Abstract
Dvl is a key protein that transmits the Wnt signal to the canonical β-catenin pathway and the noncanonical planar cell polarity (PCP) pathway. We studied the roles of Rho-associated kinase (Rho-kinase), which is activated by Dvl in the PCP pathway of mammalian cells. The expression of Dvl-1, Wnt-1, or Wnt-3a activated Rho-kinase in COS cells, and this activation was inhibited by the Rho-binding domain of Rho-kinase. The expression of Dvl-1 in PC12 cells activated Rho and inhibited nerve growth factor (NGF)-induced neurite outgrowth. This inhibition was reversed by a Rho-kinase inhibitor but not by a c-Jun N-terminal kinase inhibitor. Dvl-1 also inhibited serum starvation-dependent neurite outgrowth of N1E-115 cells, and expression of the Rho-binding domain of Rho-kinase reversed this inhibitory activity of Dvl-1. Dvl-1 mutants that did not activate Rho-kinase did not inhibit the neurite outgrowth of N1E-115 cells. Furthermore, the purified Wnt-3a protein activated Rho-kinase and inhibited the NGF-dependent neurite outgrowth of PC12 cells. Wnt-3a-dependent neurite retraction was also prevented by a Rho-kinase inhibitor and a Dvl-1 mutant that suppresses Wnt-3a-dependent activation of Rho-kinase. These results suggest that Wnt-3a and Dvl regulate neurite formation through Rho-kinase and that PC12 and N1E-115 cells are useful for analyzing the PCP pathway.
Wnt proteins constitute a large family of cysteine-rich secreted ligands that control development in organisms ranging from nematode worms to mammals (59). Wnt regulates axis formation, organ development, and cellular proliferation, morphology, motility, and fate (40, 46). Binding of the Wnt ligand to its receptors can stimulate several distinct intracellular signaling pathways, including the canonical β-catenin and noncanonical planar cell polarity-convergent extension (PCP-CE) pathways. For the activation of these pathways, the common mediator Dvl, which transmits signals from receptors to different effector molecules, is required.
Dvl is a cytoplasmic protein that acts downstream of Frizzled (Fz) and is a key protein for regulation of the Wnt signal (56). Three Dvl genes, Dvl-1, -2, and -3, have been isolated from mammals. Dvl homologs are conserved in Drosophila melanogaster (Dishevelled [Dsh]) and Xenopus laevis (Xenopus dishevelled [Xdsh]). All Dvl and Dsh family members contain three highly conserved domains: a DIX domain, a PDZ domain, and a DEP domain. The expression of Dvl in cells induces the accumulation of β-catenin in the canonical pathway and the activation of Rho and Rac in the PCP-CE pathway (7, 16, 17, 29, 41, 46, 49). The DIX and PDZ domains are important for the activation of the canonical β-catenin pathway, whereas the DEP domain is essential for the activation of the noncanonical PCP-CE pathway.
In the canonical pathway, the protein level of free cytoplasmic β-catenin is controlled by the Wnt signal (40, 46, 59). Cytoplasmic β-catenin is destabilized by a multiprotein complex containing Axin (or its homolog Axil/conductin), glycogen synthase kinase 3β (GSK-3β), casein kinase Iα (CKIα), and adenomatous polyposis coli in unstimulated cells (22, 27, 30, 36, 61). β-Catenin is phosphorylated efficiently by CKIα and GSK-3β in this complex, and phosphorylated β-catenin is ubiquitinated and degraded by the proteasome (1, 31). When Wnt binds to its cell surface receptor, consisting of Frizzled and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), Dvl and CKIɛ antagonize GSK-3β-dependent phosphorylation of β-catenin (21). Once the phosphorylation of β-catenin is reduced, β-catenin is no longer degraded, resulting in its accumulation in the cytoplasm. Stabilized β-catenin is translocated into the nucleus, where it binds to transcriptional factors T-cell factor (Tcf) and lymphoid enhancer binding factor (Lef) and thereby stimulates the transcription of Wnt target genes (5, 59).
PCP is manifested in Drosophila wing, eye, and sensory bristle development (2, 48). For example, each wing cell exhibits proximal-distal polarity within the epithelial plane by elaborating a single hair at the distal vertex. Rho and the Rho-associated kinase (Drosophila Rho-kinase, or Drok) represent core PCP gene products that can act downstream of Drosophila Fz1 (Dfz1) and Dsh (50, 58). Drok mutant cells exhibit changes of photoreceptor numbers in and misrotation of ommatidia. This phenotype resembles those of PCP mutants such as fz, dsh, and rho. Other gene products implicated downstream of Dfz1 and Dsh in the PCP signaling pathway include Rac and c-Jun N-terminal kinase (JNK) (7, 50). However, a triple mutation removing the three known Drosophila rac genes does not show the PCP phenotype (18). In zebra fish and Xenopus, Wnt-11 regulates CE movement through Fz and Dvl, but not β-catenin (20, 51). Wnt-1 and Wnt-11 activate Rho and Rac through Dvl separately during gastrulation (16). Furthermore, Wnt-5a is capable of activating JNK through Rac, which regulates CE movement (62). Thus, Dvl-dependent Rho and Rac activation is important for the PCP-CE pathway in Drosophila, zebra fish, and Xenopus. However, which cellular functions are regulated by this pathway in mammals is not well understood.
Rho, Rac, and CDC42 are members of the Rho subfamily of the small-GTP-binding protein superfamily (13, 25, 42). It has been clearly demonstrated that Rho induces the assembly of contractile actin and myosin filaments (stress fibers), that Rac induces actin-rich surface protrusions (lamellipodia), and that Cdc42 promotes the formation of actin-rich, finger-like membrane extensions (filopodia). Therefore, Rho, Rac, and CDC42 regulate three distinct signal transduction pathways linking plasma membrane receptors to the assembly of actin filaments. In addition to regulating the actin cytoskeleton, they participate in the regulation of cell polarity, gene expression, G1 cell cycle progression, microtubule dynamics, and vesicle transport.
Neurons extend neurites, one of which differentiates into an axon while the others become dendrites. Rac and Cdc42 are positive regulators of neurite outgrowth, whereas Rho inhibits neurite extension (24, 38, 45, 60). It has been shown that Dvl-1 colocalizes with axonal microtubules (53) and that it regulates microtubule stability through GSK-3 (19, 34). However, the roles of Dvl-dependent activation of Rho subfamily small G proteins in neurite outgrowth have been elusive. Furthermore, the Dvl-dependent activation of Rho-kinase through Rho has not yet been studied intensively in mammalian cells, although the Dvl-dependent activation of JNK through Rac has been demonstrated (7, 41). These considerations prompted us to examine whether Dvl regulates neurite outgrowth through its downstream molecules, including small G proteins, Rho-kinase, and JNK. In this study, we demonstrate that Wnt-3a and Dvl induce neurite retraction through Rho-kinase in PC12 and N1E-115 cells.
MATERIALS AND METHODS
Materials and chemicals.
pEGFP-myosin-binding subunit (MBS) of myosin phosphatase (pEGFP/MBS), pEF-BOS-Myc/Rho-kinase, pEF-BOS-Myc/Rho-kinase catalytic domain (CAT), pEF-BOS-Myc/Rho-kinase Rho-binding domain (RB), pEF-BOS-HA-RhoG14V, and a rabbit polyclonal antibody against MBS phosphorylated at Ser-854 (pS854) were kindly supplied by Kozo Kaibuchi (Nagoya University, Nagoya, Japan). pGEX-4T/Rho-binding domain of rhotekin (RBD) was kindly provided by Manabu Negishi (Kyoto University, Kyoto, Japan). pGK-Wnt-1-Flag and pGKneoWnt-3a were provided by Shinji Takada (Center for Integrated Bioscience, Okazaki, Japan). PC12 cells stably expressing mouse Wnt-1 (PC12/Wnt-1) and PC12 cells stably expressing tTA (PC12/tTA) (tet-off system) were provided by Ming-Gui Pan (Oregon Health Science Center, Portland, Oreg.) and Hidenori Ichijo (University of Tokyo, Tokyo, Japan), respectively. PC12 cells conditionally expressing Myc-Dvl-1 in response to tetracycline hydrochloride (PC12/Dvl) were generated by selection with G418 as described previously (52). PC12/tTA cells were designated control PC12 cells for this study. An anti-Wnt-3a antibody was prepared in rabbits by immunization with a synthetic peptide corresponding to residues 139 to 155 (SSRLQGSPGEGWKWGGC) of mouse Wnt-3a. An anti-Dvl antibody was prepared as described previously (29). An anti-Myc antibody was prepared from 9E10 cells. Glutathione S-transferase (GST) and GST-RBD were purified from Escherichia coli. Alexa 488-labeled anti-rabbit immunoglobulin G (IgG), Alexa 546-labeled anti-mouse IgG, and anti-green fluorescent protein (GFP) antibody were from Molecular Probes, Inc. (Eugene, Oreg.). Anti-RhoA antibody was from Transduction Laboratories (Lexington, Ky.). Rho-kinase inhibitor (Y-27632) and JNK inhibitor I were from Calbiochem (San Diego, Calif.), GSK-3 inhibitor (SB-216763) was from TOCRIS (Aromouth, United Kingdom), and nerve growth factor (NGF) 7.5S and pTET-splice were from Invitrogen, Inc. Other materials were obtained from commercial sources.
Plasmid construction.
pCGN/hDvl-1, pEF-BOS-HA/hDvl-1-(1-250), pCGN/hDvl-1-(1-398), pCGN/hDvl-1-(140-670), pCGN/hDvl-1-(337-670), pCGN/hDvl-1-(395-670), and pCGN/hDvl-1-(Δ251-336) were constructed as described previously (21, 28, 29). A cDNA encoding Myc-tagged Dvl-1 with EcoRI and SpeI sites was inserted into pTET-splice to generate pTET-splice/Myc-Dvl-1. For the generation of pCGN/hDvl-1-(224-398), a cDNA encoding hDvl-1-(224-398) with XbaI and SmaI sites was inserted into pCGN. For the construction of a recombinant adenovirus expressing GFP-MBS, a cDNA encoding GFP-MBS was amplified by PCR. Then the fragment was subcloned into pENTR/D-TOPO and transferred into pAD/CMV/V5-DEST (Invitrogen). 293A cells (Invitrogen) were transfected with the PacI-digested pAD/CMV/V5-DEST-derived construct, and adenoviral stocks were prepared according to the manufacturer's instructions.
Cell culture.
COS, N1E-115, and 293A cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum at 37°C. PC12/Wnt-1 cells were grown in DMEM supplemented with 10% fetal calf serum and 5% horse serum at 37°C. Control PC12 and PC12/Dvl cells were grown in the same medium supplemented with 500 ng of tetracycline hydrochloride/ml. For the expression of Myc-Dvl-1, PC12/Dvl cells were cultured without tetracycline hydrochloride for 48 h.
Rho activity assay.
PC12 cells (two subconfluent 100-mm-diameter dishes) were lysed with 500 μl of ice-cold buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 30 mM MgCl2, 0.1% Triton X-100, 10% glycerol, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, and 1 μg of aprotinin/ml) containing 40 μg of GST or GST-RBD (60). Cell lysates (0.5 mg of protein) were centrifuged for 10 min at 20,000 × g at 4°C, and the supernatants were incubated with glutathione-Sepharose for 2 h at 4°C. After the glutathione-Sepharose was precipitated by centrifugation, the bound proteins were probed with an anti-RhoA antibody.
Rho-kinase assay.
To examine whether Dvl-1 activates Rho-kinase in intact cells, we transfected subconfluent COS cells (60-mm-diameter dishes) with pCGN/hDvl-1, pEGFP-MBS, and pEF-BOS-Myc/Rho-kinase. After 12 h, the cells were starved of serum for 48 h and treated with 10% (wt/vol) trichloroacetic acid (TCA) and 2 mM dithiothreitol. The resulting precipitates were washed with ice-cold acetone and 2 mM dithiothreitol three times and were probed with anti-GFP, anti-Myc, anti-hemagglutinin 1 (HA), and anti-phospho-MBS antibodies. Where indicated, pCGN-derived constructs expressing Dvl-1 deletion mutants or pEF-BOS-HA/RhoAG14V was transfected into the cells instead of pCGN/Dvl-1. When the activity of endogenous Rho-kinase in PC12 cells was assayed, GFP-MBS was expressed by an adenovirus. After the cells had been starved of serum for 48 h and then stimulated with 160 ng of Wnt-3a purified from conditioned medium/ml, the TCA precipitates were probed with anti-GFP and anti-phospho-MBS antibodies.
Neurite formation assay.
N1E-115 cells and PC12 cells were seeded onto 18-mm-wide glass coverslips coated with poly-d-lysine (Sigma, St. Louis, Mo.). For the induction of neurite outgrowth, PC12 cells were cultured with 100 ng of NGF/ml in DMEM containing 1% fetal calf serum and 0.5% horse serum for 48 h, and N1E-115 cells were starved of serum for 48 h. Where indicated, 160 ng of Wnt-3a/ml was added to PC12 cells in the presence of 100 ng of NGF/ml. The cells were observed with the phase-contrast or relief-contrast mode of an IX-70 microscope (Olympus, Tokyo, Japan) and were photographed with a DC-250 digital camera system (Leica Microsystems AG, Wetzler, Germany). Neurite initiation from PC12 and N1E-115 cells was scored by measuring the percentage of cells bearing processes of two or more cell diameters long. More than 100 cells were evaluated for each experiment.
Immunocytochemistry.
Cells grown on glass coverslips were fixed for 15 min in phosphate-buffered saline (PBS) containing 4% (wt/vol) paraformaldehyde. The cells were washed with PBS three times and then permeabilized with PBS containing 0.2% (wt/vol) Triton X-100 and 2 mg of bovine serum albumin/ml for 20 min. The cells were washed with PBS three times and incubated with an anti-HA, anti-Dvl, or anti-Myc antibody for 1 h. After being washed with PBS, they were further incubated with Alexa-546-labeled anti-mouse IgG or Alexa-488-labeled-anti-rabbit IgG for 1 h. For visualization of F-actin, the cells were incubated with fluorescein isothiocyanate-phalloidin. The coverslips were washed, mounted on glass slides, viewed with an IX-70 microscope, and photographed with a DC-250 digital camera system. All procedures were performed at room temperature.
Purification of Wnt-3a protein.
Wnt-3a was purified to near homogeneity as described previously (57), with a slight modification. One hundred milliliters of Wnt-3a-conditioned medium was adjusted to 1% Triton X-100 and applied to a Blue Sepharose HP column (1.6 by 2.5 cm) (Amersham Biosciences, Buckinghamshire, United Kingdom) equilibrated with binding buffer (20 mM Tris-HCl [pH 7.5] and 1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonic acid [CHAPS]) containing 150 mM KCl. After the column was washed with 50 ml of binding buffer, the elution was performed in a stepwise manner with 50 ml of binding buffer containing 1.5 M KCl at a flow rate of 1 ml/min. Fractions of 5 ml were collected. When an aliquot (20 μl) of each fraction was probed with the anti-Wnt-3a antibody, a single peak was seen with fractions 2 and 3. The same procedure was repeated two times. The active fractions from the Blue Sepharose column chromatography (30 ml, with 2 mg of protein) were pooled and concentrated to 2 ml by use of a Microsep (30K) ultrafiltration device (Pall Life Sciences, Ann Arbor, Mich.). The concentrate (2 ml, with 1.7 mg of protein) was applied to a HiLoad Superdex 200 column (1.6 by 60 cm) (Amersham Biosciences) equilibrated with PBS and 1% CHAPS. Elution was performed with the same buffer at a flow rate of 1 ml/min. Fractions of 1 ml were collected. When an aliquot (20 μl) of each fraction was probed with the anti-Wnt-3a antibody, a single broad peak was seen with fractions 73 to 83. The active fractions from the HiLoad Superdex 200 column chromatography (11 ml, with 0.2 mg of protein) were applied to a HiTrap Heparin column (0.75 by 2.5 cm) (Amersham Biosciences) equilibrated with PBS and 1% CHAPS. After the column was washed with 10 ml of the same buffer, elution was performed with a 10-ml linear gradient of NaCl (0 to 1 M) in PBS and 1% CHAPS at a flow rate of 0.5 ml/min. When an aliquot (20 μl) of each fraction was probed with the anti-Wnt-3a antibody, a single broad peak was seen with fractions 6 to 15. Wnt-3a in fractions 13 to 15 was nearly homogeneous, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The active fractions (1.5 ml, with 15 μg of protein) were collected and used for experiments.
Other.
Protein concentrations were determined with bovine serum albumin as a standard (8).
RESULTS
Activation of Rho-kinase by Dvl in mammalian cells.
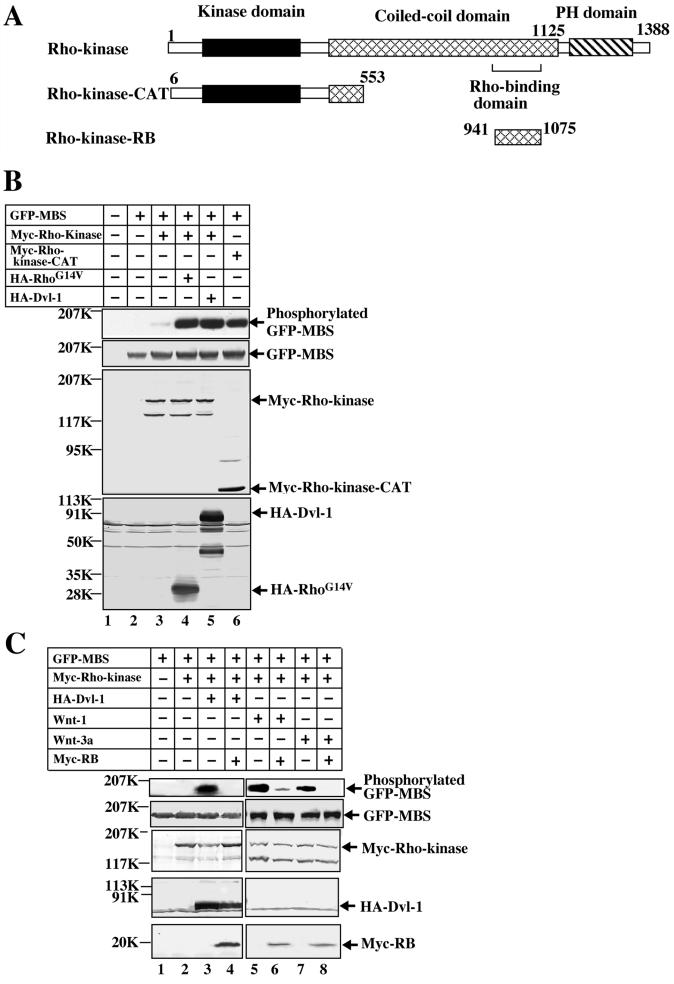
It has been demonstrated that Rho-kinase functions downstream of Dsh in Drosophila (58). Although Dvl has been shown to activate Rho in mammalian cells (17), whether Dvl activates Rho-kinase through Rho has not yet been analyzed systematically. A previous study demonstrated that Rho-kinase phosphorylates the MBS of myosin phosphatase and that Rho-kinase activation in intact cells can be assessed by using an anti-phospho-MBS antibody (26). Using this assay, we first examined whether Wnt and Dvl indeed activate Rho-kinase through Rho in mammalian cells. The expression of HA-Dvl-1 in COS cells induced the phosphorylation of GFP-MBS in the presence of Myc-Rho-kinase under conditions in which a constitutively active form of Rho (RhoG14V) activated Rho-kinase (Fig. 1B). The Dvl-1-dependent Rho-kinase activity displayed the same ability as a constitutively active form of Rho-kinase (Myc-Rho-kinase-CAT) to phosphorylate MBS (Fig. 1B). RB is known to inhibit Rho-dependent Rho-kinase activation (3). The expression of Myc-RB suppressed Dvl-dependent Rho-kinase activation (Fig. 1C). Wnt-1 and Wnt-3a have been shown to activate Rho (17). Wnt-1 and Wnt-3a also activated Rho-kinase, and the activation was suppressed by the expression of RB (Fig. 1C). These results demonstrate that Dvl-1, Wnt-1, and Wnt-3a activate Rho-kinase through Rho.
FIG. 1.
Activation of Rho-kinase by Wnt and Dvl. (A) Schematic representation of bovine Rho-kinase mutants used in this study. (B) COS cells expressing GFP-MBS (lane 2); GFP-MBS and Myc-Rho-kinase (lane 3); GFP-MBS, Myc-Rho-kinase, and HA-RhoG14V (lane 4); GFP-MBS, Myc-Rho-kinase, and HA-Dvl-1 (lane 5); and GFP-MBS and Rho-kinase-CAT (lane 6) were treated with TCA. The TCA precipitates were probed with anti-phospho-MBS, anti-GFP, anti-Myc, and anti-HA antibodies. COS cells transfected with empty vectors were used as a control (lane 1). (C) GFP-MBS and Myc-Rho-kinase were expressed in COS cells with HA-Dvl-1 (lanes 3 and 4), Wnt-1-FLAG (lanes 5 and 6), or Wnt-3a (lanes 7 and 8). Where indicated, the plasmid expressing Myc-RB was also transfected (lanes 4, 6, and 8). The cells were treated with TCA and the precipitates were probed with anti-phospho-MBS, anti-GFP, anti-Myc, and anti-HA antibodies. COS cells expressing GFP-MBS and/or Myc-Rho-kinase (lanes 1 and 2) were used as controls. The results shown are representative of three independent experiments.
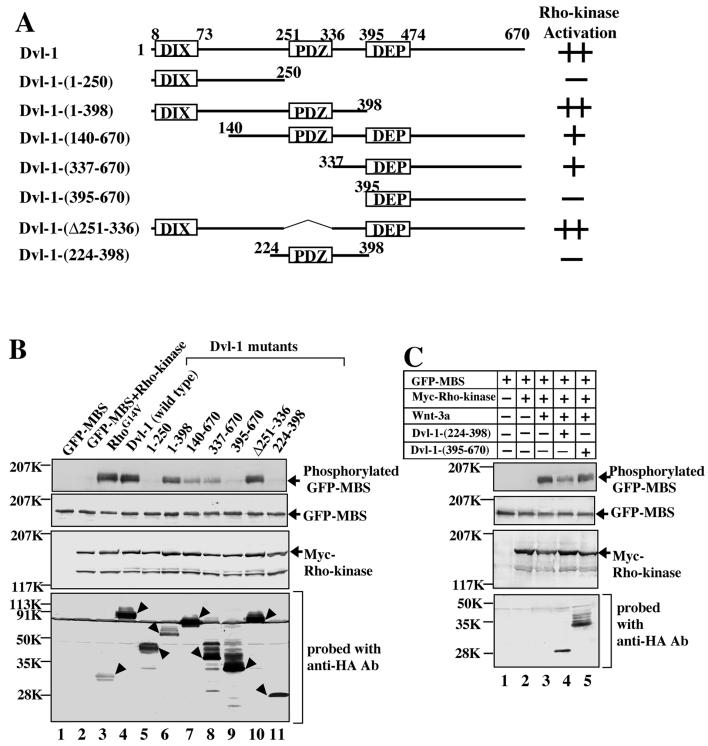
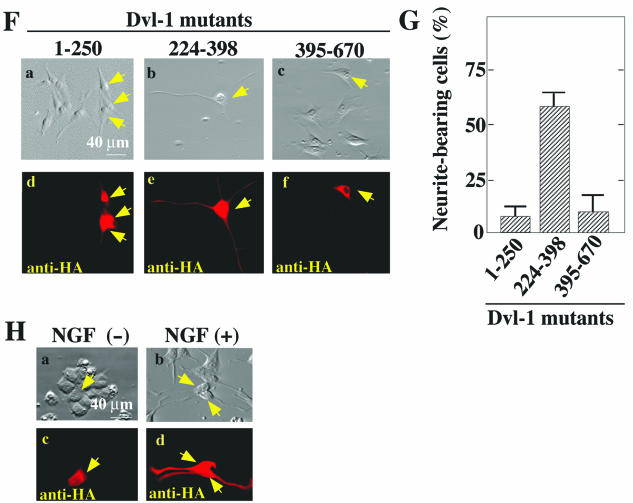
To determine which region of Dvl-1 is important for the activation of Rho-kinase, we used the expression of various deletion mutants of HA-Dvl-1 with GFP-MBS and Myc-Rho-kinase. HA-Dvl-1-(1-398) and HA-Dvl-1-(Δ251-336) activated Rho-kinase to a similar extent as wild-type HA-Dvl-1 (Fig. 2B, lanes 4, 6, and 10). HA-Dvl-1-(140-670) and HA-Dvl-1-(337-670) activated Rho-kinase to a lesser extent than wild-type HA-Dvl-1 (Fig. 2B, lanes 7 and 8), whereas HA-Dvl-1-(1-250) and HA-Dvl-1-(395-670) did not activate it (Fig. 2B, lanes 5 and 9). These results suggest that amino acid residues 337 to 394 of Dvl-1 are necessary for the activation of Rho-kinase, whereas neither the DIX, PDZ, nor DEP domain is essential. However, Dvl-1-(224-398) did not activate Rho-kinase (Fig. 2B, lane 11). Although this Dvl-1 mutant inhibited Wnt-3a-dependent Rho-kinase activation, Dvl-1-(395-670) did not (Fig. 2C, lanes 3 to 5). Taken together with previous observations regarding the activation of Rho by Dvl (17), these results suggest that the region containing the PDZ and DEP domains and residues 337 to 394, flanked by these two domains, are important for the activation of Rho-kinase by Dvl. A summary of these results is shown in Fig. 2A.
FIG. 2.
Activation of Rho-kinase by deletion mutants of Dvl. (A) Schematic representation of human Dvl-1 mutants used in this study. (B) GFP-MBS and Myc-Rho-kinase were expressed in COS cells with HA-RhoG14V (lane 3) or various deletion mutants of HA-Dvl-1 (lanes 4 to 11). The cells were treated with TCA and the precipitates were probed with anti-phospho-MBS, anti-GFP, anti-Myc, and anti-HA antibodies. COS cells expressing GFP-MBS and/or Myc-Rho-kinase (lanes 1 and 2) were used as controls. Arrowheads indicate the expression of RhoG14V and the Dvl deletion mutants. (C) Dominant-negative effect of Dvl-1-(224-398) on the activation of Rho-kinase. GFP-MBS and Myc-Rho-kinase were expressed in COS cells with Wnt-3a (lanes 3 to 5) and the indicated HA-Dvl-1 mutants (lanes 4 and 5). The cells were treated with TCA and the precipitates were probed with anti-phospho-MBS, anti-GFP, anti-Myc, and anti-HA antibodies. The results shown are representative of three independent experiments.
Inhibition of NGF-induced neurite outgrowth by Dvl in PC12 cells.
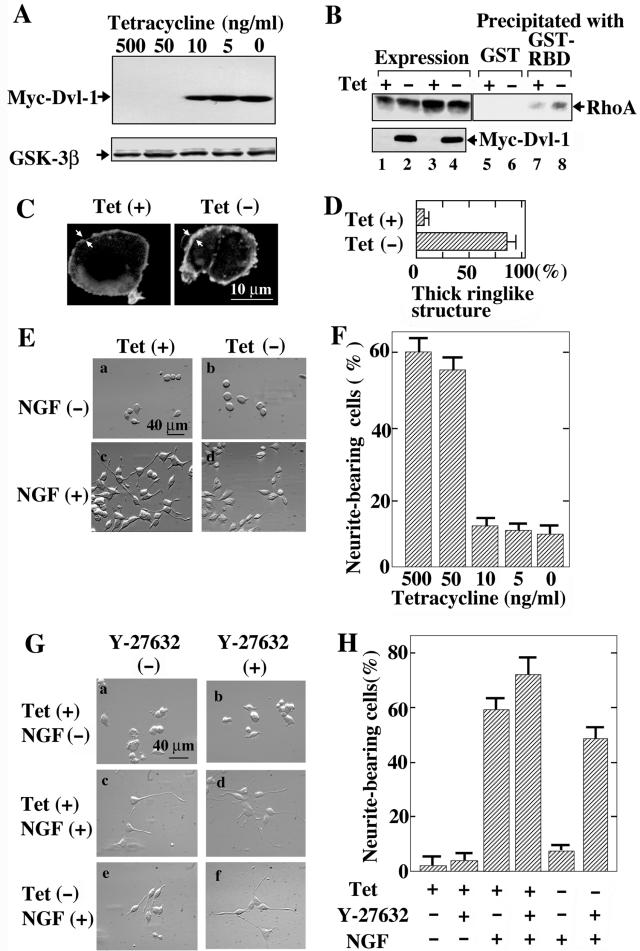
To examine the roles of Dvl in neural cells, we established PC12 cells expressing Myc-Dvl-1 (PC12/Dvl) in which the expression of Myc-Dvl-1 was under the control of a tetracycline-responsive promoter. After 2 days of incubation in a culture medium containing <10 ng of tetracycline hydrochloride/ml, expression of Myc-Dvl-1 was clearly observed (Fig. 3A). Under the same conditions, the protein levels of endogenous GSK-3β were not changed (Fig. 3A). We examined whether Dvl activates Rho and Rho-kinase in PC12 cells. The GTP-bound active form of endogenous Rho was detected by using the Rho-binding domain of rhotekin without the expression of Myc-Dvl-1 (Fig. 3B). The expression of Myc-Dvl-1 by the removal of tetracycline increased the amount of the GTP-bound form of Rho (Fig. 3B). It has been shown that NGF induces the accumulation of F-actin at protrusion sites, probably through the activation of Rac and the inhibition of Rho (24, 45), and that the activation of Rho inhibits the NGF-induced recruitment of Rac to the protrusion sites and the resultant process of neurite formation (9, 60). The expression of Myc-Dvl-1 induced the formation of a thick ringlike structure of cortical actin filaments at the cell periphery (Fig. 3C and D), which is similar to the reported effect of expression of the active form of RhoA (60). These results indicate that Dvl activates Rho in PC12 cells.
FIG. 3.
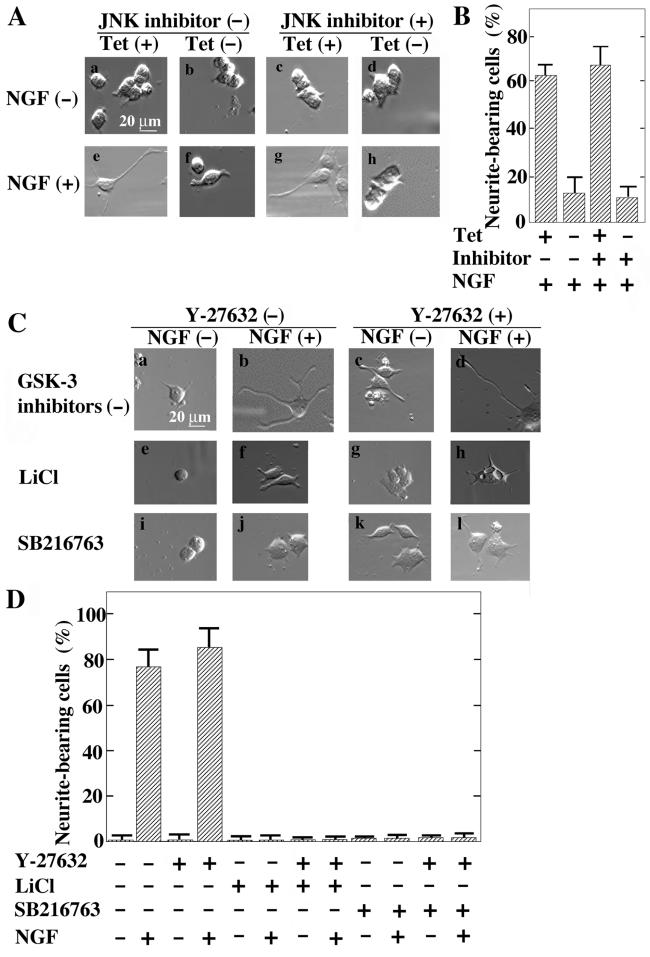
Dvl-dependent neurite retraction in PC12 cells. (A) Tetracycline-regulated Dvl-1 expression. PC12/Dvl cells were treated with various concentrations of tetracycline hydrochloride for 48 h, and the lysates were probed with anti-Myc and anti-GSK-3β antibodies. (B) Activation of Rho by Dvl-1 in PC12 cells. After PC12/Dvl cells were cultured in the presence (Tet +) (lanes 1, 3, 5, and 7) or absence (Tet −) (lanes 2, 4, 6, and 8) of 500 ng of tetracycline hydrochloride/ml for 48 h, the lysates were incubated with GST (lanes 5 and 6) or GST-RBD (lanes 7 and 8) and precipitated with glutathione-Sepharose. The bound proteins were probed with the anti-RhoA antibody. Aliquots of the lysates were probed withanti-RhoA and anti-Myc antibodies to show the expression levels of RhoA and Myc-Dvl-1 (lanes 1 to 4). (C) Effects of Dvl on cortical actin formation in PC12 cells. After PC12/Dvl cells were incubated with or without 500 ng of tetracycline hydrochloride/ml for 48 h, F-actin was stained with fluorescein isothiocyanate-labeled phalloidin. The arrows indicate the thickness of the ringlike structure of cortical actin filaments. (D) When the thickness of cortical actin filaments was >1 μm, the cells were counted as thick-ringlike-structure-bearing cells. The results shown are percentages of thick-ringlike-structure-bearing cells and are means ± standard errors (SE) of three independent experiments. (E) Tetracycline-regulated neurite retraction. PC12/Dvl cells were cultured in the presence [Tet (+)] (a and c) or absence [Tet (-)] (b and d) of tetracycline hydrochloride for 48 h and were further incubated with (c and d) or without (a and b) 100 ng of NGF/ml for 48 h. (F) Frequency of neurite formation in PC12/Dvl cells. After treatments with various doses of tetracycline hydrochloride and NGF, the numbers of neurite-bearing cells (as shown in panel E) were quantified. The results shown are means ± SE of three independent experiments. (G) Reversibility of Dvl-1-dependent neurite retraction by Rho-kinase inhibitor. PC12/Dvl cells cultured in the presence (a to d) or absence (e and f) of tetracycline hydrochloride for 48 h were further incubated in the presence (c to f) or absence (a and b) of NGF. Some cells (b, d, and f) were treated with 10 μM Y-27632. (H) The numbers of neurite-bearing cells shown in panel G were quantified. The results shown are means ± SE of three independent experiments.
We next examined whether Dvl-1 affects the neurite outgrowth of PC12 cells. NGF induced the outgrowth of neurites in the presence of tetracycline, whereas the removal of tetracycline inhibited NGF-dependent neurite extension, suggesting that the expression of Myc-Dvl-1 prevents NGF-induced neurite outgrowth of PC12 cells (Fig. 3E and F). To determine whether Rho-kinase is involved in Dvl-1-induced neurite retraction, we treated the cells with a Rho-kinase-selective inhibitor, Y-27632 (54). Under conditions in which Dvl-1 was not expressed, 10 μM Y-27632 itself did not induce neurite extension in the absence of NGF, while it slightly enhanced the NGF-induced neurite outgrowth of PC12/Dvl cells (Fig. 3G and H). Y-27632 reversed the inhibition of NGF-induced neurite outgrowth by Dvl-1 (Fig. 3G and H). These results suggest that Dvl-1 induces neurite retraction through Rho and Rho-kinase in PC12 cells.
Inhibition of serum starvation-dependent neurite outgrowth by Dvl in N1E-115 cells.
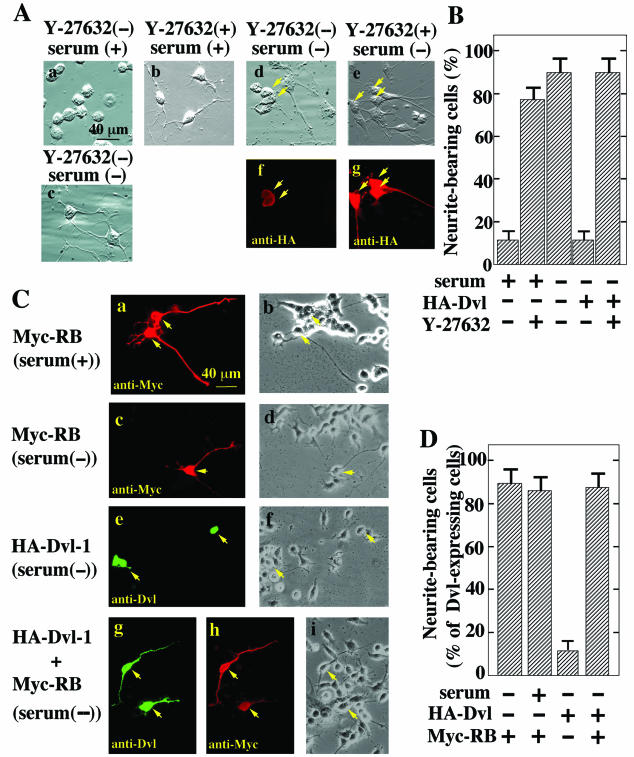
We further examined the roles of Dvl in neurite outgrowth by using another neuronal cell line, N1E-115 cells. N1E-115 neuroblastoma cells exhibit neurite outgrowth in response to serum deprivation, and Rho is responsible for causing this serum-dependent neurite retraction (32, 44, 55). Consistent with these results, Y-27632 induced neurite outgrowth in the presence of serum (Fig. 4A and B). The transient expression of HA-Dvl-1 abolished the neurite outgrowth induced by serum withdrawal, and Y-27632 reversed Dvl-1-dependent neurite retraction (Fig. 4A and B). RB induced neurite outgrowth in the presence of serum and did not affect neurite extension induced by serum deprivation (Fig. 4C and D). As expected, it reversed Dvl-1-dependent neurite retraction (Fig. 4C and D). Furthermore, Dvl-1-(1-398) and Dvl-1-(337-670), which activate Rho-kinase, reduced neurite extension, and the inhibitory activities of these Dvl-1 mutants coincided with their abilities to activate Rho-kinase (Fig. 4E and F). Dvl-1-(1-250) and Dvl-1-(395-670), which do not activate Rho-kinase, did not induce neurite retraction (Fig. 4E and F). These results also suggest that Dvl induces neurite retraction through Rho-kinase in N1E-115 cells.
FIG. 4.
Dvl-dependent neurite retraction in N1E-115 cells. (A) Inhibition of Dvl-1-dependent neurite retraction by Y-27632. N1E-115 cells cultured with (a and b) or without (c) serum were incubated with (b) or without (a and c) 10 μM Y-27632. N1E-115 cells transiently expressing HA-Dvl-1 (d to g) were cultured without serum in the presence (e and g) or absence (d and f) of 10 μM Y-27632. The cells were observed by relief-contrast microscopy (a to e) and were stained with an anti-HA antibody (f and g). Arrows indicate cells expressing HA-Dvl-1. (B) The numbers of neurite-bearing cells shown in panel A were quantified. When HA-Dvl-1 was expressed in N1E-115 cells, neurite-bearing cells among HA-Dvl-1-expressing cells were counted. The results shown are means ± SE of three independent experiments. (C) Inhibition of Dvl-1-dependent neurite retraction by the RB of Rho-kinase. Myc-RB (a to d), HA-Dvl-1 (e and f), or HA-Dvl-1 and Myc-RB (g to i) were transiently expressed in N1E-115 cells. After the cells were cultured with (a and b) or without (c to i) serum for 48 h, they were stained with anti-Dvl (e and g) and anti-Myc (a, c, and h) antibodies and observed by phase-contrast microscopy (b, d, f, and i). Arrows indicate cells expressing HA-Dvl-1 or Myc-RB. (D) The numbers of neurite-bearing cells shown in panel C were quantified. When HA-Dvl-1 was expressed in N1E-115 cells, neurite-bearing cells among HA-Dvl-1-expressing cells were counted. The results shown are means ± SE of three independent experiments. (E) Neurite retraction induced by Dvl-1 mutants. N1E-115 cells transiently expressing HA-Dvl-1-(1-398) (a and c), HA-Dvl-1-(337-670) (b and d), HA-Dvl-1-(1-250) (e and g), or HA-Dvl-1-(395-670) (f and h) were starved of serum for 48 h. The cells were observed by relief-contrast microscopy (a, b, e, and f) and were stained with an anti-HA antibody (c, d, g, and h). Arrows indicate cells expressing the Dvl-1 mutants. (F) The numbers of neurite-bearing cells shown in panel E were quantified. The results shown are percentages of neurite-bearing cells among cells expressing HA-Dvl-1 deletion mutants and are means ± SE of three independent experiments.
Effects of JNK and GSK-3 inhibitors on neurite extension.
Since the expression of Dvl-1 has been shown to activate Rac and JNK (7, 50), we examined whether JNK is involved in Dvl-1-dependent neurite retraction. In contrast to the Rho-kinase inhibitor, a JNK inhibitor did not abolish the neurite retraction induced by the expression of Myc-Dvl-1 in PC12 cells (Fig. 5A and B) under conditions in which the JNK inhibitor suppressed JNK activity (data not shown).
FIG. 5.
Involvement of JNK and GSK-3 in regulation of neurite extension. (A) Inability of a JNK inhibitor to suppress Dvl-1-dependent neurite retraction. PC12/Dvl cells were cultured in the presence (a, c, e, and g) or absence (b, d, f, and h) of tetracycline hydrochloride for 48 h. The cells were further incubated with (e to h) or without (a to d) NGF for 48 h in the presence (c, d, g, and h) or absence (a, b, e, and f) of 10 μM JNK inhibitor. (B) The numbers of NGF-induced neurite-bearing cells shown in panel A were quantified. The results are means ± SE of three independent experiments. (C) Inability of a Rho-kinase inhibitor to prevent GSK-3 inhibitor-dependent neurite retraction. Control PC12 cells were incubated with (b, d, f, h, j, and l) or without (a, c, e, g, i, and k) NGF for 48 h in the presence (e to h [10 mM LiCl] and i to l [10 μM SB216763]) or absence (a to d) of GSK-3 inhibitors. Where indicated, the cells were treated with (c, d, g, h, k, and l) or without (a, b, e, f, i, and j) 10 μM Y-27632 at the same time. (D) The numbers of neurite-bearing cells shown in panel C were quantified. The results are means ± SE of three independent experiments.
GSK-3 is an important component of the Wnt signaling pathway that functions downstream of Dvl (40, 46, 59). LiCl is known to inhibit GSK-3 and to activate the canonical β-catenin pathway (15). LiCl has also been shown to inhibit NGF-dependent neurite outgrowth in PC12 cells, probably through inhibition of the phosphorylation of microtubule-associated proteins (10). As shown in Fig. 3G and H, Y-27632 did not induce neurite extension in the absence of NGF and slightly enhanced NGF-dependent neurite outgrowth in control PC12 cells (Fig. 5C and D). Y-27632 did not prevent LiCl-dependent neurite retraction (Fig. 5C and D). SB216763, a GSK-3-specific inhibitor (12), also suppressed NGF-dependent neurite extension in control PC12 cells, and this inhibition was not reversed by Y-27632 (Fig. 5C and D). These results suggest that GSK-3 regulates neurite outgrowth and that the inhibition of Rho-kinase is not sufficient for the rescue of neurite retraction induced by GSK-3 inactivation.
Inhibition of NGF-dependent neurite extension by purified Wnt-3a.
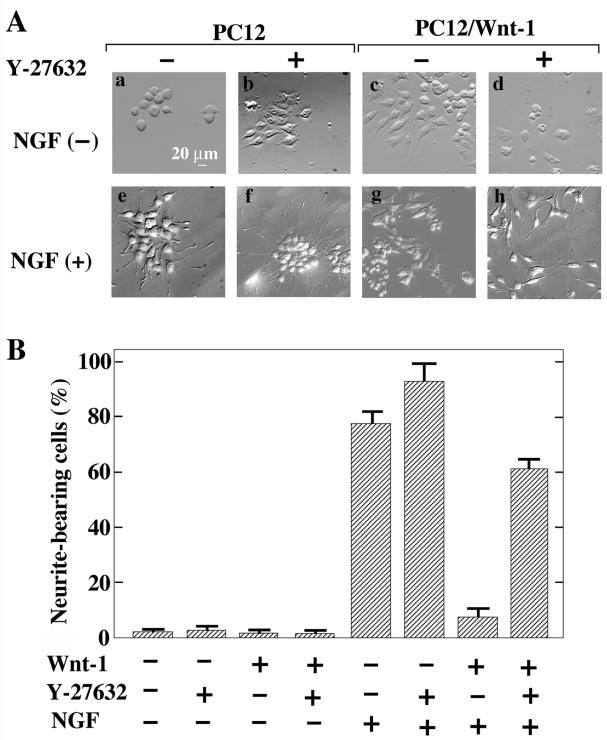
As shown in Fig. 1C, expression of Wnt-1 and Wnt-3a activate Rho-kinase. We examined whether these Wnt proteins are involved in the regulation of neurite extension through Rho-kinase. It has been previously demonstrated that PC12 cells expressing Wnt-1 (PC12/Wnt-1) fail to extend neurites after treatment with NGF (47). Indeed, NGF induced neurite formation weakly in PC12/Wnt-1 cells (Fig. 6). Y-27632 did not induce neurite outgrowth in control PC12 cells and PC12/Wnt-1 cells in the absence of NGF (Fig. 6). However, Y-27632 enhanced the NGF-dependent neurite extension of control PC12 cells and caused PC12/Wnt-1 cells to respond to NGF, resulting in the outgrowth of neurites (Fig. 6). These results suggest that Wnt-1 inhibits neurite outgrowth through Rho-kinase.
FIG. 6.
Neurite retraction by Wnt-1 in PC12 cells. (A) Control PC12 cells (a, b, e, and f) or PC12/Wnt-1 cells (c, d, g, and h) were incubated with (e to h) or without (a to d) NGF in the presence (b, d, f, and h) or absence (a, c, e, and g) of 10 μM Y-27632 for 48 h. (B) The numbers of neurite-bearing cells shown in panel A were quantified. The results are means ± SE of three independent experiments.
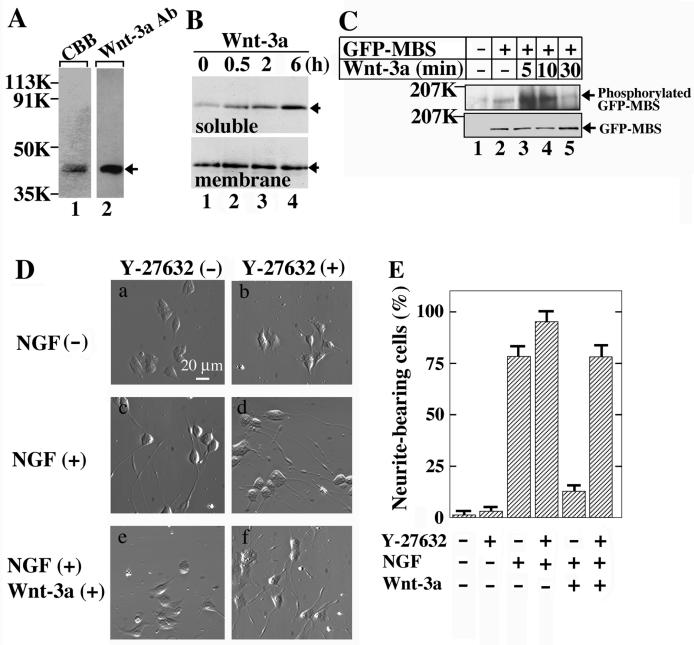
In addition, we used the purified Wnt-3a protein in this assay. Wnt-3a was purified to near homogeneity by three successive types of column chromatography (Fig. 7A). As was shown for mouse fibroblast L cells (57), the treatment of PC12 cells with purified Wnt-3a protein induced the accumulation of β-catenin in a time-dependent manner in the soluble fraction but not in the membrane fraction (Fig. 7B). The basal activity of endogenous Rho-kinase was detected in PC12 cells (Fig. 7C). Rho-kinase was activated 5 and 10 min after stimulation with the purified Wnt-3a protein, and the activity returned to the basal level after 30 min (Fig. 7C). Thus, Wnt-3a activates both the β-catenin and PCP pathways in PC12 cells.
FIG. 7.
Neurite retraction by Wnt-3a in PC12 cells. (A) Purification of Wnt-3a. The purified Wnt-3a protein was stained with Coomassie brilliant blue (CBB; 200 ng) (lane 1) and probed with an anti-Wnt-3a antibody (100 ng) (lane 2). An arrow indicates the position of Wnt-3a. (B) Accumulation of β-catenin in PC12 cells by purified Wnt-3a protein. After PC12 cells (in a 35-mm-diameter dish) were incubated with 250 ng of Wnt-3a/ml for 0.5 h (lane 2), 2 h (lane 3), or 6 h (lane 4), they were suspended in 250 μl of PBS containing 1 mM phenylmethylsulfonyl fluoride and sonicated on ice. The homogenate was centrifuged at 100,000 × g for 30 min at 4°C and the clear supernatant was used as the soluble fraction. The precipitate was suspended in PBS and used as the membrane fraction. Aliquots (4 μg) of the soluble (upper panel) and membrane (lower panel) fractions were probed with an anti-β-catenin antibody. PC12 cells without the Wnt-3a treatment were used as controls (lane 1). Arrows indicate the position of β-catenin. (C) Activation of Rho-kinase in PC12 cells by purified Wnt-3a protein. For detection of the endogenous activity of Rho-kinase, PC12 cells were infected with a recombinant adenovirus expressing GFP-MBS (lanes 2 to 5). After the cells were incubated with 160 ng of Wnt-3a/ml for the indicated times, the TCA precipitates were probed with anti-phospho-MBS and anti-GFP antibodies. PC12 cells infected with empty vectors were used as a control (lane 1). (D) Inhibition of neurite extension by purified Wnt-3a protein. PC12 cells were incubated with NGF (c and d) or NGF and 160-ng/ml Wnt-3a (e and f) in the presence (d and f) or absence (c and e) of 10 μM Y-27632 for 48 h. PC12 cells that were not treated with NGF were used as a control (a and b). (E) The numbers of neurite-bearing cells shown in panel D were quantified. The results are means ± SE of three independent experiments. (F) Inhibition of Wnt-3a-dependent neurite retraction by Dvl-1 mutants. PC12 cells transiently expressing HA-Dvl-1-(1-250) (a and d), HA-Dvl-1-(224-398) (b and e), or HA-Dvl-1-(395-670) (c and f) were incubated with NGF and 160-ng/ml Wnt-3a for 48 h. The cells were observed by relief-contrast microscopy (a to c) and were stained with an anti-HA antibody (d to f). Arrows indicate cells expressing the Dvl mutants. (G) The numbers of neurite-bearing cells among the HA-Dvl-1-expressing cells shown in panel F were quantified. The results are means ± SE of three independent experiments. (H) Effects of Dvl-1-(224-398) on neurite extension. PC12 cells transiently expressing HA-Dvl-1-(224-398) were incubated with (b and d) or without (a and c) NGF for 48 h. The cells were observed by relief-contrast microscopy (a and b) and were stained with an anti-HA antibody (c and d). Arrows indicate cells expressing HA-Dvl-1-(224-398).
When PC12 cells were incubated with NGF and purified Wnt-3a protein, neurite outgrowth was highly suppressed compared to that in cells incubated with NGF alone (Fig. 7D and E). The Wnt-3a-dependent inhibition of neurite extension was prevented by Y-27632 (Fig. 7D and E). The expression of HA-Dvl-1-(224-398), but not of HA-Dvl-1-(1-250) or HA-Dvl-1-(395-670), inhibited Wnt-3a-dependent neurite retraction (Fig. 7F and G). These results are consistent with observations that these Dvl-1 mutants did not activate Rho-kinase and that Dvl-1-(224-398), but not Dvl-1-(395-670), inhibited the Wnt-3a-dependent activation of Rho-kinase. In addition, Dvl-1-(224-398) neither induced neurite outgrowth in the absence of NGF nor affected NGF-dependent neurite extension (Fig. 7H). Therefore, Dvl-1-(224-398) functions as a dominant-negative form of the protein in the PCP pathway of Wnt signaling. From these results, we conclude that Wnt-3a inhibits NGF-dependent neurite outgrowth via Dvl and Rho-kinase.
DISCUSSION
Genetic studies have implicated DFz1, Dsh, RhoA, Rac, and Drok in the PCP pathway in Drosophila (2, 48, 50), and biochemical studies have shown that Wnt-1, Wnt-11, Frizzled1, and Dvl regulate Rho activity in mammalian cells (16, 17). Thus, the activation of Rho is a direct response to Wnt and likely represents a key pathway for Wnt-dependent regulation of the cytoskeleton. There are multiple effector proteins of Rho, including Rho-kinase (Rock), protein kinase N, the MBS of myosin phosphatase, rhophilin, rhotekin, citron, and mDia (4, 25, 42). Although Rho-kinase has been shown to function downstream of Dvl in Drosophila and zebra fish (39, 58), which effector proteins of Rho are activated by Wnt signaling in mammalian cells has not yet been clarified. Our findings that Wnt-1, Wnt-3a, and Dvl-1 activate Rho-kinase in intact mammalian cells provide a direct biochemical link from the Wnt signal to Rho-kinase activation.
Neither the DIX, PDZ, nor DEP domain was required for the activation of Rho-kinase by Dvl. Both the DIX and PDZ domains are necessary for the activation of the canonical β-catenin pathway (29, 49), and the DEP domain is essential for the activation of JNK by Dvl (7, 41). Our results suggest that the region from residues 337 to 394 of Dvl-1 is necessary for the activation of Rho-kinase. Taken together with previous observations concerning the activation of Rho by Dvl (17), these findings suggest that the region containing the PDZ and DEP domains and residues 337 to 394, flanked by these two domains, are important for the activation of Rho-kinase by Dvl. Therefore, it is likely that the Dvl-dependent Rho-kinase activation pathway is distinct from the Dvl-β-catenin pathway and the Dvl-JNK pathway. In addition, we showed that Dvl-1-(224-398), but not Dvl-1-(395-670), inhibits the Wnt-3a-dependent activation of Rho-kinase. These results are consistent with the observation that Dvl activates Rho and Rac via distinct mechanisms (16).
Our results show that Wnt and Dvl regulate neurite outgrowth through Rho-kinase in PC12 and N1E-115 cells. This conclusion was supported by five findings. Firstly, Wnt-1-, Wnt-3a-, and Dvl-1-dependent neurite retraction was suppressed by a Rho-kinase inhibitor but not by a JNK inhibitor. Secondly, Dvl-1-dependent neurite retraction was suppressed by expression of the RB of Rho-kinase. Thirdly, the expression of Dvl-1 induced the formation of a thick ringlike structure of cortical actin filaments at the periphery of PC12 cells. Fourthly, the Dvl-1 mutants that activated Rho-kinase induced neurite retraction, but other mutants that did not activate Rho-kinase did not. Fifthly, Wnt-3a activated Rho-kinase in PC12 cells and Wnt-3a-dependent neurite retraction was inhibited by Dvl-1-(224-398). Taken together with the observation that Dvl associates with actin stress fibers in mouse embryonic kidney cells (53), these findings imply that Wnt and Dvl activate Rho and Rho-kinase, thereby inhibiting neurite formation through regulation of the actin-myosin system.
Y-27632 did not induce neurite outgrowth significantly at a concentration of 10 μM but induced neurites at 200 μM in the absence of NGF in the PC12 cells used for this study (data not shown). These results are not consistent with previously reported observations that 10 μM Y-27632 itself induces neurites. One study showed that 10 μM Y-27632 induces neurite extension in 20 to 30% of PC12 cells (14) and another study demonstrated that it induces neurites in 70% of cells (6). We used PC12 cells stably expressing tTA (tet-off system). Since it is known that there are many variants of PC12 cells, the PC12 cells used for this study might have exhibited a relatively low level of sensitivity to Y-27632. Although we could detect the basal activities of endogenous Rho and Rho-kinase in these PC12 cells, we do not know whether these activities were high enough to prevent Y-27632 from extending neurites. Alternatively, the basal activities of Rho and Rho-kinase may be low in these cells and these activities may not play major roles in the inhibition of neurite outgrowth in the absence of NGF. In any case, this PC12 cell line is useful for showing that Wnt and Dvl inhibit neurite outgrowth via Rho-kinase.
Dvl-1 has been shown to colocalize with axonal microtubules and to sediment with brain microtubules (34). Dvl-1 stabilizes microtubules by inhibiting GSK-3β in differentiated neuroblastoma cells, thereby preventing the cells from retracting axons in the presence of nocodazole, which depolymerizes microtubules, and this effect of Dvl-1 is mimicked by LiCl, which is known to inhibit GSK-3. However, Wnt-7a and Dvl-1 induce axonal spreading and branching as well as a decrease in axon length in granule cell neurons (37). LiCl also mimics Wnt-7a in granule cells and induces the loss or disorganization of stable microtubules. The reasons for these opposite actions of Dvl-1 on microtubule stability in neuroblastoma cells and granule cell neurons are not known at present. Consistent with previous observations (10), LiCl inhibited NGF-induced neurite outgrowth in PC12 cells. We also confirmed these findings with SB216763, a GSK-3-specific inhibitor. Y-27632 did not suppress the effects of LiCl and SB216763 on NGF-induced neurite outgrowth. These results suggest that GSK-3 and Rho-kinase regulate the dynamics of microtubules and actin-myosin, respectively, downstream of Dvl and that both pathways are necessary for the formation of neurites. Although it has been shown that Dvl regulates axon length through GSK-3 (34), our results demonstrate that the Dvl and Rho-kinase pathway, in addition to the Dvl and GSK-3 pathway, is also involved in the regulation of neurite formation. Since β-catenin did not affect the NGF-induced neurite outgrowth (data not shown), it is unlikely that β-catenin-dependent transcriptional activation is involved in the regulation of neurite outgrowth.
The expression of Wnt-1 or Wnt-3a in PC12 cells inhibits NGF-induced neurite outgrowth (11, 47). The effects of targeted inactivation of Wnt-1 and Wnt-3a in mice suggest their critical role in neuronal development, including remodeling of the axon (23). However, the molecular mechanisms of these effects have remained unclear. It was recently reported that Wnt-3a was purified to near homogeneity and that purified Wnt-3a protein induces self-renewal of hematopoietic stem cells (43, 57). We showed that the purified Wnt-3a protein can inhibit NGF-induced neurite outgrowth via Dvl and Rho-kinase (PCP pathway) in PC12 cells. The PCP pathway has been shown to play a role in the embryogenesis of Drosophila and Xenopus, but the physiological significance of the PCP pathway in mammals has not been well characterized except for the finding that this pathway activates JNK biochemically. Our results demonstrate for the first time that NGF and Wnt-3a regulate the neurite outgrowth of PC12 cells cooperatively. Furthermore, it has been shown that Wnt-3-conditioned medium reduces axon length in neurotropin-3-responsive spinal sensory neurons but not in NGF-responsive neurons (33). Therefore, Wnt-3a and Wnt-3 may regulate axonal remodeling of different types of neuronal cells.
Studies in Drosophila have provided a conceptual framework about the PCP pathway, while the roles of the PCP pathway in mammalian cells are less understood. Although an example of PCP in mammals has been provided by the sensory hair cells of the inner ear (35), cultured PC12 and N1E-115 cells will be helpful for understanding the PCP pathway in mammalian cells.
Acknowledgments
We are grateful to Kozo Kaibuchi for helpful discussions as well as for antibodies and plasmids. We also thank Shinji Takada, Ming-Gui Pan, Hidenori Ichijo, and Manabu Negishi for plasmids and cells.
This work was supported by grants-in-aid for scientific research and for scientific research on priority areas from the Ministry of Education, Science, and Culture, Japan (2002, 2003), and by grants from the Yamanouchi Foundation for Research on Metabolic Disorders (2002, 2003) and the Uehara Memorial Foundation (2002).
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, P. N. 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2:525-535. [DOI] [PubMed] [Google Scholar]
- 3.Amano, M., K. Chihara, K. Kimura, Y. Fukata, N. Nakamura, Y. Matsuura, and K. Kaibuchi. 1997. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275:1308-1311. [DOI] [PubMed] [Google Scholar]
- 4.Amano, M., Y. Fukata, and K. Kaibuchi. 2000. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261:44-51. [DOI] [PubMed] [Google Scholar]
- 5.Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. [DOI] [PubMed] [Google Scholar]
- 6.Birkenfeld, J., H. Betz, and D. Roth. 2001. Inhibition of neurite extension by overexpression of individual domains of LIM kinase 1. J. Neurochem. 78:924-927. [DOI] [PubMed] [Google Scholar]
- 7.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Bradke, F., and C. G. Dotti. 1999. The role of local actin instability in axon formation. Science 283:1931-1934. [DOI] [PubMed] [Google Scholar]
- 10.Burstein, D. E., P. J. Seeley, and L. A. Greene. 1985. Lithium ion inhibits nerve growth factor-induced neurite outgrowth and phosphorylation of nerve growth factor-modulated microtubule-associated proteins. J. Cell Biol. 101:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou, A. H., and B. D. Howard. 2002. Inhibition by Wnt-1 or Wnt-3a of nerve growth factor-induced differentiation of PC12 cells is reversed by bisindolylmaleimide-I but not by several other PKC inhibitors. Oncogene 21:6348-6355. [DOI] [PubMed] [Google Scholar]
- 12.Coghlan, M. P., A. A. Culbert, D. A. Cross, S. L. Corcoran, J. W. Yates, N. J. Pearce, O. L. Rausch, G. J. Murphy, P. S. Carter, L. Roxbee Cox, D. Mills, M. J. Brown, D. Haigh, R. W. Ward, D. G. Smith, K. J. Murray, A. D. Reith, and J. C. Holder. 2000. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 7:793-803. [DOI] [PubMed] [Google Scholar]
- 13.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 14.Fujita, A., Y. Hattori, T. Takeuchi, Y. Kamata, and F. Hata. 2001. NGF induces neurite outgrowth via a decrease in phosphorylation of myosin light chain in PC12 cells. Neuroreport 12:3599-3602. [DOI] [PubMed] [Google Scholar]
- 15.Gurvich, N., and P. S. Klein. 2002. Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol. Ther. 96:45-66. [DOI] [PubMed] [Google Scholar]
- 16.Habas, R., I. B. Dawid, and X. He. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17:295-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habas, R., Y. Kato, and X. He. 2001. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell 107:843-854. [DOI] [PubMed] [Google Scholar]
- 18.Hakeda-Suzuki, S., J. Ng, J. Tzu, G. Dietzl, Y. Sun, M. Harms, T. Nardine, L. Luo, and B. J. Dickson. 2002. Rac function and regulation during Drosophila development. Nature 416:438-442. [DOI] [PubMed] [Google Scholar]
- 19.Hall, A. C., F. R. Lucas, and P. C. Salinas. 2000. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100:525-535. [DOI] [PubMed] [Google Scholar]
- 20.Heisenberg, C. P., M. Tada, G. J. Rauch, L. Saude, M. L. Concha, R. Geisler, D. L. Stemple, J. C. Smith, and S. W. Wilson. 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405:76-81. [DOI] [PubMed] [Google Scholar]
- 21.Hino, S.-I., T. Michiue, M. Asashima, and A. Kikuchi. 2003. Casein kinase Iɛ enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of β-catenin. J. Biol. Chem. 278:14066-14073. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda, S., S. Kishida, H. Yamamoto, H. Murai, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17:1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeya, M., S. M. Lee, J. E. Johnson, A. P. McMahon, and S. Takada. 1997. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389:966-970. [DOI] [PubMed] [Google Scholar]
- 24.Jalink, K., E. J. van Corven, T. Hengeveld, N. Morii, S. Narumiya, and W. H. Moolenaar. 1994. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 126:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaibuchi, K., S. Kuroda, and M. Amano. 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68:459-486. [DOI] [PubMed] [Google Scholar]
- 26.Kawano, Y., Y. Fukata, N. Oshiro, M. Amano, T. Nakamura, M. Ito, F. Matsumura, M. Inagaki, and K. Kaibuchi. 1999. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 147:1023-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikuchi, A. 1999. Roles of axin in the Wnt signalling pathway. Cell. Signal. 11:777-788. [DOI] [PubMed] [Google Scholar]
- 28.Kishida, M., S.-I. Hino, T. Michiue, H. Yamamoto, S. Kishida, A. Fukui, M. Asashima, and A. Kikuchi. 2001. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iɛ. J. Biol. Chem. 276:33147-33155. [DOI] [PubMed] [Google Scholar]
- 29.Kishida, S., H. Yamamoto, S.-I. Hino, S. Ikeda, M. Kishida, and A. Kikuchi. 1999. DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol. Cell. Biol. 19:4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishida, S., H. Yamamoto, S. Ikeda, M. Kishida, I. Sakamoto, S. Koyama, and A. Kikuchi. 1998. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J. Biol. Chem. 273:10823-10826. [DOI] [PubMed] [Google Scholar]
- 31.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, K.-I. Nakayama, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozma, R., S. Sarner, S. Ahmed, and L. Lim. 1997. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell. Biol. 17:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krylova, O., J. Herreros, K. E. Cleverley, E. Ehler, J. P. Henriquez, S. M. Hughes, and P. C. Salinas. 2002. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron 35:1043-1056. [DOI] [PubMed] [Google Scholar]
- 34.Krylova, O., M. J. Messenger, and P. C. Salinas. 2000. Dishevelled-1 regulates microtubule stability: a new function mediated by glycogen synthase kinase-3β. J. Cell Biol. 151:83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis, J., and A. Davies. 2002. Planar cell polarity in the inner ear: how do hair cells acquire their oriented structure? J. Neurobiol. 53:190-201. [DOI] [PubMed] [Google Scholar]
- 36.Liu, C., Y. Li, M. Semenov, C. Han, G.-H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 37.Lucas, F. R., R. G. Goold, P. R. Gordon-Weeks, and P. C. Salinas. 1998. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 111:1351-1361. [DOI] [PubMed] [Google Scholar]
- 38.Luo, L. 2000. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 1:173-180. [DOI] [PubMed] [Google Scholar]
- 39.Marlow, F., J. Topczewski, D. Sepich, and L. Solnica-Krezel. 2002. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 12:876-884. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J. R., A. M. Hocking, J. D. Brown, and R. T. Moon. 1999. Mechanism and function of signal transduction by the Wnt/β-catenin and Wnt/Ca2+ pathways. Oncogene 18:7860-7872. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi, T., K. Kawachi, S. Kamakura, N. Masuyama, H. Yamanaka, K. Matsumoto, A. Kikuchi, and E. Nishida. 1999. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274:30957-30962. [DOI] [PubMed] [Google Scholar]
- 42.Narumiya, S., T. Ishizaki, and N. Watanabe. 1997. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 410:68-72. [DOI] [PubMed] [Google Scholar]
- 43.Reya, T., A. W. Duncan, L. Ailles, J. Domen, D. C. Scherer, K. Willert, L. Hintz, R. Nusse, and I. L. Weissman. 2003. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409-414. [DOI] [PubMed] [Google Scholar]
- 44.Sarner, S., R. Kozma, S. Ahmed, and L. Lim. 2000. Phosphatidylinositol 3-kinase, Cdc42, and Rac1 act downstream of Ras in integrin-dependent neurite outgrowth in N1E-115 neuroblastoma cells. Mol. Cell. Biol. 20:158-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebok, A., N. Nusser, B. Debreceni, Z. Guo, M. F. Santos, J. Szeberenyi, and G. Tigyi. 1999. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J. Neurochem. 73:949-960. [DOI] [PubMed] [Google Scholar]
- 46.Seidensticker, M. J., and J. Behrens. 2000. Biochemical interactions in the Wnt pathway. Biochim. Biophys. Acta 1495:168-182. [DOI] [PubMed] [Google Scholar]
- 47.Shackleford, G. M., K. Willert, J. Wang, and H. E. Varmus. 1993. The Wnt-1 proto-oncogene induces changes in morphology, gene expression, and growth factor responsiveness in PC12 cells. Neuron 11:865-875. [DOI] [PubMed] [Google Scholar]
- 48.Shulman, J. M., N. Perrimon, and J. D. Axelrod. 1998. Frizzled signaling and the developmental control of cell polarity. Trends Genet. 14:452-458. [DOI] [PubMed] [Google Scholar]
- 49.Smalley, M. J., E. Sara, H. Paterson, S. Naylor, D. Cook, H. Jayatilake, L. G. Fryer, L. Hutchinson, M. J. Fry, and T. C. Dale. 1999. Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J. 18:2823-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strutt, D. I., U. Weber, and M. Mlodzik. 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature 387:292-295. [DOI] [PubMed] [Google Scholar]
- 51.Tada, M., and J. C. Smith. 2000. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127:2227-2238. [DOI] [PubMed] [Google Scholar]
- 52.Takeda, K., T. Hatai, T. S. Hamazaki, H. Nishitoh, M. Saitoh, and H. Ichijo. 2000. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275:9805-9813. [DOI] [PubMed] [Google Scholar]
- 53.Torres, M. A., and W. J. Nelson. 2000. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149:1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uehata, M., T. Ishizaki, H. Satoh, T. Ono, T. Kawahara, T. Morishita, H. Tamakawa, K. Yamagami, J. Inui, M. Maekawa, and S. Narumiya. 1997. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389:990-994. [DOI] [PubMed] [Google Scholar]
- 55.van Leeuwen, F. N., H. E. Kain, R. A. Kammen, F. Michiels, O. W. Kranenburg, and J. G. Collard. 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wharton, K. A. J. 2003. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev. Biol. 253:1-17. [DOI] [PubMed] [Google Scholar]
- 57.Willert, K., J. D. Brown, E. Danenberg, A. W. Duncan, I. L. Weissman, T. Reya, J. R. I. Yates, and R. Nusse. 2003. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448-452. [DOI] [PubMed] [Google Scholar]
- 58.Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess, J. D. Axelrod, and L. Luo. 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105:81-91. [DOI] [PubMed] [Google Scholar]
- 59.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14:59-88. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi, Y., H. Katoh, H. Yasui, K. Mori, and M. Negishi. 2001. RhoA inhibits the nerve growth factor-induced Rac1 activation through Rho-associated kinase-dependent pathway. J. Biol. Chem. 276:18977-18983. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto, H., S. Kishida, T. Uochi, S. Ikeda, S. Koyama, M. Asashima, and A. Kikuchi. 1998. Axil, a member of the Axin family, interacts with both glycogen synthase kinase-3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol. Cell. Biol. 18:2867-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamanaka, H., T. Moriguchi, N. Masuyama, M. Kusakabe, H. Hanafusa, R. Takada, S. Takada, and E. Nishida. 2002. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]