Abstract
Autophagy is an intracellular process leading to vacuolar degradation of cytoplasmic components, which is important for nutrient recycling. Autophagic degradation of chloroplastic proteins via Rubisco-containing bodies is activated in leaves upon low sugar availability in Arabidopsis and our recent study reveals the contribution of autophagy to nighttime energy availability for growth. Whereas metabolic analysis supports that autophagic proteolysis provides a supply of alternative energy sources such as amino acids during sugar deficit, changes in a large number of metabolites due to autophagy deficiency are also observed. Here, we performed statistical characterization of that metabolic data. Principal component analysis clearly separated wild type and autophagy-deficient atg5 mutant samples, pointing to significant effects of autophagy deficiency on metabolite profiles in Arabidopsis leaves. Thirty-six and four metabolites were significantly increased and decreased in atg5 compared with wild type, respectively. These results imply that autophagic proteolysis is linked to plant metabolic processes.
Keywords: Arabidopsis, autophagy, chloroplast, energy availability, metabolite profiling, Rubisco-containing body
Autophagy is a ubiquitous recycling system by which proteins and organelles in eukaryotic cells are transported for degradation in the vacuoles of yeast and plants or the lysosomes of animals (for a review, see refs. 1–3). During autophagy, a portion of the cell’s cytoplasmic contents is sequestered in a double-membraned vesicle called an autophagosome and delivered to the vacuole/lysosome. The outer membrane of the autophagosome then fuses with the vacuolar/lysosomal membrane and the inner membrane structure (called the autophagic body) is degraded by resident hydrolases. The amino acids (AAs) released due to autophagic proteolysis are supplied to the AA pool for new protein biosynthesis and/or energy production in yeast and mice.4,5 Autophagy is also considered to play an important role in nutrient recycling, particularly under starvation conditions, in plants.1-3
We have demonstrated that chloroplastic proteins are degraded by autophagy via Rubisco-containing bodies (RCBs), a type of autophagic body containing chloroplast stroma.6,7 Chloroplasts are the predominant source of material for autophagic recycling in plants because the majority of plant nutrients are distributed to chloroplasts, such that chloroplastic proteins account for 75 to 80% of total leaf nitrogen in C3 plants.8 We previously investigated the effects of nutrient factors on the appearance of RCBs in Arabidopsis leaves.9 We found that RCB appearence in excised leaves is strongly suppressed by metabolic sugars, whether added externally or produced during photosynthesis in the light. RCB production is activated under conditions of low sugar availability, such as in leaves at the end of the night in a diurnal cycle or in leaves lacking starch due to starchless mutations. These results suggest a role of RCB/autophagy for energy production via degradation of chloroplastic proteins when photosynthesitic carbon assimilation is restricted. Although the importance of autophagy in plant carbon recycling for energy production had also been suggested based on the observed activation of autophagy during sugar starvation and the sensitivity of autophagy deficient (atg) mutants to continuous darkness,10,11 a substantial role for autophagy in plant energy availability had not been established.
Energy availability is a primary factor influencing plant growth and survival under both adequate growth conditions and several stress conditions. Plants normally obtain energy via photosynthetic carbon assimilation during the day and a portion of the photoassimilate is retained, mainly as starch, for respiration during the night.12 The effects of disrupted energy availability are revealed in Arabidopsis starchless mutants, in which the lack of starch accumulation causes a transient sugar deficit at night.13,14 Sugar deficit causes starvation symptoms, leading to growth interruption. Thus, plants adjust their metabolism to fluctuations or disruptions of sugar availability due to changes in day-night periods or suboptimal conditions. Enzymatic pathways for catabolism of alternative respiratory substrates such as AAs for energy production seem to exist in plant metabolic pathways.15
Our recent study indicates that autophagy makes a substantial contribution to energy availability for plant growth.16 The reduced growth of Arabidopsis atg mutants under short-day conditions was largely relieved under continuous light or under short-day conditions combined with feeding of exogenous sucrose, suggesting that autophagy contributes to nighttime energy availability. To investigate further the role of autophagy at night, we generated starchless atg double mutants because nighttime sugar availability is reduced in starchless mutants. The double mutants showed severe starvation symptoms and phenotypes: reduced growth and early cell death in leaves were observed under short-day conditions. To examine changes in the AA pool, we performed metabolic analysis using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS). The contents of free AAs were increased in starchless mutant leaves compared with those of WT and the increases in branched chain AA and aromatic AA contents were partially compromised in starchless atg double mutants. This metabolic difference indicates that autophagy can contribute to energy availability by supplying alternative energy sources such as AAs.
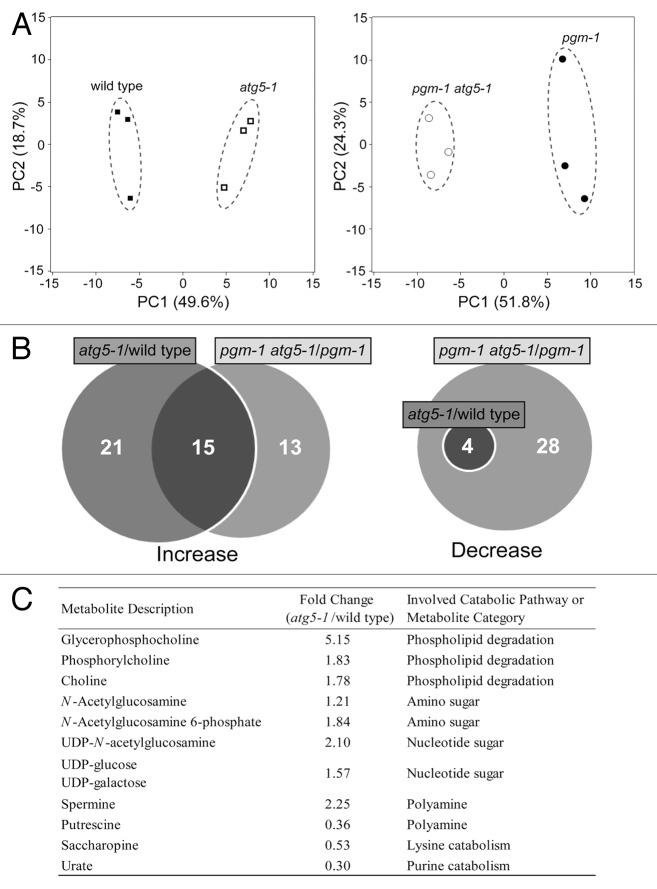
The growth retardation under shortened photoperiods in atg single mutants is similar to the phenotype observed in starchless mutants, indicating a substantial contribution of autophagy to nighttime energy availability.16,17 However, decreases of free AA contents in the atg5 single mutant compared with WT plants were not observed in our CE-TOFMS analysis.16 Thus, we still do not have clear metabolic evidence indicating that autophagy provides AAs at night when sugars are not exhausted in WT plants. The activity of autophagy each night in WT is likely to be lower than during a sugar deficit in starchless mutants. It is noteworthy that the contents of many metabolites other than AAs were changed in atg5 plants compared with WT.16 To test for differences in metabolic profiles due to autophagy deficiency in an unbiased manner, we performed principal component analysis (PCA) (Fig. 1A). The PCA score scatterplot showed that metabolic profiles substantially differed between WT and atg5 as well as between starchless pgm mutants and pgm atg5 double mutants. The first principal component (PC1), accounting for 49.6% of the variation in the data, clearly separated atg5 and WT plants. Metabolic profiles in pgm and pgm atg5 were also separated by PC1, accounting for 51.8% of the data variation.
Figure 1. Significant changes in metabolic profiles due to autophagy deficiency observed by CE-TOFMS analysis. (A) Principal component analysis score scatterplots of metabolic profiles of the wild type ecotype Columbia (closed squares) and autophagy-deficient atg5-1 mutant (open squares) sample set (left) and the starchless pgm-1 mutant (closed circles) and pgm-1 atg5-1 double mutant (open circles) sample set (right). (B) The amounts of individual metabolites were subjected to t-test comparisons between wild type and atg5-1 or between pgm-1 and pgm-1 atg5-1. Venn diagrams of the number of metabolites significantly increased (left) or decreased (right) at the 5% level are shown. (C) Selected metabolites from catabolic pathways of possible energy sources or common metabolite categories. The metabolites shown here were selected from among the significantly increased or decreased metabolites in leaves of atg5-1 compared with wild type. All data were obtained from a previously performed CE-TOFMS analysis.16 Statistical analysis was performed using JMP software (SAS Institute).
To characterize further the differences in the annotated metabolites, we compared the amounts of metabolites by t-test analysis between WT and atg5 or between pgm and pgm atg5 and produced Venn diagrams illustrating the number of metabolites significantly increased or decreased at the 5% level (Fig. 1B). For the atg5 mutation, 36 metabolites were significantly increased in the WT background and 28 were significantly increased in the pgm background; 15 of the increased metabolites were common between the two backgrounds. In addition, some metabolites that were increased in atg5 could be classified into common pathways or similar metabolite categories (Fig. 1C). Phospholipid-related intermediates, including choline, glycerophosphocholine and phosphorylcholine, increased in atg5 compared with WT, similar to the difference between pgm atg5 and pgm.16 These metabolites are derived from phospholipase-mediated degradation of phosphatidylcholine,18 a major phospholipid in plants and lipids can be alternative respiratory substrates during sugar starvation.19 Such a catabolic process may be alternatively activated due to autophagy deficiency. The contents of several amino sugars and nucleotide sugars, such as N-acetylglucosamine (GlcNAc) and GlcNAc 6-phosphate, UDP-GlcNAc, UDP-glucose and UDP-galactose, significantly increased in atg5 compared with WT (Fig. 1C). These metabolites are involved in biological processes including sucrose metabolism, cell wall metabolism and protein modification.20,21
Four metabolites were decreased in atg5 compared with WT and all of them were among the 28 metabolites that were significantly decreased in pgm atg5 compared with pgm (Fig. 1B). The contents of urate and saccharopine significantly decreased in atg5 relative to WT (Fig. 1C), again similar to the difference between pgm atg5 and pgm.16 These metabolites are possible intermediates of the purine and lysine catabolic pathways, respectively.22,23 Such catabolic pathways may be linked to autophagy. Polyamines, which mainly consist of putrescine, the triamine spermidine and the tetraamine spermine, are natural compounds involved in plant responses to several types of abiotic stress.24 The putrescine content decreased and the spermine content increased in atg5 compared with WT (Fig. 1C).
Recent work has established the role of autophagy during the development of plant vegetative organs. Autophagy contributes to Rubisco degradation during natural leaf senescence,25 and autophagy is involved in nitrogen remobilization at the whole-plant level in Arabidopsis.26 Autophagy deficiency causes excessive accumulation of salicylic acid and oxidative damage, leading to an early cell death phenotype during leaf development.27 On the other hand, the link between autophagy and plant metabolism has not been well characterized because of scant metabolic profiling focusing on plant autophagy, despite its ubiquitous role in proteolysis and catabolism in eukaryotic cells. Here, statistical characterization of a large data set acquired from CE-TOFMS analysis indicates that there are significant effects of autophagy deficiency on the metabolism of Arabidopsis leaves (Fig. 1). Although the precise mechanisms underlying the changes in specific substances and intermediates are unclear, these phenomena provide insights to guide future analyses of autophagy in plants. Further metabolite analyses using atg mutants at a large or fine scale, such as characterization of differences according to developmental stages or growth conditions, may be effective to elucidate the importance of autophagy in plant metabolism.
Acknowledgments
This work was supported by KAKENHI (Grant Number 24-3942 to M.I. and Grant Number 24380037 to H.I.), a Research Fellowship for Young Scientists (to M.I.) from the Japan Society for the Promotion of Science and a Scientific Research on Innovative Area (Planned Research No. 23119503 to H.I.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/25023
References
- 1.Li FQ, Vierstra RD. Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012;17:526–37. doi: 10.1016/j.tplants.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Liu YM, Bassham DC. Autophagy: pathways for self-eating in plant cells. Annu Rev Plant Biol. 2012;63:215–37. doi: 10.1146/annurev-arplant-042811-105441. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimoto K. Beginning to understand autophagy, an intracellular self-degradation system in plants. Plant Cell Physiol. 2012;53:1355–65. doi: 10.1093/pcp/pcs099. [DOI] [PubMed] [Google Scholar]
- 4.Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011;7:727–36. doi: 10.4161/auto.7.7.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem. 2005;280:31582–6. doi: 10.1074/jbc.M506736200. [DOI] [PubMed] [Google Scholar]
- 6.Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, et al. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 2008;148:142–55. doi: 10.1104/pp.108.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, et al. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol. 2009;149:885–93. doi: 10.1104/pp.108.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makino A, Sakuma H, Sudo E, Mae T. Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant Cell Physiol. 2003;44:952–6. doi: 10.1093/pcp/pcg113. [DOI] [PubMed] [Google Scholar]
- 9.Izumi M, Wada S, Makino A, Ishida H. The autophagic degradation of chloroplasts via rubisco-containing bodies is specifically linked to leaf carbon status but not nitrogen status in Arabidopsis. Plant Physiol. 2010;154:1196–209. doi: 10.1104/pp.110.158519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung T, Phillips AR, Vierstra RD. ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A AND ATG12B loci. Plant J. 2010;62:483–93. doi: 10.1111/j.1365-313X.2010.04166.x. [DOI] [PubMed] [Google Scholar]
- 11.Suttangkakul A, Li FQ, Chung T, Vierstra RD. The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell. 2011;23:3761–79. doi: 10.1105/tpc.111.090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stitt M, Zeeman SC. Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol. 2012;15:282–92. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, et al. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004;39:847–62. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- 14.Graf A, Schlereth A, Stitt M, Smith AM. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA. 2010;107:9458–63. doi: 10.1073/pnas.0914299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araújo WL, Tohge T, Ishizaki K, Leaver CJ, Fernie AR. Protein degradation - an alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011;16:489–98. doi: 10.1016/j.tplants.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Izumi M, Hidema J, Makino A, Ishida H. Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol. 2013;161:1682–93. doi: 10.1104/pp.113.215632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caspar T, Huber SC, Somerville C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985;79:11–7. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bargmann BO, Munnik T. The role of phospholipase D in plant stress responses. Curr Opin Plant Biol. 2006;9:515–22. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, et al. The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell. 2009;21:2733–49. doi: 10.1105/tpc.108.064857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleczkowski LA, Kunz S, Wilczynska M. Mechanisms of UDP-glucose synthesis in plants. Crit Rev Plant Sci. 2010;29:191–203. doi: 10.1080/07352689.2010.483578. [DOI] [Google Scholar]
- 21.Olszewski NE, West CM, Sassi SO, Hartweck LM. O-GlcNAc protein modification in plants: Evolution and function. Biochim Biophys Acta. 2010;1800:49–56. doi: 10.1016/j.bbagen.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galili G, Tang GL, Zhu XH, Gakiere B. Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr Opin Plant Biol. 2001;4:261–6. doi: 10.1016/S1369-5266(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 23.Zrenner R, Stitt M, Sonnewald U, Boldt R. Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol. 2006;57:805–36. doi: 10.1146/annurev.arplant.57.032905.105421. [DOI] [PubMed] [Google Scholar]
- 24.Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34:35–45. doi: 10.1007/s00726-007-0501-8. [DOI] [PubMed] [Google Scholar]
- 25.Ono Y, Wada S, Izumi M, Makino A, Ishida H. Evidence for contribution of autophagy to Rubisco degradation during leaf senescence in Arabidopsis thaliana. Plant Cell Environ. 2013;36:1147–59. doi: 10.1111/pce.12049. [DOI] [PubMed] [Google Scholar]
- 26.Guiboileau A, Yoshimoto K, Soulay F, Bataillé MP, Avice JC, Masclaux-Daubresse C. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol. 2012;194:732–40. doi: 10.1111/j.1469-8137.2012.04084.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, et al. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–27. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]