466. [DOI] [PubMed] [Google Scholar]
- 35.Sundvold, V., K. M. Torgersen, N. H. Post, F. Marti, P. D. King, J. A. Rottingen, A. Spurkland, and T. Lea. 2000. T cell-specific adapter protein inhibits T cell activation by modulating Lck activity. J. Immunol. 165:2927-2931. [DOI] [PubMed] [Google Scholar]
- 36.Tang, J., S. Sawasdikosol, J. H. Chang, and S. J. Burakoff. 1999. SLAP, a dimeric adapter protein, plays a functional role in T cell receptor signaling. Proc. Natl. Acad. Sci. USA 96:9775-9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson, M. G., J. Lin, and A. Weiss. 2000. Lymphocytes with a complex: adapter proteins in antigen receptor signaling. Immunol. Today 21:584-591. [DOI] [PubMed] [Google Scholar]
- 38.Wu, L., Z. Yu, and S. H. Shen. 2002. SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T cell receptor activation. J. Biol. Chem. 277:40420-40427. [DOI] [PubMed] [Google Scholar]
- 39.Xing, L., C. Ge, R. Zeltser, G. Maskevitch, B. J. Mayer, and K. Alexandropoulos. 2000. c-Src signaling induced by the adapters Sin and Cas is mediated by Rap1 GTPase. Mol. Cell. Biol. 20:7363-7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]
- 41.Yasuda, K., M. Nagafuku, T. Shima, M. Okada, T. Yagi, T. Yamada, Y. Minaki, A. Kato, S. Tani-Ichi, T. Hamaoka, and A. Kosugi. 2002. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 169:2813-2817. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323-332. [DOI] [PubMed] [Google Scholar]
- 44.Zhumabekov, T., P. Corbella, M. Tolaini, and D. Kioussis. 1995. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods 185:133-140. [DOI] [PubMed] [Google Scholar]