Abstract
Background
Nicotine dependence is associated with an increased risk of mood and anxiety disorders and suicide. The primary hypothesis of this study was to identify whether the polymorphisms of two glutathione-S-transferase enzymes (GSTM1 and GSTT1 genes) predict an increased risk of mood and anxiety disorders in smokers with nicotine dependence.
Materials and methods
Smokers were recruited at the Centre of Treatment for Smokers. The instruments were a sociodemographic questionnaire, Fagerström Test for Nicotine Dependence, diagnoses of mood disorder and nicotine dependence according to DSM-IV (SCID-IV), and the Alcohol, Smoking and Substance Involvement Screening Test. Anxiety disorder was assessed based on the treatment report. Laboratory assessment included glutathione-S-transferases M1 (GSTM1) and T1 (GSTT1), which were detected by a multiplex-PCR protocol.
Results
Compared with individuals who had both GSTM1 and GSTT1 genes, a higher frequency of at least one deletion of the GSTM1 and GSTT1 genes was identified in anxious smokers [odds ratio (OR)=2.21, 95% confidence interval (CI)=1.05–4.65, P=0.034], but there was no association with bipolar and unipolar depression (P=0.943). Compared with nonanxious smokers, anxious smokers had a greater risk for mood disorders (OR=4.67; 95% CI=2.24–9.92, P<0.001), lung disease (OR=6.78, 95% CI=1.95–23.58, P<0.003), and suicide attempts (OR=17.01, 95% CI=2.23–129.91, P<0.006).
Conclusion
This study suggests that at least one deletion of the GSTM1 and GSTT1 genes represents a risk factor for anxious smokers. These two genes may modify the capacity for the detoxification potential against oxidative stress.
Keywords: anxiety disorder, genetic polymorphisms, glutathione-S-transferase, mood disorder, nicotine dependence
Introduction
The relationship between nicotine dependence and anxiety disorders has been established in epidemiological and clinical studies. Cigarette smoking is associated with an increased risk of anxiety disorders. In the National Comorbidity Survey, nearly 61.3% of individuals with a lifetime history of panic disorder and 68.4% with generalized anxiety disorders were current or past smokers, whereas only 39% of smokers had no mental disorders (Lasser et al., 2000; Ziedonis et al., 2008).
The nexus between co-occurring disorders such as smoking, depression, and anxiety is not clear. Depression and anxiety may increase the risks of smoking, and smoking may increase the risks of depression and anxiety. A history of previous psychiatric disorders increases the risk of early onset of smoking, and contributes to progression from daily smoking to nicotine dependence (Breslau et al., 2004). Depression and anxiety symptoms have been associated with a greater risk for the onset of smoking behaviors (Patton et al., 1998). In contrast, other studies have found evidence that smoking increases the risk for the onset of depression and anxiety disorders (Pasco et al., 2008; Boden et al., 2010; Moylan et al., 2012). Smoking appears to increase the risk of the development of mood disorders, anxiety disorders, and suicide (Pedersen and von Soest, 2009; Berlin et al., 2011). Nicotine dependence is a vulnerability factor for the development of severe depressive and anxiety symptoms and is associated with slower recovery (Jamal et al., 2012).
Previous studies have shown a link between anxiety and depressive disorders and chronic obstructive pulmonary disease (COPD); these associations are partly attributable to cigarette smoking and nicotine dependence (Goodwin et al., 2012). Anxiety and depression are prevalent comorbidities in COPD and are related to a worsened course of disease. Genetic variation might also be an influence in smoking behavior and susceptibility for diseases induced by tobacco use (Quaak et al., 2009).
It is known that genes coding for the glutathione-S-transferase M1 (GSTM1) and theta 1 (GSTT1) are involved in detoxification of many toxic compounds and products of oxidative stress (Hayes and Pulford, 1995). Benzo(a)pyrene present in cigarettes is metabolized by glutathione-S-transferase enzymes. Therefore, glutathione systems protect the cell against xenobiotics and oxidative and nitrosative stress (Berk et al., 2008). There are increased oxidative stress damage biomarkers in mood disorders and nicotine dependences (Nunes et al., 2013; Vargas et al., 2013a). Glutathione-S-transferase genes are involved in cellular protection against inflammation and oxidative stress damage from various toxic substances in tobacco smoke (Kim et al., 2006). A reduced antioxidant capacity has been described in depressed smokers (Vargas et al., 2013a).
The absence of GSTM1/GSTT1 activity is the result of homozygosity for an inherited deletion of these genes, termed the null genotype (Hayes and Pulford, 1995), which combined with cigarette smoking has been implicated as a risk factor for a wide range of tobacco-related diseases, including susceptibility to coronary heart disease (Li et al., 2000; Wang et al., 2008; Wang et al., 2010), cerebrovascular diseases (Um et al., 2006), lung cancer (Schneider et al., 2004; Lam et al., 2009), and COPD (Xue et al., 2012). In contrast, another study did not find an association between GSTM1/GSTT1 null genotype and risk of ischemic heart disease (Norskov et al., 2011).
In the light of these findings, the current study examines the role of GSTM1 and GSTT1 genes in smokers with anxiety and mood disorders. These two genes may modify the capacity for the detoxification potential against oxidative stress. We hypothesized that at least one deletion of the GSTM1 and GSTT1 genes compared with people who had both GSTM1 and GSTT1 genes is associated with a greater risk for anxiety and mood disorders in smokers.
Materials and methods
Study population
Smokers (n=151) were recruited from outpatients at the Centre of Treatment for Smokers, at University Hospital of Londrina, State University of Londrina (UEL) (Paraná, Brazil). The controls were never-smokers (n=191) and were recruited from staff at UEL. This research was approved by the Ethics Research Committee at State University of Londrina, and all participants gave written informed consent to participate in the study. The study was conducted from March 2011 to July 2012. Smokers were men and women aged 18–60 years of all ethnicities.
Instruments
Questionnaire
A self-reported questionnaire was used to obtain information on smoking status and demographic information. Clinical characteristics were obtained through an interviewer-administered structured questionnaire.
Nicotine dependence, mood and anxiety disorders
The diagnoses of major depressive disorder, bipolar disorder, and nicotine dependence were obtained using the Structured Clinical Interview for DSM-IV, axis I (SCID-IV) translated and validated into Portuguese (Del Ben et al., 2001). The diagnosed anxiety disorders included panic disorder and generalized anxiety disorders that were reported for ongoing treatment.
Smoking status
The Fagerström Test for Nicotine Dependence (FTND) was described by Fagerström and Schneider (1989). This instrument was translated and adapted into Portuguese (Carmo and Pueyo, 2002). The FTND is a self-administered six-item questionnaire and scores range from 0 to 10.
The number of pack years was calculated according to the definition: the number of cigarettes smoked per day×number of years smoked/20 (one pack contains 20 cigarettes) (Huxley et al., 2012).
The Alcohol, Smoking and Substance Involvement Screening Test
The Alcohol, Smoking and Substance Involvement Screening Test is a questionnaire to screen for risk of alcohol, smoking, and substance use in adults. Smokers whose alcohol involvement scores were between 0 and 10 were considered at low risk, and those with scores between 11 and 26 were at moderate risk of harm and were offered a brief intervention. Smokers whose sedatives or sleeping pills scores were between 0 and 3 were at low risk of harm, and those with scores between 4 and 26 were at moderate risk of harm and were offered a brief intervention. Smokers whose score was 27 or more were considered at high risk of harm and substance dependence and required intensive intervention (World Health Organization, 2002).
Genotyping
Genomic DNA was extracted from 200 μl of peripheral blood cells of all participants using the Biopur Kit (Biometrix Diagnostic, Curitiba, Brazil) according to the manufacturer’s instructions. After precipitation with ethanol, the DNA pellet was resuspended in 50 μl of Biopur Kit specific buffer, quantified by spectrophotometry, and stored at freezer −80°C for later use in genotyping analyses.
The genetic polymorphisms were studied using multiplex PCR protocol (Abdel-Rahman et al., 1996) with modifications: 80–100 ng of DNA was amplified in a total volume of 25 μl reaction containing 20 mmol/l Tris-HCl; 50 mmol/l KCl; 1.5 mmol/l MgCl2; 2 mmol/l of each deoxynucleotide triphosphate; 1 pmol of each primer; and 1.25 U of AmpliTaq DNA polymerase. PCR was carried out in a PTC-100 Termocycler (MJ Research Inc., Minnesota, USA), after 5 min of pretreatment at 94°C, 30 cycles of 1 min at 94°C, 1 min at 59°C, and 30 s at 72°C, followed by 5 min at 72°C. The PCR products were analyzed by electrophoresis on 10% acrylamide gel and detected by a nonradioisotopic technique using a commercially available silver staining method.
The genotype was coded according to GSTM1/GSTT1 complete gene deletion polymorphisms: (a) GSTT1 present and GSTM1 absent or GSTT1 absent and GSTM1 present (at least one gene deleted), (b) both genes present, and (c) both genes deleted.
The multiplex PCR assay cannot discriminate the heterozygous presence of the allele from the homozygous presence. However, the distributions of these two genotypes were similar to those reported in another study (Cornelis et al., 2007).
The absence of a 215 bp fragment in the electrophoretic profile indicates the GSTM1 null genotype, and the absence of a 480 bp fragment indicates the GSTT1 null genotype. A fragment of 312 bp related to a nonpolymorphic fragment of the CYP1A1 gene was used as an internal control in all reactions. Negative controls were analyzed with each experiment.
Statistical analyses
Comparisons were made between smokers with and without anxiety disorders for sociodemographic and clinical characteristics, and the laboratory measurements, using appropriate parametric tests where data were normally distributed and nonparametric statistical tests for categorical or excluded non-normal data. All associations between smokers with and without anxiety disorders and genetic polymorphisms, mood disorders, lung disease, and suicide attempts were performed using contingency tables to calculate the odds ratios (OR) and the 95% confidence interval (CI). All tests were two-tailed and a P-value of 0.05 was used for statistical significance.
Results
Characteristics of participants
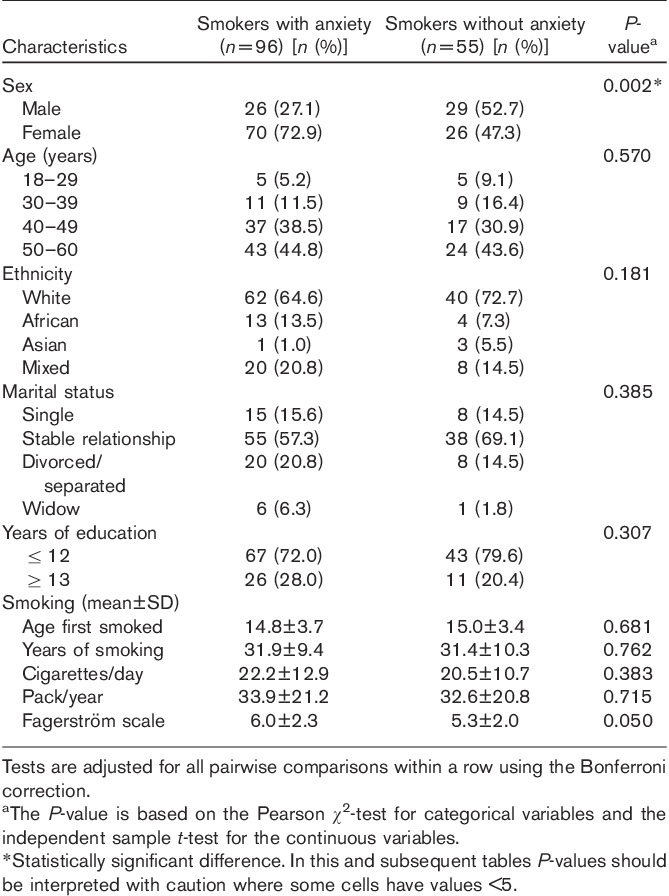
In examining sociodemographic variables, anxious smokers did not differ with respect to marital status, age, years of education, and ethnicity. There were significantly more women among the anxious smokers compared with the nonanxious smokers (P<0.01). Women sought more treatment for smoking cessation and exhibited higher rates of anxiety disorders than men. The mean age for all groups was 46.25 years. There were no significant differences with respect to years smoked, pack years, scores on FTND scale, and age of onset of smoking between anxious and nonanxious smokers (Table 1).
Table 1.
Sociodemographic characteristics of smokers with and without anxiety disorders
Clinical characteristics
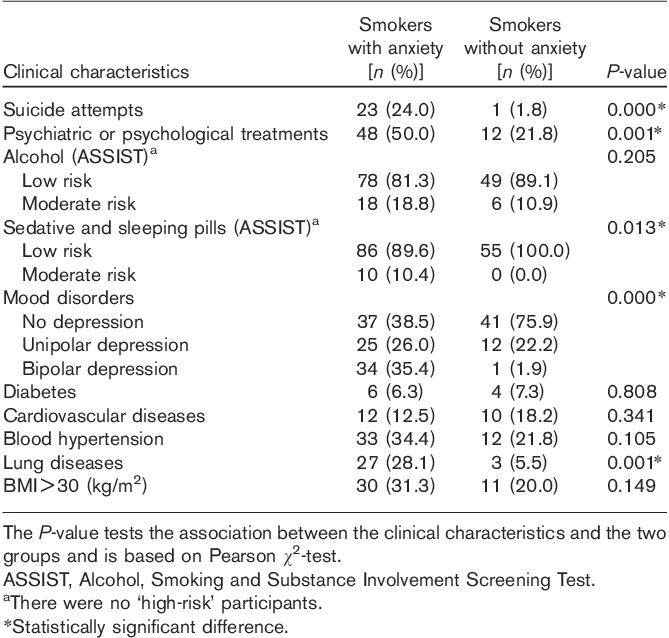
Anxious smokers had significantly more lung disease, suicide attempts, psychiatric and psychological treatment, use of sedative and sleeping pills, and mood disorders than nonanxious smokers (P<0.01). There were no significant differences with respect to diabetes, hypertension, cardiovascular diseases, alcohol risk, and BMI (kg/m2) between anxious and nonanxious smokers (Table 2).
Table 2.
Clinical characteristics of smokers with and without anxiety disorders
Genotyping
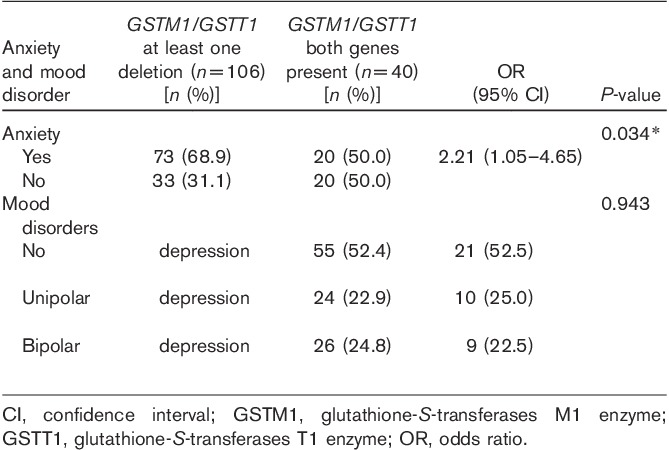
There was a higher frequency of at least one deletion, compared with the presence of both of the GSTT1 and the GSTM genes, in anxious smokers (OR=2.21, 95% CI=1.05–4.65, P=0.034). However, there were no significant associations between GSTM1/GSTT1 genotypes and unipolar and bipolar smokers (P=0.0943) (Table 3).
Table 3.
GSTM1/GSTT1 genetic polymorphism assessments of smokers with anxiety and mood disorders
The relationship between smoking, mood disorders, lung disease, suicide attempts, GSTM1/GSTT1 genetic polymorphism, and anxiety disorders is shown in Table 4.
Table 4.
Relationship between smoking, mood disorders, lung disease, and suicide attempts
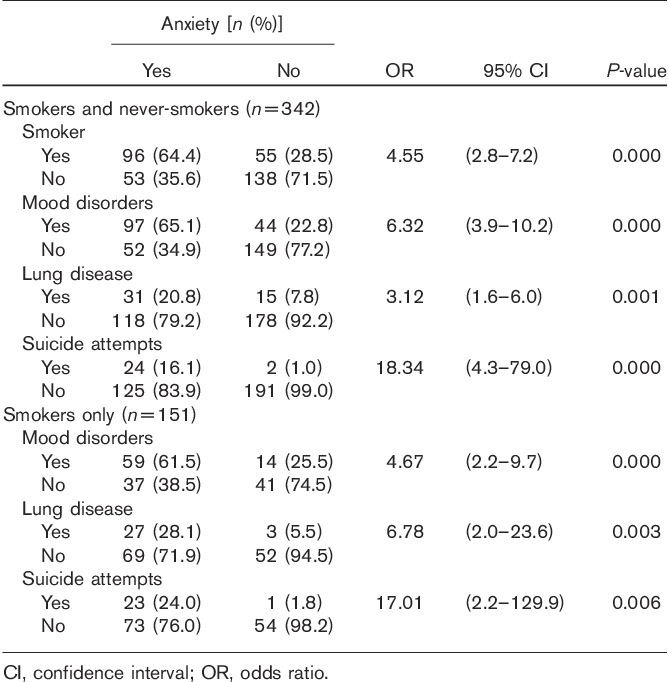
The results showed that anxiety disorders were significantly associated with mood disorders (OR=4.67, 95% CI=2.24–9.92, P<0.0001), lung disease (OR=6.78, 95% CI=1.95–23.58, P<0.0026), and suicide attempts (OR=17.01, 95% CI=2.23–129.91, P<0.006).
Comparisons between the anxious and nonanxious groups were performed, using pooled smokers and never-smokers (n=342), to estimate OR with 95% CI and the combined effects of GST genotypes. There was a positive association between anxiety and smokers (OR=4.55, 95% CI=2.8–7.19, P<0.000), mood disorders (OR=6.32, 95% CI=3.9–10.17, P<0.000), lung disease (OR=3.12, 95% CI=1.61–6.03, P<0.001), and suicide attempts (OR=18.34, 95% CI=4.26–78.96, P<0.000).
Discussion
Our data confirm results from previous studies in showing that anxious smokers exhibited having higher rates of suicide attempts than nonanxious smokers. Smokers who attempted suicide exhibited 17.0 more likelihood of having anxiety compared with smokers who did not attempt suicide. This striking finding is consistent with the hypothesis that individuals who are nicotine dependent are more likely to be anxious, depressive, and suicidal (Pedersen and von Soest, 2009; Berlin et al., 2011). Previous studies have reported that smoking is associated with a risk of suicidal behavior and depressive disorders (Malone et al., 2003; Breslau et al., 2005; McGee et al., 2005; Hughes, 2008; Goodwin et al., 2013). Other studies have found associations between prior smoking or nicotine dependence and subsequent suicidal behavior, which were independent of depression (Boden et al., 2008; Bronisch et al., 2008).
The smoking and suicide nexus may be explained, in part, by inflammation and oxidative stress. Individuals with suicidal behavior had significantly higher levels of nitric oxide metabolites (products of nitrates and nitrites) and lipid hydroperoxides (a biomarker of oxidative damage to lipids or lipid peroxidation) and lower levels of plasma total antioxidant potential TRAP (a biomarker of total antioxidant defenses) than those without a history of suicide attempts (Vargas et al., 2013b, in press). Proinflammatory cytokines have been implicated in depressive smokers (Nunes et al., 2012). Inflammation and oxidative stress is a mechanism activating production of kynurenine, which may deplete tryptophan, leading to reduced levels of serotonin. Increased plasma kynurenine levels were found in suicide attempters (Sublette et al., 2011). Decreased levels of serotonin were related to suicidal behavior in depressed smokers (Malone et al., 2003).
Co-occurring affective, anxiety disorders, and nicotine dependence share pathways that may explain these disorders. First, monoamine oxidases (MAO) catalyze the metabolism of dopamine, norepinephrine, and serotonin. Cigarette smoke inhibits the activity of MAO type A and type B (Benowitz, 2010). Lowered MAO activity, which may play a role in central nervous system serotonin metabolism, could modulate, in part, the link between cigarette smoking and suicidal behavior (Breslau et al., 2005). Second, this comorbidity can potentially be explained by one disorder being an epiphenomenon of the other and by a partly shared genetic etiology (Middeldorp et al., 2005). Finally, there are alterations of neurotransmitters by inflammation and oxidative stress, and cigarette smoking and mood disorders are associated with increased levels of inflammation and oxidative stress (Rytilä et al., 2006; Yanbaeva et al., 2007; Berk et al., 2011; Maes et al., 2011).
Smokers who had mood disorders exhibited 4.67 times the risk of having anxiety compared with smokers who did not have a mood disorder. The relationship between mood and anxiety disorders was associated with several markers of clinical severity, including earlier age of onset, greater number of depressive episodes, and higher prevalence of attempted suicide, when compared with mood disorder without comorbid anxiety (Goes et al., 2012). Comorbidity of anxiety and bipolar/unipolar disorders, suicidal behavior, substance abuse, and familial history may be explained by heavy familial-genetic loading for affective illness (Dilsaver et al., 2006). Individuals with anxiety and depressive disorders have increased rates of smoking compared with those without mental disorders or depression (Lasser et al., 2000).
In the present study, smokers who had lung disease exhibited 6.78 times the risk of having anxiety compared with smokers who did not have lung disease. This finding was consistent with previous studies that have reported a link between anxiety, depression, and lung disease (Di Marco et al., 2006; Goodwin et al., 2012). Therefore, it is necessary to consider the cumulative effects of depressive and anxiety disorders, physical illnesses, and smoking on suicide rates. Patients with some pulmonary diseases have an increased risk of mood and anxiety disorders (Dome et al., 2010). The association between lung disease (COPD) and anxiety in smokers may be accounted for by the higher rate of the null variant of the enzyme genes in the anxiety group. Because the association between the polymorphisms and anxiety in smokers is uncertain it is probably most reasonable to conjecture that the increased rate of lung disease is due to the enzyme deficiency rather than to anxiety.
Our study also provides evidence for the association between at least one deletion of the GSTM1 and GSTT1 genes in anxious smokers, compared with those who had the presence of both GSTM1 and GSTT1 genes. However, we did not find significant differences for these polymorphisms in unipolar and bipolar smokers. These results are in accordance with another study that did not find an association of nicotine acetylcholine receptor gene of smokers with depression, but found that it was positively associated with the prevalence of both anxious and depressive smokers (Bjørngaard et al., 2012).
Some limitations of the study need to be mentioned. First, these results should be confirmed using a larger sample size. Because of the small sample size, the possibility of type II statistical errors cannot be excluded. Second, all participants were recruited from the Centre for Smoking Cessation. We did not compare smokers with nonsmokers recruited from the same geographic area and rigorously matched with the smokers for age and sex of the GSTM1 and GSTT1 genes. However, there were no significant differences between smokers and nonsmokers for the GSTM1/GSTT1 gene polymorphisms reported in another study (Saadat and Mohabatkar, 2004). Third, there is a considerable overlap between symptoms of anxiety and mood disorders that needs to be interpreted with caution, and assessments of anxiety disorders were based on reported treatment. We did not find differences for mood disorders; however, further research is needed to clarify this issue. Finally, this study was conducted on cross-sectional data, and thus results can only determine associations, not causality.
A bidirectional relationship might exist between neurotransmitter activity, inflammation, and oxidative stress status in nicotine dependence and depressive disorders (Nunes et al., 2013). Our data strengthen the hypothesis of a role of GSTM1 and GSTT1 for the detoxification potential against oxidative stress in anxious smokers that exhibited higher rates of suicide attempts and mood disorders than nonanxious smokers.
Taken all together, the results suggest that both or at least one deletion of the GSTM1 and GSTT1 genes represent a risk factor for anxious smokers with co-occurrence with lung diseases, suicide attempts, sedative use, and mood disorders. The impact on health risks of this comorbidity suggests that we need to aggressively target smoking cessation as a part of routine care (Berk, 2007). Moreover, it is necessary to develop more effective therapeutic targets to reduce morbidity and mortality due to the co-occurrence of nicotine dependence and anxiety and mood disorders and sedative use.
Acknowledgements
The authors gratefully acknowledge the Health Sciences Postgraduate Program, the genetic laboratory and clinical analyses laboratory at State University of Londrina (Brazil), and the Barwon Psychiatric Research Unit, Deakin University (Geelong, Australia).
This study was supported by the Health Sciences Postgraduate Program at Londrina State University, Paraná, Brazil (UEL), the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES), and the Ministry for Science and Technology of Brazil (CNPq).
Conflicts of interest
There are no conflicts of interest.
References
- Abdel-Rahman SZ, El-Zein RA, Anwar WA, Au WW.A multiplex PCR procedures for polymorphic analysis of GSTM1 and GSTT1 genes in population studies.Cancer Lett 1996;107:229–233 [DOI] [PubMed] [Google Scholar]
- Benowitz NL.Nicotine addiction.N Engl J Med 2010;362:2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M.Should we be targeting smoking as a routine intervention?Acta Neuropsychiatrica 2007;19:131–132 [Google Scholar]
- Berk M, Ng F, Dean O, Dodd S, Bush AI.Glutathione: a novel treatment target in psychiatry.Trends Pharmacol Sci 2008;29:346–351 [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors.Neurosci Biobehav Rev 2011;35:804–817 [DOI] [PubMed] [Google Scholar]
- Berlin I, Covey LS, Donohue MC, Agostiv V.Duration of smoking abstinence and suicide-related outcomes.Nicotine Tob Res 2011;13:887–893 [DOI] [PubMed] [Google Scholar]
- Bjørngaard JH, Gunnell D, Elvestad MB, Davey Smith G, Skorpen F, Krokan H, et al. The causal role of smoking in anxiety and depression: a Mendelian randomization analysis of the HUNT study.Psychol Med 2012;12:1–9 [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood LJ.Cigarette smoking and suicidal behaviour: results from a 25-year longitudinal study.Psychol Med 2008;38:433–439 [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, Horwood J.Cigarette smoking and depression: tests of causal linkages using a longitudinal birth cohort.Br J Psychiatry 2010;196:440–446 [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC.Psychiatric disorders and stages of smoking.Biol Psychiatry 2004;55:69–76 [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Johnson E, Peterson E, Davis G.Smoking and the risk of suicidal behavior: a prospective study of a community sample.Arch Gen Psychiatry 2005;62:328–334 [DOI] [PubMed] [Google Scholar]
- Bronisch T, Höfler M, Lieb R.Smoking predicts suicidality: findings from a prospective community study.J Affect Disord 2008;108:135–145 [DOI] [PubMed] [Google Scholar]
- Carmo JT, Pueyo AA.Adaptation into Portuguese for the Fagerström test for nicotine dependence (FTND) to evaluate the dependence and tolerance for nicotine in Brazilian smokers.Rev Bras Med 2002;591/273–80 [Google Scholar]
- Cornelis MC, El-Sohemy A, Campos H.GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction.Am J Clin Nutr 2007;86:752–758 [DOI] [PubMed] [Google Scholar]
- Del Ben CM, Vilela JAA, Crippa JAS, Hallak JEC, Cybelli ML, Zuardi AW.Confiabilidade da ‘Entrevista Clínica Estruturada para o D.S.M.- IV’ – versão clínica traduzida para o português.Rev Bras Psiquiatr 2001;23:156–159 [Google Scholar]
- Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, et al. Anxiety and depression in COPD patients: the roles of gender and disease severity.Respir Med 2006;100:1767–1774 [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Akiskal HS, Akiskal KK, Benazzi F.Dose–response relationship between number of comorbid anxiety disorders in adolescent bipolar/unipolar disorders, and psychosis, suicidality, substance abuse and familiality.J Affect Disord 2006;96:249–258 [DOI] [PubMed] [Google Scholar]
- Dome P, Lazary J, Kalapos MP, Rihmer Z.Smoking, nicotine and neuropsychiatric disorders.Neurosci Biobehav Rev 2010;34:295–342 [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Schneider NG.Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire.J Behav Med 1989;12:159–182 [DOI] [PubMed] [Google Scholar]
- Goes FS, McCusker MG, Bienvenu OJ, Mackinnon DF, Mondimore FM, Schweizer B, et al. Co-morbid anxiety disorders in bipolar disorder and major depression: familial aggregation and clinical characteristics of co-morbid panic disorder, social phobia, specific phobia and obsessive-compulsive disorder.Psychol Med 2012;42:1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Lavoie KL, Lemeshow AR, Jenkins E, Brown S, Fedoronko DA.Depression, anxiety, and COPD: the unexamined role of nicotine dependence.Nicotine Tob Res 2012;14:176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Prescott MR, Tamburrino M, Calabrese JR, Liberzon I, Galea S.Cigarette smoking and subsequent risk of suicidal ideation among National Guard Soldiers.J Affect Disord 2013;145:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ.The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance.Crit Rev Biochem Mol Biol 1995;30:445–600 [DOI] [PubMed] [Google Scholar]
- Hughes JR.Smoking and suicide: a brief overview.Drug Alcohol Depend 2008;98:169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley RR, Yatsuya H, Lutsey P, Woodward M, Alonso A, Folsom AR.Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in African Americans and Whites.Am J Epidemiol 2012;175:816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Willem Van der Does AJ, Cuijpers P, Penninx BWJH.Association of smoking and nicotine dependence with severity and course of symptoms in patients with depressive or anxiety disorder.Drug Alcohol Depend 2012;126:138–146 [DOI] [PubMed] [Google Scholar]
- Kim JH, Park S, Lee K, Cho J, Ha E, Myung S, Hong Y.GSTM1and GSTP1Polymorphisms as potential factors for modifying the effect of smoking on inflammatory response.J Korean Med Sci 2006;21:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TK, Ruczinski I, Helzlsouer K, Shugart YY, Li KE, Clipp S, et al. Copy number variants of GSTM1 and GSTT1 in relation to lung cancer risk in a prospective cohort study.Ann Epidemiol 2009;19:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH.Smoking and mental illness: a population-based prevalence study.JAMA 2000;284:2606–2610 [DOI] [PubMed] [Google Scholar]
- Li R, Boerwinkle E, Olshan AF, Chambless LE, Pankow JS, Tyroler HA, et al. Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease.Atherosclerosis 2000;149:451–462 [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M.A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness.Prog Neuropsychopharmacol Biol Psychiatry 2011;35:676–692 [DOI] [PubMed] [Google Scholar]
- Malone KM, Waternaux C, Haas GL, Cooper TB, Li S, Mann JJ.Cigarette smoking, suicidal behavior, and serotonin function in major psychiatric disorders.Am J Psychiatry 2003;160:773–779 [DOI] [PubMed] [Google Scholar]
- McGee R, Williams S, Nada-Raja S.Is cigarette smoking associated with suicidal ideation among young people?Am J Psychiatry 2005;162:619–620 [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI.The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies.Psychol Med 2005;35:611–624 [DOI] [PubMed] [Google Scholar]
- Moylan S, Jacka F, Pasco J, Berk M.Cigarette smoking, nicotine dependence and anxiety disorders: a systematic review of population-based, epidemiological studies.BMC Med 2012;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norskov MS, Frikke-Schmidt R, Loft S, Sillesen H, Grande P, Nordestgaard B, et al. Copy number variation in glutathione S-transferases M1 and T1 and ischemic vascular disease: four studies and meta-analyses.Circ Cardiovasc Genet 2011;4:418–428 [DOI] [PubMed] [Google Scholar]
- Nunes SOV, Vargas HO, Brum J, Prado E, Vargas MM, de Castro MRP, et al. A comparison of inflammatory markers in depressed and non-depressed smokers.Nicotine Tob Res 2012;14:540–546 [DOI] [PubMed] [Google Scholar]
- Nunes SOV, Vargas HO, Prado E, Barbosa DS, Melo LP, Moylan S, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence.Neurosc Biobehav Rev 2013;37:1336–1345 [DOI] [PubMed] [Google Scholar]
- Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, et al. Tobacco smoking as a risk factor for major depressive disorder: population-based study.Br J Psychiatry 2008;193:322–326 [DOI] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G.Depression, anxiety, and smoking initiation: a prospective study over 3 years.Am J Public Health 1998;88:1518–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W, von Soest T.Smoking, nicotine dependence and mental health among young adults: a 13-year population-based longitudinal study.Addiction 2009;104:129–137 [DOI] [PubMed] [Google Scholar]
- Quaak M, van Schayck CP, Knaapen AM, van Schooten FJ.Genetic variation as a predictor of smoking cessation success. A promising preventive and intervention tool for chronic respiratory diseases?Eur Respir J 2009;33:468–480 [DOI] [PubMed] [Google Scholar]
- Rytilä P, Rehn T, Ilumets H, Rouhos A, Sovijärvi A, Myllärniemi M, et al. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD.Respir Res 2006;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat M, Mohabatkar H.Polymorphisms of glutathione S-transferases M1 and T1 do not account for interindividual differences for smoking behavior.Pharmacol Biochem Behav 2004;77:793–795 [DOI] [PubMed] [Google Scholar]
- Schneider J, Benges U, Philipp M, Woitowitz HJ.GSTM1, GSTT1, and GSTP1 polymorphism and lung cancer risk in relation to tobacco smoking.Cancer Lett 2004;208:65–74 [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder.Brain Behav Immun 2011;25:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JY, Kim HM, Han SH, Cho KH, Moon BS, Hong SH.Glutathione S-transferase gene polymorphism and ischemic cerebrovascular disease.Int J Neurosci 2006;116:55–65 [DOI] [PubMed] [Google Scholar]
- Vargas HO, Nunes SOV, Castro MRP, Vargas MM, Barbosa DS, Bortolasci CC, et al. Oxidative stress and inflammatory markers are associated with depression and nicotine dependence.Neurosci Lett 2013a;544:136–140 [DOI] [PubMed] [Google Scholar]
- Vargas HO, Nunes SOV, Castro MP, Bortolasci CC, Barbosa DS, Morimoto HK, et al. Oxidative stress and lowered total antioxidant status are associated with history of suicide attempts.J Affect Disorders 2013b;150:923–930 [DOI] [PubMed] [Google Scholar]
- Wang LS, Tang JJ, Tang NP, Wang MW, Yan JJ, Wang QM, et al. Association of GSTM1 and GSTT1 gene polymorphisms with coronary artery disease in relation to tobacco smoking.Clin Chem Lab Med 2008;46:1720–1725 [DOI] [PubMed] [Google Scholar]
- Wang J, Zou L, Huang SD, Lu FL, Lang XL, Han L, et al. Genetic polymorphisms of glutathione S-transferase genes GSTM1, GSTT1 and risk of coronary heart disease.Mutagenesis 2010;25:365–369 [DOI] [PubMed] [Google Scholar]
- World Health Organization The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility.Addiction 2002;97:1183–1194 [DOI] [PubMed] [Google Scholar]
- Xue H, Su J, Sun K, Xie W, Wang H.Glutathione S-transferase M1 and T1 gene polymorphism and COPD risk in smokers: an updated analysis.Mol Biol Rep 2012;39:5033–5042 [DOI] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EFM.Systemic effects of smoking.Chest 2007;131:1557–1566 [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report.Nicotine Tob Res 2008;10:1691–1715 [DOI] [PubMed] [Google Scholar]


