Abstract
Stage-specific embryonic antigen 1 (SSEA-1), an antigenic epitope defined as a Lewis x carbohydrate structure, is expressed during the 8-cell to blastocyst stages in mouse embryos and in primordial germ cells, undifferentiated embryonic stem cells, and embryonic carcinoma cells. For many years, SSEA-1 has been implicated in the development of mouse embryos as a functional carbohydrate epitope in cell-to-cell interaction during morula compaction. In a previous study, α1,3-fucosyltransferase IX (Fut9) exhibited very strong activity for the synthesis of Lewis x compared to other α1,3-fucosyltransferases in an in vitro substrate specificity assay. Fut4 and Fut9 transcripts were expressed in mouse embryos. The Fut9 transcript was detected in embryonic-day-13.5 gonads containing primordial germ cells, but the Fut4 transcript was not. In order to identify the role of SSEA-1 and determine the key enzyme for SSEA-1 synthesis in vivo, we have generated Fut9-deficient (Fut9−/−) mice. Fut9−/− mice develop normally, with no gross phenotypic abnormalities, and are fertile. Immunohistochemical analysis revealed an absence of SSEA-1 expression in early embryos and primordial germ cells of Fut9−/− mice. Therefore, we conclude that expression of the SSEA-1 epitope in the developing mouse embryo is not essential for embryogenesis in vivo.
Carbohydrate chains of glycoproteins and glycolipids on the cell surface play important roles in cell-cell interaction and recognition. Stage-specific embryonic antigen 1 (SSEA-1), an antigenic epitope which was defined as a Lewis x [Lex; Galβ1-4(Fucα1-3)GlcNAc] carbohydrate epitope, is widely expressed on the surfaces of mammalian cells and is considered to be involved in cell-cell interactions during embryogenesis, differentiation, and the neurodevelopmental process (6, 10-12, 21, 22, 44). In the developing mouse embryo, SSEA-1 appears after the third cleavage (8-cell stage), at the time of onset of compaction, and disappears around the 32-cell stage, after completion of the compaction process. There is some evidence that the polylactosamine backbone carrying SSEA-1 facilitates the close contact and sorting of SSEA-1-positive blastomeres to the inner cell mass during the formation of blastocysts at this stage (6, 12). SSEA-1 is also implicated in cell-cell interactions, such as the differentiation of mouse F9 embryonic carcinoma (EC) cells (12, 17) and TERA-2-derived human EC cells (2, 3), and in the early neural development of chicken embryos (1). In the gonads of humans, mice, pigs, and chickens, an anti-SSEA-1 monoclonal antibody (MAb) is used as a specific marker for primordial germ cells (PGC) (20, 29, 45).
The expression of Lex epitopes is determined by α1,3-fucosyltransferase(s) (α1,3FUT or α1,3Fut). The genes encoding α1,3FUTs form a family. To date, human genes encoding six α1,3FUTs, FUT3 (Fuc-TIII) (25), FUT4 (Fuc-TIV) (16, 26, 28), FUT5 (Fuc-TV) (49), FUT6 (Fuc-TVI) (23, 48), FUT7 (Fuc-TVII) (33, 42), and FUT9 (Fuc-TIX) (18), have been cloned and characterized. Distinct acceptor specificity patterns are emerging among the α1,3FUTs. FucT-III, FucT-IV, FucT-V, FucT-VI, and FucT-IX can synthesize the Lex structure, while FucT-VII cannot. FucT-III, FucT-IV, FucT-V, FucT-VI, and FucT-VII can synthesize the sialyl Lex structure, which is the minimal ligand for all three selectins known as cell adhesion molecules for leukocyte-endothelium interaction (5, 13, 31). Detailed analysis of the substrate specificity of the polylactosamine acceptor has revealed Fuc-TIX to have more-efficient activity for synthesis of the Lex carbohydrate epitope than other α1,3FUTs (34) and to fucosylate the remote internal N-acetyllactosamine units of α2,3-sialylated polylactosamine (47). Mice have only three functional α1,3Fut genes, encoding Fut4, Fut7, and Fut9, corresponding to human FUT4, FUT7, and FUT9, respectively. The mouse gene orthologous to the ancestral gene for human FUT3, FUT5, and FUT6 seems to be a pseudogene (9, 15). The FUT9 gene sequence is highly conserved among humans, mice, rats, and hamsters (4, 18, 24, 38), indicating that it has been under strong selective pressure during evolution and thus suggesting that it has an essential role in these organisms. The chromosomal positions of the six human α1,3FUT genes have been assigned: the FUT3, FUT5, and FUT6 genes form a cluster at 19p13.3 (30, 36), while the FUT4 and FUT7 genes are located at 11q21 and 9q34.3, respectively (30, 39, 40). The mouse Fut4 gene was mapped to chromosome 9, which is syntenic to 11q21 in humans (15). The human FUT9 gene was mapped to 6q16 by fluorescent in situ hybridization (19).
Previously, Kudo et al. cloned and characterized a cDNA encoding mouse Fuc-TIX from a mouse brain cDNA library by use of an expression cloning method (24). The transcript for Fut9 was expressed mainly in the brain and kidney of the adult mouse (24). The Fut9 gene is probably a candidate for the Lex synthase gene, expressed during the neural differentiation of P19 EC cells (37). Very recently, Nishihara et al. reported that Fut9 determines Lex expression in the adult mouse brain (35). FUT4 and FUT9 genes were suspected to control the biosynthesis of SSEA-1 during the embryonic stage of human development (8). In mice, the Fut4 transcript was expressed in all preimplantation embryos from the 1-cell to the blastocyst stage and was not correlated with the pattern of SSEA-1 expression (27).
To investigate directly if SSEA-1 contributes to the compaction events or germ line development in mammals, we carried out the construction and initial characterization of Fut9-deficient mice. Fut9-deficient mice are viable and do not express SSEA-1 in the developing embryo between the 8-cell and the blastocyst stage or in PGC. These observations imply that SSEA-1 plays nonessential roles in compaction events or PGC development.
MATERIALS AND METHODS
Recovery and handling of embryos.
Female C57BL/6N mice (age, 3 to 4 weeks) were made to superovulate by administration of intraperitoneal injections of 5 IU of pregnant mare serum gonadotropin (PMSG) and 5 IU of human chorionic gonadotropin (hCG) 48 h later. To obtain embryos, females were paired individually overnight with a male and inspected for vaginal plugs the next day as an indication of successful mating. The uteri were dissected, and embryos were flushed out with M2 medium containing bovine serum albumin (BSA) at 4 mg/ml. Late-2-cell and sometimes early-4-cell embryos were flushed from oviducts at 46 to 50 h post-hCG treatment. To recover 4-cell, 8-cell, and compaction embryos, the embryos were cultured in M16 medium under paraffin oil in a humidified atmosphere of 5% CO2 in air at 37°C.
Quantitative analysis of Fut4 and Fut9 transcripts in early embryos and gonads containing PGC.
About 100 embryos were lysed in 50 μl of ISOGEN (Nippon Gene Co., Ltd., Tokyo, Japan) and 10 μg of yeast tRNA. Total cellular RNA from embryos in various stages of development was extracted from the lysate according to the manual supplied and then dissolved with 12 μl of diethylpyrocarbonate (DEPC)-treated water. cDNAs were made from the total RNAs by using the Superscript first-strand system (Invitrogen). All of the total RNAs obtained from embryos were heated to 70°C for 10 min in 14 μl of DEPC-treated water containing 0.5 μg of oligo(dT) primer. Then 2 μl of 10× synthesis buffer (200 mM Tris-HCl [pH 8.4], 500 mM KCl, 25 mM MgCl2, and 1 mg of BSA/ml), 1 μl of 10 mM deoxynucleoside triphosphate mix, 2 μl of 0.1 M dithiothreitol, and 1 μl of Superscript II were added to the sample. The resulting reaction mixture was incubated at 42°C for 50 min. The reaction was terminated by incubating at 90°C for 5 min. This first-strand cDNA was diluted 10-fold as a PCR template. A 50-μl volume of the PCR mixture contained 2 to 10 μl of diluted first-strand cDNA solution. The competitive reverse transcription-PCR (RT-PCR) methods used for measurement of the transcripts of the Fut4, Fut9, and β-actin genes have been described in detail previously (24). The Fut9 gene was amplified with 5′-CAGCTGGGATCTGACTAACTTACC-3′ as a forward primer and 5′-CCACATGAATGAATGAATCAGCTGG-3′ as a reverse primer. The Fut4 gene was amplified with 5′-TTGCAGCCTGCGCTTCAACATCAG-3′ and 5′-ACTCAGCTGGTGGTAGTAACGGAC-3′. The β-actin gene, used as an internal control, was amplified with 5′-GATATCGCTGCGCTGGTCGTCGAC-3′ and 5′-CAAGAAGGAAGGCTGGAAAAGAGC-3′.
PCR conditions were as follows: a pre-PCR heating step at 95°C for 11 min, followed by 50 cycles of 1 min at 95°C, 1.5 min at 65°C, and 2 min at 72°C. After the competitive RT-PCR, a 10-μl aliquot was electrophoresed in a 1% agarose gel, and the bands were visualized by staining with ethidium bromide. The intensities of the bands of amplified fragments were quantified by scanning the positive signals by use of an NIH Image system. To ascertain the efficiency of the method of preparing cDNA from total RNA, the β-actin transcripts in each sample were measured.
Cloning of the genomic DNA and chromosomal mapping of the Fut9 locus.
A cDNA fragment obtained by rapid amplification of the 5′ end (5′ RACE) in this study and the cDNA encoding the open reading frame (ORF) of the Fut9 gene obtained in the previous study were used as probes to screen a λFix II phage genomic library of a 129/Sv mouse (Stratagene, La Jolla, Calif.). We isolated several distinct clones with inserts of different sizes and signals. All inserts were the subcloned into pBluescript SK(−) with a SalI site and then mapped by using restriction endonucleases BamHI, SacI, and SpeI.
For chromosomal mapping using an interspecific backcross panel, genomic DNA was amplified by using primers CB-197 (5′-AAGCCTAATGCTTGCTCTCAGTCG-3′) and CB-56 (5′-CCACATGAATGAATGAATCAGCTGG-3′), corresponding to the sequences in intron 2 and the ORF, respectively, of the Fut9 gene, with the following parameters: 35 cycles of 94°C for 1 min, 64°C for 1 min, and 72°C for 3 min. With this primer pair, a PCR product of approximately 3.0 kb was obtained from both C57BL/6J and Mus spretus genomic DNAs. Following digestion with the RsaI enzyme, five fragments were found in C57BL/6 DNA, whereas only three bands were seen in M. spretus DNA.
Generation of Fut9−/− mice.
A replacement-type gene targeting vector was constructed in which exon 3, containing the entire ORF for the Fut9 gene, was replaced with a neomycin gene from the pMC-Neo PolyA vector (Stratagene). Positive-negative selection was achieved by flanking the 3′ end of the targeting vector with a herpes simplex virus thymidine kinase gene. v17-2 embryonic stem (ES) cells were cultured on mouse embryonic fibroblast feeder layers in Dulbecco's modified Eagle medium containing 15% fetal bovine serum, 1,000 U of leukemia inhibitory factor/ml, and 100 μM β-mercaptoethanol. The ES cells were transfected with 25 μg of NotI-linearized targeting vector by using a Bio-Rad Gene Pulser set to 0.35 kV and 250 μF with a 0.2-cm electrode gap. Transfected cells were cultured with 250 μg of Geneticin (Invitrogen)/ml and 2 μM ganciclovir (Synex) for 8 to 10 days. DNA derived from Geneticin- and ganciclovir-resistant ES clones was screened by PCR and Southern blot analysis by means of digestion with the restriction enzyme SacI using a 5′ probe external to the targeting vector sequence in Fig. 3A. Targeted ES cells were injected into C57BL/6 blastocysts to generate chimeras. To achieve germ line transmission, chimeric male mice with a high degree of chimerism (as judged by the agouti coat color) were mated with C57BL/6 females. Mixed-background mice carrying a targeting allele of Fut9 were backcrossed four times with C57BL/6N mice. Comparisons of litter sizes were performed by the unpaired t test using StatView for Macintosh (HULINKS Inc., Tokyo, Japan). Mice were bred in a specific-pathogen-free facility with food and water available ad libitum.
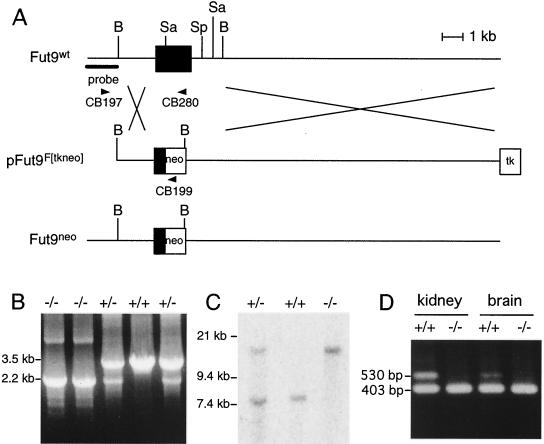
FIG. 3.
Targeted disruption of the Fut9 genomic locus. (A) Schematic representations of the Fut9 locus (top), the targeting vector (center), and the disrupted allele generated by homologous recombination (bottom). Solid boxes, indicate exon 3 containing the entire Fut9 ORF. The location of the probe for Southern blot analysis is indicated. Arrowheads indicate primers for genotyping by PCR. Abbreviations for restriction sites: B, BamHI; Sa, SacI; Sp, SpeI. (B) Routine genotyping of mice was performed with a mixture of three oligonucleotides, allowing detection of the mutated and wild-type alleles. The CB197-CB280 was able to amplify a 3.5-kb band from the wild-type Fut9 gene, whereas the CB197-CB199 primer pair was designed to amplify a 2.2-kb band from the mutated, targeted allele. (C) Southern blot analysis of DNA extracted from a mouse tail. DNA was digested with SacI and hybridized with the probe shown in panel A. The probe detects an 8-kb fragment in wild-type (+/+) mice and a 15-kb fragment in mutant (−/−) mice. Mice heterozygous for the mutation (+/−) show both the 8- and 15-kb bands. (D) Quantitative analysis of Fut9 transcripts in the kidneys and brains of adult wild-type and Fut9−/− mice by competitive RT-PCR. PCR was performed with a mixture of cDNA and 10 fg of the Fut9 competitor DNA.
Genotyping using targeted ES cells and tail biopsy specimens.
We extracted genomic DNA from ES cells, as well as from tail biopsy specimens, with a DNeasy tissue kit (QIAGEN, Tokyo, Japan). The mice or ES cells were genotyped by PCR with primers CB199 (5′-ACATTGGGTGGAAACATTCCAG-3′), CB197 (5′-AAGCCTAATGCTTGCTCTCAGTCG-3′), and CB280 (5′-TTTGCTACATCAATTAGCTCCCCT-3′) and with LA-Taq (Takara, Kyoto, Japan). After a denaturing step for 1 min at 94°C, DNA was amplified for 50 cycles (consisting of 98°C for 1 s and 58°C for 5 min). PCR fragments were resolved on a 1% agarose gel. CB197 and CB280 are primers specific for the Fut9 gene, designed to produce a 3.5-kb DNA fragment when amplifying the wild-type allele. Oligonucleotide CB199 (hybridizing to the neo gene), together with CB197, is supposed to amplify a smaller, 2.2-kb band from the mutant allele.
Proper insertion of the targeting construct was monitored by Southern blot analysis. Twenty-five micrograms of genomic DNA from tail biopsy specimens was digested with SacI and electrophoresed in a 0.8% agarose gel. Methods for transfer to the membrane, hybridization, and detection of hybridization signals have been described in detail previously (24).
Immunostaining of mouse embryos.
Fut9-deficient embryos were collected 48 to 68 h post-hCG treatment from superovulated Fut9−/− female mice mated with Fut9−/− males as described above. After embryo collection, zonae pellucidae were removed with acid Tyrode's solution (7). The zona pellucida-free embryos were analyzed by indirect immunofluorescence microscopy. Anti-SSEA-1 was used as a primary antibody. Controls for indirect immunofluorescence experiments included the use of preimmune serum as a primary serum and the omission of the primary serum (use of the secondary antibody alone).
Isolation and immunohistochemical staining of mouse embryonic gonads.
We isolated gonads from embryos at embryonic day 13.5 (E13.5), and they were defined as being in a postmigratory stage. Three antibodies, an anti-SSEA-1 MAb, an anti-EMA-1 MAb, and an anti-mouse Vasa homologue (anti-MVH) antibody, were used for immunohistochemical analysis. For detection of MVH protein in developing gonads, we used a rabbit polyclonal antiserum against MVH, kindly provided by T. Noce of the Mitsubishi Kasei Institute of Life Science (14). All specimens were fixed in 10% formaldehyde and embedded in paraffin. Deparaffinized 4-μm-thick sections were washed in phosphate-buffered saline (PBS) three times and treated with 0.3%(vol/vol) H2O2 in methanol for 15 min to block endogenous peroxidase. The sections were again washed in PBS three times and then incubated for 20 min with 0.5% normal swine serum in PBS at room temperature to avoid nonspecific staining. Antigens were detected by applying the primary antibody at a concentration of 10 μg/ml for 12 h at room temperature, followed by the streptavidin-biotin complex method (Elite ABC kit; Vectastain; Vector Laboratories, Burlingame, Calif.). Glass slides were washed with PBS between each step, and 0.1 mg of 3,3′-diaminobenzidine 4HCl (Dojin, Kumamoto, Japan)/ml in 0.1 M Tris-HCl buffer (pH 7.6) was used for the peroxidase reaction. Nuclei were counterstained with Mayer's hematoxylin.
Nucleotide sequence accession number.
The sequence data in this article have been deposited in the GenBank/EMBL data libraries under accession no. AB015426.
RESULTS AND DISCUSSION
Gene expression of α1,3Fut in mouse embryos and PGC.
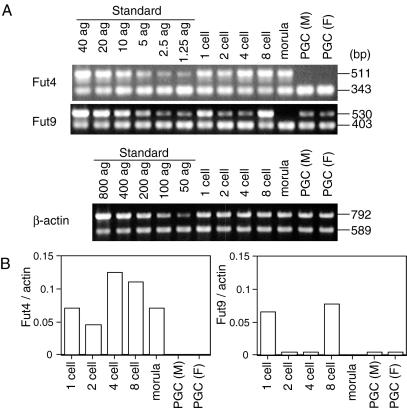
In order to elucidate the correlation of the expression of the two α1,3Fut genes with the expression of SSEA-1 in preimplantation embryos (1-, 2-, 4-, and 8-cell embryos, and morulae) and male and female gonads (E13.5) containing PGC, expression of the Fut4 and Fut9 transcripts was examined by competitive RT-PCR (Fig. 1). The Fut4 transcript was expressed in all preimplantation embryos. These results confirmed the data of Liu et al. (27), which suggested that the expression pattern of the Fut4 transcript is not correlated with that of SSEA-1. Expression of the Fut9 transcript was detected in 1-cell embryos and then decreased dramatically in 2- and 4-cell embryos. It increased transiently in 8-cell embryos and then vanished completely at the morula stage. This pattern of expression during the 1- to 8-cell stages is typical of many genes that are expressed before implantation and reflects the replacement of the maternal mRNAs with embryonically expressed transcripts (43). The transient augmentation of Fut9 transcription in the 8-cell embryo correlated with the appearance of SSEA-1. In both male and female gonads containing PGC, the Fut9 transcript was expressed but the Fut4 transcript was not. These results reveal that Fuc-TIX is a likely candidate for the α1,3-Fut responsible for the synthesis of SSEA-1 in preimplantation embryos and PGC.
FIG. 1.
Quantitative analysis of Fut4, Fut9, and β-actin transcripts in developing early embryos and PGC. (A) Electrophoretic patterns of each product by competitive RT-PCR. The standard control for the PCR was performed with a mixture of standard plasmid (Fut4, Fut9 [1.25 to 40 ag], β-actin [50 to 800 ag]) and a fixed concentration of each gene competitor DNA (Fut4, Fut9 [10 ag], β-actin [100 ag]). The coamplified DNA fragments derived from standard DNA or cDNA and from competitor DNA were subjected to electrophoresis in a 1% agarose gel and then visualized by ethidium bromide staining. (B) Bar chart showing expression levels of Fut4 and Fut9. Amounts of Fut4 and Fut9 transcripts were normalized by the amount of the β-actin transcript.
Genomic organization and physical localization of the mouse Fut9 gene.
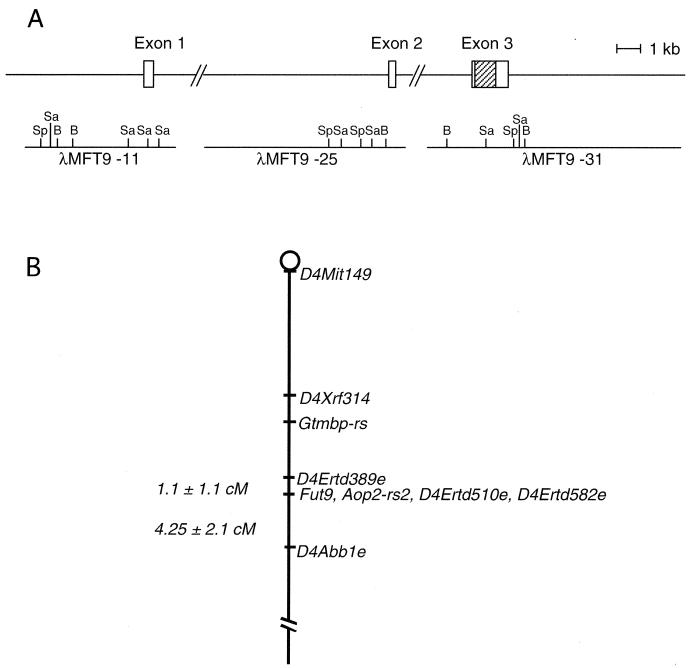
We first determined the 5′ end of the Fut9 transcript by using the 5′ RACE method with a brain mRNA of an adult mouse. The cDNA fragment obtained by 5′ RACE and the cDNA encoding the ORF of the Fut9 gene obtained in a previous study (24) were used as probes to screen a λFix II phage genomic library of the 129/Sv mouse. We isolated three independent phage clones, λMFT9-31, λMFT9-25, and λMFT9-11, which possessed inserts of approximately 20, 16, and 12 kb, respectively. Restriction mapping and nucleotide sequencing revealed that the λMFT9-31 clone encompassed the ORF and 3′ untranslated region (3′ UTR) of Fut9 cDNA but encoded only 8 bp in the 5′ UTR. The DNA fragments in the λMFT9-25 and λMFT9-11 clones, which hybridized with cDNA probes, were subcloned into a pBluescript SK(−) vector with appropriate restriction sites for sequencing. As shown in Fig. 2, λMFT9-25 and λMFT9-11 were determined to encode exons 2 and 1, respectively. All junction sequences were consistent with the GT-AG rule at intron-exon splice junctions. Thus, the genomic organization of the Fut9 gene was found to be similar to that of other members of the α1,3FUT gene family, except for the FUT7 gene.
FIG. 2.
Genomic organization and chromosomal localization of the mouse Fut9 gene. (A) Schematic diagram of Fut9 gene structure. The three exons of the Fut9 gene are shown. Hatched rectangle, translated region; open rectangles, 5′ and 3′ UTRs. The three genomic clones (λMFT9-11, λMFT9-25, and λMFT9-31) isolated from a λFix II phage library are diagramed below the exons. Restriction sites are indicated. B, BamHI; Sp, SpeI; Sa, SacI. (B) Map showing part of mouse chromosome 4 and the location of the Fut9 gene in relation to other linked loci mapped on the Jackson Laboratory BSS backcross panel. Missing points were inferred from surrounding data when the assignment was unambiguous. Raw data, locus definitions, and references for this cross are available at http://www.jax.org/resources/documents/cmdata.
To examine the relationship between the Fut9 locus and known mouse mutations, we mapped the Fut9 gene by using an interspecific backcross panel as described in Materials and Methods. The Fut9 gene was unambiguously mapped on chromosome 4 by typing the Jackson Laboratory BSS Interspecific Backcross panel (41). The position of the gene relative to other markers is mapped in Fig. 2B: 1.1 ± 1.1 cM distal to D4Edtd389e and 4.25 ± 2.1 cM proximal to D4Abble. Aop2-rs2, D4Ertd510e, and D4Ertd582e cosegregate with the Fut9 gene. This genome region is syntenic to human 6q14-q21, where the human homologue of the Fut9 gene is located. A neurological mutation, waddler (wd), which shows an ataxic phenotype, was assigned near the Fut9 gene (32). Unfortunately, however, the mutant strain is probably extinct. It is of interest that the position of the Fut9 gene was mapped approximately 11.5 cM proximal to Ggtb, encoding β1,4-galactosyltransferase I (β4Gal-TI), since both glycosyltransferases Fuc-TIX and β4Gal-TI are required for synthesis of the Lex structure.
Generation of Fut9-deficient mice.
To generate ES cells with one disrupted Fut9 allele, we constructed a replacement-targeting vector, pFut9F[tkneo] (Fig. 3A). A neomycin resistance expression cassette replaced 3 kb of the Fut9 genomic DNA, which contains the third exon of the partial cDNA of Fut9 (Fig. 3A). ES cells that underwent homologous recombination were selected in the presence of Geneticin and ganciclovir. Figure 3 shows the external probe in the position of intron 2 used to identify Fut9 homologous recombinations by Southern blot analysis, which showed an 8-kb fragment in wild-type ES cells and a 15-kb fragment in mutated ES cells. Mice heterozygous for the mutation show both the 8- and 15-kb bands.
Mutated ES cells were injected into C57BL/6 blastocysts to generate chimeric mice. Three male chimeras with high degrees of chimerism (more than 80%) were bred with C57BL/6 females to produce agouti progeny. Fut9 heterozygous mice were identified by Southern blotting and PCR. These Fut9+/− mice were interbred, and tail DNA from progeny was analyzed by Southern blotting and PCR. Genotyping of animals resulting from the crosses was first achieved by routine PCR, with oligonucleotides hybridizing to the 5′ end of the Neo cassette (CB199), as well as to regions located before (CB197) or after (CB280) the Neo insertion site in the wild-type allele. The presence of mutant Fut9 alleles in adult animals was made clear by amplification of a diagnostic band of 2.2 kb (Fig. 3B). Proper homologous recombination was checked by Southern blot analysis. The results of Southern blotting using SacI are shown in Fig. 3C. It is evident that Fut9 wild-type mice harbor only the 8-kb fragment, as expected, and that Fut9−/− mice harbor only the 15-kb fragment. Heterozygous mice harbor both fragments.
Analysis of the offspring resulting from crosses among Fut9 heterozygotes revealed a Mendelian distribution of wild-type, heterozygous, and homozygous offspring (Table 1). There was little difference between the numbers of offspring resulting from crosses among wild-type mice (average litter size ± standard error of the mean from at least 10 mating pairs, 6.8 ± 2.2), among heterozygous mice (6.2 ± 1.9), and among null mice (6.6 ± 1.2). This establishes the absence of a relationship of Fut9 deficiency with insemination or embryonic development. These results, as well as the absence of any gross phenotypes, demonstrated that the Fut9−/− mouse is viable.
TABLE 1.
Genotype analysis of offspring from Fut9 heterozygous intercrosses
| Sex | No. of offspring of the following genotype:
|
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| Male | 53 | 89 | 59 |
| Female | 42 | 97 | 45 |
| Total | 95 | 186 | 104 |
| % of total | 24.7 | 48.3 | 27.0 |
Expression of SSEA-1 in the preimplantation stage in Fut9−/− mice.
To confirm the generation of a null mutation of the Fut9 locus, we analyzed the expression of the Fut9 gene in normal and mutant mice. Since the Fut9 transcript has been reported to show high levels of expression in the brain and kidney (24), we analyzed the expression of the Fut9 transcript in these tissues by competitive RT-PCR. The results confirmed the complete absence in tissues of Fut9−/− mice of a band that was detected in the corresponding tissues of wild-type mice (Fig. 3D).
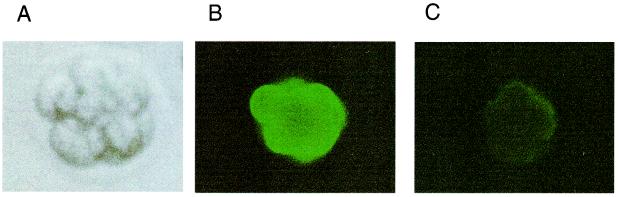
Fuc-TIX was judged to be the most likely candidate for SSEA-1 synthase expressed at the implantation stage, based on the expression pattern of the Fut9 transcript (Fig. 1). To analyze the expression of SSEA-1 in the embryos of Fut9−/− mice, preimplantation embryos were isolated from wild-type and Fut9−/− mice and cultured in vitro. Morphologically normal eggs were cultured in vitro for both wild-type and Fut9−/− mice. Expression of SSEA-1 in preimplantation embryos of wild-type or Fut9−/− mice at different stages of development was examined by indirect immunofluorescence microscopy using an anti-SSEA-1 MAb. For wild-type mice, SSEA-1 immunostaining was detected at the cell surface in 8- to 16-cell embryos (Fig. 4B) but not at other stages (data not shown). For Fut9−/− mice, SSEA-1 expression was dramatically diminished in 8- to 16-cell embryos (Fig. 4C). The faint signals on the periphery of Fut9−/− 8-cell embryos were at the background level as much as those of negative-control staining using only a fluorescein isothiocyanate-conjugated second antibody. Nishihara et al. (34) reported that Fuc-TIX exhibited 15-times-stronger activity for Lewis x (SSEA-1) synthesis than Fuc-TIV when oligosaccharides were used as acceptors. Moreover, when glycolipids were used as acceptors, Fuc-TIX synthesized the SSEA-1 epitope on the glycolipids with 200-times-stronger efficiency than Fuc-TIV. We also observed that SSEA-1 completely disappeared in the kidneys and brains of Fut9−/− mice (unpublished data), although Fut4 transcripts were expressed in such tissues. These facts strongly indicated that Fuc-TIX is a major enzyme responsible for SSEA-1 expression in preimplantation embryos and that the involvement of Fuc-TIV is negligible.
FIG. 4.
Expression of SSEA-1 in developing embryos of wild-type and Fut9−/− mice. Immunofluorescent detection was performed by using an anti-SSEA-1 MAb with an 8-cell embryo of a wild-type mouse. (A) Results with a phase-contrast microscope. (B and C) Results for wild-type (B) and Fut9−/− (C) mice with an immunofluorescence microscope.
Expression of SSEA-1 on PGC in Fut9-deficient mice.
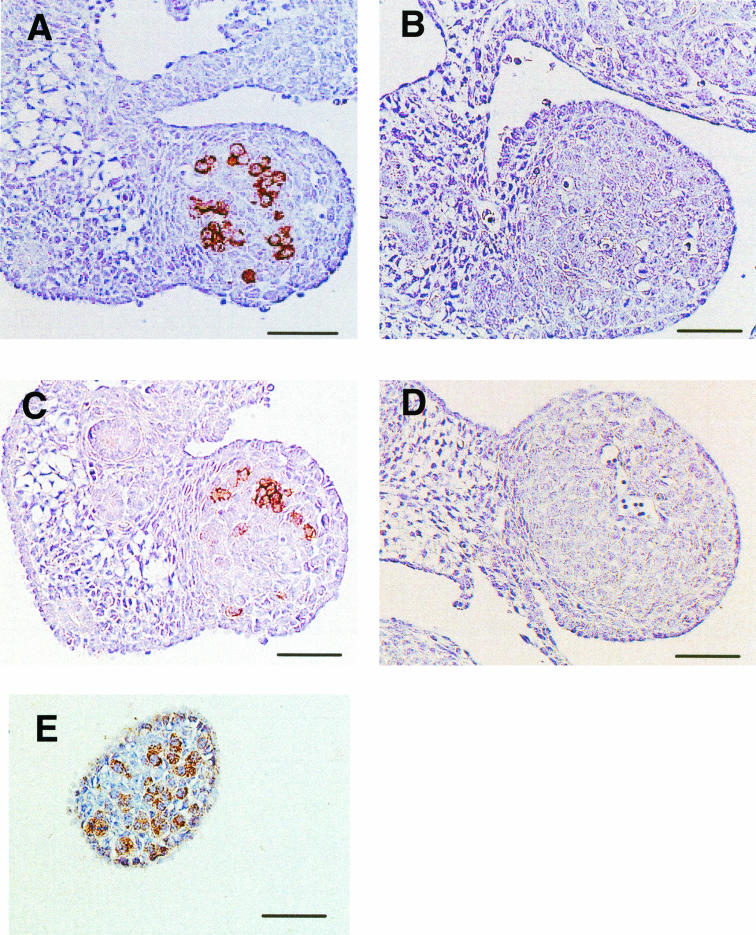
SSEA-1 and EMA-1 can be used as reliable markers for identifying PGC in mice, goats, chicks, etc. The EMA-1 MAb was produced against a glycoprotein cell surface antigen of the EC Nulli SCC1. Expression of these antigenic epitopes in PGC of both wild-type and Fut9−/− embryos was examined at E13.5. Anti-SSEA-1 and anti-EMA-1 MAbs strongly stained PGC in wild-type gonads (Fig. 5A and C), but the reactivities to both antibodies were completely lost in the PGC of Fut9−/− mice (Fig. 5B and D). To examine the presence of PGC in the null mutant, we used an anti-MVH antibody.
FIG. 5.
Expression of SSEA-1 in PGC of E13.5 gonads. Images show immunohistochemical staining of E13.5 gonads from wild-type (A and C) and Fut9−/− (B, D, and E) mice with the anti-SSEA-1 MAb (A and B), the EMA-1 MAb (C and D), and the anti-MVH antibody (E).
The Mvh gene is required for male germ cell development and is specifically expressed in PGC in the gonad, thus representing a specific marker for PGC (14, 46). MVH expression was clearly observed in the gonad of the Fut9−/− embryo (Fig. 5E). This finding suggests that although SSEA-1 is absent, PGC development is normal in Fut9−/− embryos.
In conclusion, SSEA-1 expressed both in preimplantation embryos and in PGC is synthesized by Fut9, which is not essential for early mouse development.
Acknowledgments
We thank Keishin Hayashida and Tsutomu Inoue of the Animal House, Institute of Life Science, Soka University, for assistance in mouse breeding. Takayuki Sakurai of the Laboratory of Mammalian Development, Genetic Strains Research Center, National Institute of Genetics, kindly provided instructions for the handling and culture of early embryos. We are grateful to Lucy B. Rowe of the Jackson Laboratory for analysis of the backcross mapping data.
This work was performed as a part of the R&D Project of Industrial Science and Technology Frontier Program (R&D for Establishment and Utilization of a Technical Infrastructure for Japanese Industry), supported by the New Energy and Industrial Technology Development Organization.
REFERENCES
- 1.Andressen, C., K. Moertter, and J. K. Mai. 1996. Spatiotemporal expression of CD15 in the developing chick retina. Brain Res. Dev. Brain Res. 95:263-271. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, P. W., I. Damjanov, D. Simon, G. S. Banting, C. Carlin, N. C. Dracopoli, and J. Fogh. 1984. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab. Investig. 50:147-162. [PubMed] [Google Scholar]
- 3.Andrews, P. W., E. Nudelman, S. Hakomori, and B. A. Fenderson. 1990. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamethylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR). Differentiation 43:131-138. [DOI] [PubMed] [Google Scholar]
- 4.Baboval, T., T. Henion, E. Kinnally, and F. I. Smith. 2000. Molecular cloning of rat α1,3-fucosyltransferase IX (Fuc-TIX) and comparison of the expression of Fuc-TIV and Fuc-TIX genes during rat postnatal cerebellum development. J. Neurosci. Res. 62:206-215. [DOI] [PubMed] [Google Scholar]
- 5.Berg, E. L., J. Magnani, R. A. Warnock, M. K. Robinson, and E. C. Butcher. 1992. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Le(x) and sialyl Le(a). Biochem. Biophys. Res. Commun. 184:1048-1055. [DOI] [PubMed] [Google Scholar]
- 6.Bird, J. M., and S. J. Kimber. 1984. Oligosaccharides containing fucose linked α(1-3) and α(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev. Biol. 104:449-460. [DOI] [PubMed] [Google Scholar]
- 7.Bornslaeger, E. A., and R. M. Schultz. 1985. Adenylate cyclase activity in zona-free mouse oocytes. Exp. Cell Res. 156:277-281. [DOI] [PubMed] [Google Scholar]
- 8.Cailleau-Thomas, A., P. Coullin, J. J. Candelier, L. Balanzino, B. Mennesson, R. Oriol, and R. Mollicone. 2000. FUT4 and FUT9 genes are expressed early in human embryogenesis. Glycobiology 10:789-802. [DOI] [PubMed] [Google Scholar]
- 9.Costache, M., P. A. Apoil, A. Cailleau, A. Elmgren, G. Larson, S. Henry, A. Blancher, D. Iordachescu, R. Oriol, and R. Mollicone. 1997. Evolution of fucosyltransferase genes in vertebrates. J. Biol. Chem. 272:29721-29728. [DOI] [PubMed] [Google Scholar]
- 10.Eggens, I., B. Fenderson, T. Toyokuni, B. Dean, M. Stroud, and S. Hakomori. 1989. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J. Biol. Chem. 264:9476-9484. [PubMed] [Google Scholar]
- 11.Fenderson, B. A., E. M. Eddy, and S. Hakomori. 1990. Glycoconjugate expression during embryogenesis and its biological significance. Bioessays 12:173-179. [DOI] [PubMed] [Google Scholar]
- 12.Fenderson, B. A., U. Zehavi, and S. Hakomori. 1984. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J. Exp. Med. 160:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxall, C., S. R. Watson, D. Dowbenko, C. Fennie, L. A. Lasky, M. Kiso, A. Hasegawa, D. Asa, and B. K. Brandley. 1992. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J. Cell Biol. 117:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara, Y., T. Komiya, H. Kawabata, M. Sato, H. Fujimoto, M. Furusawa, and T. Noce. 1994. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila Vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. USA 91:12258-12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gersten, K. M., S. Natsuka, M. Trinchera, B. Petryniak, R. J. Kelly, N. Hiraiwa, N. A. Jenkins, D. J. Gilbert, N. G. Copeland, and J. B. Lowe. 1995. Molecular cloning, expression, chromosomal assignment, and tissue-specific expression of a murine α-(1,3)-fucosyltransferase locus corresponding to the human ELAM-1 ligand fucosyl transferase. J. Biol. Chem. 270:25047-25056. [DOI] [PubMed] [Google Scholar]
- 16.Goelz, S. E., C. Hession, D. Goff, B. Griffiths, R. Tizard, B. Newman, G. Chi-Rosso, and R. Lobb. 1990. ELFT: a gene that directs the expression of an ELAM-1 ligand. Cell 63:1349-1356. [DOI] [PubMed] [Google Scholar]
- 17.Gooi, H. C., T. Feizi, A. Kapadia, B. B. Knowles, D. Solter, and M. J. Evans. 1981. Stage-specific embryonic antigen involves α1 goes to 3 fucosylated type 2 blood group chains. Nature 292:156-158. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko, M., T. Kudo, H. Iwasaki, Y. Ikehara, S. Nishihara, S. Nakagawa, K. Sasaki, T. Shiina, H. Inoko, N. Saitou, and H. Narimatsu. 1999. α1,3-Fucosyltransferase IX (Fuc-TIX) is very highly conserved between human and mouse; molecular cloning, characterization and tissue distribution of human Fuc-TIX. FEBS Lett. 452:237-242. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko, M., T. Kudo, H. Iwasaki, T. Shiina, H. Inoko, T. Kozaki, N. Saitou, and H. Narimatsu. 1999. Assignment of the human α1,3-fucosyltransferase IX gene (FUT9) to chromosome band 6q16 by in situ hybridization. Cytogenet. Cell Genet. 86:329-330. [DOI] [PubMed] [Google Scholar]
- 20.Karagenc, L., Y. Cinnamon, M. Ginsburg, and J. N. Petitte. 1996. Origin of primordial germ cells in the prestreak chick embryo. Dev. Genet. 19:290-301. [DOI] [PubMed] [Google Scholar]
- 21.Knowles, B. B., D. P. Aden, and D. Solter. 1978. Monoclonal antibody detecting a stage-specific embryonic antigen (SSEA-1) on preimplantation mouse embryos and teratocarcinoma cells. Curr. Top. Microbiol. Immunol. 81:51-53. [DOI] [PubMed] [Google Scholar]
- 22.Kojima, N., B. A. Fenderson, M. R. Stroud, R. I. Goldberg, R. Habermann, T. Toyokuni, and S. Hakomori. 1994. Further studies on cell adhesion based on Le(x)-Le(x) interaction, with new approaches: embryoglycan aggregation of F9 teratocarcinoma cells, and adhesion of various tumour cells based on Le(x) expression. Glycoconj. J. 11:238-248. [DOI] [PubMed] [Google Scholar]
- 23.Koszdin, K. L., and B. R. Bowen. 1992. The cloning and expression of a human α-1,3 fucosyltransferase capable of forming the E-selectin ligand. Biochem. Biophys. Res. Commun. 187:152-157. [DOI] [PubMed] [Google Scholar]
- 24.Kudo, T., Y. Ikehara, A. Togayachi, M. Kaneko, T. Hiraga, K. Sasaki, and H. Narimatsu. 1998. Expression cloning and characterization of a novel murine α1,3-fucosyltransferase, mFuc-TIX, that synthesizes the Lewis x (CD15) epitope in brain and kidney. J. Biol. Chem. 273:26729-26738. [DOI] [PubMed] [Google Scholar]
- 25.Kukowska-Latallo, J. F., R. D. Larsen, R. P. Nair, and J. B. Lowe. 1990. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group α(1,3/1,4)fucosyltransferase. Genes Dev. 4:1288-1303. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, R., B. Potvin, W. A. Muller, and P. Stanley. 1991. Cloning of a human α(1,3)-fucosyltransferase gene that encodes ELFT but does not confer ELAM-1 recognition on Chinese hamster ovary cell transfectants. J. Biol. Chem. 266:21777-21783. [PubMed] [Google Scholar]
- 27.Liu, N., C. Jin, Z. M. Zhu, J. Zhang, H. Tao, C. Ge, S. Yang, and S. Zhang. 1999. Stage-specific expression of α1,2-fucosyltransferase and α1,3-fucosyltransferase (FT) during mouse embryogenesis. Eur. J. Biochem. 265:258-263. [DOI] [PubMed] [Google Scholar]
- 28.Lowe, J. B., L. M. Stoolman, R. P. Nair, R. D. Larsen, T. L. Berhend, and R. M. Marks. 1990. ELAM-1-dependent cell adhesion to vascular endothelium determined by a transfected human fucosyltransferase cDNA. Cell 63:475-484. [DOI] [PubMed] [Google Scholar]
- 29.Marani, E., J. W. van Oers, P. A. Tetteroo, R. E. Poelmann, J. van der Veeken, and M. G. Deenen. 1986. Stage specific embryonic carbohydrate surface antigens of primordial germ cells in mouse embryos: FAL (S.S.E.A.-1) and globoside (S.S.E.A.-3). Acta Morphol. Neerl. Scand. 24:103-110. [PubMed] [Google Scholar]
- 30.McCurley, R. S., A. Recinos III, A. S. Olsen, J. C. Gingrich, D. Szczepaniak, H. S. Cameron, R. Krauss, and B. W. Weston. 1995. Physical maps of human α(1,3)fucosyltransferase genes FUT3-FUT6 on chromosomes 19p13.3 and 11q21. Genomics 26:142-146. [DOI] [PubMed] [Google Scholar]
- 31.Mebius, R. E., and S. R. Watson. 1993. L- and E-selectin can recognize the same naturally occurring ligands on high endothelial venules. J. Immunol. 151:3252-3260. [PubMed] [Google Scholar]
- 32.Mock, B. A., and M. C. Hirano. 1998. Encyclopedia of the mouse genome VII. Mouse chromosome 4. Mamm. Genome 8:S68-S90. [DOI] [PubMed] [Google Scholar]
- 33.Natsuka, S., K. M. Gersten, K. Zenita, R. Kannagi, and J. B. Lowe. 1994. Molecular cloning of a cDNA encoding a novel human leukocyte α-1,3-fucosyltransferase capable of synthesizing the sialyl Lewis x determinant. J. Biol. Chem. 269:16789-16794. [PubMed] [Google Scholar]
- 34.Nishihara, S., H. Iwasaki, M. Kaneko, A. Tawada, M. Ito, and H. Narimatsu. 1999. α1,3-Fucosyltransferase 9 (FUT9; Fuc-TIX) preferentially fucosylates the distal GlcNAc residue of polylactosamine chain while the other four α1,3FUT members preferentially fucosylate the inner GlcNAc residue. FEBS Lett. 462:289-294. [DOI] [PubMed] [Google Scholar]
- 35.Nishihara, S., H. Iwasaki, K. Nakajima, A. Togayachi, Y. Ikehara, T. Kudo, Y. Kushi, A. Furuya, K. Shitara, and H. Narimatsu. 2003. α1,3-Fucosyltransferase IX (Fut9) determines Lewis X expression in brain. Glycobiology 13:445-455. [DOI] [PubMed] [Google Scholar]
- 36.Nishihara, S., M. Nakazato, T. Kudo, H. Kimura, T. Ando, and H. Narimatsu. 1993. Human α-1,3 fucosyltransferase (FucT-VI) gene is located at only 13 kb 3′ to the Lewis type fucosyltransferase (FucT-III) gene on chromosome 19. Biochem. Biophys. Res. Commun. 190:42-46. [DOI] [PubMed] [Google Scholar]
- 37.Osanai, T., W. Chai, Y. Tajima, Y. Shimoda, Y. Sanai, and C. T. Yuen. 2001. Expression of glycoconjugates bearing the Lewis X epitope during neural differentiation of P19 EC cells. FEBS Lett. 488:23-28. [DOI] [PubMed] [Google Scholar]
- 38.Patnaik, S. K., A. Zhang, S. Shi, and P. Stanley. 2000. α(1,3)Fucosyltransferases expressed by the gain-of-function Chinese hamster ovary glycosylation mutants LEC12, LEC29, and LEC30. Arch. Biochem. Biophys. 375:322-332. [DOI] [PubMed] [Google Scholar]
- 39.Reguigne, I., M. R. James, C. W. Richard III, R. Mollicone, A. Seawright, J. B. Lowe, R. Oriol, and P. Couillin. 1994. The gene encoding myeloid α-3-fucosyl-transferase (FUT4) is located between D11S388 and D11S919 on 11q21. Cytogenet. Cell Genet. 66:104-106. [DOI] [PubMed] [Google Scholar]
- 40.Reguigne-Arnould, I., J. Wolfe, N. Hornigold, S. Faure, R. Mollicone, R. Oriol, and P. Coullin. 1996. Fucosyltransferase genes are dispersed in the genome: FUT7 is located on 9q34.3 distal to D9S1830. C. R. Acad. Sci. Ser. III 319:783-788. [PubMed] [Google Scholar]
- 41.Rowe, L. B., J. H. Nadeau, R. Turner, W. N. Frankel, V. A. Letts, J. T. Eppig, M. S. Ko, S. J. Thurston, and E. H. Birkenmeier. 1994. Maps from two interspecific backcross DNA panels available as a community genetic mapping resource. Mamm. Genome 5:253-274. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki, K., K. Kurata, K. Funayama, M. Nagata, E. Watanabe, S. Ohta, N. Hanai, and T. Nishi. 1994. Expression cloning of a novel α1,3-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis x carbohydrate determinants in leukocytes. J. Biol. Chem. 269:14730-14737. [PubMed] [Google Scholar]
- 43.Schultz, R. M. 1993. Regulation of zygotic gene activation in the mouse. Bioessays 15:531-538. [DOI] [PubMed] [Google Scholar]
- 44.Solter, D., and B. B. Knowles. 1978. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75:5565-5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi, Y., N. C. Talbot, C. E. Rexroad, Jr., and V. G. Pursel. 1997. Identification of pig primordial germ cells by immunocytochemistry and lectin binding. Mol. Reprod. Dev. 46:567-580. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, S. S., Y. Toyooka, R. Akasu, Y. Katoh-Fukui, Y. Nakahara, R. Suzuki, M. Yokoyama, and T. Noce. 2000. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 14:841-853. [PMC free article] [PubMed] [Google Scholar]
- 47.Toivonen, S., S. Nishihara, H. Narimatsu, O. Renkonen, and R. Renkonen. 2002. Fuc-TIX: a versatile α1,3-fucosyltransferase with a distinct acceptor- and site-specificity profile. Glycobiology 12:361-368. [DOI] [PubMed] [Google Scholar]
- 48.Weston, B. W., R. P. Nair, R. D. Larsen, and J. B. Lowe. 1992. Isolation of a novel human α(1,3)fucosyltransferase gene and molecular comparison to the human Lewis blood group α(1,3/1,4)fucosyltransferase gene. Syntenic, homologous, nonallelic genes encoding enzymes with distinct acceptor substrate specificities. J. Biol. Chem. 267:4152-4160. [PubMed] [Google Scholar]
- 49.Weston, B. W., P. L. Smith, R. J. Kelly, and J. B. Lowe. 1992. Molecular cloning of a fourth member of a human α(1,3)fucosyltransferase gene family. Multiple homologous sequences that determine expression of the Lewis x, sialyl Lewis x, and difucosyl sialyl Lewis x epitopes. J. Biol. Chem. 267:24575-24584. [PubMed] [Google Scholar]