Abstract
Although the regulation of hemidesmosome dynamics during processes such as epithelial migration, wound healing, and carcinoma invasion is important, the mechanisms involved are poorly understood. The integrin α6β4 is an essential component of the hemidesmosome and a target of such regulation. Epidermal growth factor (EGF) can induce hemidesmosome disassembly by a mechanism that involves serine phosphorylation of the β4 integrin subunit. Using a combination of biochemical and mutational analyses, we demonstrate that EGF induces the phosphorylation of three specific serine residues (S1356, S1360, and S1364) located within the connecting segment of the β4 subunit and that phosphorylation on these residues accounts for the bulk of β4 phosphorylation stimulated by EGF. Importantly, phosphorylation of these serines is critical for the ability of EGF to disrupt hemidesmosomes. Using COS-7 cells, which assemble hemidesmosomes type II upon exogenous expression of the α6β4 integrin, we observed that expression of a β4 construct containing Ser→Ala mutations of S1356, S1360, and S1364 reduced the ability of EGF to disrupt hemidesmosomes and that this effect appears to involve cooperation among these phosphorylation sites. Moreover, expression of Ser→Asp mutants that mimic constitutive phosphorylation reduced hemidesmosome formation. Protein kinase C-α (PKC-α) is the kinase responsible for phosphorylating at least two of these serines, based on in vitro kinase assays, peptide mapping, and mutational analysis. Together, these results highlight the importance of serine phosphorylation in regulating type II hemidesmosome disassembly, implicate a cluster of serine residues within the connecting segment of β4, and argue for a key role for PKC-α in regulating these structures.
The interaction of epithelial cells with the basal lamina is a dynamic process. Healthy, intact epithelia form stable adhesive contacts with the basal lamina. In contrast, epithelial injury and consequent wound healing, as well as malignant transformation and invasion, induce dynamic interactions with the basal lamina that underlie epithelial migration (12, 20, 41). Understanding how such interactions are regulated at the molecular level is an area of considerable importance and is often approached by studying the behavior of specific cell adhesion receptors.
One of the most important molecules mediating stable cell attachment to the basal lamina is the integrin α6β4, a laminin receptor that links the basal lamina to the intermediate filament network and that is a main component of a multiprotein structure known as the hemidesmosome (4, 14, 24). This integrin interacts with several hemidesmosomal proteins directly or indirectly, namely HD1/plectin, bullous pemphigoid antigen 1 (BPAG1), and BPAG2, and it is through the first two of these proteins that the β4 subunit is linked indirectly to the cytokeratins (4, 14). More specifically, the β4 subunit contains several structural and functional regions within its unique 1,000-amino-acid-long cytoplasmic tail that are important for its association with these hemidesmosomal proteins (16, 31, 42). These regions include two pairs of FN type III repeats separated by a connecting segment. The β4 subunit has at least two areas where it binds to plectin: one encompassing part of the FN type III repeat 2 plus a region of the connecting segment and another one encompassing the last FN repeat plus the carboxy-terminal residues (27, 28, 30, 36). The FN type III repeats 3 and 4 are also important for binding BPAG1 and -2 (3, 18). Two types of hemidesmosomes have been described. Type I hemidesmosomes are present in skin and several types of epithelia, and they are composed of α6β4, HD1/plectin, BPAG1, and BPAG2 (4, 14). Type II hemidesmosomes are present in intestinal epithelia and they contain only α6β4 and HD1/plectin (11, 43).
The α6β4 integrin is critical for the formation of the hemidesmosome, and the loss of its expression in certain genetic diseases or in β4 null mice results in the disappearance of these structures, producing an epithelium that is mechanically and functionally deficient (8, 29, 44). In the absence of β4 mutations, hemidesmosome disassembly also occurs during wound healing and carcinoma invasion and it facilitates cell migration, even though cells can still express all of the hemidesmosomal proteins (13, 19, 35). Growth factors such as epidermal growth factor (EGF) have been used to induce hemidesmosome disassembly and to study the mechanisms involved (7, 21, 35). EGF stimulates phosphorylation of the β4 subunit on both serine and tyrosine residues (21, 35). Phosphorylated serine residues, which proportionally constitute the vast majority of the phosphorylated residues in the β4 subunit, are dependent on a functional protein kinase C (PKC) pathway, and the inhibition of conventional PKCs results in the inhibition of both hemidesmosome disassembly and β4 phosphorylation (35). None of these serine residues have been identified. In this study, we identify three phosphoserine residues located in the connecting segment of the β4 subunit that constitute approximately 50% of total phosphorylation of β4 that is induced by EGF. We show that at least two of these serines are phosphorylated by PKC-α, an isoform that has been previously shown to play a major role in hemidesmosome dynamics (35). Site-specific mutations of these sites that mimic constitutive phosphorylation reduce the formation of type II hemidesmosomes. In contrast, mutations that nullify phosphorylation of these serines reduce the ability of EGF to disrupt hemidesmosomes.
MATERIALS AND METHODS
Cells and reagents.
HaCat cells, immortalized, human keratinocytes, were obtained from S. La Flamme (Albany Medical College, Albany, N.Y.). COS-7 cells were provided by J. P. Kinet (Beth Israel Deaconess Medical Center, Boston, Mass.). All cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2.
The following antibodies were used in this study: rat GoH3 monoclonal antibody (MAb [integrin α6 specific]) from Chemicon and rat MAb 439-9B (integrin β4 specific) (9) provided by Rita Falcioni (Regina Elena Cancer Institute, Rome, Italy). A peptide-specific antiserum elicited against the last 20 amino acids of the COOH terminus of the β4 subunit was prepared commercially. The HD1/plectin MAb was obtained from Becton-Dickinson (San Diego, Calif.). Tetramethyl rhodamine isothiocyanate- and Cy2-conjugated secondary antibodies were purchased from Jackson Immunolabs.
Laminin-1, prepared from the EHS sarcoma, was provided by Hynda Kleinman (National Institute of Dental Research, Bethesda, Md.). Collagen type I was purchased from Collagen Corp. (Palo Alto, Calif.). Human recombinant EGF, α-chymotrypsin, Asp-N-endoproteinase, and V8 protease were purchased from Sigma Chemical (St. Louis, Mo.). Purified PKC isoforms were purchased from Calbiochem (San Diego, Calif.). Gö6976 was from Alexis Biochemical.
Metabolic radiolabeling.
Cells were plated on laminin-1-coated dishes for 2 h and then washed several times with phosphate-free Dulbecco's modified Eagle's medium and starved for 1 h before adding 32PO4 (1.0 mCi/ml; NEN, Boston, Mass.) and incubating for 3 more h. The cells were then stimulated with EGF (100 ng/ml) for 15 min, washed several times with phosphate-buffered saline (PBS) and extracted in radioimmunoprecipitation assay buffer containing 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 0.5% deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 50 mM sodium pyrophosphate, 100 mM sodium fluoride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 10-μg/ml each leupeptin, pepstatin A, and aprotinin, and 50 mM Tris-HCl (pH 7.5). The samples were immunoprecipitated using the 439-9B antibody, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Micropore).
In vitro PKC kinase assay.
After immunoprecipitation of α6β4 with the 439-9b antibody and antirat agarose in radioimmunoprecipitation assay buffer and washing several times with kinase buffer (50 mM Tris/HCl [pH 7.5], 10 mM MgCl2), the beads were resuspended in 50 μl of kinase buffer containing 1 μg of a PKC isoform (PKC-α, -βΙ, -βΙΙ, and -δ from Calbiochem), 50 μM ATP, 140 μM phosphatidylserine, 4 μM 1,2-dioleoyl-SN-glycerol (DOG; Avanti), and 10 μCi of [γ-32]ATP (NEN). The reaction was carried out for 20 min at 30°C. The samples were centrifuged, washed, and eluted in sample buffer before PAGE and electrotransfer onto a PVDF membrane.
Analysis of the radiolabeled β4 integrin subunit.
The PVDF membranes containing the β4 immunoprecipitates were exposed to a phosphor screen (Bio-Rad), and the area of the membrane that contained the β4 subunit was excised with a razor. For phosphoamino acid analysis, the excised band (or eluted phosphopeptide [see below]) was acid hydrolyzed and resolved using two-dimensional thin-layer electrophoresis (TLE) and exposed to a phosphor screen following standard techniques (25). For phosphopeptide mapping, the band was digested with trypsin as described previously (25) and run on a thin-layer chromatography (TLC) plate (cellulose) using a pH 1.9 buffer during the electrophoresis separation in the first dimension (TLE) and then a standard chromatography buffer in the second dimension (TLC), followed by exposure of the plate to a phosphor screen. The phosphopeptide profile of β4 was compared with a prediction profile generated by PhosphoPepsort4 (www.genestream.org) to identify candidate sequences. The cellulose area corresponding to the spots of interest was scraped off, and the phosphopeptides were eluted for further analysis. Manual Edman degradation was performed using standard techniques (25). The eluted phosphopeptides were subject to 2 to 5 cycles of degradation and then resolved using TLE and exposed to a phosphor screen. Diagnostic secondary digestions from the phosphopeptides were performed as described previously (25) by digesting the eluted phosphopeptides with either α-chymotrypsin (1 μg/ml in ammonium bicarbonate buffer at pH 8 [see above]), V8 protease, or Asp-N-endoproteinase (1 μg/ml, pH 7.6). The digests were inactivated and resolved using TLE alongside undigested controls.
Site-directed mutagenesis and cDNA transfections.
Single or multiple amino acid substitutions on human wild-type β4 cDNA were made by generating PCR fragments into which the desired mutations had been introduced by means of appropriately designed primers following standard techniques (6). The resulting point mutations were confirmed by dideoxy sequencing. The vectors (pcDNA4 plasmid; InVitrogen) containing the mutant β4 cDNAs (1 μg) were cotransfected with an α6 cDNA (1 μg) (39) into the COS-7 cells by using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Myristylated PKC-α (35) was kindly provided by Alex Toker (Beth Israel Deaconess Medical Center, Boston, Mass.).
Indirect immunofluorescence.
COS-7 transfectants were plated on coverslips for 3 h before overnight stimulation with EGF (100 ng/ml). In some cases, cells were incubated in the presence of Gö6976 (1 μM), a conventional PKC inhibitor (23). The cells were then fixed using 1% paraformaldehyde in PBS for 20 min, followed by permeabilization for 10 min using 0.5% Triton X-100 in PBS. The cells were rinsed with PBS and blocked and double stained as described previously (34). The preparations were analyzed using standard fluorescence microscopy. A quantitative analysis was performed by counting the number of cells showing hemidesmosomal plaques as a percentage of the total number of cells expressing similar levels of α6β4.
RESULTS
Peptide mapping of the 32P-labeled β4 integrin subunit.
To identify phosphorylation sites in the β4 integrin subunit, HaCat cells were metabolically labeled with 32PO4 and then stimulated with EGF for 15 min, and detergent extracts were immunoprecipitated with a β4 antibody. Analysis of the immunoprecipitated proteins by autoradiography revealed a relatively low level of constitutive phosphorylation of the β4 subunit that increased 3.5-fold in response to EGF stimulation (Fig. 1A). Phosphorylation of the α6 subunit, which is also immunoprecipitated by the β4 antibody, was not observed (data not shown). The radiolabeled bands corresponding to the β4 subunit were excised and subjected to phosphoamino acid analysis. As shown in Fig. 1B, the bulk of the phosphorylation induced by EGF was on serine (96%) with significantly less phosphorylation on tyrosine and threonine (3% and 1%, respectively), a pattern similar to that observed for EGF stimulation of A431 cells (35). Subsequently, phosphopeptide mapping was performed by digesting the excised β4 band with trypsin and resolving the sample by using TLE in one dimension and TLC in the second dimension. This analysis yielded approximately 20 discernible phosphopeptides from both HaCat cells (Fig. 2A [only 10 are labeled for reference]) and A431 cells (data not shown). Most of the radioactivity (∼50%), however, was contained in three phosphopeptides (pp0, pp1, and pp2, below the reference line in Fig. 2A). The spatial relationship (i.e., diagonal laddering) of these three peptides suggested that they are isoforms of the same multilabeled phosphopeptide (5).
FIG. 1.
EGF stimulates phosphorylation of the β4 integrin largely on serine. (A) HaCat cells plated on laminin-1 were labeled with 32PO4 as described in Materials and Methods and stimulated with EGF (100 ng/ml) for 15 min. Cells were lysed, and the α6β4 integrin was immunoprecipitated, separated using SDS-PAGE, blotted with the β4-specific polyclonal antibody, and exposed to a phosphor screen. (B) Phosphoamino acid analysis of 32PO4-labeled β4. The radiolabeled band that corresponded to the β4 subunit obtained from cells stimulated with either 0 (−) or 100-ng/ml (+) EGF was cut from the membrane, subjected to acid hydrolysis, and resolved using two-dimensional TLE as described in Materials and Methods. The plate was then exposed to a phosphor screen. S, phosphoserine; T, phosphothreonine; Y, phosphotyrosine.
FIG. 2.
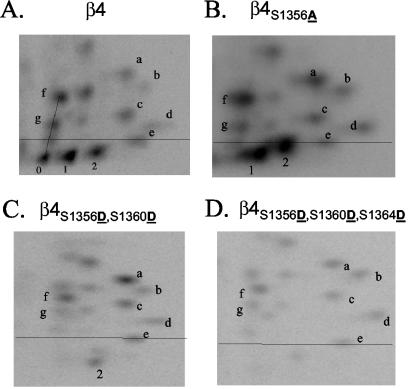
Peptide mapping analysis of phosphorylated wild-type and mutant β4 integrin. Excised bands containing 32P-radiolabeled wild-type β4 from HaCat cells (A) or mutated β4 from COS-7 transfectants (B to D) were digested using trypsin, and the peptides were resolved in two dimensions using a combination of TLE and TLC and exposed to a phosphor screen. The phosphopeptides of interest for this study (pp0, pp1, and pp2) are located below a line defined by a control dye. Notice the disappearance of pp0 and both an increase and relocation of pp1 and pp2 to more hydrophobic (higher) positions in β4S1356A (B); the disappearance of pp0 and pp1, as well as the relocation of pp2 to a more hydrophilic (lower) position in the double mutant β4S1356D S1360D (C); and the disappearance of pp0, pp1, and pp2 in the triple mutant β4S1356D S1360D S1364D (D).
Identification of the major β4 phosphorylation sites.
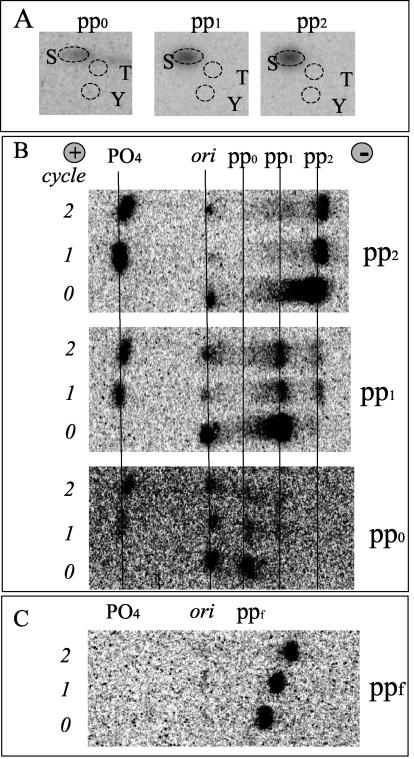
The major phosphopeptides (pp0, pp1, and pp2) were recovered from the TLC plate and subjected to a sequential analysis that included phosphoamino acid analysis, manual Edman degradation, diagnostic secondary digests, and analysis of their relative electrophoretic and chromatographic mobility. Phosphoamino acid analysis revealed that all of the phosphopeptides contained only phosphoserine (Fig. 3A). It was apparent from Edman degradation that the three phosphopeptides were phosphorylated on the first position, because phosphate (PO4) is shed during the first cycle by the cleaved amino acid (Fig. 3B). Moreover, after the first round of degradation, pp0 produced a residual phosphopeptide that ran similar to pp1 and pp1 produced a phosphopeptide similar to pp2, further supporting their relationship as isoforms of the same multilabeled phosphopeptide. As a control, we used ppf (Fig. 2A), which did not shed phosphate during the first two cycles (Fig. 3C). Additional information on the phosphopeptides was obtained by comparing their digestion patterns with specific enzymes (V8 protease, Asp-N-endoproteinase, and α-chymotrypsin). As shown in Table 1, pp1 and pp2 were sensitive to V8 protease (cuts the E-X bond, where X is any amino acid) and Asp-N-endoproteinase (cuts the X-D bond) but not chymotrypsin (cuts the F-X, W-X, or Y-X bond). In contrast, the ppf peptide, used as a control, was digested by α-chymotrypsin but not the other proteases.
FIG. 3.
Analysis of phosphorylated peptides (pp0, pp1, and pp2) derived from radiolabeled β4 integrin. 32P radiolabeled peptides were eluted from TLE or TLC plates and subjected to phosphoamino acid analysis (A) or Edman degradation (B and C). The peptides were degraded for at least 2 cycles. Notice that the phosphate is shed during the first cycle for pp0, pp1, and pp2 (B). In contrast, ppf (C), used as a control, did not shed any phosphate (PO4 position) during the first 2 cycles. ori, sample origin; +, anode; −, cathode; S, phosphoserine; T, phosphothreonine; Y, phosphotyrosine.
TABLE 1.
Diagnostic digestion of β4 integrin phosphopeptides
| Enzyme | Digestion ofa:
|
||
|---|---|---|---|
| pp1 | pp2 | ppf | |
| V8 protease | + | + | − |
| Asp-N-endoproteinase | + | + | − |
| α-Chymotrypsin | − | − | + |
Phosphopeptides were eluted from TLC plates and subjected to digestion as described in Materials and Methods. +, susceptibility to the enzyme.
The PepSort4 program (www.genestream.org) was used to identify putative sequences for the major phosphopeptides of β4 based on their mobility in TLE or TLC. This analysis revealed two phosphopeptides (designated PP-I and PP-II), both of which are contained within β4, whose sequences were consistent with all (PP-I) or most (PP-II) of our data (Fig. 4A). Namely, these peptides contained a serine at the first position (as predicted by Edman degradation) and at least two additional serine residues (as predicted by “diagonal laddering” observed in peptide mapping) and could potentially be cut with V8 (E in both PP-I and -II) and Asp-N-endoproteinase (D only in PP-I) but not α-chymotrypsin (no F, W, or Y in either candidate). To assess the validity of the identified sequences, site-specific mutagenesis and subsequent biochemical analyses were performed. Initially, we mutated (underlined residues below) the first serine (S1356 or S1427 Ser→Ala or Ser→Asp) of the two candidate phosphopeptides (PP-I and PP-II) within the full-length β4 cDNA (Fig. 4B). The β4 wild-type, β4S1356A (PP-I), and β4S1427A (PP-II) plasmids were transiently transfected into COS-7 cells, and 2 days later, the cells were metabolically labeled with 32PO4, stimulated with EGF, and processed for peptide mapping. The phosphopeptide map of wild-type β4 was very similar to that observed for HaCat and A431 cells (shown for HaCat in Fig. 2A). Analysis of Ser→Ala mutants at the first position of PP-I and PP-II revealed that pp0 disappeared only for the PP-I (β4S1356A in Fig. 2B and 4B) and not for PP-II (Fig. 4B). Moreover, an alteration in the mobility of pp1 and pp2 from β4S1356A (PP-I) towards more hydrophobic regions was observed, consistent with the gain of hydrophobicity predicted for a multiphosphorylated peptide that has one of its serines mutated to alanine. These results suggested that PP-I is the candidate phosphopeptide and rule out PP-II.
FIG. 4.
Identification of S1356, S1360, and S1364 as the major sites of β4 phosphorylation. (A) Two candidate peptides (PP-I and PP-II) were identified based on a combined analysis that included phosphopeptide mobility, Edman degradation, phosphoamino acid analysis, and diagnostic enzymatic digestion. (B) Point mutations introduced into the candidate serines in the full-length β4 were analyzed by peptide mapping as described in Fig. 2. The table notes the presence or disappearance of the three major peptides of interest after peptide mapping. The peptide maps of mutants critical for the identification process are shown on Fig. 2. (C) Position of S1356, S1360, and S1364 within the β4 integrin cytoplasmic tail. FNn, fibronectin type III repeats; CS, connecting segment. (D) Structure of β4 integrin point mutations (S1356, S1360, and S1364) constructed on a full-length β4 for this study. Two sets of mutants were built: Ser→Ala, to block phosphorylation and Ser→Asp to mimic constitutive phosphorylation.
To identify the two other predicted phosphorylation sites within PP-I, we constructed a series of double and triple mutants (Fig. 4B). Mutation of S1356 and S1360 in PP-I (Fig. 2C, β4S1356D S1360D; and Fig. 4B) resulted in the loss of both pp0 and pp1 and the altered mobility of the remaining pp2 towards more hydrophilic (lower) regions as expected for Ser→Asp mutations. Potential phosphorylation of S1358 or S1366 was excluded because triple-mutant constructs containing these mutations, either as S1356 S1358 S1360 or S1356 S1360 S1366, failed to eliminate all three peptides of interest (Fig. 4B). In contrast, the triple mutation of S1356 S1360 S1364 eliminated all three phosphopeptides (Fig. 2D, β4S1356D S1360D S1364D; and Fig. 4B). Taken together, these results strongly indicate that S1356, S1360, and S1364, which are located in the connecting segment (Fig. 4C), are the major residues phosphorylated on β4 after EGF stimulation.
PKC-α phosphorylates pp1 and pp2 in vitro.
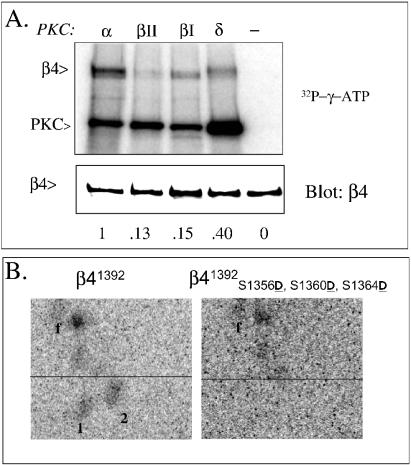
We had shown previously that PKC-α is involved in EGF-induced serine phosphorylation of the β4 integrin subunit, although we did not assess whether it phosphorylated β4 directly (35). Two of the identified phosphorylation sites, S1360 and S1364, are located within a consensus sequence for PKC (Prosite) (32). Therefore, we examined the possibility that these serines are directly phosphorylated by PKC using immune complexes of β4 obtained from α6β4-transfected COS-7 cells as substrates for purified PKC-α. As shown in Fig. 5A, no significant phosphorylation of β4 was seen in the absence of enzyme but the addition of PKC-α resulted in significant phosphorylation. Other PKC isoforms were able to phosphorylate β4, albeit to a lesser extent (0.15×, 0.13×, and 0.40× for PKC-βI, -βII, and -δ, respectively; Fig. 5A), suggesting isoform preference.
FIG. 5.
PKC-α phosphorylates pp1 and pp2 in vitro. Lysates were obtained from COS-7 cells transfected with wild-type β4 (A), truncated β41392 (B, left) or triple Ser→Asp mutant β41392 S1356D S1360D S1364D (B, right) were immunocomplexed with anti-β4 antibodies and antirat agarose and labeled with [γ-32P]ATP and either PKC-α, -βΙ, -βΙΙ, or -δ as described in Materials and Methods. Radiolabeled immunocomplexes were eluted and separated by SDS-PAGE, electrotransferred to a PVDF membrane, and exposed to a phosphor screen (A, top panel) and later blotted with an anti-β4 polyclonal antibody (A, bottom panel) Autophosphorylation of PKC indicates the activity of the enzymes used in the assay. In panel B, radiolabeled β4 was excised from the PVDF membrane, trypsinized, and analyzed by peptide mapping as described in the legend to Fig. 2.
To address whether S1356, S1360, and S1364 are phosphorylated by PKC-α, we used a β4 construct (β41392D1356 D1360 D1364) that carries a triple Ser→Asp mutation on a truncated β4 (residues 1 to 1392) to perform immune-complex kinase assays with PKC-α and subsequent phosphopeptide analysis. The truncation was used to reduce the number of unrelated peptides. As a control, we used the truncated cDNA lacking the serine mutations (β41392). The peptide-mapping analysis revealed that pp1 and pp2 are present in the control construct but eliminated by the triple mutation (Fig. 5B). These results indicate that at least two of the three major serines are phosphorylated by PKC-α, most likely S1360 and S1364, because they are located within a consensus PKC site (Prosite) (32). Conventional PKC members prefer substrates with basic residues at positions −6, −4, −2, +2, or + 3 and a hydrophobic residue at + 1 (32).
Serine phosphorylation on S1356, S1360, and S1364 mediates the effects of EGF on hemidesmosomal plaques.
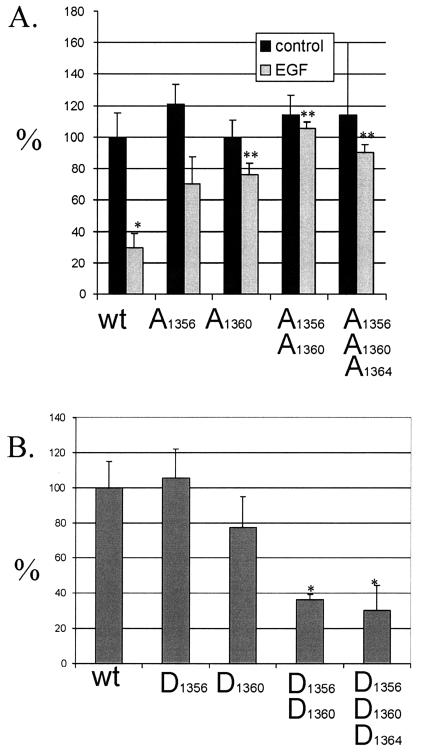
COS-7 cells have been used as a model to study the formation of type II hemidesmosomes because they express plectin and intermediate filaments but lack α6β4 (27). Expression of this integrin results in the recruitment of plectin into “plaque-patterned” hemidesmosomes (27) which can be detected by colocalization of α6β4 and HD1/plectin using indirect immunofluorescence (Fig. 6A to C). We have previously shown that EGF disrupts hemidesmosomes and induces serine phosphorylation of the β4 integrin (35). To test whether phosphorylation of serines S1356, S1360, and S1364 mediates the disruptive action of EGF on hemidesmosomes, we expressed single, double, or triple Ser→Ala mutations (β4S1356A, β4S1360A, β4S1356A S1360A, and β4S1356A S1360A S1364A) in COS-7 cells, predicting that these mutations would prevent hemidesmosome disruption induced by EGF. In the absence of EGF, the mutation of these serines did not significantly alter the ability of exogenous β4 to form hemidesmosomal plaques (Fig. 7A). In the presence of EGF, the frequency of hemidesmosomal plaque formation by wild-type β4 was reduced significantly (Fig. 7A), confirming previous results (21, 35). Mutations in S1356, S1360, and S1364, in contrast, impeded the ability of EGF to disrupt hemidesmosomal plaques. The double mutant β4S1356A S1360A was more effective than the single mutants in this regard. These results suggest that cooperative phosphorylation on S1356, S1360, and S1364 mediates the disruption of type II hemidesmosomes in response to EGF. The fact that the Ser→Ala mutation of these serines did not affect hemidesmosome formation suggests that they are not essential for the formation of the hemidesmosome itself.
FIG. 6.
Expression of hemidesmosomal plaques in COS-7 transfectants. Examples of COS-7 transfectants determined to be positive (A, B, C) or negative (D, E, and F) for expression of hemidesmosomal plaques. Panels A to C show COS-7 cells transfected with wild-type β4 + α6 integrin subunits. Panels C to E show COS-7 cells transfected with β4S1356D S1360D S1364D + α6 integrin subunits. The cells were grown overnight on coverslips and processed for indirect immunofluorescence. The cells were stained for HD1/plectin (B and E [red in panel C or F]) and α6β4 (A and D [green in panel C or F]) and analyzed for colocalization (yellow in panel C or F). Notice the hemidesmosomal plaques in panel C and only a thread-like pattern in panel F. Bar, 10 μm.
FIG. 7.
Quantitation of the effect of serine mutations on hemidesmosome dynamics. (A and B) COS-7 cells transfected with the indicated Ser→Ala (A) or Ser→Asp (B) phosphorylation mutants as described in Materials and Methods. (A) The Ser→Ala mutants were incubated in the absence or presence of EGF (100 ng/ml) overnight and processed for indirect immunofluorescence as described in the legend to Fig. 6 and Materials and Methods. The graph describes the percentage of positive cells showing the presence of hemidesmosomal plaques in relation to the wild type β4 (wt; 100%). (B) The Ser→Asp mutants were incubated overnight on coverslips and then processed for indirect immunofluorescence and analyzed as described for panel A. *, P < 0.05 versus wild type; **, P < 0.05 versus wild type + EGF.
The hypothesis that phosphorylation of S1356, S1360, and S1364 influences hemidesmosome dynamics was evaluated further by constructing single, double, or triple Ser→Asp mutants that mimic constitutive phosphorylation in the absence of exogenous stimuli (β4S1356D, β4S1360D, β4S1356D S1360D, and β4S1356D S1360D S1364D). The ability to form hemidesmosomal plaques differed among these mutants (Fig. 7B). The double and triple phosphorylation-mimicking mutants β4S1356D S1360D and β4S1356D S1360D S1364D exhibited a substantial reduction in the plaque pattern, although some recruitment of plectin was still observed in threadlike structures (Fig. 6D to F and Fig. 7B). Expression of the single mutations did not significantly differ from that in the wild type. These results indicate that cooperative phosphorylation on S1356, S1360, and S1364 inhibits hemidesmosome formation.
The major β4 serine phosphorylation sites (S1356, S1360, and S1364) mediate the effects of PKC-α on hemidesmosomal plaques.
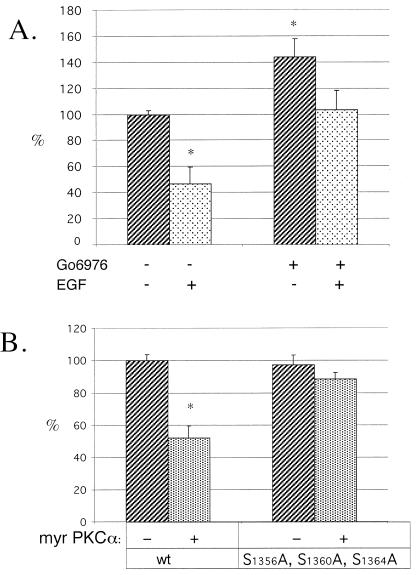
We evaluated the hypothesis that the inhibition of conventional PKCs either modifies the formation of hemidesmosomal plaques or prevents the disruption of these structures by EGF stimulation. COS-7 cells cotransfected with α6 and β4 were treated with the conventional PKC inhibitor Gö6976 before stimulation with EGF. As shown in Fig. 8A, this inhibition significantly increased the formation of hemidesmosomal plaques. Moreover, the effects of EGF were substantially, although not completely, reduced by the inhibitor, indicating that conventional PKCs play an important role in the overall stability of the hemidesmosome even in nonstimulated cells. Subsequently, we tested the possibility that PKC-α is sufficient to induce the disruption of hemidesmosomal plaques by cotransfecting α6β4 with a myristylated form of PKC-α (myrPKC-α) that acts as a constitutively active variant of this isoform. As shown in Fig. 8B, expression of myrPKC-α reduced the number of hemidesmosomal plaques substantially. Moreover, mutation of S1356, S1360, and S1364 in β4 impeded the effects of myrPKC-α on hemidesmosomal plaques, indicating that phosphorylation of these serines is important in the overall stability of the plaque.
FIG. 8.
β4 serines S1356, S1360, and S1364 mediate the effects of PKC-α on hemidesmosomal plaques. (A). COS-7 cells expressing exogenous α6β4 were incubated overnight on coverslips in the presence or absence of Gö6976 and/or EGF and then analyzed and processed for indirect immunofluorescence as described in the legend to Fig. 6 and Materials and Methods. The graph describes the percentage of positive cells showing the presence of hemidesmosomal plaques in relation to the unstimulated cells (100%). (B) COS-7 cells were cotransfected with α6, β4, and myrPKC-α. The cells were plated on coverslips overnight and processed and analyzed as described for panel A.
DISCUSSION
Strong adhesion to the substrate can hinder cell migration. Such is the case for hemidesmosomes, which provide epithelial cells with a strong and stable anchor to the basal lamina. It is clear from wound healing assays that hemidesmosomes need to be disassembled to allow cell migration (13). The α6β4 integrin, a component of the hemidesmosome, plays a pivotal role in the formation and stability of this multiprotein structure and, as a consequence, the ability of epithelial cells to migrate (4, 24). Previously we and others had shown that EGF, acting as a chemotactic factor, induces mobilization of the α6β4 integrin and promotes hemidesmosome disassembly (21, 35). EGF stimulates a PKC-α-dependent pathway that results in the phosphorylation of the β4 integrin subunit on serine residues and its redistribution to actin-rich structures (35). Subsequent to our work, other groups have supported the notion that PKC mediates the serine phosphorylation of β4 (1, 37). To understand how EGF induces disassembly of the hemidesmosome, it was essential to identify the main serine phosphorylation sites, assess whether these sites are critical for disassembly, and determine the kinase(s) responsible for their phosphorylation. We now have resolved all of these key issues by showing that EGF induces the phosphorylation of the β4 subunit on a cluster of serines (S1356, S1360, and S1364) located within the connecting segment of the cytoplasmic tail. The combined phosphorylation of S1356, S1360, and S1364 is significant quantitatively because it accounts for ∼50% of the total phosphorylation of β4 after EGF stimulation. Moreover, the fact that these serines are evolutionarily conserved in the sequences of human, rat, and mouse β4 (10, 17, 42) suggests they are critical for the function of the α6β4 integrin.
The cytoplasmic tail of β4 is essential for both hemidesmosome formation and disassembly (8, 31, 38, 40). Our results substantiate the importance of serine phosphorylation of the β4 cytoplasmic tail in hemidesmosome disassembly. Specifically, we demonstrate that the ability of EGF to disrupt hemidesmosomes is reduced significantly by mutations that prevent the phosphorylation of S1356, S1360, and S1364. Double or triple mutations of these serines were more efficient than were single mutations in preventing EGF action on hemidesmosomes, suggesting that there is cooperation among these phosphorylation sites to regulate hemidesmosome disassembly. S1356, S1360, and S1364 are located immediately after the minimal region considered necessary for the recruitment of HD1/plectin to the hemidesmosome, and preventing the phosphorylation of S1356, S1360 and S1364 by mutating these residues to alanine had no significant effect on the ability of α6β4 to recruit plectin and form hemidesmosomal plaques. This result is consistent with a previous study demonstrating that a truncated form of β4 (nucleotides 1 to 1355) that excludes the serine cluster is still capable of recruiting HD1/plectin (27). The sustained phosphorylation of S1356, S1360, and S1364, however, probably affects the formation or the stability of the hemidesmosome, as evidenced by the reduced number of hemidesmosomal plaques observed for the phosphorylation-mimicking mutants. This effect also appears to involve cooperation of phosphorylation of the three serine residues because the double and triple Ser→Asp mutations were more effective than the single mutations at reducing hemidesmosomal plaques.
Although mutations in the major serine phosphorylation sites that we identified had a significant effect on hemidesmosome dynamics, none of these mutants resulted in complete elimination of hemidesmosomes and recruitment of plectin. This observation suggests that additional mechanisms of disruption exist, such as other phosphorylation sites. Previous studies had shown that preventing the phosphorylation of two tyrosines in the connecting segment (Y1422 and Y1440) caused a partial inhibition of hemidesmosome disassembly induced by EGF (7). Taken together, these results suggest that the phosphorylation of multiple residues is needed for complete abrogation of the disruptive effects of EGF on the hemidesmosome. Given that our peptide mapping analyses identified approximately 20 potential phosphorylation sites in the β4 subunit, this assumption seems valid. Moreover, a complex regulation is also indicated by the multiplicity of interaction sites between β4 and HD1/plectin (27, 28, 30, 36). The β4 integrin has at least two different binding sites for HD1/plectin (36), and it is reasonable to expect that additional phosphorylation sites might be located throughout the molecule, probably at the carboxy terminus where the second HD1/plectin site is located (36).
The biochemical mechanisms by which phosphorylation of S1356, S1360, and S1364 induce hemidesmosome disruption merit discussion. In addition to a potential inhibition of plectin/β4 association, phosphorylation of S1356, S1360, and S1364 could also destabilize the hemidesmosome by interfering with β4 self-association. Both intermolecular and intramolecular interactions of β4 involving the connecting segment have been reported (36, 38) and could be influenced by phosphorylation of the serine cluster. Disruption of intermolecular interactions by serine phosphorylation may reduce plaque formation. On the other hand, intramolecular interactions may affect the recruitment of other hemidesmosomal components (18, 38). The folding of the carboxy terminus of β4 onto the connecting segment has been hypothesized to regulate the recruitment of BPAG1 and BPAG2 into type I hemidesmosomes, a process that is facilitated by the binding of HD1/plectin (18). It will be interesting to address whether S1356, S1360, and S1364 regulate the recruitment of BPAG1 and BPAG2 directly or indirectly by regulating β4-plectin interactions.
The destabilization of the hemidesmosome by the phosphorylation of S1356, S1360, and S1364 could result from conformational changes caused by the electrostatic charges of the added phosphate groups. This possibility is evidenced by the Ser→Asp mutations that increased the number of charged residues and resulted in hemidesmosome instability. Alternatively, serine phosphorylation may provide the binding site for other destabilizing molecules. It is interesting to note in this regard that other authors have observed PKC-α-mediated serine phosphorylation on unknown residues in the connecting segment that induces the association of 14-3-3 proteins and, in concert with signals from the Ron receptor, may contribute to the destabilization of the hemidesmosome (37). Ron may also induce tyrosine phosphorylation of β4 through src-fyn, a pathway also implicated in EGF-mediated disassembly of hemidesmosomes (22).
A key finding in this study is that PKC-α phosphorylates the β4 subunit directly and that at least two of the three serines we identified are substrates for this kinase. These findings are consistent with several observations including our previous study implicating PKC-α in the EGF-induced phosphorylation of β4 (35), the fact that EGF can activate PKC through the phospholipase C-γ-mediated formation of diacylglycerol and inositol triphosphate (15, 33), and a recent study demonstrating that PKC-α phosphorylates the connecting segment of β4 (37). Our study does not rule out the possibility, however, that other kinases might be involved in the phosphorylation of the identified serines as well. For example, PKC-δ has been shown to phosphorylate β4 (1). Consistent with this study, we observed that PKC-δ phosphorylates β4 in vitro, although to a lesser extent than PKC-α. In contrast to our study and that by Santoro et al. (37), a study by Alt et al. (1) was unable to detect β4 phosphorylation with PKC-α or show its disruptive effects on hemidesmosomes. One explanation for the latter finding is that these authors overexpressed wild-type PKC-α instead of a constitutively active form. Some studies have shown that the cellular effects produced by overexpression of PKC-α may depend on the presence of modulators, such as phorbol myristate acetate (26).
Previous studies have shown that hemidesmosomes type I (α6β4, plectin, and BPAG1 and -2) impede the migration of stratified epithelial cells and that the PKC-mediated disruption of these structures facilitates migration (7, 22, 35). A431 cells, for example, assemble type I hemidesmosomes, and their migration closely correlates with hemidesmosome disassembly induced by EGF (35). Hemidesmosome disassembly and migration of these cells require PKC-α activation and are associated with serine phosphorylation of the β4 subunit (35). Interestingly, we demonstrate in the present study that S1356, S1360, and S1364 are the major sites of EGF-stimulated β4 serine phosphorylation in A431 cells. This result links phosphorylation of these key serine residues with migration. In contrast, however, we also observed that COS-7 cell expression of the triple-serine mutation of β4, which resists the disruption of the hemidesmosomal plaque (type II α6β4 and plectin) after EGF stimulation, did not affect migration (data not shown). This result raises the possibility that the anchor provided by type II hemidesmosomes is different from that of type I hemidesmosomes in terms of resisting migration, the latter being more efficient because it contains at least two more proteins. This assessment is consistent with the fact that cells that normally express type II hemidesmosomes, such as intestinal epithelial cells, are constantly migrating (2).
In summary, we have identified a cluster of serines in the β4 integrin subunit that are targeted directly by PKC-α in response to growth factor stimulation and whose phosphorylation status regulates the dynamics of hemidesmosomes. This mechanism has important implications for the function of the α6β4 integrin in migration and carcinoma invasion.
Acknowledgments
This work was supported by NIH grants CA88919 (I.R.) and CA80789 (A.M.M.) and the Harvard Digestive Diseases Center.
We thank Kaylene Simpson, Elizabeth Lipscomb, and Leslie Shaw for valuable discussions.
REFERENCES
- 1.Alt, A., M. Ohba, L. Li, M. Gartsbein, A. Belanger, M. F. Denning, T. Kuroki, S. H. Yuspa, and T. Tennenbaum. 2001. Protein kinase Cdelta-mediated phosphorylation of alpha6beta4 is associated with reduced integrin localization to the hemidesmosome and decreased keratinocyte attachment. Cancer Res. 61:4591-4598. [PubMed] [Google Scholar]
- 2.Beaulieu, J. F. 1997. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 31:1-78. [DOI] [PubMed] [Google Scholar]
- 3.Borradori, L., P. J. Koch, C. M. Niessen, S. Erkeland, M. R. van Leusden, and A. Sonnenberg. 1997. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the beta4 integrin subunit. J. Cell Biol. 136:1333-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borradori, L., and A. Sonnenberg. 1999. Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Investig. Dermatol. 112:411-418. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110-149. [DOI] [PubMed] [Google Scholar]
- 6.Cormack, B. 2003. Directed mutagenesis using the polymerase chain reaction. Unit 8.5. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, 9th ed. John Wiley & Sons, Inc., New York, N.Y. [Online.]
- 7.Dans, M., L. Gagnoux-Palacios, P. Blaikie, S. Klein, A. Mariotti, and F. G. Giancotti. 2001. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J. Biol. Chem. 276:1494-1502. [DOI] [PubMed] [Google Scholar]
- 8.Dowling, J., Q. C. Yu, and E. Fuchs. 1996. Beta-4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134:559-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcioni, R., A. Sacchi, J. Resau, and S. J. Kennel. 1988. Monoclonal antibody to human carcinoma-associated protein complex: quantitation in normal and tumor tissue. Cancer Res. 48:816-821. [PubMed] [Google Scholar]
- 10.Feltri, M. L., M. Arona, S. S. Scherer, and L. Wrabetz. 1997. Cloning and sequence of the cDNA encoding the beta 4 integrin subunit in rat peripheral nerve. Gene 186:299-304. [DOI] [PubMed] [Google Scholar]
- 11.Fontao, L., S. Dirrig, K. Owaribe, M. Kedinger, and J. F. Launay. 1997. Expression of HD1—relationship with the cytoskeleton in cultured human colonic carcinoma cells. Exp. Cell Res. 231:319-327. [DOI] [PubMed] [Google Scholar]
- 12.Gailit, J., and R. A. Clark. 1994. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 6:717-725. [DOI] [PubMed] [Google Scholar]
- 13.Gipson, I. K., S. Spurr-Michaud, A. Tisdale, J. Elwell, and M. A. Stepp. 1993. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp. Cell Res. 207:86-98. [DOI] [PubMed] [Google Scholar]
- 14.Green, K. J., and J. C. R. Jones. 1996. Desmosomes and hemidesmosomes—structure and function of molecular components. FASEB J. 10:871-881. [DOI] [PubMed] [Google Scholar]
- 15.Hepler, J. R., N. Nakahata, T. W. Lovenberg, J. DiGuiseppi, B. Herman, H. S. Earp, and T. K. Harden. 1987. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J. Biol. Chem. 262:2951-2956. [PubMed] [Google Scholar]
- 16.Hogervorst, F., I. Kuikman, A. E. von dem Borne, and A. Sonnenberg. 1990. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 9:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennel, S. J., V. Godfrey, L. Y. Ch'ang, T. K. Lankford, L. J. Foote, and A. Makkinje. 1992. The beta 4 subunit of the integrin family is displayed on a restricted subset of endothelium in mice. J. Cell Sci. 101:145-150. [DOI] [PubMed] [Google Scholar]
- 18.Koster, J., D. Geerts, B. Favre, L. Borradori, and A. Sonnenberg. 2003. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J. Cell Sci. 116:387-399. [DOI] [PubMed] [Google Scholar]
- 19.Larjava, H., T. Salo, K. Haapasalmi, R. H. Kramer, and J. Heino. 1993. Expression of integrins and basement membrane components by wound keratinocytes. J. Clin. Investig. 92:1425-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liotta, L. A., M. L. Stracke, S. A. Aznavoorian, M. E. Beckner, and E. Schiffmann. 1991. Tumor cell motility. Semin. Cancer Biol. 2:111-114. [PubMed] [Google Scholar]
- 21.Mainiero, F., A. Pepe, M. Yeon, Y. Ren, and F. G. Giancotti. 1996. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J. Cell Biol. 134:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariotti, A., P. A. Kedeshian, M. Dans, A. M. Curatola, L. Gagnoux-Palacios, and F. G. Giancotti. 2001. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 155:447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martiny-Baron, G., M. G. Kazanietz, H. Mischak, P. M. Blumberg, G. Kochs, H. Hug, D. Marme, and C. Schachtele. 1993. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268:9194-9197. [PubMed] [Google Scholar]
- 24.Mercurio, A. M. 1995. Laminin receptors—achieving specificity through cooperation. Trends Cell Biol. 5:419-423. [DOI] [PubMed] [Google Scholar]
- 25.Moore, D., and B. M. Sefton. 1997. Analysis of protein phosphorylation, p. 18.0.1-18.9.28. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, 9th ed. JohnWiley & Sons, Inc., New York, N.Y.
- 26.Nakashima, S. 2002. Protein kinase C alpha: regulation and biological function. J. Biochem. 132:669-675. [DOI] [PubMed] [Google Scholar]
- 27.Niessen, C. M., E. H. Hulsman, L. C. Oomen, I. Kuikman, and A. Sonnenberg. 1997. A minimal region on the integrin beta4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J. Cell Sci. 110:1705-1716. [DOI] [PubMed] [Google Scholar]
- 28.Niessen, C. M., E. H. Hulsman, E. S. Rots, P. Sanchez-Aparicio, and A. Sonnenberg. 1997. Integrin alpha 6 beta 4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol. Biol. Cell 8:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niessen, C. M., M. H. van der Raaij-Helmer, E. H. Hulsman, R. van der Neut, M. F. Jonkman, and A. Sonnenberg. 1996. Deficiency of the integrin beta 4 subunit in junctional epidermolysis bullosa with pyloric atresia: consequences for hemidesmosome formation and adhesion properties. J. Cell Sci. 109:1695-1706. [DOI] [PubMed] [Google Scholar]
- 30.Nievers, M. G., I. Kuikman, D. Geerts, I. M. Leigh, and A. Sonnenberg. 2000. Formation of hemidesmosome-like structures in the absence of ligand binding by the (alpha)6(beta)4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the (beta)4 integrin subunit. J. Cell Sci. 113:963-973. [DOI] [PubMed] [Google Scholar]
- 31.Nievers, M. G., R. Q. Schaapveld, L. C. Oomen, L. Fontao, D. Geerts, and A. Sonnenberg. 1998. Ligand-independent role of the beta 4 integrin subunit in the formation of hemidesmosomes. J. Cell Sci. 111:1659-1672. [DOI] [PubMed] [Google Scholar]
- 32.Nishikawa, K., A. Toker, F. J. Johannes, S. Y. Zhou, and L. C. Cantley. 1997. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 272:952-960. [DOI] [PubMed] [Google Scholar]
- 33.Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693-698. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovitz, I., and A. M. Mercurio. 1997. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 139:1873-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovitz, I., A. Toker, and A. M. Mercurio. 1999. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J. Cell Biol. 146:1147-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezniczek, G. A., J. M. de Pereda, S. Reipert, and G. Wiche. 1998. Linking integrin alpha6beta4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the beta4 subunit and plectin at multiple molecular sites. J. Cell Biol. 141:209-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santoro, M. M., G. Gaudino, and P. C. Marchisio. 2003. The MSP receptor regulates alpha 6 beta 4 and alpha 3 beta 1 integrins via 14-3-3 proteins in keratinocyte migration. Dev. Cell 5:257-271. [DOI] [PubMed] [Google Scholar]
- 38.Schaapveld, R. Q., L. Borradori, D. Geerts, M. R. van Leusden, I. Kuikman, M. G. Nievers, C. M. Niessen, R. D. Steenbergen, P. J. Snijders, and A. Sonnenberg. 1998. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J. Cell Biol. 142:271-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw, L. M., M. M. Lotz, and A. M. Mercurio. 1993. Inside-out integrin signaling in macrophages. Analysis of the role of the alpha 6A beta 1 and alpha 6B beta 1 integrin variants in laminin adhesion by cDNA expression in an alpha 6 integrin-deficient macrophage cell line. J. Biol. Chem. 268:11401-11408. [PubMed] [Google Scholar]
- 40.Spinardi, L., Y. L. Ren, R. Sanders, and F. G. Giancotti. 1993. The beta 4 subunit cytoplasmic domain mediates the interaction of alpha 6 beta 4 integrin with the cytoskeleton of hemidesmosomes. Mol. Biol. Cell 4:871-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stetler-Stevenson, W. G., S. Aznavoorian, and L. A. Liotta. 1993. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 9:541-573. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, S., and Y. Naitoh. 1990. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 9:757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uematsu, J., Y. Nishizawa, A. Sonnenberg, and K. Owaribe. 1994. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J. Biochem. 115:469-476. [DOI] [PubMed] [Google Scholar]
- 44.Vidal, F., D. Aberdam, C. Miquel, A. M. Christiano, L. Pulkkinen, J. Uitto, J. P. Ortonne, and G. Meneguzzi. 1995. Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 10:229-234. [DOI] [PubMed] [Google Scholar]