Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature monocytes and granulocytes that are potent inhibitors of T cell activation. A role for MDSCs in bacterial infections has only recently emerged and nothing is known about MDSC function in the context of Staphylococcus aureus (S. aureus) infection. Since S. aureus biofilms are capable of subverting immune-mediated clearance, we examined whether MDSCs could play a role in this process. CD11b+Gr-1+ MDSCs represented the main cellular infiltrate during S. aureus orthopedic biofilm infection, accounting for over 75% of the CD45+ population. Biofilm-associated MDSCs inhibited T cell proliferation and cytokine production, which correlated with a paucity of T cell infiltrates at the infection site. Analysis of FACS-purified MDSCs recovered from S. aureus biofilms revealed increased Arg-1, iNOS, and IL-10 expression, key mediators of MDSC suppressive activity. Targeted depletion of MDSCs and neutrophils using the mAb 1A8 (anti-Ly6G) improved bacterial clearance by enhancing the intrinsic pro-inflammatory attributes of infiltrating monocytes and macrophages. Furthermore, the ability of monocytes/macrophages to promote biofilm clearance in the absence of MDSC action was revealed with RB6-C85 (anti-Gr-1 or anti-Ly6G/Ly6C) administration, which resulted in significantly increased S. aureus burdens both locally and in the periphery, since effector Ly-6C monocytes and by extension, mature macrophages, were also depleted. Collectively, these results are the first to demonstrate that MDSCs are key contributors to the chronicity of S. aureus biofilm infection, as their immunosuppressive function prevents monocyte/macrophage proinflammatory activity, which facilitates biofilm persistence.
Keywords: S. aureus, biofilm, orthopedic infection, myeloid-derived suppressor cells, inflammatory monocytes, macrophages
INTRODUCTION
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature monocytes and granulocytes that are potent inhibitors of T cell activation (1). In mice, MDSCs are characterized by their expression of CD11b and Gr-1, but can be further subdivided into monocyte- and granulocyte-like subsets based on their differential expression of Ly6C and Ly6G, which are referred to as M-MDSC and G-MDSCs, respectively (2, 3). CD11b+Gr-1+ cells normally reside in the bone marrow prior to their differentiation into mature granulocytes, macrophages, or dendritic cells. However, MDSCs can be recruited into lymphoid and inflamed tissues during pathologic conditions by the actions of growth factors, such as G-CSF, GM-CSF, and VEGF, where disturbances in cytokine homeostasis block their differentiation into mature myeloid effector cells, resulting in MDSC expansion (3, 4). Several factors influence MDSC activation, including proinflammatory cytokines driven by MyD88-dependent signaling (i.e. IL-6), reactive oxygen species (ROS), and cyclooxygenase-2 (COX-2). These proinflammatory molecules induce the expression of arginase-1 (Arg-1) and several anti-inflammatory cytokines that not only contribute to the inhibition of T cell responses, but may also play a role in macrophage polarization towards an alternatively activated M2 phenotype (4). Although MDSCs are well-recognized for their role in tumor immunosuppression (1, 4, 5), recent evidence suggests that MDSCs can also regulate immune responses during bacterial infections (6–10).
Staphylococcus aureus (S. aureus) is a leading cause of community-acquired and nosocomial infections (11, 12). Infection risk is increased by the presence of foreign materials, and S. aureus is a leading cause of biofilm infections on indwelling medical devices and orthopedic implants (13, 14). Biofilms are heterogeneous bacterial communities encased in a self-produced matrix that represent a serious health care concern based on their chronicity and recalcitrance to antibiotic therapy (15). Previous work from our laboratory has shown that S. aureus biofilms skew macrophages toward an alternatively activated M2 anti-inflammatory phenotype, typified by robust Arg-1 expression that correlates with the failure to recruit T cells to the site of infection (16). However, Arg-1 expression was also detected in other cell types, leading us to examine the identity of alternative Arg-1+ cells associated with S. aureus biofilms. In the current study, we have identified a predominant CD11b+Gr-1+Arg-1+ MDSC infiltrate that contributes to the anti-inflammatory environment typical of S. aureus biofilm-associated infections.
Here we sought to examine the functional role of MDSCs in shaping the anti-inflammatory milieu during S. aureus orthopedic biofilm infection. Although we identified MDSCs using well-established markers (17–19), their ability to attenuate T cell proliferation was required to establish their identity as a bona fide MDSC population. Indeed, we found that MDSCs infiltrating S. aureus biofilms were capable of inhibiting T cell proliferation, which represents the first report of MDSCs in any type of staphylococcal infection. Furthermore, qRT-PCR analysis of FACS-purified MDSCs revealed increased expression of typical MDSC molecules, including Arg-1, iNOS, and IL-10. Administration of mAb 1A8 (anti-Ly6G), which specifically depleted the immunosuppressive MDSC population and mature neutrophils, significantly increased monocyte and macrophage proinflammatory activity, which translated into decreased S. aureus burdens in the infected joint. Independent evidence to support the importance of monocytes/macrophages in biofilm containment in the absence of MDSCs was demonstrated by the finding that RB6-C85 (anti-Gr-1 or anti-Ly6G/Ly6C) treatment, which depleted effector monocytes and macrophages in addition to MDSCs and granulocytes, significantly increased S. aureus burdens and proinflammatory mediator expression as well as bacterial dissemination to peripheral organs. These results indicate that MDSCs establish an anti-inflammatory milieu during S. aureus biofilm infection that thwarts monocyte and macrophage proinflammatory activity, leading to persistent colonization. This prominent MDSC infiltrate also explains the paucity of T cells associated with S. aureus biofilms. Collectively, these studies demonstrate a role for MDSCs during staphylococcal biofilm infection and preventing their immunosuppressive actions may offer novel treatment strategies to thwart these devastating, chronic infections.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice (8 weeks of age) were purchased from the National Cancer Institute (Frederick, MD). These studies were performed in strict accordance with recommendations found in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center.
Mouse model of S. aureus orthopedic biofilm infection
To simulate infectious complications in patients following surgical device placement, a mouse S. aureus orthopedic implant infection model was utilized as previously described with minor modifications (20). Animals were anesthetized with ketamine/xylazine (Hospira, Inc., Lake Forest, IL and Akorn, Inc., Decatur, IL; 100 mg/kg and 5 mg/kg, respectively) and the surgical site was disinfected with povidone-iodine. A medial parapatellar arthrotomy with lateral displacement of the quadriceps-patella was performed to access the distal femur. A burr hole was created in the femoral intercondylar notch extending into the intrameduallary canal using a 26-gauge needle, whereupon a pre-cut 0.8 cm orthopedic-grade Kirschner (K)-wire (0.6 mm diameter, Nitinol [nickel-titanium]; Custom Wire Technologies, Inc. Port Washington, WI) was inserted into the intramedullary canal, leaving approximately 1 mm protruding into the joint space. A total of 103 colony forming units (CFU) of the bioluminescent S. aureus USA300 LAC::lux isolate (16) was inoculated at the implant tip. In some experiments, control mice received sterile implants using an identical procedure. Animals received Buprenex (0.1 mg/kg s.c.; Reckitt Benckiser, Hull, England) immediately after infection and 24 h later for pain relief. After this interval, all mice exhibited normal ambulation and no discernable pain behaviors.
Scanning electron microscopy (SEM)
Mice were sacrificed at day 28 following S. aureus infection, whereupon the whole femur harboring the titanium implant was fixed in 0.1 M Sorensen’s phosphate buffer containing 2% glutaraldehyde and 2% paraformaldehyde for 1 h at room temperature and held in fixative overnight at 4° C. Fixed specimens were washed three times in Tris buffered saline followed by three rinses in ddH2O and decalcification in 14% EDTA for two days. After rinsing in ddH2O, specimens were dehydrated using a graded series of ethanol washes and critical pointed dried in a Pelco CPD2 critical point dryer (Ted Pella, Inc., Redding, CA). Dried specimens were mounted on aluminum stubs with carbon tabs and colloidal silver paste and sputter coated with gold-palladium using a Hummer VI sputter coater (Anatech, LTD, Battle Creek, MI). Samples were viewed using a Quanta 200 scanning electron microscope (FEI, Hillsboro, OR) operated at 25 Kv.
In vivo depletion studies
To deplete MDSCs in vivo, mice received i.p. injections of either 1A8 (anti-Ly6G) or RB6-C85 (anti-Gr-1) Abs (100 µg/each) one day prior to S. aureus infection and every 72 h thereafter until sacrifice. Control mice received equivalent amounts of isotype-matched control Abs (rat IgG2a and IgG2b, respectively) using the same treatment regimen. All Abs were purchased in Ultra-LEAF form (low endotoxin, azide-free) from BioLegend (San Diego, CA). Animals were euthanized at 7 or 14 days after infection to determine the impact of cell depletion on S. aureus persistence and tissue-associated leukocyte infiltrates. Bone marrow and splenocytes were also collected to determine the efficiency of Ab-mediated depletion. A separate model of S. aureus catheter-associated biofilm infection was utilized in some experiments as previously described to confirm the action of RB6-C85 Ab depletion (16, 21).
Computed tomography (CT) of S. aureus orthopedic biofilm infections
Bone integrity in the context of Gr-1+ cell depletion during S. aureus orthopedic biofilm infection was monitored using live CT scans. Briefly, mice were anesthetized with 1.5% isoflurane in a 70% nitrous oxide/30% oxygen mixture and imaged using a FLEX Triumph X-ray computed tomography/single photon emission computed tomography (CT/SPECT) system and software (TriFoil Imaging, Inc., Northridge, CA). 1024 CT projections for each image were acquired at 75kVp and reconstructed using Triumph X-O 4.1. CT images were generated using the 3D image visualization and analysis software VIVID, which is based on Amira 4.1 (TriFoil Imaging, Inc.).
Recovery of orthopedic implant and surrounding tissues for S. aureus enumeration
For collecting inflamed soft tissue surrounding the infected knee joint, the skin was removed and the subcutaneous tissue dorsal to the patellar tendon was excised, weighed, and processed for flow cytometery as described below. Muscle and tendon tissues were excluded from the analysis. After processing, a small aliquot was removed for quantitation of bacterial burdens. Next, the implant was extracted from the femur and sonicated for 5 min in 1 ml of PBS to dislodge bacteria from the implant. The knee joint (including cartilage and ligaments) and femur were homogenized using two sequential procedures due to the resilient nature of these tissues; initially a 30 sec dispersal using a hand-held homogenizer, followed by disruption in a Bullet Blender (Next Advance, Inc. Averill Park, NY) using 100 µm stainless steel beads (0.9–2.0 mm stainless steel blend). After centrifugation, serial 10-fold dilutions of tissue, knee, or femur homogenates as well as implant sonicates were plated on trypticase soy agar with 5% sheep blood (Remel Products, Lenexa, KS). Titers are expressed as CFU per gram of tissue or per ml for titanium implants. Remaining homogenates were centrifuged (20,000 × g, 20 min) and frozen at −80°C for further analysis by MILLIPLEX bead arrays as described below.
Morphologic and histologic analysis
To confirm that FACS-purified CD11b+Gr-1+ and Ly6G+Ly6C+ cells recovered from infection sites appeared morphologically similar to MDSCs, cells were adhered to glass slides by cyto-centrifugation (Cytopro, Wescor; Logan, UT) and stained with StainRITE (Polysciences, Inc., Warrington, PA). Images were obtained using a Zeiss Axioskop 40 microscope (Zeiss, Thornwood, NY). For hemotoxylin and eosin (H&E) staining, implant-associated tissues were fixed in 10% formalin and washed with ddH2O prior to decalcification (Super Decalcification I-Delicate Decalcifier, Polysciences, Inc., Warrington, PA), according to the manufacturer’s instructions. Decalcified tissue was washed thoroughly with ddH2O before an incision was made in the quadriceps muscle and the femur to remove the implant. Tissues were then embedded in paraffin, with 4 µm sections mounted for H&E staining. H&E stained tissues were evaluated for inflammatory changes by a board certified pathologist (J.A.K.) with the degree of inflammation determined using a scoring scale (where (0), no observable pathology; (1), low; (2), moderate; and (3), severe pathology). To evaluate splenic architecture following Ab-mediated cell depletion, spleens were fixed in 10% formalin, paraffin embedded, and sectioned for H&E staining.
MILLIPLEX multi-analyte bead array
To evaluate the effects of Ly6G-versus Gr-1-mediated cell depletion on the inflammatory milieu during S. aureus orthopedic biofilm infection, a custom-designed mouse microbead array was used (MILLIPLEX; Millipore Corporation, Billerica, MA), which detects the following mediators: G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, CCL2, CCL3, CCL5, CXCL1, CXCL2, CXCL9, CXCL10, TNF-α and VEGF. A Bio-Plex workstation (Bio-Rad, Hercules, CA) was used to analyze results and values were normalized to the total amount of protein recovered from each sample.
Flow Cytometry
To characterize leukocyte infiltrates in inflamed soft tissues surrounding the knee joint during S. aureus biofilm infection, tissues were excised, dissociated using the rubber end of a plunger from a 3 cc syringe, and passed through a 35 µm filter (BD Falcon, Bedford, MA). The resulting filtrate was washed with 1× PBS and cells were collected by centrifugation (300 × g, 10 min), whereupon RBCs were lysed using BD Pharm Lyse (BD Biosciences; San Diego, CA). After lysis, cells were resuspended in PBS containing 2% FBS, followed by incubation in Fc Block (BD Biosciences, San Diego, CA) to minimize non-specific antibody binding. Cells were stained with CD45-APC, Ly6G-PE, Ly6C-PerCPCy5.5, F4/80-PE Cy7, CCR2-FITC (R&D Systems; Minneapolis, MN), and CD11b-eFluor450. All fluorochrome-conjugated antibodies were purchased from BD Biosciences (San Diego, CA) or eBioscience (San Diego, CA) unless otherwise indicated. An aliquot of cells was stained with isotype-matched control antibodies to assess the degree of non-specific staining and fluorescence minus one was used to identify gating thresholds (22). The number of events analyzed ranged from 20,000–100,000 per sample, depending on the experimental setup. Analysis was performed using BD FACSDiva software with cells gated on the total leukocyte population (CD45+).
MDSC recovery from S. aureus orthopedic biofilm infections for T cell proliferation assays
MDSCs were collected from the soft tissues surrounding infected knee joints as described above, using either Gr-1-PE and CD11b-FITC or Ly6G-PE, Ly6C-PerCP Cy5.5 and CD11b-eFluor450 depending on the experimental setup. For comparisons, CD11b+Gr-1+ MDSCs were isolated from the spleens of naïve and S. aureus-infected animals. The purity of MDSC populations was not examined post-sort due to limiting cell numbers. However, cytospins and gene expression analysis revealed that sorted MDSCs were highly enriched, as they displayed characteristic markers and nuclear morphologies consistent with that reported for MDSCs in the literature. For CD4+ T cell isolation, spleens from naïve mice were pressed through a 250 µm Nitex filter (Genesee, San Diego, CA) to generate a single cell suspension. RBCs were lysed using BD Pharm Lyse and splenocytes were incubated in Fc Block and subsequently stained with CD4-Pacific blue (BD Biosciences). CD4+ T cells collected by FACS were greater than 95% pure and were immediately labeled with efluor670 Cell Proliferation Dye (eBioscience) according to the manufacturer’s instructions.
For establishing the functional activity of MDSCs associated with S. aureus orthopedic biofilm infections, T cell proliferation assays were performed. Briefly, efluor670-labeled CD4+ T cells were plated at 1.5×105 cells/well in a 96-well round bottom plate in RPMI-1640 with 10% FBS, supplemented with 100 ng/ml recombinant mouse IL-2 (Invitrogen; Frederick, MD). FACS-purified Gr-1+CD11b+, Ly6GhighLy6C+, Ly6GlowLy6Clow, or Ly6G−Ly6C+ cells were added at 1:1 or 1:5 ratios to CD4+ T cells subjected to polyclonal stimulation with CD3/CD28 Dynabeads (Gibco; Oslo, Norway), since TCR immunodominant epitopes for S. aureus are not defined. Cells were incubated at 37°C for 72 h, whereupon the extent of T cell proliferation was determined by flow cytometry and supernatants were saved for cytokine evaluation by MILLIPLEX analysis.
Quantitative real-time reverse transcription-PCR (qRT-PCR)
Ly6GhighLy6C+ MDSCs and Ly6G−Ly6C+ inflammatory monocytes from S. aureus infected tissues were purified by FACS, whereupon total RNA was immediately isolated using the TaqMan Gene Expression Cells-to-CT™ Kit (Ambion, Austin, TX). qRT-PCR was performed using TaqMan primer/probe mixes (Foster City, CA) for the following genes of interest: iNOS, Arg-1, COX-2, IL-10, IL-13, and IL-12p40. Gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and are presented as the fold-induction (2−ΔΔCt) value for Ly6GhighLy6C+ MDSCs relative to Ly6G−Ly6C+ monocyte fraction.
In vitro macrophage and MDSC experiments
Ly6GhighLy6C+ MDSCs were isolated from S. aureus implant-associated tissues or spleens at day 14 after infection by FACS as described above and bone marrow-derived macrophages were prepared as previously reported (23). Cells were plated at 5×104 cells/well in a 96-well plate and stimulated with either PGN (10 µg/ml) or heat-inactivated S. aureus (107/well) for 24 h. After a 24 h incubation period, supernatants were collected and stored at −80°C until MILLIPLEX analysis.
Statistics
Significant differences between experimental groups were determined by an unpaired two-tailed Student t-test or a one-way ANOVA with Bonferroni’s multiple comparison post-hoc analysis using GraphPad Prism version 4 (LaJolla, CA). For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Accumulation of CD11b+Gr-1+ cells during S. aureus orthopedic biofilm infection
We recently reported that S. aureus biofilms skew infiltrating macrophages towards an alternatively activated M2 state typified by Arg-1 expression (16, 24). However, other Arg-1+ cells distinct from macrophages were also observed, which led us to investigate their identity. A likely candidate was MDSCs based on their robust Arg-1 expression and well described anti-inflammatory attributes in cancer (2, 4, 5). Here we utilized a mouse model of orthopedic biofilm infection (20) to demonstrate the presence and functional importance of MDSCs in shaping the anti-inflammatory biofilm milieu in an immunocompetent host. Biofilm formation on the orthopedic implant was confirmed by SEM, which revealed S. aureus attachment to a dense matrix deposited on the implant surface and bacterial tower formation (Fig 1). A prominent CD11b+Gr-1+ infiltrate was observed, which accounted for approximately 75% of the total CD45+ leukocyte population by day 14 after infection (Fig 2A and B). Co-expression of CD11b and Gr-1 is used to define MDSCs and cytospin preparations of FACS-purified CD11b+Gr-1+ cells recovered from the site of S. aureus biofilm infection confirmed their heterogeneous composition of both granulocytic and monocytic morphologies (Fig 2C). In particular, cells with ringed nuclei suggested the presence of immature granulocytes, and immature monocytes with large rounded nuclei and little cytoplasm were also observed (Fig 2C). CD11b+Gr-1+ cells were also detected in mice receiving sterile implants, which was not unexpected, since MDSCs have been reported in virtually every inflammatory environment and are associated with wound healing responses under normal conditions (19, 25); however, their numbers were significantly lower compared to S. aureus infected animals (Fig 2B). The abundance of CD11b+Gr-1+ cells during early S. aureus orthopedic infection may be one mechanism that contributes to the establishment of chronic disease.
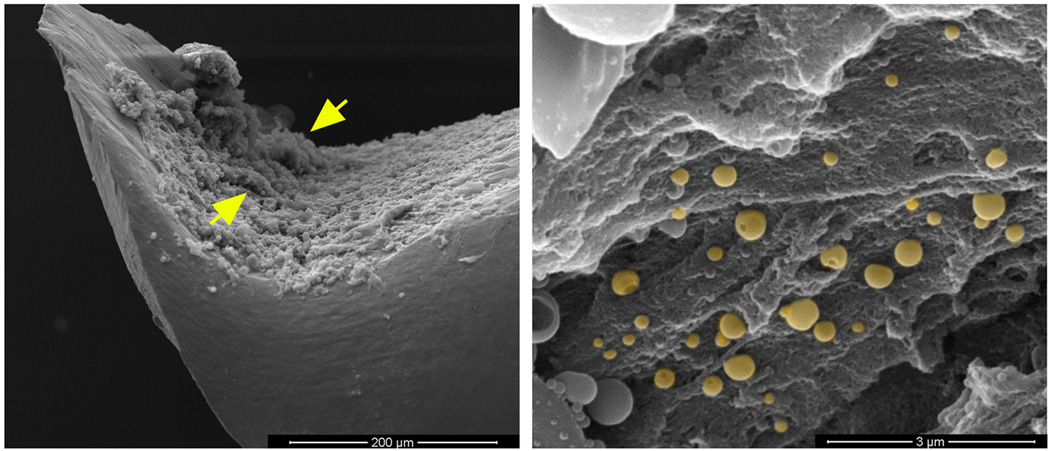
Figure 1. Demonstration of S. aureus biofilm formation in vivo on orthopedic implants.
Titanium orthopedic implants were isolated from wild type mice at day 28 following S. aureus infection and processed for SEM analysis. (A) Biofilm formation is visible on the concave surface of the implant (300× magnification) demonstrating the irregular pattern of the biofilm surface with tower structures visible (arrows); (B) Higher magnification of the biofilm surface revealing numerous cocci interspersed with matrix material (20,000× magnification). The image has been pseudocolored to highlight S. aureus (gold).
Figure 2. Accumulation of CD11b+Gr-1+ cells during S. aureus orthopedic biofilm infection.
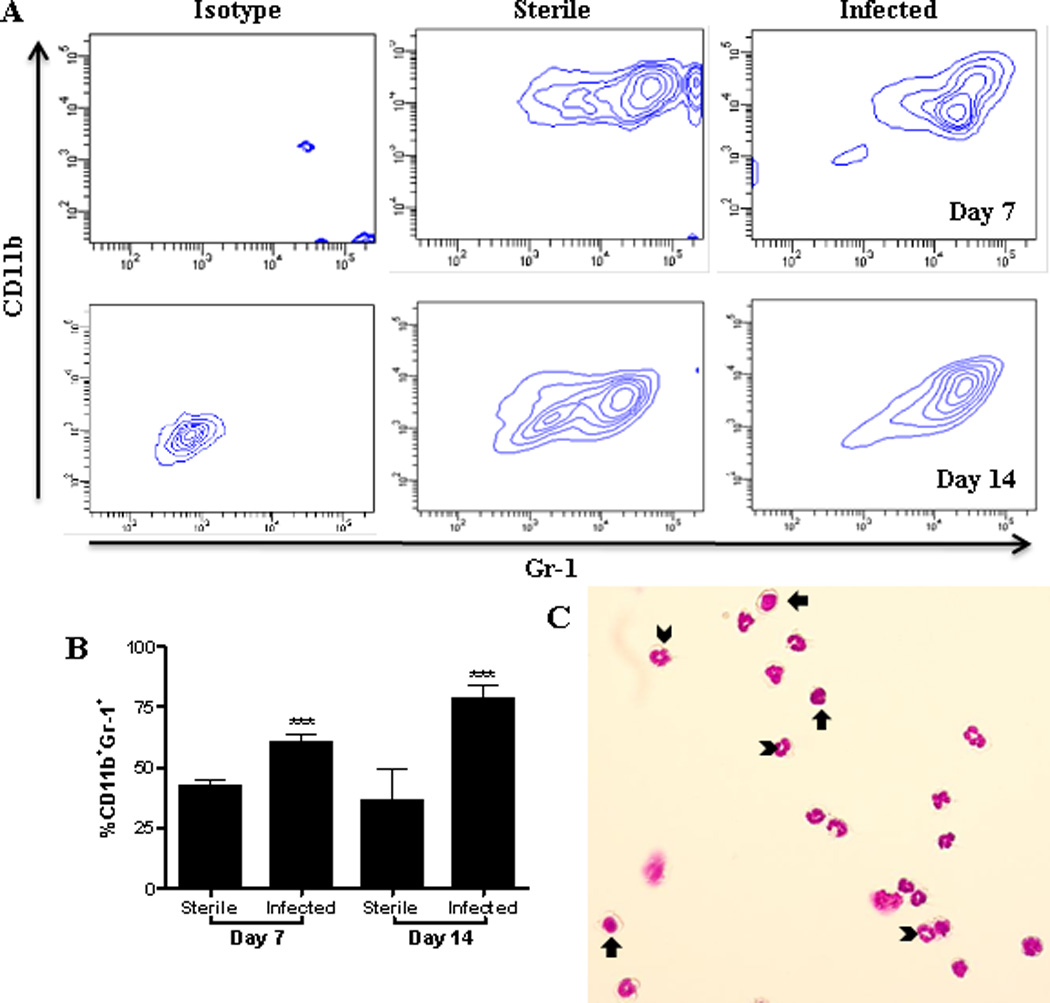
Implant-associated tissues were collected from sterile and infected mice and analyzed by flow cytometry for CD11b+Gr-1+ cells at the indicated time points. (A) Representative contour plots and (B) CD11b+Gr-1+ infiltrates expressed as a percent of the total CD45+ leukocyte population. (C) Cytospin preparations of FACS-purified CD11b+Gr-1+ cells from infected tissues at day 14. Arrowheads and arrows indicate cells suggestive of immature granulocytes and monocytes, respectively. Significant differences are denoted by asterisks (***, p < 0.001; unpaired two-tailed Student t-test) and are representative of 3 sterile and 5 infected mice per group.
CD11b+Gr-1+ MDSCs recruited to the site of S. aureus orthopedic biofilm infection inhibit T cell activation
A hallmark of MDSCs is their ability to inhibit antigen-specific and polyclonal T cell activation (4, 18). This is a critical attribute based on the promiscuity in surface marker expression between MDSCs and other myeloid lineages (25, 26). To determine whether S. aureus biofilm-associated CD11b+Gr-1+ infiltrates were bona fide MDSCs, we examined their ability to inhibit polyclonal CD4+ T cell activation, since S. aureus immunodominant TCR epitopes have not yet been identified. MDSCs were recovered from tissues at day 14, which coincided with maximum cell numbers at the infection site (Fig 2B). CD11b+Gr-1+ cells from S. aureus-infected tissues significantly suppressed T cell proliferation (Fig 3A), establishing their identity as MDSCs. The inhibitory activity of biofilm-associated MDSCs was further demonstrated by their ability to significantly impair T cell cytokine secretion, including TNF-α, IFN-γ, IL-17, and IL-4 (Fig 3B–E).
Figure 3. CD11b+Gr-1+ infiltrates from the site of S. aureus biofilm infection inhibit T cell proliferation.
FACS-purified CD11b+Gr-1+ cells recovered from infected joint tissues at day 14 were immediately cultured ex vivo with efluor670-labeled CD4+ T cells at a 1:1 ratio for proliferation assays. (A) Representative histograms of fluorescence intensity, with percent proliferation reported. (B-E) Supernatants from MDSC-CD4+ T cell co-cultures were collected at 72 h to quantitate TNF-α (B), IFN-γ (C), IL-17 (D), and IL-4 (E) by MILLIPLEX. Significant differences are denoted by asterisks (*, p < 0.05; ***, p < 0.001; unpaired two-tailed Student t-test). ND; Not detected; (−) T cells only; (+) T cells incubated with CD3/CD28 Dynabeads. Results are representative of three to nine independent experiments.
We next determined whether the immunosuppressive nature of MDSCs was restricted to the biofilm infection site or if they were also suppressive in the periphery, which has been reported for MDSCs in tumor-bearing animals (27, 28). CD11b+Gr-1+ cells from the spleens of either naïve or infected animals were unable to suppress CD4+ T cell proliferation (data not shown). It was not unexpected that MDSCs from naïve animals failed to inhibit T cell activation, as pathologic conditions are known to elicit MDSC expansion and activation (2, 4, 29, 30). Several groups have reported that MDSCs only acquire suppressive function after exposure to factors in inflammatory environments (25, 27, 31) and our results suggest that these signals are only present within in the local biofilm milieu.
Since the Gr-1 Ab RB6-C85 recognizes both Ly6G and Ly6C epitopes (32), we stained for both markers and identified three distinct populations associated with S. aureus orthopedic biofilms, namely Ly6GhighLy6C+, Ly6GlowLy6Clow, and Ly6G−Ly6C+ (Fig 4A). Each subset was purified by FACS to determine which was responsible for the observed CD4+ T cell suppression of the original Gr-1+ population (Fig 3). Ly6GhighLy6C+ cells significantly inhibited CD4+ T cell proliferation in a ratio-dependent manner, confirming their identity as MDSCs (Fig 4E). Similar to observations with the bulk CD11b+Gr-1+ population (Fig 3), Ly6GhighLy6C+ cells decreased TNF-α and IL-17 expression (Fig 4F and G, respectively). Cytospins of the Ly6GhighLy6C+ population revealed an immature granulocytic morphology characterized by numerous ringed nuclei (Fig 4B), which when taken together with their suppressive action, is highly suggestive of these cells as G-MDSCs. The Ly6G−Ly6C+ population was typified by a relatively homogeneous monocyte-like morphology that was unable to suppress CD4+ T cell activation (Fig 4D and E), suggesting these cells are inflammatory monocytes. Collectively, these results are the first to demonstrate the recruitment of a bona fide MDSC population in any model of staphylococcal infection or biofilm formation caused by any bacterial species.
Figure 4. Ly6GhighLy6C+ cells infiltrating S. aureus biofilms are bona fide MDSCs.
Leukocyte infiltrates associated with S. aureus-infected joints were collected at day 14 and analyzed for Ly6C and Ly6G expression by flow cytometry. Representative contour plot (A) and cytospin preparations of FACS-purified Ly6GhighLy6C+ (B), Ly6GlowLy6Clow (C), and Ly6G−Ly6C+ (D) cells. (E) Analysis of ex vivo polyclonal CD4+ T cell proliferation following a 1:1 and 1:5 co-culture with Ly6GhighLy6C+s, Ly6GlowLy6Clow, and Ly6G−Ly6C+ cells for 72 h, whereupon conditioned supernatants were assessed for TNF-α (F) and IL-17 (G) expression by MILLIPLEX. Significant differences are denoted by asterisks (*, p < 0.05; ***, p < 0.001; one-way ANOVA with Bonferroni’s multiple comparison post-hoc analysis) and results are representative of three to nine replicates. ND, Not detected; (−) T cells only; (+) T cells incubated with CD3/CD28 Dynabeads.
Studies by other groups have reported neutrophil infiltrates in mouse models of S. aureus orthopedic infection (33–35). However, these reports utilized either immunostaining with Ly6G, Ly6G depletion, or LysM-GFP mice to identify neutrophils and, as our results demonstrate, these approaches cannot differentiate between neutrophils and MDSCs (36). It is possible that the Ly6GlowLy6Clow cells observed in our model of S. aureus orthopedic biofilm infection are neutrophils based on their cytospin morphology, revealing fewer immature cells compared to the MDSC population (Figs 4C and B, respectively), and lack of T cell suppressive activity (Fig 4E).
Ly6GhighLy6C+ cells recruited to sites of S. aureus orthopedic biofilm infection express genes characteristic of MDSCs
Due to the differential immunosuppressive properties of the Ly6GhighLy6C+ and Ly6G−Ly6C+ subsets associated with S. aureus orthopedic biofilm infection, we next examined gene expression profiles of FACS-purified populations immediately ex vivo by qRT-PCR as further confirmation of their identity. The Ly6GhighLy6C+ MDSC subset displayed increased iNOS, Arg-1, COX-2, and IL-10 concomitant with reduced IL-12p40 expression compared to the Ly6G−Ly6C+ monocytic fraction (Fig 5), similar to MDSC profiles described in other disease models (1, 37–40).
Figure 5. Ly6GhighLy6C+ biofilm-associated infiltrates express genes characteristic of MDSCs.
FACS-purified Ly6GhighLy6C+ MDSCs and Ly6G−Ly6C+ inflammatory monocytes were recovered from infected joint tissues at day 14, whereupon RNA was immediately isolated for qRT-PCR analysis. Gene expression levels in Ly6GhighLy6C+ MDSCs were calculated after normalizing signals against GAPDH and are presented as the fold-change relative to the Ly6G−Ly6C+ monocyte population. Results represent the mean ± SEM of three independent experiments.
MDSCs play an important role in regulating inflammatory processes through their production of several pro- and anti-inflammatory cytokines (7, 25). To assess the inflammatory status of MDSCs, cells were recovered from the site of biofilm infection or the spleen and immediately stimulated ex vivo with heat-inactivated S. aureus or PGN. We found that regardless of their origin, Ly6GhighLy6C+ MDSCs were inherently less proinflammatory than macrophages (Supplemental Fig 1). Collectively, these results provide further evidence to support the identity of infiltrating Ly6GhighLy6C+ cells into S. aureus orthopedic biofilm infections as MDSCs.
Depletion of Ly6G+ MDSCs increases monocyte infiltrates and their intrinsic proinflammatory activity, resulting in enhanced S. aureus biofilm clearance
To assess the functional role of Ly6GhighLy6C+ MDSCs in orchestrating the anti-inflammatory biofilm milieu to facilitate bacterial persistence, mice were treated with the mAb 1A8 to target Ly6G+ cells (37, 41, 42). This approach would deplete MDSCs, leaving the Ly6C+ monocyte and macrophage populations intact and able to combat S. aureus infection, presumably in the absence of immunosuppression. We confirmed that 1A8 was effective at depleting the Ly6GhighLy6C+ MDSC population, which was more robust at day 7 compared to day 14 (Fig 6A and B). Interestingly, the frequency of Ly6C+ monocytes was significantly increased at day 7 (Fig 6C) and we predicted that the absence of immunosuppressive Ly6G+ MDSCs would promote the proinflammatory attributes of these Ly6C+ mononuclear phagocytes. To address this possibility, we examined the activation state of FACS-purified Ly6G−Ly6C+ cells from the infection site of 1A8-treated versus isotype control mice by qRT-PCR. In the context of MDSC depletion with 1A8, iNOS, IL-12p40, and IL-6 expression was increased in Ly6G−Ly6C+ cells at day 7 (Fig 7). Increased Arg-1 and IL-10 expression was also observed in Ly6G−Ly6C+ cells (Fig 7) and although both possess anti-inflammatory properties, they may be important in maintaining a balanced inflammatory environment at the site of infection, due to the absence of normally immunosuppressive MDSCs.
Figure 6. MDSC depletion augments monocyte recruitment during S. aureus orthopedic biofilm infection.
Implant-associated tissues from 1A8- and isotype control-treated mice were collected at the indicated time points after infection and analyzed by flow cytometry. (A) Representative contour plots of Ly6C and Ly6G staining and (B) quantitation of Ly6G+Ly6C+ MDSCs and (C) Ly6C+ inflammatory monocytes. Results are presented as a percentage of the total CD45+ leukocyte infiltrate and are representative of two independent experiments (n = 10 mice per group). Significant differences are denoted by asterisks (**, p < 0.01; ***, p < 0.001; unpaired two-tailed Student t-test).
Figure 7. MDSC depletion enhances intrinsic proinflammatory gene expression in Ly6C+ monocytes during S. aureus biofilm infection.
Ly6G−Ly6C+ monocytes were purified from tissues surrounding the infected joints of 1A8- and isotype control-treated mice at days 7 and 14 post-infection by FACS, whereupon RNA was immediately isolated for qRT-PCR analysis. Gene expression levels in Ly6G−Ly6C+ monocytes recovered from MDSC depleted animals were calculated after normalizing signals against GAPDH and are presented as the fold-change relative to Ly6G−Ly6C+ cells from isotype control mice. Results represent the mean ± SEM of two independent experiments.
Because Ly6C+ monocyte infiltrates were increased in the context of MDSC depletion and displayed intrinsic proinflammatory activity, we next examined whether this would translate into superior anti-biofilm activity. This prediction was confirmed, since Ly6G+ cell depletion with 1A8 significantly reduced S. aureus burdens in both the tissue and knee joint at days 7 and 14 compared to isotype control animals (Fig 8B), which correlated with less gross evidence of exudate formation in MDSC-depleted mice (Fig 8A). Ly6G+ cell depletion did not cause S. aureus dissemination from the primary site of infection (Fig 8C) and histopathologic analysis of H&E stained tissues showed no dramatic differences in the degree of joint inflammation or splenic architecture (as a measure of extramedullary hematopoiesis) between 1A8-treated and isotype control animals (data not shown).
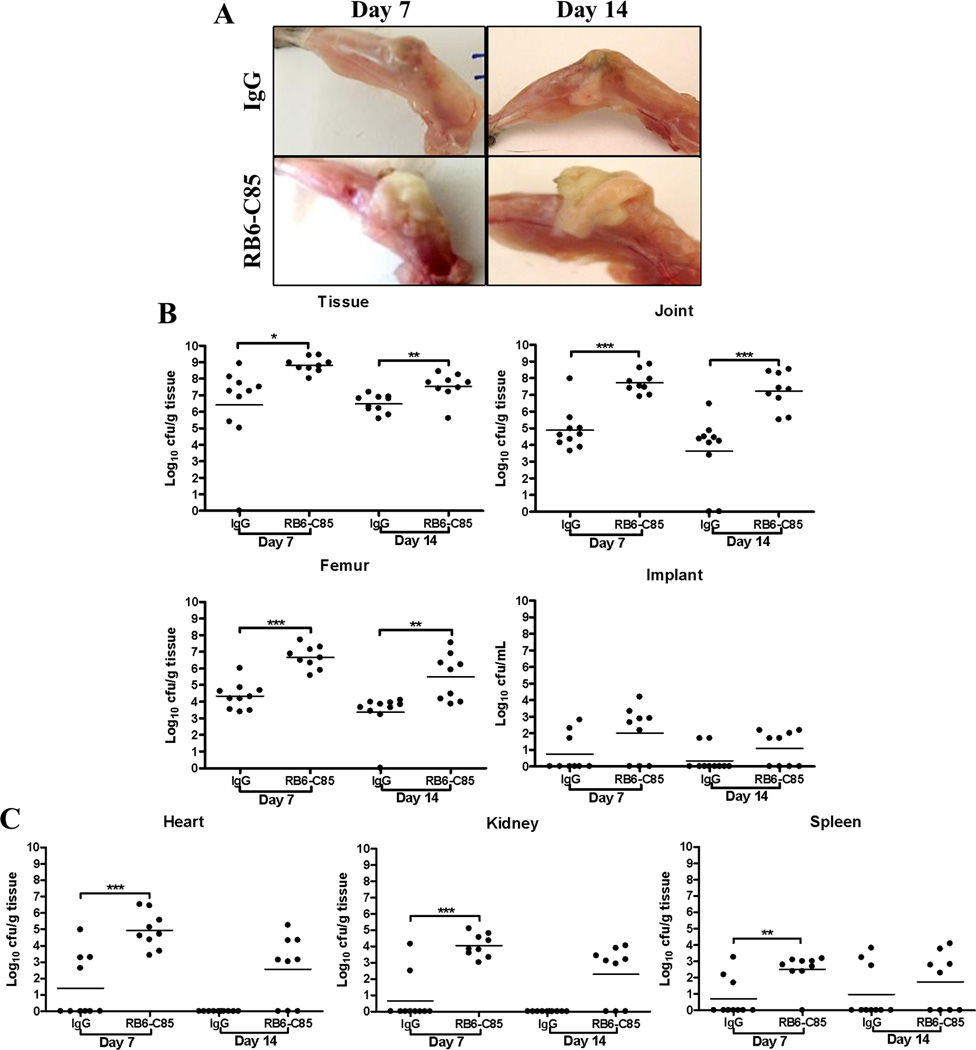
Figure 8. MDSC depletion reduces S. aureus burdens during orthopedic biofilm infection.
(A) Gross appearance of infected tissues from animals receiving 1A8 or an isotype-matched IgG. (B) Bacterial burdens associated with the knee joint, surrounding soft tissue, femur, and orthopedic implant and (C) heart and kidney of IgG control or 1A8-treated animals at days 7 and 14 post-infection. Results are expressed as the number of CFU per ml for orthopedic implants or CFU per gram tissue to correct for alterations in tissue sampling sizes. Significant differences in bacterial burdens between IgG and 1A8-treated mice are denoted by asterisks (*p < 0.05; **, p < 0.01; unpaired two-tailed Student t-test).
To investigate the impact of Ly6G+ cell depletion on the inflammatory milieu during S. aureus orthopedic biofilm infection, soft tissues surrounding the knee, knee joint, and femur were analyzed using MILLIPLEX arrays. Several cytokines (G-CSF, IL-1β, IL-6) and chemokines (CXCL1, CXCL9 and CCL3) were dramatically reduced in 1A8-treated compared to isotype control mice primarily at day 7 (Fig 9), in agreement with increased bacterial clearance in the former (Fig 8B). Collectively, these results demonstrate that during S. aureus orthopedic biofilm infection, Ly6GhighLy6C+ MDSCs elicit a local microenvironment that restricts monocyte/macrophage proinflammatory activity, facilitating the establishment of an anti-inflammatory milieu that favors bacterial persistence. We propose that these effects were not significantly influenced by neutrophil loss following 1A8 treatment, since the majority of Ly6G+ leukocytes infiltrating infected joints (i.e. ~ 75%) were MDSCs.
Figure 9. 1A8 treatment attenuates inflammatory mediator production during S. aureus orthopedic biofilm infection.
Tissue homogenates surrounding orthopedic implants were prepared at days 7 and 14 post-infection from 1A8- and isotype control-treated mice, whereupon G-CSF (A), IL-1β (B), IL-6 (C), CXCL1 (D), CXCL9 (E) and CCL3 (F) expression was quantitated by MILLIPLEX. Results were normalized to the amount of total protein recovered to correct for alterations in tissue sampling size. Significant differences are denoted by asterisks (*, p < 0.05; unpaired two-tailed Student t-test) and are representative of five mice per group.
Gr-1+ cell depletion confirms the inhibitory action of MDSCs on monocytes/macrophages to prevent S. aureus biofilm clearance
Our results have established that MDSC depletion with 1A8 facilitated S. aureus biofilm clearance, in part, due to decreased immunosuppressive effects that promoted the proinflammatory attributes of infiltrating monocytes and macrophages. To further demonstrate that monocytes/macrophages were critical for anti-biofilm activity in the absence of an MDSC infiltrate, we treated mice with the mAb RB6-C85. Similar to 1A8, RB6-C85 depletes Ly6G+ MDSCs and neutrophils, but also targets monocytes based on its reactivity with Ly6C, which would also impact macrophage numbers by default (29, 37, 41–43). Therefore, any differences between 1A8 and RB6-C85 depletion would further support a role for monocytes/macrophages in mediating biofilm clearance without the suppressive MDSC population. As previously demonstrated, Ly6G/Ly6C staining detected three cell populations in implant-associated tissues of isotype control animals at days 7 and 14 following infection, namely Ly6GhighLy6C+ MDSCs, a Ly6GlowLy6Clow granulocyte-like population, and a Ly6G−Ly6C+ inflammatory monocyte subset (Fig 10A). However, at day 7 RB6-C85-treated animals only displayed one cell population that shifted from Ly6GlowLy6Clow to Ly6GhighLy6C+ at day 14 after infection (Fig 10B). The percentage of Ly6GlowLy6Clow cells in RB6-C85-treated animals was significantly higher than isotype-treated controls at day 7; however, no differences were apparent at day 14, since the population had shifted to Ly6GhighLy6C+ (Fig 10B). Although Ly6GhighLy6C+ infiltrates were increased in RB6-C85 treated animals at day 14 post-infection (Fig 10B), they were unable to inhibit T cell proliferation (Supplemental Fig 2), and as such, do not represent a true MDSC phenotype. Therefore, we suggest that the presence of Ly6GlowLy6Clow and Ly6GhighLy6C+ populations in the infected joint of RB6-C85 treated mice results from increased demand from the overwhelming infection (Fig 11) which agrees with the results to follow demonstrating extensive extramedullary hematopoiesis in the spleens of these animals. This is supported by the finding that Ly-6G+Ly-6C+ cells were significantly lower in RB6-C85 treated mice receiving sterile implants compared to isotype control antibody (data not shown).
Figure 10. RB6-C85 administration alters leukocyte infiltrates during S. aureus orthopedic biofilm infection.
Implant-associated tissues from RB6-C85- and isotype control-treated mice were collected at the indicated time points after infection and analyzed by flow cytometry. (A) Representative contour plots of Ly6C and Ly6G staining and quantitation of (B) Ly6GhighLy6C+ MDSCs and Ly6GlowLy6Clow neutrophils and (C) inflammatory monocytes (CCR2+) and macrophages (F4/80+) present in infected animals receiving RB6-C85 or isotype control Ab. Results are expressed as a percent of the total CD45+ leukocyte population. Significant differences are denoted by asterisks (*, p < 0.05; **, p < 0.01; unpaired two-tailed Student t-test) and are representative of 10 mice per group from two independent experiments.
Figure 11. RB6-C85 treatment enhances S. aureus biofilm burdens and dissemination.
(A) Gross appearance of infected tissues from animals receiving RB6-C85 or an isotype-matched IgG revealed a marked caseous exudate in the former. (B) Bacterial burdens associated with the knee joint, surrounding soft tissue, femur, and orthopedic implant and (C) heart, kidney and spleen of control IgG or RB6-C85-treated animals at days 7 and 14 post-infection. Results are expressed as CFU per ml for orthopedic implants or CFU per gram of tissue to correct for differences in tissue sampling size. Significant differences between IgG and RB6-C85 animals are denoted by asterisks (*p < 0.05; **, p < 0.01; ***, p < 0.001; unpaired two-tailed Student t-test) and are representative of 10 mice per group from two independent experiments.
We also examined CCR2 and F4/80 expression as markers for inflammatory monocytes and macrophages, respectively (44–46). Because RB6-C85 also recognizes the Ly6C epitope, we expected both of these cell populations to be decreased, as CCR2+ inflammatory monocytes express Ly6C and differentiate into F4/80+ macrophages once they have migrated into tissues (45). As expected, the percentage of Ly6C+CCR2+ cells was significantly decreased in RB6-C85-treated animals compared to isotype controls at days 7 and 14 after infection (Fig 10C). Likewise, there were significantly fewer F4/80+ macrophages in RB6-C85 depleted mice at day 7 post-infection, and only a very small percentage of cells remained at day 14 (Fig 10C).
Gr-1+ cell depletion exacerbates S. aureus orthopedic biofilm infection due to the loss of monocyte/macrophage effectors
Strikingly, S. aureus-infected mice treated with RB6-C85 displayed a grossly visible caseous exudate (Fig 11A), which was typified by significantly increased bacterial burdens in the knee joint, surrounding soft tissue, and femur at days 7 and 14 after infection compared to infected animals receiving an isotype-matched control Ab (Fig 11B). Histological analysis of tissues collected from RB6-C85-treated mice revealed increased inflammation in the joint space, surrounding soft tissue, and bone compared to isotype control animals (Table 1). Furthermore, the degree of osteolysis was more severe in RB6-C85 treated animals, as evidenced by CT imaging (Fig 12) and femurs were more brittle upon harvest. These results are in stark contrast with those obtained during 1A8 depletion where biofilm burdens were reduced, indicating monocytes and macrophages are able to promote bacterial clearance in the absence of an immunosuppressive MDSC population, since the only difference between the Ab-depletion strategies was the targeting of monocytes/macrophages.
Table 1.
Degree of inflammation and extramedullary hematopoiesis associated with RB6-C85 mAb treatment during S. aureus orthopedic infection
| Spleen |
Average Extramedullary Hematopoiesis Score |
| Naive Spleen | 0.5 |
| D7 IgG | 1 |
| D7 RB6-C85 | 3 |
| D7 IgG | 2 |
| D7 RB6-C85 | 3 |
| D14 IgG | 1.5 |
| D14 RB6-C85 | 3 |
| D14 IgG | 1.5 |
| D14 RB6-C85 | 2.5 |
| Tissue |
Average Inflammatory Score |
| D7 IgG | 2 |
| D7 RB6-C85 | 3 |
| D7 IgG | 2.5 |
| D7 RB6-C85 | 2.5 |
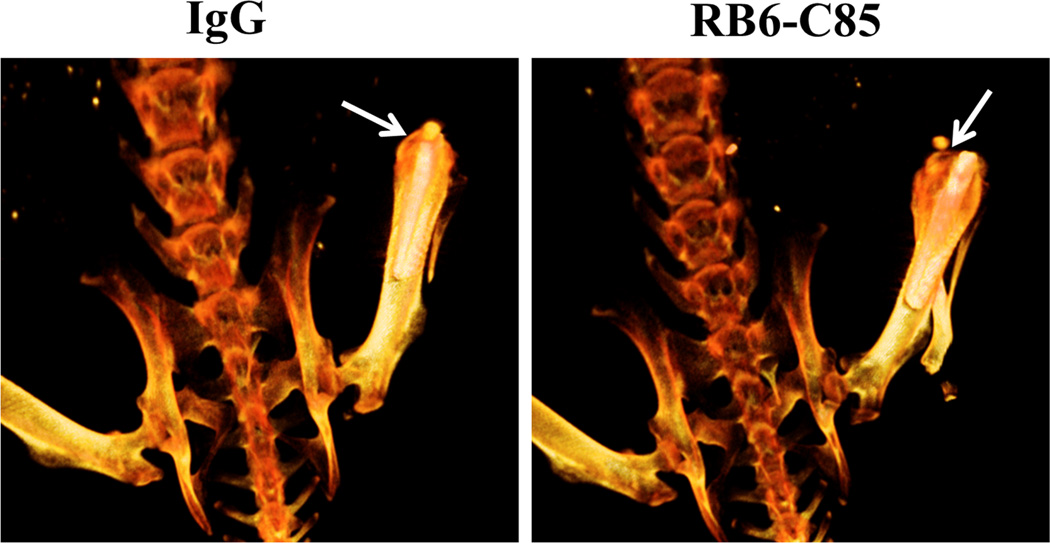
Figure 12. RB6-C85 administration results in increased osteolysis during S. aureus orthopedic biofilm infection.
CT images (dorsal view) are presented at day 14 post-infection from mice receiving RB6-C85 or isotype control Ab. Arrows indicate the region of bone loss near the implant tip at the patella. Color intensity is indicative of bone density, where white = most dense, dark orange = least dense. Images are representative of 4 individual animals per group.
Targeting Gr-1+ cells by RB6-C85 administration not only increased bacterial burdens and inflammation at the site of S. aureus orthopedic biofilm infection, but also led to significant systemic effects. First, Gr-1+ depletion enhanced S. aureus dissemination, as bacterial burdens in the heart, kidney, and spleen of RB6-C85-treated animals were significantly elevated at day 7 after infection compared to the isotype control group (Fig 11C). Second, RB6-C85-treated animals displayed significant splenomegaly (Fig 13A and B). Histopathology revealed marked expansion of the splenic sinuses and red pulp with extensive extramedullary hematopoiesis, typified by numerous erythroid islands, megakaryoctyes, and leukocyte islands in RB6-C85-treated animals, which was not observed in infected isotype control mice (Fig 13C).
Figure 13. RB6-C85 treatment leads to splenomegaly and extrameduallary hematopoiesis during S. aureus orthopedic biofilm infection.
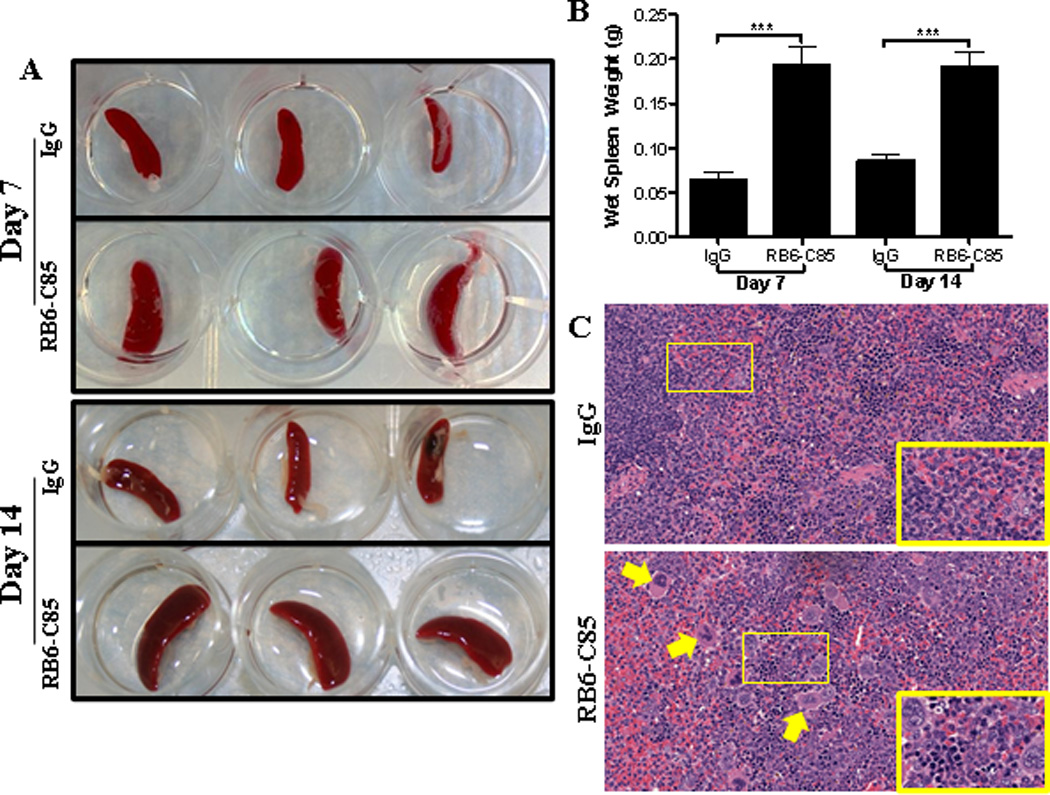
Gross appearance (A) and weight (B) of spleens from RB6-C85- or IgG-treated mice at days 7 and 14 after S. aureus orthopedic biofilm infection (n = 10 per group). (C) H&E-stained sections of spleens from IgG- and RB6-C85-treated mice at day 14 post-infection (n = 3/group; 40× magnification; zoomed images (60×) depict areas delineated by rectangles in the 40× field of view). Arrows indicate presence of megakaryocytes in RB6-C85-treated spleens. Significant differences between IgG and RB6-C85 animals are denoted by asterisks (***, p < 0.001; unpaired two-tailed Student t-test).
To examine changes in the inflammatory milieu after RB6-C85 treatment, inflammatory mediator expression was assessed. Numerous cytokines (IL-1β, G-CSF, and IL-17) and chemokines (CXCL1, CXCL2, and CCL3) were significantly increased at days 7 and 14 in RB6-C85-treated animals compared to isotype controls (Fig 14). Similar changes were observed in the infected knee joint and femur (data not shown). Taken together with the results from 1A8 depletion, these findings demonstrate that MDSCs are critical for limiting the proinflammatory activity of monocytes and macrophages during S. aureus biofilm infection, which sets the stage for bacterial persistence.
Figure 14. RB6-C85 administration exacerbates inflammatory mediator production during S. aureus orthopedic biofilm infection.
Tissue homogenates surrounding orthopedic implants were prepared at days 7 and 14 post-infection from RB6-C85- and isotype control-treated mice, whereupon IL-1β (A), G-CSF (B), IL-17 (C), CXCL1 (D), CXCL2 (E), and CCL3 (F) expression was quantitated by MILLIPLEX. Results were normalized to the amount of total protein recovered to correct for alterations in tissue sampling size. Significant differences are denoted by asterisks (*p < 0.05; **, p < 0.01; ***, p < 0.001; unpaired two-tailed Student t-test) and are representative of five mice per group.
DISCUSSION
An emerging role for MDSCs has been described in several diseases aside from cancer, most recently to include bacterial infections (7–10, 42, 47). Using a mouse model of S. aureus orthopedic biofilm infection, we demonstrate that a population of CD11b+Gr-1+ MDSCs accumulate in the joint tissue and depletion of this population results in improved bacterial clearance by promoting the proinflammatory attributes of infiltrating monocytes and macrophages. This is the first study to report a functional role for MDSCs in any type of staphylococcal or biofilm infection and suggests that MDSCs are key contributors to the chronicity of S. aureus biofilms through their modulation of the host immune response.
Our previous studies have shown that S. aureus biofilms augment Arg-1 expression and polarize macrophages toward an M2 anti-inflammatory state (16). The current study has expanded the repertoire of immune suppressive effectors to include MDSCs. MDSCs are notable for their robust Arg-1 expression, which depletes extracellular arginine, causing T cell dysfunction at multiple levels, including cell cycle arrest, reduced expression of the CD3ζ chain, and a global reduction in several proteins essential for T cell activity (48–51). Limited numbers of CD4+ T cells were detected in implant-associated tissues during S. aureus biofilm infection (i.e. 2–5%). We expected T cell infiltrates to be enhanced following Gr-1 and Ly6G depletion originating from the loss of MDSC activity; however, this was not the case. One possibility to explain this finding is that the combined action of MDSCs and regulatory cytokines serve to limit T cell numbers at the site of biofilm infection, which remains to be determined. Besides actions on T cells, arginine depletion via MDSC Arg-1 activity reduces its availability for iNOS, which thwarts M1 classical macrophage activation, as we have previously shown in S. aureus biofilms (16). By extension, the significant MDSC infiltrate associated with S. aureus biofilms in vivo is likely an important factor in skewing monocytes/macrophages towards a M2 anti-inflammatory phenotype that promotes bacterial persistence and our studies confirmed that MDSCs recovered from the site of orthopedic biofilm infection express Arg-1 and IL-10. By extension, we predicted that depletion of the suppressive MDSC population would allow infiltrating monocytes to act as true effector cells. This was confirmed by the finding that Ly6C+ monocytes recovered from MDSC depleted animals expressed a wide array of proinflammatory genes compared to monocytes recovered from IgG treated mice where the MDSC population remained intact. In addition, MDSC depletion significantly decreased biofilm burdens, confirming the importance of this population in orchestrating the anti-inflammatory biofilm milieu to facilitate infection persistence. Besides MDSCs, regulatory T cells (Tregs) also possess anti-inflammatory attributes similar to MDSCs (52). However, we did not detect any CD4+CD25+Foxp3+ cells associated with S. aureus biofilm infections (data not shown), whereas another group has reported Treg involvement in biofilm clearance (33). The reasons for these discrepancies are not clear but may arise from differences in experimental models and/or S. aureus strains tested. Based on our analysis, we propose that MDSCs represent the main immunosuppressive effector cell during S. aureus orthopedic biofilm infection. The signals controlling MDSC recruitment, activation, and suppressive activity during S. aureus biofilm infection remain ill-defined and are ongoing topics of investigation in our laboratory.
Our RB6-C85 depletion studies revealed significant increases in bacterial dissemination from the orthopedic infection site. As mentioned previously, RB6-C85 recognizes both Ly6G and Ly6C epitopes, effectively depleting MDSCs, neutrophils, monocytes, and by extension, macrophages. Therefore, although MDSC infiltrates were reduced, effector populations were also targeted, leaving fewer leukocytes either locally or systemically to prevent S. aureus dissemination to peripheral organs. By extension, we propose that the function of each leukocyte subset differs depending on the local microenvironment; namely although the inhibitory actions of MDSCs were negated following RB6-C85 treatment, this coincided with a local reduction in inflammatory monocytes/macrophages, such that biofilm growth could not be held in check at the primary infection site (i.e. joint). When biofilm-associated bacteria seeded peripheral sites, the paucity of systemic neutrophils likely accounted for the failure to effectively clear the infection, which is essential since neutrophils are a main effector cell against planktonic S. aureus (53–56). Dissemination was not observed with anti-Ly6G Ab treatment, which was attributed to the local monocyte/macrophage population that remained intact and exhibited heightened proinflammatory activity. MDSC infiltrates were also detected in a S. aureus catheter-associated biofilm infection model (Supplemental Fig 3) and RB6-C85 treatment similarly increased bacterial burdens and dissemination (Supplemental Fig 4), providing independent confirmation that MDSCs are a hallmark of S. aureus biofilm infection.
One notable finding in the current study was the extensive extramedullary hematopoiesis observed in the spleens of RB6-C85-treated animals compared to isotype controls. Extramedullary hematopoiesis is frequently seen during chronic inflammatory diseases and cancer (19), and expansion of CD11b+Gr-1+ MDSCs has been reported in tumor and polymicrobial sepsis models (7, 57, 58). During infection, the requirement for myeloid cells dramatically increases in response to an expanding infectious burden, which creates a need for emergency myelopoiesis and the mobilization of immature myeloid cells from the bone marrow and spleen (19). The targeted reduction in Gr-1+ cells coincident with increasing biofilm burdens with RB6-C85 treatment likely explains the extensive extramedullary hematopoiesis observed in the spleens of these animals. Another unexpected finding was that Gr-1+ (Ly6G/Ly6C) infiltrates were increased at the site of orthopedic infection following RB6-C85 administration. However, this was likely a compensatory mechanism in response to elevated bacterial burdens both locally and systemically in Gr-1-depleted mice, since these newly recruited Ly6G+Ly6C+ cells were unable to suppress CD4+ T cell proliferation, which agrees with reports of polymicrobial sepsis (7). In addition, we also observed enhanced levels of G-CSF, IL-6, and VEGF in the serum of RB6-C85-treated mice, all of which contribute to the expansion of immature myeloid cell populations (2, 4). Alternatively, the failure to deplete Ly6G+Ly6C+ infiltrates at later intervals could be explained by the induction of anti-rat IgG antibodies that would be expected to impair the efficacy of RB6-C85 treatment (rat anti-mouse Gr-1). However, this appears less likely because RB6-C85 was still capable of significantly reducing inflammatory monocyte and macrophage infiltrates into S. aureus infected joints two weeks after repeated Ab administration.
Live CT scans revealed significantly more osteolysis in the femurs of RB6-C85-treated animals compared to 1A8 and isotype control mice, which may be attributed to the increased bacterial burdens in the former. The exact mechanisms of osteolysis are still not completely understood, and differ depending on pathologic conditions (59, 60). However, several studies suggest that proinflammatory mediators, such as IL-1β, could play a role in the initiation and progression of osteolysis (61–63) and numerous proinflammatory mediators were significantly elevated in the joint and surrounding soft tissue following RB6-C85 treatment, including IL-1β that coincided with increased bone destruction. Additionally, S. aureus is not only capable of colonizing the bone matrix, but can also invade osteoblasts, which could contribute to chronicity (64). Furthermore, S. aureus internalization by osteoblasts can lead to apoptosis and disrupt the balance of osteoblast and osteoclast activities, which could facilitate bone destruction (64). Interestingly, a recent study identified phenol-soluble modulins as a key inducer of osteoblast proliferation in a S. aureus osteomyelitis model (65); however, effects on osteoclasts remain to be defined.
Our studies are just beginning to explore the role of MDSCs during S. aureus infection. By manipulating these cells with Ab depletion strategies, we demonstrated that their immunosuppressive function prevents monocytes/macrophages from eliminating biofilm-associated bacteria by attenuating their proinflammatory properties. Our findings do not exclude the possibility that the biofilm matrix may also play a role in thwarting immune recognition in vivo; however, this remains an area of debate. Although it is clear that intact biofilms do afford some degree of protection against macrophage phagocytosis as previously shown by our laboratory and others (16, 23, 66–69), it is clear that neutrophils are fully capable of invading and phagocytosing biofilm-associated bacteria (23, 70–72), yet there is no apparent impact on biofilm growth. The fact that staphylococcal biofilms polarize macrophages towards an alternatively activated M2 phenotype does suggest that macrophage surface receptors are triggered to elicit this programming event; however, the identity of these receptor(s) remains unknown. Future studies examining a S. aureus biofilm-defective mutant would be valuable for determining whether signals from the biofilm itself are responsible for MDSC recruitment and immunosuppressive activities. Preventing the presumed immunosuppressive action of infiltrating MDSCs may offer a novel therapeutic strategy to thwart these devastating, chronic infections.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Katherine Estes in the UNMC Small Animal Imaging Core for assistance with CT scans, Dr. Charles Kuszynski and Victoria Smith in the UNMC Cell Analysis Facility for support with FACS analysis, Roxanne Alter for assistance with cytospin stains, and the UNMC Tissue Sciences Facility for imaging of H&E stained tissues. We also thank John Varrone and Kohei Nishitani at the University of Rochester School of Medicine and Dentistry for providing the protocol for processing infected orthopedic devices for SEM analysis.
Footnotes
This work was supported by the National Institutes of Health National Institute of Allergy and Infectious Disease (NIAID) P01 AI083211 Project 4 to T.K. and an American Heart Association (AHA) predoctoral fellowship to C.E.H. (13PRE16910040).
REFERENCES
- 1.Zhang C, Lei GS, Shao S, Jung HW, Durant PJ, Lee CH. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infection and immunity. 2012;80:3634–3641. doi: 10.1128/IAI.00668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in cancer biology. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. The Journal of clinical investigation. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 6.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. The Journal of experimental medicine. 2010;207:1453–1464. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. The Journal of experimental medicine. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, Levy DE, Lee JS, Mallampalli RK, Chan YR, Ray A, Ray P. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal immunology. 2013;6:189–199. doi: 10.1038/mi.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. Journal of immunology. 2013;190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 10.Obregon-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1(int)CD11b(+) Myeloid-Derived Suppressor Cells in Mycobacterium tuberculosis Infection. PloS one. 2013;8:e80669. doi: 10.1371/journal.pone.0080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel RP. Health care-associated infections: major issues in the early years of the 21st century. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(Suppl 1):S85–S88. doi: 10.1086/518136. [DOI] [PubMed] [Google Scholar]
- 12.Watkins RR, David MZ, Salata RA. Current concepts on the virulence mechanisms of meticillin-resistant Staphylococcus aureus. Journal of medical microbiology. 2012;61:1179–1193. doi: 10.1099/jmm.0.043513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmon DR, Hanssen AD, Patel R. Prosthetic joint infection: criteria for future definitions. Clinical orthopaedics and related research. 2005:89–90. [PubMed] [Google Scholar]
- 14.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infection and immunity. 2011;79:1789–1796. doi: 10.1128/IAI.01386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical microbiology reviews. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. Journal of immunology. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Medical microbiology and immunology. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- 18.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends in immunology. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Molecular medicine. 2011;17:281–292. doi: 10.2119/molmed.2010.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PloS one. 2010;5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PloS one. 2012;7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nature immunology. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 23.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. Journal of immunology. 2013;190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke ML, Kielian T. Deciphering mechanisms of staphylococcal biofilm evasion of host immunity. Frontiers in cellular and infection microbiology. 2012;2:62. doi: 10.3389/fcimb.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infection and immunity. 2012;80:2026–2034. doi: 10.1128/IAI.00239-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. European journal of immunology. 2011;41:749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A, Boumpas D, Verginis P. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. Journal of immunology. 2012;188:1136–1146. doi: 10.4049/jimmunol.1101816. [DOI] [PubMed] [Google Scholar]
- 29.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Current opinion in immunology. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer research. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maenhout SK, Van Lint S, Emeagi PU, Thielemans K, Aerts JL. Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared to their peripheral counterparts. International journal of cancer. Journal international du cancer. 2013 doi: 10.1002/ijc.28449. [DOI] [PubMed] [Google Scholar]
- 32.Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.0113014. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infection and immunity. 2011;79:5010–5018. doi: 10.1128/IAI.05571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niska JA, Meganck JA, Pribaz JR, Shahbazian JH, Lim E, Zhang N, Rice BW, Akin A, Ramos RI, Bernthal NM, Francis KP, Miller LS. Monitoring bacterial burden, inflammation and bone damage longitudinally using optical and muCT imaging in an orthopaedic implant infection in mice. PloS one. 2012;7:e47397. doi: 10.1371/journal.pone.0047397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archer NK, Harro JM, Shirtliff ME. Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infection and immunity. 2013;81:2070–2075. doi: 10.1128/IAI.00084-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and molecular life sciences : CMLS. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saiwai H, Kumamaru H, Ohkawa Y, Kubota K, Kobayakawa K, Yamada H, Yokomizo T, Iwamoto Y, Okada S. Ly6C+ Ly6G− Myeloid-derived suppressor cells play a critical role in the resolution of acute inflammation and the subsequent tissue repair process after spinal cord injury. Journal of neurochemistry. 2013;125:74–88. doi: 10.1111/jnc.12135. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. The Journal of experimental medicine. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. International journal of cancer. Journal international du cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE(2) catabolism in myeloid cells. Journal of leukocyte biology. 2010;88:839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. The Journal of general virology. 2010;91:2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 42.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. European journal of immunology. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribes S, Regen T, Meister T, Tauber SC, Schutze S, Mildner A, Mack M, Hanisch UK, Nau R. Resistance of the brain to Escherichia coli K1 infection depends on MyD88 signaling and the contribution of neutrophils and monocytes. Infection and immunity. 2013;81:1810–1819. doi: 10.1128/IAI.01349-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature immunology. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 45.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews. Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 46.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. European journal of immunology. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 47.Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. British journal of cancer. 2013;108:2281–2290. doi: 10.1038/bjc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X, Pribis JP, Rodriguez PC, Morris SM, Jr, Vodovotz Y, Billiar TR, Ochoa JB. The Central Role of Arginine Catabolism in T-Cell Dysfunction and Increased Susceptibility to Infection After Physical Injury. Annals of surgery. 2013 doi: 10.1097/SLA.0b013e31828611f8. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. Journal of immunology. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 51.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. Journal of immunology. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 52.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. Journal of immunology. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 54.Palazzolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, Kreiswirth BN, Skaar EP, DeLeo FR. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. Journal of immunology. 2008;180:500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 55.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS pathogens. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Seminars in immunopathology. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. Journal of immunology. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 58.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. Journal of leukocyte biology. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 59.Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. The Journal of bone and joint surgery. British volume. 2012;94:10–15. doi: 10.1302/0301-620X.94B1.28047. [DOI] [PubMed] [Google Scholar]
- 60.Abu-Amer Y. Inflammation, cancer, and bone loss. Current opinion in pharmacology. 2009;9:427–433. doi: 10.1016/j.coph.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clinical orthopaedics and related research. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 62.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, Santambrogio L, Ross FP, Goldring SR, Purdue PE. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31:73–80. doi: 10.1002/jor.22190. [DOI] [PubMed] [Google Scholar]
- 63.Epstein NJ, Warme BA, Spanogle J, Ma T, Bragg B, Smith RL, Goodman SB. Interleukin-1 modulates periprosthetic tissue formation in an intramedullary model of particle-induced inflammation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23:501–510. doi: 10.1016/j.orthres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Shi S, Zhang X. Interaction of Staphylococcus aureus with osteoblasts (Review) Experimental and therapeutic medicine. 2012;3:367–370. doi: 10.3892/etm.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell host & microbe. 2013;13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kristian SA, Birkenstock TA, Sauder U, Mack D, Gotz F, Landmann R. Biofilm formation induces C3a release and protects Staphylococcus epidermidis from IgG and complement deposition and from neutrophil-dependent killing. The Journal of infectious diseases. 2008;197:1028–1035. doi: 10.1086/528992. [DOI] [PubMed] [Google Scholar]
- 67.Cerca F, Andrade F, Franca A, Andrade EB, Ribeiro A, Almeida AA, Cerca N, Pier G, Azeredo J, Vilanova M. Staphylococcus epidermidis biofilms with higher proportions of dormant bacteria induce a lower activation of murine macrophages. Journal of medical microbiology. 2011;60:1717–1724. doi: 10.1099/jmm.0.031922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, Rohde H. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infection and immunity. 2011;79:2267–2276. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiliopoulou AI, Kolonitsiou F, Krevvata MI, Leontsinidis M, Wilkinson TS, Mack D, Anastassiou ED. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcus epidermidis. FEMS microbiology letters. 2012;330:56–65. doi: 10.1111/j.1574-6968.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 70.Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, Kielian T. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infection and immunity. 2013;81:4363–4376. doi: 10.1128/IAI.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunther F, Wabnitz GH, Stroh P, Prior B, Obst U, Samstag Y, Wagner C, Hansch GM. Host defence against Staphylococcus aureus biofilms infection: phagocytosis of biofilms by polymorphonuclear neutrophils (PMN) Molecular immunology. 2009;46:1805–1813. doi: 10.1016/j.molimm.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Graves SF, Kobayashi SD, DeLeo FR. Community-associated methicillin-resistant Staphylococcus aureus immune evasion and virulence. Journal of molecular medicine. 2010;88:109–114. doi: 10.1007/s00109-009-0573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.