Abstract
A family of orphan transporters has been discovered that are structurally related to the Na+-Cl−-dependent neurotransmitter transporters, including the dopamine transporter. One member of this family, the mouse XT2 gene, is predominantly expressed in the kidney and has 95% homology to rat ROSIT (renal osmotic stress-induced Na+-Cl− organic solute cotransporter). To study the physiological functions of this transporter, we generated XT2-knockout mice by gene targeting. XT2−/− mice develop and survive normally with no apparent abnormalities. To attempt to identify potential substrates for XT2, we screened urine from XT2-knockout mice by high-pressure liquid chromatography and mass spectrometry and found significantly elevated concentrations of glycine. To study glycine handling, XT2+/+ and XT2−/− mice were injected with radiolabeled glycine, and urine samples were collected to monitor glycine excretion. After 2 h, XT2−/− mice were found to excrete almost twice as much glycine as the XT2+/+ controls (P = 0.03). To determine whether the absence of the XT2 transporter affected sodium and fluid homeostasis, we measured systolic blood pressure by computerized tail-cuff manometry. Systolic blood pressure was significantly higher in XT2−/− mice (127 ± 3 mmHg) than in wild-type controls (114 ± 2 mmHg; P < 0.001). This difference in systolic blood pressure was maintained on high and low salt feeding. To examine whether the alteration in blood pressure and the defect in glycine handling were related, we measured systolic blood pressure in the XT2−/− mice during dietary glycine supplementation. Glycine loading caused systolic blood pressure to fall in the XT2−/− mice from 127 ± 3 to 115 ± 3 mmHg (P < 0.001), a level virtually identical to that of the wild-type controls. These data suggest that the XT2 orphan transporter is involved in glycine reabsorption and that the absence of this transporter is sufficient to cause hypertension.
The transport of hydrophilic substances across cell membranes is mediated by substrate-specific transporter proteins that can be classified into several families of related genes. Within these families, there are sequence homology and conservation of transmembrane domain topology. For example, the superfamily of sodium- and chloride-dependent transporter is comprised of transporters for neurotransmitters, amino acids, and organic osmolytes (11, 31, 36). Based on the homology of transmembrane domains with those of the neurotransmitter family, a group of putative transporters (17) has been identified that includes XT2/ROSIT (16, 33), XT3/rB21a (16, 27), NTT4/XT1 (4, 12), NTT5 (6), and NTT7/v7-3 (24, 32). However, studies in transfected cells and oocyte expression systems failed to determine conclusive substrate specificity (4, 16, 27) for this family of orphan transporters. Although sharing relatively high amino acid similarity (28 to 41%) with classical neurotransmitter transporter family members, these orphan transporters have larger second and fourth extracellular loops. In addition, relative to the family of neurotransmitter transporters, the cellular localization of these orphan transporters is more diverse (8, 13, 14, 16). Based on sequence homology, we have cloned the XT2 gene in mouse (16). We found that the XT2 gene is highly homologous to the ROSIT (renal osmotic stress-induced Na-Cl-organic solute cotransporter) that had previously been identified in rat (33). ROSIT is expressed in the S3 segment of proximal tubules and has been shown to be upregulated during hypernatremic state and postischemic renal injury (18, 33). Mouse XT2 cDNA is also expressed predominantly in the kidney. However, the substrate and physiological function of this orphan transporter is not known. In order to define the physiological functions of XT2, we generated mice with targeted disruption of XT2 gene. These studies have identified nonredundant functions of XT2 to promote renal glycine transport and to regulate blood pressure.
MATERIALS AND METHODS
Animals.
XT2−/− and XT2+/− mice, generated as described below, and wild-type mice with a mixed background, 3 to 6 months old, were obtained. Experimental protocols were in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Md. [publication no. 865-23]) and approved by the Institutional Animal Care and Use Committee.
Generation of XT2-null mouse.
The mouse XT2 gene was isolated from a 129/Sv genomic library. A targeting vector was constructed by using an 8-kb genomic DNA fragment containing exon 1 and intron 1. A DNA fragment containing the whole exon 1 and part of intron 1 was replaced by a 3.5-kb loxP-flanked Neo-TK selection cassette (Fig. 1A), and a PGK-DT cassette was inserted upstream for negative selection. The vector was linearized at a unique NotI site and electroporated into RW4 embryonic stem (ES) cells. Homologous recombinants were screened from the G418-resistant colonies by PCR and confirmed by Southern blot analysis. Male chimeras produced by injection of targeted ES cells into C57BL/6J blastocysts were bred with C57BL/6J females. Germ line transmission of the targeted mutation was screened by PCR and verified by Southern blot analysis of tail DNA. Heterozygotes were then mated with transgenic C57BL/6J mice carrying a CMV-Cre transgene (a gift from Yuan Zhuang at Duke University). Constitutively expressed Cre recombinase excises the loxP-flanked Neo-TK cassette, which renders heterozygous animals without the Neo-TK selection cassette (XT2+/−). Crossing XT2+/− mice produces homozygous mutant, heterozygous, and wild-type mice. Animals of this generation were used for the studies reported. Mice were group-housed at two to five mice per cage with free access to food and water under a 12-h light-dark cycle.
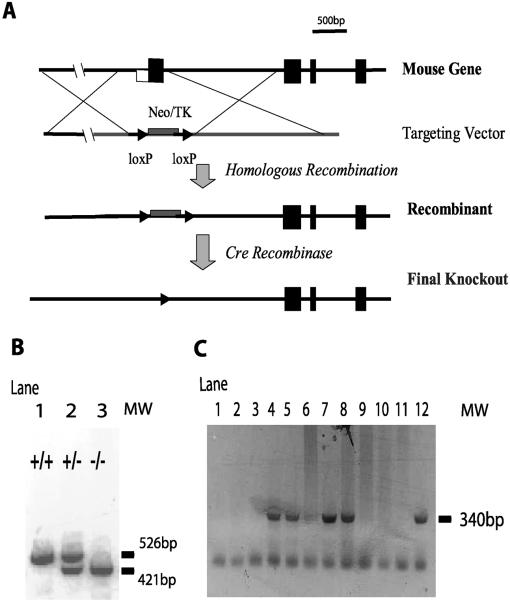
FIG. 1.
Generation of XT2-null mutant mice. (A) Targeting strategy for disruption of the XT2 gene; (B) analysis of mouse XT2 genomic PCR product in a 1.0% agarose gel stained with ethidium bromide. A PCR product of 526 bp is seen in wild-type (lane 1) and heterozygous mutants (lane 2), whereas a 421-bp fragment is seen in homozygous (lane 3) and heterozygous (lane 2) mutants; (C) analysis of mouse XT2 RT-PCR product in a 1.0% agarose gel stained with ethidium bromide. An amplification product of the predicted size (340 bp) is seen in RT-PCR with RNA (2 μg) from kidneys of the XT2 wild-type mice (lanes 4 to 6) and heterozygous mutants (lanes 7 and 8) but absent in homozygous mutants (lanes 9 to 11). The positive control is XT2 cDNA (lane 12). Negative controls include genomic DNA (lanes 1 and 2) and no reverse transcriptase (lane 3).
Genotyping PCR and RT-PCR.
The genotype of each individual in the knockout mouse colonies was determined by PCR. Two XT2 sequence-specific oligonucleotides were used for genotyping PCR: forward primer (5′-CAGTAGATGCGGGTGTTGAACA-3′; 139 bp upstream of the transcription start of XT2 gene) and reverse primer (5′-ACCAGGATGGGACTCAAAGACA-3′; 115 bp downstream of the 3′ end of the first exon of XT2 gene). The expected PCR product is 526 bp in wild-type mice and 421 bp in knockout mice. To examine the expression of XT2 gene in mice, reverse transcription-PCR (RT-PCR) was performed with the RNAs from mouse kidney tissues. Kidney tissues were obtained from adult male mice sacrificed by decapitation. Total RNA was prepared by the TRIzol method as recommended by the manufacturer (Gibco-BRL). Reverse-transcribed mouse RNAs were obtained by using the OmniScript RNA PCR kit (Qiagen). The following XT2 sequence-specific oligonucleotides were used: forward primer 5′-CCTCAGGGATGGACCCGCTTGTG-3′ (corresponding to nucleotides 11 to 33 of the XT2 cDNA coding sequence) and reverse primer 5′-ACCGTGTTGTAGTACAAACTGA-3′ (complementary to nucleotides 327 to 350 of the XT2 cDNA coding sequence) (16). RT was performed with 2 μg of total RNA in a buffer containing 10 mM Tris-HCl (pH 8.3); 50 mM KCl; 5 mM MgCl2; 2.5 μM TTTTTT primer; 1 mM (each) dGTP, dATP, dTTP, and dCTP; and 50 U of Moloney murine leukemia virus reverse transcriptase incubated at 37°C for 1 h. The PCR amplifications were performed in the presence of 2 mM MgCl2, 0.15 μM forward and reverse primers, and 2.5 U of Taq polymerase. After an initial incubation for 2 min at 95°C, samples were subjected to 40 cycles of 30 s at 92°C, 1 min at 56°C, and 1 min at 65°C. The PCR products were electrophoresed through 1.0% agarose gels containing ethidium bromide. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sequence-specific primers were used to control for the quality and amount of RNA.
Urine collection.
Individual XT2−/− or wild-type mice were housed separately in metabolic cages with free access to food and water. Urine samples were collected during a 24-h period in a 6-ml tube with 1 μl of concentrated (100 mg/ml) ampicillin-streptomycin solution at the bottom. A 1-ml urine sample was taken from each tube and centrifuged at 3,000 rpm at room temperature for 1 min to remove insoluble particles. An equal volume of methanol was added to the urine supernatant. After vigorous mixing, the white cloudy mixture was centrifuged at 14,000 rpm at room temperature for 2 min. The resulting clear supernatant was used for subsequent high-pressure liquid chromatography (HPLC) analysis or mass spectrometry measurement.
HPLC analysis.
Urine samples were first derivatized with O-phthalaldehyde (OPA) solution before they were loaded to a C18 Ultrasphere reverse-phase column (Beckman, Palo Alto, Calif.) according to the standard procedure of Fernstrom and Fernstrom (7). Briefly, OPA was added to the methanol-treated urine to reach a final concentration of 10 mM. After a 2-min incubation at room temperature, the urine sample was centrifuged 10 min at 20,000 rpm, and the supernatant was filtered through a 0.2-μm-pore-size filter. A 20-μl portion of the filtered urine was injected into a HPLC system equipped with a calibrated C18 column and a fluorescence detector. The column was eluted with a linear gradient of 20 to 40% acetonitrile.
Tandem mass spectrometry.
A standard amino acid analysis protocol (26) was used for the analysis of urine samples. Blood samples from the orbital sinus of anesthetized mice were collected into a heparin-coated capillary tube. Plasma was separated by centrifugation. A 20- to 30-mg plasma sample was used for amino acid analysis. A total of 5 nmol of glycine and 1 nmol of synthetic peptide AVLMFYDER were added to each spot as an internal control.
Renal [14C]glycine autoradiography.
Individually housed adult male XT2−/− and wild-type mice received an intraperitoneal injection of 5 μCi of [14C]glycine. Mice were anesthetized 15 min later and perfused thoroughly with phosphate-buffered saline (Gibco-BRL). Fresh kidneys were removed and frozen immediately in liquid nitrogen. Kidneys were cut on a cryostat into 20-μm sections. Autoradiography of kidney sections was analyzed after 1 week exposure at −80°C.
BBMV uptake.
Brush-border membrane vesicles (BBMV) were isolated from the kidney cortex of wild-type or knockout mice according to the method of Zelikovic and Budreau-Patters as described previously (37), with minor modifications. Briefly, kidney cortex slices from three mice of the same genotype were homogenized in 15 ml of cold isolation buffer (50 mM mannitol, 1 mM MgSO4, 2 mM Tris, 3 mM HEPES [pH 7.1]). MgCl2 (final concentration, 10 mM) was added to precipitate the intracellular and basolateral membranes. The final vesicle preparation was suspended in 296 mM mannitol-1 mM MgSO4-2 mM HEPES-Tris (pH 7.35), and the protein concentrations were ∼10 mg of protein/ml. Uptake of the radioactive amino acids was assayed by a Millipore filtration technique (35).
Systolic blood pressure measurements in conscious mice.
Systolic blood pressures were measured in conscious mice by using a computerized tail-cuff system (Visitech Systems, Cary, N.C.) that determines systolic blood pressure by using a photoelectric sensor. This system allows pressures to be measured in four mice simultaneously and minimizes the potential for observer bias. Before the study was initiated, mice were adapted to the apparatus for at least 5 days. The validity of this system has been established previously, and we have demonstrated its correlation with intra-arterial pressure measurements in several experimental systems (1).
Effects of reduced dietary sodium or glycine supplement on systolic blood pressures.
To determine the effects of glycine treatment on blood pressure, we measured systolic blood pressures in mice during glycine treatment. Glycine was added to drinking water at a concentration of 130 mM. Systolic blood pressures were measured at least five times per week during the 2 weeks of glycine treatment.
Renal hemodynamic studies.
Clearances of inulin and glycine were measured in mice by using a procedure that has been described previously (5). On the day of the study, animals were anesthetized with 0.04 mg pentobarbital and isofurane/g. The left carotid artery and left jugular vein were cannulated with polyethylene catheters (PE-10) for intravenous infusions to monitor mean arterial pressure and to allow intermittent sampling of arterial blood. After surgery, normal saline (2% of the body weight) was infused intravenously over 20 min to replace surgical losses. A priming dose of carboxyl-14C-inulin and [3H]glycine was given, followed by infusion of pentobarbitol, carboxyl-14C-inulin and [3H]glycine in normal saline at a rate of 25 μl/min/100 g of body weight. The bladder was cannulated via a suprapubic incision with a PE-50 catheter. After 30 min of calibration, renal function was measured during three consecutive 30-min clearance periods. Carboxyl-14C-inulin and [3H]glycine in plasma and urine were measured in a liquid scintillation counter (Nuclear Chicago-TM Analytical, Inc., Elk Grove, Ill.). Clearances of inulin and glycine were calculated by using standard formulas. Using this method, the preparation was hemodynamically stable for up to 4 h, and renal function measurements were highly reproducible.
Statistical analysis.
The value for each parameter within a group is expressed as the mean ± the standard error of the mean. For comparisons between the groups, statistical significance was assessed by using an unpaired Student t test. A paired Student t test was used for comparisons within groups. Survival analysis during low-salt feeding was determined by chi-square.
RESULTS
Generation of XT2-null mice.
To disrupt the gene encoding mouse XT2, we used a targeting construct in which exon 1 and a part of the first intron of the mouse XT2 gene were replaced by a loxP-flanked PGK-Neo/HSK-TK selection cassette (Fig. 1A). Correctly targeted ES cells were identified, cloned, and expanded. Chimeric animals were generated by injection of blastocysts with the targeted ES cell lines. These chimeras transmitted the targeted XT2 locus to their progeny. These XT2+/− animals were crossed with transgenic CMV-Cre mice to remove the PGK-neo cassette to ensure that its presence would not interfere with the expression of neighboring genes. The deletion of the first exon of mouse XT2 gene in the homozygote knockout mice was confirmed by genotyping PCR (Fig. 1B).
Among 186 offspring from heterozygous XT2+/− matings, the ratios of wild-type, heterozygous, and homozygous offspring were similar to the 1:2:1 Mendelian ratio (21% [+/+], 52% [+/−], and 27% [−/−]), suggesting that the absence of XT2 gene function was not detrimental to fetal development or perinatal survival. Expression of XT2 mRNA was examined by RT-PCR and compared in the XT2-deficient and wild-type mice. As shown in Fig. 1C, while the XT2 cDNA was easily detected in wild-type mice, the specific PCR product could not be detected in XT2−/− mice.
The XT2−/− mice developed normally, and they could not be distinguished from their wild-type littermates based on their external appearance. Similarly, pathological examination of a number of organs including the kidney did not reveal significant abnormalities (data not shown).
Abnormal urinary glycine excretion in XT2-deficient mice.
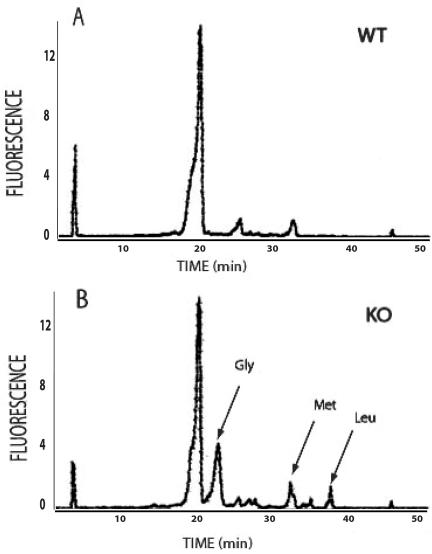
The specific substrate for the XT2 transporter protein is unknown. Since it is most highly expressed in the kidney, we reasoned that the absence of the transporter might be associated with an abnormal loss of the substrate in urine. To examine this possibility, we collected urine from XT2+/+ and XT2−/− animals and performed a whole-range analysis of mouse urine by using a HPLC-mass spectrometry. Although the excretory profiles were generally similar between the groups, we found an elution peak in the XT2−/− mice that was not present in the wild-type controls. By mass spectrometry, this HPLC elution peak was demonstrated to consist of glycine (Fig. 2). To determine whether XT2 deficiency caused a more generalized defect in amino acid handling, we used tandem mass spectrometry to assess the amino acid content of blood and urine in XT2+/+ and XT2−/− mice (Table 1). Concentrations of the 10 amino acids in blood that were examined were similar between the two groups. However, urinary glycine levels were consistently increased in the urine of XT2-deficient mice, and the urinary glycine/creatinine ratio was approximately three times higher in the XT2-deficient mice than wild-type controls (24.7 mg of glycine/mg of creatinine versus 5.8 mg of glycine/mg of creatinine, P < 0.05). In contrast, the XT2 mutation did not affect excretion of any of the other amino acids that were tested.
FIG. 2.
Urinary amino acid content analysis. The amino acid content in urine sample from wild-type XT2 mice (A) and homozygous mutants (B) was analyzed by reversed-phase HPLC after a 2-min OPA derivatization procedure. The arrows in the knockout mice profile (B) denote peaks that coeluted with the external amino acid standards.
TABLE 1.
Amino acid content in plasma and urinea
| Amino acid | Amino acid content (mM) in:
|
Reabsorption efficiency (%)
|
||||
|---|---|---|---|---|---|---|
| Plasma
|
Urine
|
|||||
| Wild type | Knockout | Wild type | Knockout | Wild type | Knockout | |
| Gly | 0.26 | 0.25 | 0.82 | 3.11 | 94.2 | 75.3 |
| Ala | 0.66 | 0.48 | 0.077 | 0.252 | 99.8 | 99.5 |
| Val | 0.46 | 0.51 | 0.065 | 0.16 | 99.9 | 99.7 |
| Pro | 1.67 | 1.67 | 0.13 | 0.24 | 99.9 | 99.9 |
| Leu/Ile | 0.24 | 0.26 | 0.027 | 0.26 | 99.9 | 99.2 |
| Met | 0.07 | 0.07 | 0.014 | 0.08 | 99.7 | 98.2 |
| Phe | 0.09 | 0.09 | 0.011 | 0.08 | 99.6 | 98.6 |
| Tyr | 0.07 | 0.07 | 0.011 | 0.076 | 99.5 | 97.5 |
| Asp | 0.06 | 0.06 | 0.019 | 0.026 | 99.2 | 99.2 |
| Glu | 0.06 | 0.06 | 0.023 | 0.21 | 99.9 | 99.7 |
Plasma and urine samples were collected as described in Materials and Methods. Results are the average of six littermates of the same genotype.
Renal accumulation of glycine is impaired in XT2-deficient mice.
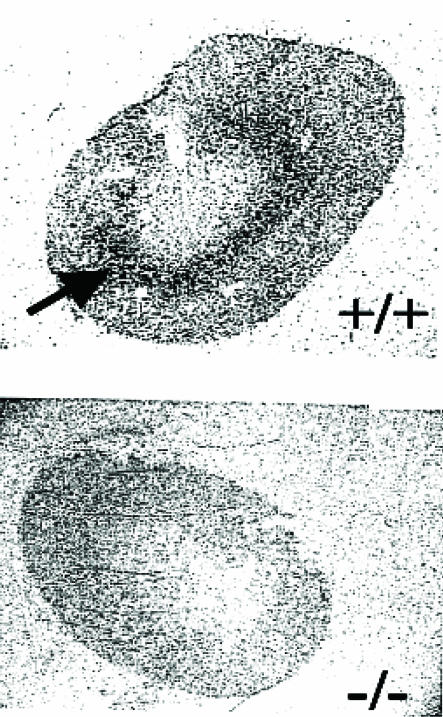
To determine whether the XT2 mutation affects renal handling of glycine, mice were injected with [14C]glycine intraperitoneally and kidneys were perfused thoroughly with phosphate-buffered saline, removed, sectioned, and [14C]glycine accumulation was determined by autoradiography. As can be seen in Fig. 3, significant glycine accumulation is easily visualized in the outer medulla. However, in kidneys from XT2-deficient mice, no accumulation of [14C]glycine could be detected above background levels.
FIG. 3.
In vivo tracing of renal glycine reabsorption. Mice were injected with 14C-labeled glycine 15 min before the subsequent anesthesia and perfusion. Kidneys were sectioned into 20-μm slices and subjected to autoradiography.
Amino acids uptake by BBMV.
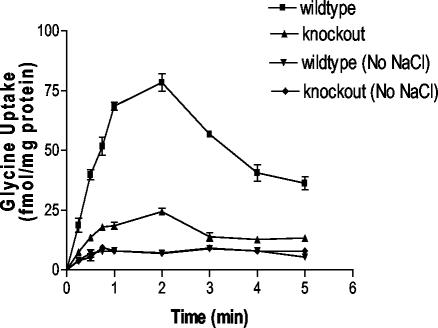
To further examine the role of XT2 in amino acids reabsorption in the kidney, we compared the kinetic properties of amino acid uptake by BBMV prepared from XT2+/+ and XT2−/− mice. Based on the methods used for the preparation of BBMV, this fraction was highly enriched for apical membranes. As shown in Fig. 4, typical time-dependent accumulation of glycine into BBMV under Na+ gradient conditions was seen in the BBMV from wild-type animals with an overshoot of uptake above equilibrium at about 2 min. Although glycine reabsorption depended on sodium concentrations in both groups, the absolute rate of glycine reabsorption was significantly reduced in the XT2-deficient mice compared to wild-type control after 1, 2, 3, 4, and 5 min of incubation (P < 0.001).
FIG. 4.
[3H]glycine uptake by BBMV. Vesicles were prepared in 1 mM MgSO4, 2 mM HEPES-Tris (pH 7.35), 50 mM K2SO4, and 250 mM mannitol. Vesicles were preincubated with valinomycin (10 mg/mg of protein). External medium contained 1 mM MgSO4, 2 mM HEPES-Tris, and either 100 mM NaCl, 200 mM mannitol, or 250 mM mannitol. Vesicles were incubated at 37°C in an uptake buffer containing 20 nM [3H]glycine.
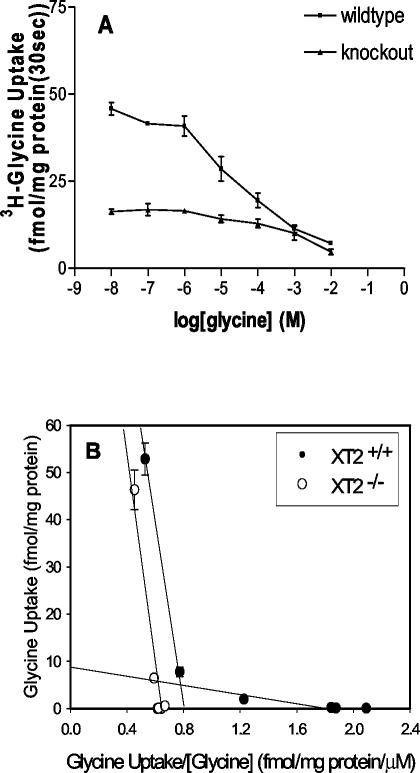
We further examined the initial rate of glycine uptake at different concentrations of substrate (Fig. 5A), the results are presented in an Eadie-Hofstee plot (Fig. 5B). At all concentrations of glycine in the experiment, BBMV from knockout mice showed only a fraction of glycine uptake activity in contrast to that from wild-type mice. Nevertheless, the difference became smaller as the glycine concentration approached 1 mM. The Eadie-Hofstee plot revealed a two-component uptake system for wild-type sample. The high-affinity component had a Km of 8.9 μM and a Vmax of 7.8 fmol/mg of protein, while the low-affinity component had a Km of 820 μM and a Vmax of 690 fmol/mg of protein. In comparison, the XT2−/− samples had no high-affinity component, and its low-affinity component was similar to that of the wild-type samples (Km = 880 μM), which suggested the vesicles from knockout mice might still have low-affinity glycine uptake capacity. The high-affinity uptake component was also dependent on the presence of sodium in the uptake solution since the substitution of sodium with mannitol abolished the time-dependent accumulation of radioactively labeled glycine by the BBMV preparation.
FIG. 5.
Kinetics of glycine uptake by mouse kidney BBMV preparations. The conditions were the same as in Fig. 3 except that different concentrations of unlabeled glycine were added to the uptake mixture. The reaction was terminated after 30 s, and the residual radioactivity inside the vesicles was taken as the initial velocity of glycine uptake. (A) [3H]glycine uptake was plotted against the concentrations of unlabeled glycine added; (B) the initial velocity of glycine uptake was plotted against the ratio of glycine uptake velocity and the total concentrations of glycine.
Experiments to assess the glycine uptake activity of XT2 were also conducted in transfected cell culture system. Each of the six splice variants of XT2 (16) was transfected into HEK 293 cells or MDCK cells. However, none of the splice variants confer detectable plasma membrane glycine uptake activity to the cells (data not shown).
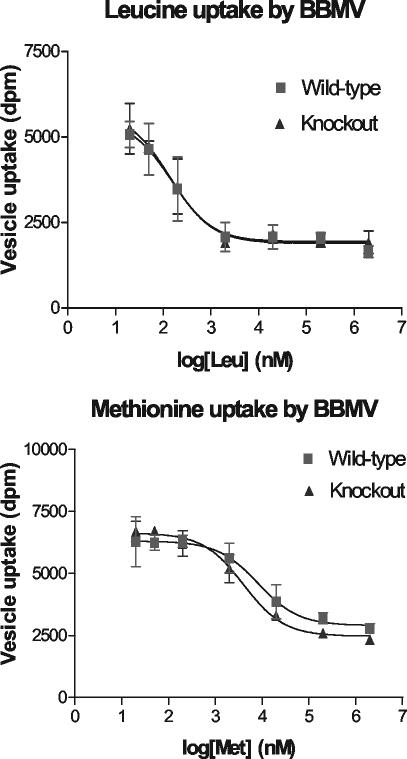
For comparison, uptake of leucine and methionine by BBMV was also examined (Fig. 6). At all concentrations, no significant differences were detected in leucine and methionine transport between BBMV from wild-type and XT2-deficient mice. Thus, the absence of XT2 protein was associated with a specific defect in glycine transport by renal BBMV preparations.
FIG. 6.
Uptake of leucine or methionine by mouse kidney BBMV preparations. The conditions were the same as Fig. 4, except that different concentrations of unlabeled leucine or methionine were added to the uptake mixture. Reaction was initiated by the addition of 20 nM of [3H]leucine or [3H]methionine.
Elevated blood pressure in XT2-deficient mice.
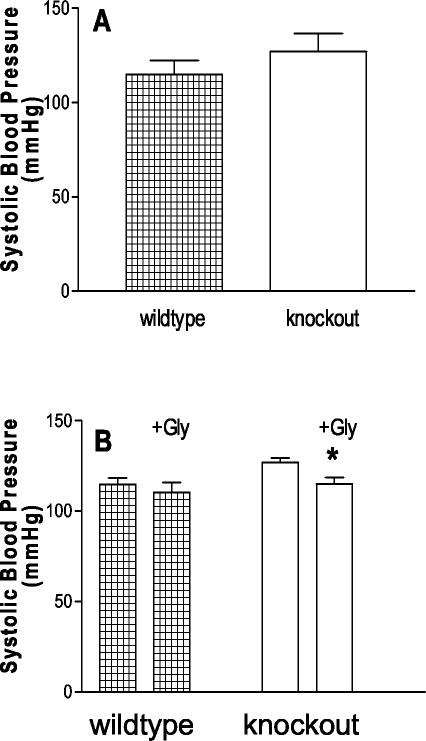
To determine whether the XT2 gene plays a role in physiological regulation, we measured blood pressures in XT2+/+ and XT2−/− mice by using computerized tail-cuff manometer. As shown in Fig. 7A, systolic blood pressure was significantly higher in XT2-deficient mice than in wild-type controls (127 ± 3 versus 114 ± 2 mmHg; P < 0.001). The elevated blood pressure in XT2-deficient mice was also maintained on both low-salt (<0.04% NaCl) and high-salt (6% NaCl) diets (Table 2).
FIG. 7.
XT2−/− mice show mild hypertension. (A) Both XT2−/− mice and their littermates were trained and recorded as described in Materials and Methods. The differences in their average blood pressures were evaluated by the Student t test (P < 0.005). (B) The same groups of mice were given 0.1 g of glycine/ml in the drinking water. The blood pressure was measured at least five times during the 2-week testing period.
TABLE 2.
Systolic blood pressure during dietary salt alterationa
| Dietary condition | Mean systolic blood pressure (mmHg) ± SD in:
|
|
|---|---|---|
| Wild-type mice | XT2−/− mice | |
| Normal salt | 120 ± 2 | 132 ± 3 |
| Low salt | 118 ± 2 | 131 ± 3 |
| High salt | 120 ± 2 | 135 ± 2 |
Both wild-type and XT2 knockout mice were fed with diets containing different salts (as described in Materials and Methods). The mean blood pressures are the averages of daily measurements of blood pressure during a 2-week period (n = 12 for each genotype).
Dietary glycine supplement lowers blood pressure in XT2-deficient mice.
We next performed studies to determine whether there was an association between abnormal glycine handling and the development of hypertension in the XT2-deficient mice. To this end, we compared blood pressures in a group of XT2−/− mice before and during supplementation with oral glycine. As shown in Fig. 7B, blood pressure fell significantly in XT2−/− mice with glycine supplement from 127 ± 3 to 115 ± 3 mmHg (P < 0.005). A similar level of glycine supplement did not significantly affect blood pressure in wild-type mice (Fig. 7B). Furthermore, two-way analysis of variance test confirmed that glycine causes a significant change to the systolic blood pressure of knockout mice (P = 0.03).
DISCUSSION
As a prototypic orphan transporter, mouse XT2 has evaded previous efforts of substrate identification (16). In the present study we disrupted the XT2 gene in mice in an attempt to identify its substrate specificity and physiological role. The knockout strategy utilized a Cre recombinase-mediated cassette deletion of the targeted locus of mouse XT2 gene where exon 1 and part of intron 1 were replaced. XT2-null mice exhibited no gross abnormalities and grew to adulthood, which facilitates the analysis of physiological consequence of XT2 gene knockout in adult mice.
Phenotypic characterization of the XT2 knockout mice revealed that the XT2 transporter played an nonredundant role in renal glycine recycling. In contrast to their wild-type littermates, XT2 knockout mice excreted four times more glycine in their urine, and their kidneys had a reabsorption efficiency of ∼75%. The deficiency of renal glycine reabsorption was also visualized by the absence of the glycine reabsorption band in the renal cortex from XT2 knockout mice. The apical membrane-enriched BBMV preparation from knockout mice showed dramatically decreased glycine uptake activity at substrate concentrations lower than 1 mM. Further analysis of the uptake kinetics by the Eadie-Hofstee method showed that the glycine uptake process was mediated by a two-component system. The two-component kinetics of glycine uptake in our experiments is consistent with previous studies in rats (3, 15, 30, 37) and rabbits (23, 35). The high-affinity component of renal glycine reabsorption was found to be absent in the knockout mice. Therefore, these results suggest that XT2 mediates the sodium-dependent high-affinity reuptake of glycine on the brush border membrane of renal proximal tubule. However, none of the six splice variants of the XT2 gene increased the plasma membrane glycine uptake activity in culture cells. These results may not be totally unexpected as XT2 may require the coexpression of other cotransporters or accessory proteins to exhibit full activity. In fact, the renal cysteine transporter was shown to be a multimer consisting of different subunits (2, 22, 25). Identification of such cofactors would be one of the major interests for future studies.
Glycine is a nonessential glucogenic amino acid that can be readily converted from serine. It is the simplest of all of the amino acids and can be synthesized from acetic acid and folic acid. Spillover of glycine in XT2 knockout mice does not affect their levels of glycine in plasma, which suggests that glycine can be generated sufficiently to compensate the continual loss of glycine. Nevertheless, plasma glycine is recycled in the kidney, and only a trace amount of glycine can be detected in urine of normal animals. Renal absorption of glycine has been detected in the S1, S2, and S3 segments of the proximal tubule. A recent study by Parks and Barfuss (20) suggested that proximal tubular transport of glycine consists of active luminal transport and passive paracellular transport across basolateral membrane. However, the passive transport of glycine in the proximal tubule can be bidirectional depending on the gradient of luminal and peritubular fluid. Since the concentration of glycine in the paracellular fluid rises as a consequence of active luminal glycine transport, its concentration may exceed that of luminal glycine, causing the back-leak of glycine into the lumen. The S3 segment of the proximal tubule exhibited the least paracellular blood to lumen flux of glycine compared to the other portions of proximal tubules. This would explain the modest reduction of renal glycine reabsorption in XT2-deficient mice.
Our data also suggest that the XT2-containing glycine uptake complex is specific for glycine. Previous studies have hypothesized that proline may share a transport system with glycine based on the observation that there is severe spillover of proline in the urine of humans with hyperglycinuria (9, 28). However, no apparent decrease in the reabsorption of proline was observed in our study, which suggests that XT2 might not contribute to the renal proline clearance. Despite the increase of leucine and methionine in the urine from XT2 knockout mice, detailed analysis of the renal reabsorption efficiency and in vitro uptake experiments showed that these two amino acids were reabsorbed into proximal tubules of knockout mice almost as efficiently as in wild-type mice.
Our animal model revealed an unexpected connection between renal glycine handling and blood pressure regulation. Blood pressures were increased by 15 to 20% in XT2-deficient mice compared to their wild-type littermates. The development of hypertension in the mice lacking XT2 is independent of dietary sodium intake since the disparity in blood pressure persisted in the face of extreme variations in dietary sodium intake. A causal role for glycine was demonstrated by our findings that the blood pressure of XT2-deficient mice fell significantly with the glycine supplements, whereas the same treatment had little effect on the blood pressure of wild-type mice.
A role for glycine in blood pressure regulation is not completely surprising. For example, glycine is a renal vasodilator that can modulate vascular tone and glomerular filtration (10, 19, 29). These hemodynamic responses to glycine are lost in experimental hypertension (21). Moreover, it has been suggested that deficiency of glycine during fetal development may cause blood pressure elevation (34). The progeny of pregnant rats fed a low-protein diet develop hypertension (34) that can be prevented if the diet is supplemented with glycine. In contrast, supplementing the diet with alanine or urea does not ameliorate hypertension. Thus, we speculate that a relative deficiency of glycine in the kidneys of XT2-deficient mice may lead to elevated blood pressure because of impaired vasodilator mechanisms and/or through developmental effects that convey susceptibility to hypertension.
In summary, we have found that functional deletion of the XT2 gene produces hypertension in mice. XT2 gene deletion causes the elimination of high-affinity renal reabsorption of glycine and a decreased intrarenal glycine concentration. The elevated blood pressure appears to be attributable to a decreased renal glycine level. The exact molecular mechanism leading to the increase in blood pressure is unclear. However, the physiological events described here resulting from XT2 gene deletion may provide new insights into mechanisms for regulation of blood pressure by renal glycine and may lead to new therapeutic approaches for the treatment of hypertension.
Acknowledgments
We thank Susan Suter for excellent work with mouse breeding and husbandry.
M.G.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by NIH grant NIH60451.
REFERENCES
- 1.Athirakul, K., H. S. Kim, L. P. Audoly, O. Smithies, and T. M. Coffman. 2001. Deficiency of COX-1 causes natriuresis and enhanced sensitivity to ACE inhibition. Kidney Int. 60:2324-2329. [DOI] [PubMed] [Google Scholar]
- 2.Chairoungdua, A., H. Segawa, J. Y. Kim, K. Miyamoto, H. Haga, Y. Fukui, K. Mizoguchi, H. Ito, E. Takeda, H. Endou, and Y. Kanai. 1999. Identification of an amino acid transporter associated with the cysteinuria-related type II membrane glycoprotein. J. Biol. Chem. 274:28845-28848. [DOI] [PubMed] [Google Scholar]
- 3.Chesney, R. W., I. Zelikovic, A. Budreau, and D. Randle. 1991. Chloride and membrane potential dependence of sodium ion-proline symport. J. Am. Soc. Nephrol. 2:885-893. [DOI] [PubMed] [Google Scholar]
- 4.El Mestikawy, S., B. Giros, M. Pohl, M. Hamon, S. F. Kingsmore, M. F. Seldin, and M. G. Caron. 1994. Characterization of an atypical member of the Na+/Cl−-dependent transporter family: chromosomal localization and distribution in GABAergic and glutamatergic neurons in the rat brain. J. Neurochem. 62:445-455. [DOI] [PubMed] [Google Scholar]
- 5.Fan, P. Y., C. R. Albrightson, D. N. Howell, C. Best, A. M. Badger, and T. M. Coffman. 1993. The azaspirane SKF 105685 ameliorates renal allograft rejection in rats. J. Am. Soc. Nephrol. 3:1680-1685. [DOI] [PubMed] [Google Scholar]
- 6.Farmer, M. K., M. J. Robbins, A. D. Medhurst, D. A. Campbell, K. Ellington, Duckworth M, A. M. Brown, D. N. Middlemiss, G. W. Price, and M. N. Pangalos. 2000. Cloning and characterization of human NTT5 and v7-3: two orphan transporters of the Na+/Cl−-dependent neurotransmitter transporter gene family. Genomics 70:241-252. [DOI] [PubMed] [Google Scholar]
- 7.Fernstrom, M. H., and J. D. Fernstrom. 1981. Rapid measurement of free amino acids in serum and CSF using high-performance liquid chromatography. Life Sci. 29:2119-2130. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, J., V. Bancila, P. Mailly, J. Masson, M. Hamon, S. El Mestikawy, and M. Conrath. 1999. Immunocytochemical evidence of vesicular localization of the orphan transporter RXT1 in the rat spinal cord. Neuroscience 92:729-743. [DOI] [PubMed] [Google Scholar]
- 9.Greene, M. L., P. S. Lietman, L. E. Rosenberg, and J. E. Seegmiller. 1973. Familial hyperglycinuria: new defect in renal tubular transport of glycine and imino acids. Am. J. Med. 54:265-271. [DOI] [PubMed] [Google Scholar]
- 10.Heyman, S. N., M. Brezis, F. H. Epstein, K. Spokes, and S. Rosen. 1992. Effect of glycine and hypertrophy on renal outer medullary hypoxic injury in ischemia reflow and contrast nephropathy. Am. J. Kidney Dis. 19:578-586. [DOI] [PubMed] [Google Scholar]
- 11.Koepsell, H. 1998. Organic cation transporters in intestine, kidney, liver, and brain. Annu. Rev. Physiol. 60:243-266. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Q. R., S. Mandiyan, B. Lopez-Corcuera, H. Nelson, and N. Nelson. 1993. A rat brain cDNA encoding the neurotransmitter transporter with an unusual structure. FEBS Lett. 315:114-118. [DOI] [PubMed] [Google Scholar]
- 13.Masson, J., X. Langlois, L. Lanfumey, C. Gerard, Z. Aidouni, B. Giros, M. Hamon, and S. el Mestikawy. 1995. Immunolabeling of the Na+/Cl−-dependent “orphan” transporter Rxt1 in the rat central nervous system. J. Neurosci. Res. 42:423-432. [DOI] [PubMed] [Google Scholar]
- 14.Masson, M. J., Riad, F. Chaudhry, M. Darmon, Z. Aidouni, M. Conrath, B. Giros, M. Hamon, J. Storm-Mathisen, L. Descarries, and S. El Mestikawy. 1999. Unexpected localization of the Na+/Cl−-dependent-like orphan transporter, Rxt1, on synaptic vesicles in the rat central nervous system. Eur. J. Neurosci. 11:1349-1361. [DOI] [PubMed] [Google Scholar]
- 15.McNamara, P. D., L. M. Pepe, and S. Segal. 1979. Sodium gradient dependence of proline and glycine uptake in rat renal brush-border membrane vesicles. Biochim. Biophys. Acta 556:151-160. [DOI] [PubMed] [Google Scholar]
- 16.Nash, S. R., B. Giros, S. F. Kingsmore, K. M. Kim, S. El Mestikawy, Q. Dong, F. Fumagalli, M. F. Seldin, and M. G. Caron. 1998. Cloning, gene structure and genomic localization of an orphan transporter from mouse kidney with six alternatively spliced isoforms. Receptors Channels 6:113-128. [PubMed] [Google Scholar]
- 17.Nelson, N. 1998. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 71:1785-1803. [DOI] [PubMed] [Google Scholar]
- 18.Obermuller, N., B. Kranzlin, R. Verma, N. Gretz, W. Kriz, and R. Witzgall. 1997. Renal osmotic stress-induced cotransporter: expression in the newborn, adult and post-ischemic rat kidney. Kidney Int. 52:1584-1592. [DOI] [PubMed] [Google Scholar]
- 19.Olsen, N. V., J. M. Hansen, S. D. Ladefoged, N. Fogh-Andersen, S. L. Nielsen, and P. P. Leyssac. 1990. Overall renal and tubular function during infusion of amino acids in normal man. Clin. Sci. 78:497-501. [DOI] [PubMed] [Google Scholar]
- 20.Parks, L. D., and D. W. Barfuss. 2002. Transepithelial transport and metabolism of glycine in S1, S2, and S3 cell types of the rabbit proximal tubule. Am. J. Physiol. Renal Physiol. 283:F1208-F1215. [DOI] [PubMed] [Google Scholar]
- 21.Qiu, C., K. Engels, L. Samsell, and C. Baylis. 1995. Renal effects of acute amino acid infusion in hypertension induced by chronic nitric oxide blockade. Hypertension 25:61-66. [DOI] [PubMed] [Google Scholar]
- 22.Rajan, D. P., R. Kekuda, W. Huang, H. Wang, L. D. Devoe, F. H. Leibach, P. D. Prasad, and V. Ganapathy. 1999. Cloning and expression of a b(0,+)-like amino acid transporter functioning as a heterodimer with 4F2hc instead of rBAT: a new candidate gene for cysteinuria. J. Biol. Chem. 274:29005-29010. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran, V. M., J. A. Barry, J. G. Kleinman, and K. Ramaswamy. 1987. Proton gradient-dependent transport of glycine in rabbit renal brush-border membrane vesicles. J. Biol. Chem. 262:14974-14977. [PubMed] [Google Scholar]
- 24.Sakata, K., S. Shimada, T. Yamashita, K. Inoue, and M. Tohyama. 1999. Cloning of a bovine orphan transporter and its short splicing variant. FEBS Lett. 443:267-270. [DOI] [PubMed] [Google Scholar]
- 25.Sato, H., M. Tamba, T. Ishii, and S. Bannai. 1999. Cloning and expression of a plasma membrane cysteine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274:11455-11458. [DOI] [PubMed] [Google Scholar]
- 26.Shah, A. J., V. de Biasi, S. G. Taylor, C. Roberts, P. Hemmati, R. Munton, A. West, C. Routledge, and P. Camilleri. 1999. Development of a protocol for the automated analysis of amino acids in brain tissue samples and microdialysates. J. Chromatogr. B Biomed. Sci. Appl. 735:133-140. [DOI] [PubMed] [Google Scholar]
- 27.Smith, K. E., S. G. Fried, M. M. Durkin, E. L. Gustafson, L. A. Borden, T. A. Branchek, and R. L. Weinshank. 1995. Molecular cloning of an orphan transporter: a new member of the neurotransmitter transporter family. FEBS Lett. 357:86-92. [DOI] [PubMed] [Google Scholar]
- 28.Statter, M., A. Ben-Zvi, A. Shina, R. Schein, and A. Russell. 1976. Familial iminoglycinuria with normal intestinal absorption of glycine and imino acids in association with profound mental retardation, a possible “cerebral phenotype.” Helv. Paediatr. Acta 31:173-182. [PubMed] [Google Scholar]
- 29.Thomsen, K., C. B. Nielsen, and A. Flyvbjerg. 2002. Effects of glycine on glomerular filtration rate and segmental tubular handling of sodium in conscious rats. Clin. Exp. Pharmacol. Physiol. 29:449-454. [DOI] [PubMed] [Google Scholar]
- 30.Torres, A. M., E. J. Ochoa, E. Guibert, J. V. Rodriguez, and M. M. Elias. 1993. Renal transport of glycine during glutathione replenishment in rats. Biochem. Med. Metab. Biol. 50:159-168. [DOI] [PubMed] [Google Scholar]
- 31.Torres, G. E., R. R. Gainetdinov, and M. G. Caron. 2003. Plasma membrane monoamine transporters: structure, regulation, and function. Nat. Rev. Neurosci. 4:13-25. [DOI] [PubMed] [Google Scholar]
- 32.Uhl, G. R., S. Kitayama, P. Gregor, E. Nanthakumar, A. Persico, and S. Shimada. 1992. Neurotransmitter transporter family cDNAs in a rat midbrain library: “orphan transporters” suggest sizable structural variations. Brain Res. Mol. Brain Res. 16:353-359. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman, J. C., E. Delpire, W. Tonidandel, R. Kojima, and S. R. Gullans. 1994. Molecular characterization of ROSIT, a renal osmotic stress-induced Na+-Cl−-organic solute cotransporter. Am. J. Physiol. 267(Pt. 2):F688-F694. [DOI] [PubMed] [Google Scholar]
- 34.Woods, L. L., J. R. Ingelfinger, J. R. Nyengaard, and R. Rasch. 2001. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 49:460-467. [DOI] [PubMed] [Google Scholar]
- 35.Wunz, T. M., and S. H. Wright. 1993. Betaine transport in rabbit renal brush-border membrane vesicles. Am. J. Physiol. 264(Pt. 2):F948-F955. [DOI] [PubMed] [Google Scholar]
- 36.Zahniser, N. R., and S. Doolen. 2001. Chronic and acute regulation of Na+/Cl−-dependent neurotransmitter transporters: drugs, substrates, presynaptic receptors, and signaling systems. Pharmacol. Ther. 92:21-55. [DOI] [PubMed] [Google Scholar]
- 37.Zelikovic, I., and A. Budreau-Patters. 1999. Cl− and membrane potential dependence of amino acid transport across the rat renal brush border membrane. Mol. Genet. Metab. 67:236-247. [DOI] [PubMed] [Google Scholar]