Abstract
Objectives
Readmission is associated with high mortality, morbidity, and cost. We used the ACS-NSQIP to determine risk factors for readmission following lower extremity bypass (LEB).
Methods
We identified all patients who received LEB in the 2011 ACS-NSQIP database. Multivariable logistic regression was used to assess independent predictors of 30-day readmission. We also identified our institutional contribution of LEB patients to the ACS-NSQIP from 2005–2011 to determine our institution’s rate of readmission and readmission indications.
Results
Among 5018 patients undergoing LEB, ACS-NSQIP readmission analysis was performed on 4512, excluding those whose readmission data was unavailable, suffered a death on index admission, or remained in the hospital at 30 days. Overall readmission rate was 18%, and readmission rate of those with NSQIP captured complications was 8%. Multivariable predictors of readmission were dependent functional status (OR 1.40, 95% CI: 1.08–1.79), dyspnea (OR 1.28, 95% CI: 1.02–1.60), cardiac comorbidity (OR 1.46, 95% CI: 1.16–1.84), dialysis dependence (OR 1.44, 95% CI: 1.05–1.97), obesity (OR 1.28, 95% CI: 1.07–1.53), malnutrition (OR 1.42, 95% CI 1.12–1.79), CLI operative indication (OR 1.40, 95% CI: 1.10–1.79), and return to the operating room on index admission (OR 8.0, 95% CI: 6.68–9.60). The most common post-discharge complications occurring in readmitted patients included wound complications (56%), multiple complications (22%), and graft failure (5%). Our institutional data contributed 465 LEB patients to the ACS-NSQIP from 2005–2012, with an overall readmission rate of 14%. Unplanned readmissions related to the original LEB (related unplanned) made up 75% of cases. The remainder 25% included readmissions that were planned staged procedures related to the original LEB (related planned, 11%) and admissions for a completely unrelated reason (unrelated unplanned, 14%). The most common readmission indications included wound infection (40%) and graft failure (13%). Readmissions were attributable to NSQIP captured post-discharge complications in 44% of cases, an additional 44% had a non-NSQIP defined reason for readmission, and the remainder (12%) included patients admitted for complications described in NSQIP but not meeting strict NSQIP criteria.
Discussion
Readmissions are common after lower extremity bypass. Optimization of select chronic conditions, closer follow-up of patients in poor health and those who required return to the OR, and early detection of surgical site infections may improve readmission rates. Our finding that 25% of readmissions after LEB are not procedure related informs the broader discussion on how a readmission penalty affects vascular surgery in particular.
INTRODUCTION
The Medicare Payment Advisory Commission (MedPAC) estimates Medicare expenditures for potentially preventable rehospitalizations to be as high as $12 billion annually.1 As a result, MedPAC has put forth recommendations to determine payments partially based on readmission rates.1,2 According to these recommendations, hospitals with high risk-adjusted rehospitalization rates would receive lower average per-case payments. This has led to increased interest in studying rehospitalization rates and contributing factors. Readmission data have already changed healthcare review in other countries. The Australian Council on Healthcare Standards uses readmission rates as an internal marker of problems in patient management and outcomes, eventually leading to review of one-third of hospitals.3 In the British National Health Service, acute readmissions are known as “failed discharges,” indicating a deficit in the quality of care received during the index admission.2 Studies have concluded lower quality of inpatient care to be associated with higher readmission rates.4
A 2009 New England Journal of Medicine study of 12 million Medicare beneficiaries by Jencks and colleagues reported a 30-day readmission rate of 24% for patients undergoing surgery for peripheral vascular disease; third highest of any diagnosis related group behind only congestive heart failure (CHF) and psychoses.5 Subsequent studies further explored readmission rates after vascular surgery procedures. Jackson et al reported low rehospitalization rates for vascular surgery patients at a single institution with 12% overall readmission rate and 9% unplanned rehospitalization rate.6 Other authors have similarly looked at readmission rates after specific procedures such as amputations, carotid endarterectomy and abdominal aortic aneurysm, though the precise relationship between quality and readmission rates in the area of vascular surgery remains uncertain.7–15
For lower extremity arterial occlusive disease, Goodney et al showed a readmission rate of 19% for Medicare beneficiaries undergoing lower extremity bypass (LEB) in the late 1990s as part of a larger study evaluating the impact of hospital volume on readmission.16 More recently, McPhee found a 23% unplanned readmission rate for patients undergoing open LEB at a single institution,17 while Vogel found a 30% readmission rate after endovascular tibioperoneal interventions in Medicare beneficiaries with critical limb ischemia (CLI).18 While these studies have shed light on the issue of readmission in this population, readmission following open LEB, including risk factors for readmission, has not been studied on the national level using clinical data but rather only administrative data intended for billing purposes. With a prospectively collected, clinical design making use of direct medical record review and patient contact, we now aim to add the perspective of the American College of Surgeons National Surgery Quality Improvement Program (ACS-NSQIP) database to study the incidence of and risk factors for readmission in patients undergoing LEB.
METHODS
Data Source
We utilized data from the 2011 ACS-NSQIP, a national, prospectively collected clinical database with over 350 member institutions with a mission of facilitating quality-control review of perioperative outcomes. Clinical nurse reviewers are trained and certified by the ACS to input data at each participating institution using standardized definitions created by the ACS. While not all cases from participating institutions are included in the database, cases are selected for NSQIP inclusion based on a systematic sampling system developed to prevent bias in case selection. The NSQIP data are subject to a rigorous annual audit process with validated accuracy.19–21 Thirty-day postoperative outcomes, including post-hospitalization information, are collected from hospital records, clinic visits, and follow-up. In 2011, the ACS-NSQIP introduced a variable for 30-day readmission and for this reason analysis was restricted to that year only. Given the methodology of NSQIP follow up, readmission to any institution, including non-NSQIP institutions other than where the procedure occurred, was captured. For patients in whom contact regarding 30-day outcomes is not possible, the readmission field is marked as unavailable. While the ACS-NSQIP captures 30-day readmission for all patients, indication for readmission is not available. As a result, we retrospectively reviewed our institutional contribution of LEB patients to the ACS-NSQIP from 2005–2012 to determine the rate of readmission following LEB and better delineate readmission indication. Readmission and indication for readmission to our own institution was determined for all patients via physician review of the medical record. After the addition of readmission as a formal NSQIP parameter in late 2010, readmission to any institution was recorded. This study was approved by the Institutional Review Board approval at the Beth Israel Deaconess Medical Center.
Patients
Patients undergoing infra-inguinal LEB as a principal operative procedure were identified via query of the 2011 ACS-NSQIP Participant User File and our institutional NSQIP contribution from 2005–2012 using the following Current Procedural Terminology (CPT) codes: 35556, 35566, 35571, 35583, 35585, 35587, 35651, 35656, 35666 and 35671. Inclusion criteria required that a LEB CPT code be listed as the principal operative procedure. Baseline patient demographics, comorbidities, operative details and postoperative course were extracted from the database. Definitions for NSQIP collected data points may be found in the NSQIP user guide available online.22
Outcomes
Our primary outcome measure was overall readmission to any hospital within 30-days of surgery. In the ACS-NSQIP analyses, overall readmission referred to any readmission event. A readmission was deemed “complication associated” if the patient had both a readmission event and a NSQIP defined post-discharge complication. Readmission analysis excluded patients not at risk for 30-day readmission including those with deaths during index admission and those remaining in the hospital at 30 days. Secondary outcome measures included overall 30-day morbidity and mortality stratified as either pre or post discharge. Using our institutional data, physician chart review was used to determine readmission to our own institution as well as the indication for readmission. Readmission indication was then utilized to classify our institutional readmissions as related or unrelated and planned or unplanned. Planned readmissions were those admissions scheduled prior to a patient’s discharge from their index admission. Related readmissions included readmissions that could possibly be attributed to the index procedure.
Measures/Terms
While definitions for all NSQIP terms may be found in the NSQIP user guide, this study also utilized newly created terms defined here. Patients were considered to have CLI if they met NSQIP criteria for “rest pain/gangrene” or an “open wound” at the time of surgery. Obesity denoted any patient with a body mass index greater than or equal to 30 kg/m2. Malnutrition referred to patients with preoperative albumin < 3.5 g/dL. Patients were listed as having a cardiac comorbidity if they met NSQIP criteria for CHF within previous 30 days, history of myocardial infarction within 6 months prior to surgery, history of cardiac surgery or history of percutaneous cardiac intervention. Any wound complication refers to a composite variable inclusive of any NSQIP defined surgical site infection (SSI) including superficial SSI, deep SSI, organ space SSI and dehiscence.
Statistical Analysis
All analyses were conducted using IBM SPSS Statistics version 21.0.0 for Macintosh (IBM Corp., Armonk, NY). Categorical variables were analyzed using the chi-square or Fisher’s exact test where appropriate. Continuous variables were compared using two tailed independent samples t-test. Cases missing data for any given parameter were eliminated from consideration for the purposes of bivariate analysis. Multivariable logistic regression was performed to determine independent predictors of readmission. Predictive models for readmission included only information available at the time of patient discharge which excluded post-discharge complications. All variables with a p-value less than .10 on bivariate analysis were included in the model. Any candidate covariate with missing data was analyzed to assess whether the missingness was associated with the primary outcome, readmission. Missingness was found not to be associated with the primary outcome and thus, dummy variables were created to signify missing data. All dummy variable effect estimates were then found to be non-significant. The lone exception to this was the variable indicating albumin < 3.5 g/dL which was found to be missing not at random. The non-random missingness of albumin measurements has been well documented and for this reason albumin was removed from consideration in our multivariable model.23 Backward stepwise elimination was used to determine final independent predictors. Throughout all analyses, statistical significance was as determined by a criterion of p < .05.
RESULTS
Demographics/Clinical Details - NSQIP
As shown in Table 1, 5018 patients underwent LEB in the 2011 NSQIP. Mean age was 68 years with male gender (64.6%), non-black ethnicity (83%) and non-smokers (58.3%) making up the majority of the cohort. Comorbidities are outlined in Table 1. A minority had an infra-popliteal LEB target (42%) and approximately one third (32%) utilized non-vein graft material. Further operative detail may be found in Table 2. Discharge was to home in 71% of cases.
Table 1.
Demographic characteristics and comorbidities of LEB patients in the 2011 NSQIP database.
| All Patients (N = 5,018) N (%) |
|
|---|---|
|
| |
| Age, years; Mean (SD) | 67.5 (11.5) |
|
| |
| Male | 3,241 (64.8) |
|
| |
| Race | |
| - White | 3,690 (74.3) |
| - Black | 841 (16.9) |
| - Asian | 50 (1.0) |
| - Native American | 13 (0.3) |
| - Native Hawaiian/Pacific Islander | 10 (0.2) |
| - Unknown | 414 (8.3) |
|
| |
| Chronic Nursing Home Residence | 149 (3.0) |
|
| |
| Dependent Functional Status | 528 (10.6) |
|
| |
| Diabetes Mellitus | 2,188 (43.6) |
|
| |
| Chronic Obstructive Pulmonary Disease | 641 (12.8) |
|
| |
| Smoking | 2,092 (41.7) |
|
| |
| Cardiac Comorbidity | 941 (29.0)* |
|
| |
| History of Revascularization or Amputation | 1,304 (49.0) |
|
| |
| CLI | 2,315 (66.0)* |
|
| |
| Dialysis | 23 (0.5) |
|
| |
| Preoperative SIRS/sepsis | 231 (4.6) |
|
| |
| ASA Class ≥ 4 | 1,056 (20.9) |
|
| |
| Obesity | 1,375 (7.8) |
|
| |
| Malnutrition | 1,027 (30.1) |
Percents reflect valid percent given missing data.
Table 2.
Operative Variables for LEB Patients Who Underwent Readmission Analysis in the 2011 NSQIP Database.
| Overall (N=4,512) | Readmitted (N= 826) | Not Readmitted (N=3,686) | p-value | |
|---|---|---|---|---|
|
| ||||
| Mean OR Time, minutes (SD) | 225 (110) | 243 (117) | 221 (108) | <.001 |
|
| ||||
| Popliteal Inflow (vs. Femoral Inflow) | 374 (8.3) | 82 (9.9) | 292 (7.9) | .069 |
|
| ||||
| Popliteal Target (vs. Infra-Popliteal Target) | 1,888 (41.8) | 403 (48.8) | 1485 (40.3) | <.001 |
|
| ||||
| Synthetic Graft (vs. Vein Graft) | 1,554 (34.4) | 276 (33.4) | 1278 (34.7) | .517 |
|
| ||||
| Operative Time > 225 min (vs. < 225 min) | 2,057 (42.1) | 422 (51.2) | 1470 (40.0) | <.001 |
|
| ||||
| Emergency Case* | 252 (5.6) | 55 (6.7) | 197 (5.3) | .154 |
|
| ||||
| Elective Surgery** | 2,988 (67.2) | 489 (60.1) | 2499 (68.8) | <.001 |
|
| ||||
| Additional Procedure | ||||
| - Fasciotomy | 57 (1.3) | 9 (1.1) | 48 (1.3) | .732 |
| - Minor Amputation | 151 (3.3) | 36 (4.4) | 115 (3.1) | .086 |
| - Endovascular Procedure | 536 (11.9) | 97 (11.7) | 439 (11.9) | .953 |
| - Additional Bypass | 132 (2.9) | 20 (2.4) | 112 (3.0) | .423 |
Cases reported by surgeon and anesthesiologist as emergent; Surgery must occur within 12 hours of admission or onset of related preoperative symptomatology.
Patient admitted from home or current living situation on day of previously scheduled surgery.
Morbidity/Mortality – NSQIP
The 30-day mortality rate in the NSQIP cohort was 2.6% (N = 129/5018), of which approximately one third (33%; N = 42/129) occurred after hospital discharge. Overall morbidity rate was 44%. Pre-discharge complications occurred in 38% of patients while 11% experienced a post-discharge complication within 30-days of surgery. Temporal distribution of complications varied according to specific complications. SSI’s were five times more likely to occur post-discharge than pre-discharge (8.6% vs. 1.7%), though all other NSQIP defined complications were more likely to occur pre-discharge. Further, post-discharge wound complications were seen to occur well after discharge with mean date of diagnosis for post-discharge superficial SSI, deep SSI and dehiscence occurring on post-discharge days 12.6, 14.4 and 10.0, respectively. Patients with post-discharge complications had varying rates of readmission depending on the nature of the complication.
Bivariate Analysis – Readmission – NSQIP
Readmission analyses were performed on 4,512 patients (Tables 2 and 3). Exclusions were made for patients not at risk for 30-day readmission at the time of discharge (death on index admission or in hospital at 30 days; N = 96) and those for whom readmission data was unavailable (N = 410) (Table 4). Overall readmission rate was 18% while complication associated readmission rate was 8%. Factors associated with readmission on bivariate analysis were primarily patient characteristics and operative details rather than in-hospital postoperative adverse events. The only pre-discharge complication associated with readmission on bivariate analysis was postoperative hemorrhage defined as transfusion of 5 or more units of packed red cells within 72 hours postoperatively. Readmitted patients were more likely to have a longer length of postoperative stay and a discharge to a location other than home than were non-readmitted patients (Table 4).
Table 3.
Demographic characteristics and co-morbidities for LEB patients who underwent readmission analysis in the 2011 NSQIP database.
| Overall (N = 4,512) N (%) |
Readmitted (N = 831) N (%) |
Not Readmitted (N = 3,763) N (%) |
p-Value | |
|---|---|---|---|---|
|
| ||||
| Age, years; Mean (SD) | 67.4 (11.5) | 67.8(11.5) | 67.3 (11.5) | .330 |
|
| ||||
| Male | 2,921 (64.8) | 510 (61.7) | 2411 (65.5) | .044 |
|
| ||||
| Race | .040 | |||
| - White | 3,321 (80.1) | 592 (76.7) | 2,729 (80.8) | |
| - Black | 772 (18.6) | 173 (22.4) | 599 (17.7) | |
| - Asian | 38 (0.9) | 5 (0.6) | 33 (1.0) | |
| - Native American | 10 (0.2) | 1 (0.1) | 9 (0.3) | |
| - Native Hawaiian/Pacific Islander | 7 (0.2) | 1 (0.1) | 6 (0.2) | |
|
| ||||
| Chronic Nursing Home Residence | 137 (3.0) | 39 (4.7) | 98 (2.7) | .003 |
|
| ||||
| Dependent Functional Status | 473 (10.5) | 136 (16.5) | 337 (9.2) | <.001 |
|
| ||||
| Diabetes Mellitus | 1,976 (43.8) | 416 (50.4) | 1560 (42.3) | <.001 |
|
| ||||
| Smoker | 1,911 (42.4) | 327 (39.6) | 1,584 (43.0) | .08 |
|
| ||||
| Chronic Obstructive Pulmonary Disease | 572 (12.7) | 127 (15.4) | 445 (12.1) | .011 |
|
| ||||
| Cardiac Comorbidity | 849 (34.3) | 202 (44.2) | 647 (32.0) | <.001 |
|
| ||||
| History of Revascularization or Amputation | 1,201 (49.5) | 244 (55.3) | 957 (48.2) | .007 |
|
| ||||
| CLI | 2,092 (65.7) | 467 (76.8) | 1625 (63.1) | <.001 |
|
| ||||
| Dialysis Dependence | 270 (6.0) | 86 (10.4) | 184 (5.0) | <.001 |
|
| ||||
| Systemic Sepsis | .001 | |||
|
| ||||
| SIRS | 151 (3.3) | 46 (5.6) | 105 (2.8) | |
|
| ||||
| Sepsis | 42 (0.9) | 6 (0.7) | 36 (1.0) | |
|
| ||||
| Septic shock | 1 (0) | 0 (0) | 1 (0) | |
|
| ||||
| ASA Class ≥ 4 | 923 (20.5) | 236 (28.6) | 687 (18.6) | <.001 |
|
| ||||
| Obesity | 1,243 (27.9) | 268 (32.8) | 975 (26.8) | .001 |
|
| ||||
| Malnutrition2 | 922 (38.6) | 246 (51.7) | 676 (35.3) | <.001 |
SD, Standard deviation; SIRS, systemic inflammatory response syndrome; ASA Class, American Society of Anesthesiologists classification
Table 4.
Complications & Postoperative Outcomes in LEB Patients Who Underwent Readmission Analysis in the 2011 NSQIP Database.
| Overall (N=4,512) | Readmitted (N=826) | Not Readmitted (N=3,686) | p-Value | |
|---|---|---|---|---|
| Length of Postoperative Stay, days; Mean (SD) | 5.9 (5.4) | 6.3 (4.3) | 5.9 (5.7) | .033 |
| Non-Home Discharge | 1,238 (27.6) | 291 (35.4) | 947 (25.9) | <.001 |
| Any Complication | 1,996 (44.2) | 678 (82.1) | 1318 (35.8) | <.001 |
| Any Wound Complication | 515 (11.4) | 306 (37.0) | 209 (5.7) | <.001 |
| Overall Mortality | 71 (1.6) | 31 (3.8) | 40 (1.1) | <.001 |
| Myocardial Infarction | 93 (2.1) | 38 (4.6) | 55 (1.5) | <.001 |
| Bleeding | 1,235 (27.4) | 287 (34.7) | 948 (25.6) | <.001 |
| Graft Failure | 79 (1.8) | 42 (5.1) | 37 (1.0) | <.001 |
| Acute Kidney Injury | 24 (0.5) | 15 (1.8) | 9 (0.2) | <.001 |
| Sepsis | 105 (2.3) | 57 (6.9) | 48 (1.3) | <.001 |
| Deep Vein Thrombosis | 30 (0.7) | 18 (2.2) | 12 (0.3) | <.001 |
| Pulmonary Embolism | 9 (0.2) | 8 (1.0) | 1 (0) | <.001 |
| Pneumonia | 62 (1.4) | 29 (3.5) | 33 (0.9) | <.001 |
Multivariable Analysis – Readmission – NSQIP
Multivariable predictors of overall readmission are shown in Figure 1. Independent predictors of readmission were primarily patient related. Postoperative stay longer than 6 days, the study mean, and return to the operating room (OR) on index admission were the lone postoperative predictors. Return to the OR also had the largest effect size with an odds ratio of 24.9 (95% CI: 18.6 – 33.3). Reason for return to the OR on index admission (N = 728/4922) was not specifically available, though 45% (N = 39/87) of patients with a pre-discharge wound complication and 83% (N = 38/46) of patients with pre-discharge graft failure were taken back to the OR.
Figure 1.
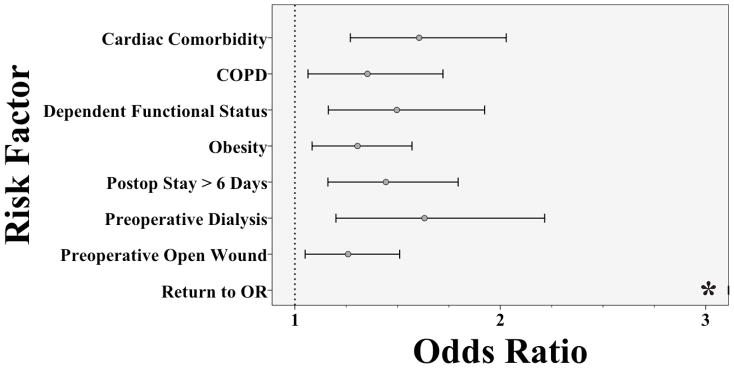
Independent Predictors of Readmission for LEB Patients in the 2011 NSQIP
*Odds ratio for Return to OR = 24.9 (95%CI: 18.6 – 33.3)
Readmission Indications – NSQIP
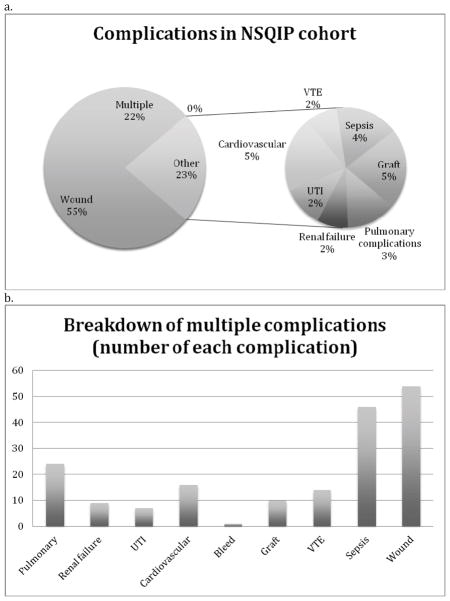
Approximately half of overall readmissions (N = 373/826; 45%) were defined as complication associated in the setting of a NSQIP defined post-discharge complication. The most common post-discharge events occurring in readmitted patients included wound complications (56%), multiple complications (22%) and graft failure (5%) (Figure 2a). Of those with multiple complications, wound infections were also the most common (N = 54/82; 66%) followed by sepsis (N = 46/82; 56%) and pneumonia (N = 12/82; 15%) (Figure 2b).
Figure 2.
2a. Complications by type among LEB patients who were readmitted as recorded in the 2011 NSQIP cohort. 2b. Breakdown of patients with multiple complications.
Readmission Indications – Institutional
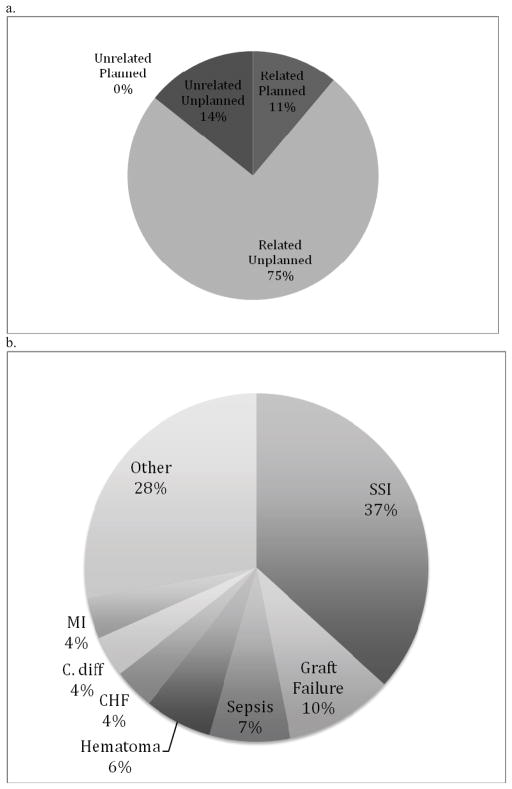
Our institution contributed 465 patients undergoing infra-inguinal LEB to the NSQIP from 2005–2012. Excluding 12 patients for either pre-discharge death or index hospital stay longer than 30 days, the overall readmission rate for our institutional patients was 14% (N = 63/453). NSQIP identified readmission to any institution (captured after late 2010) was 16% (N = 18/113), though only one of these readmissions was to an outside institution. Cases performed prior to this time were evaluated for readmission to our own institution via physician chart review yielding a rate of 13% (N = 45/340). The characteristics of our institutional cohort did differ from the NSQIP cohort with respect to certain patient demographics. Our institutional patients were older (70 vs. 68 years; p<.001) and more likely to have CLI (77% vs. 66%; p<.001) though less likely to be ASA class 4 or above (15% vs. 21%; p=.002). Readmissions were unplanned in 89% of cases (N = 56/63) and planned in the remainder (Figure 3a). Of the 47 patients who required unplanned readmission, reoperation occurred in 7 cases (15%) including bypass revision in 3 cases, new bypass in 2 cases, angioplasty in 1 case and operative debridement in 1 case.
Figure 3.
3a. Institutional LEB Readmissions, by Relationship to Index Procedure. 3b. Breakdown of Institutional Related Unplanned LEB Readmissions by Diagnosis.
The most common readmission indications (Figure 3b) in our patients included wound infection (40%) and graft failure (13%). Readmissions were attributable to NSQIP captured post-discharge complications in 44% of cases (N = 28/63). An additional 44% had a non-NSQIP defined reason for readmission. The remaining 12% included patients admitted for complications described in NSQIP but not strictly meeting NSQIP criteria. Some reasons for readmission not captured by NSQIP were procedure-related complications not defined in NSQIP while others included concern for a complication or monitoring. Unrelated readmission indications included a number of varied complaints such as vascular complaints related to the contralateral lower extremity (N = 3/9), complication of tooth extraction (N = 1/9) and mechanical fall (N = 1/9), among others. Notably, complaints related to the contralateral lower extremity, though unrelated to the operated limb, are often indistinguishable through ICD-9 (International Classification of Disease) diagnosis codes, which lack laterality.
DISCUSSION
This study represents the first investigation of a national, multi-center, prospective clinical database for risk factors for readmission following LEB. In contradistinction to prior studies using administrative billing data, such as Medicare, NSQIP methodology includes direct medical record review and patient telephone contact specifically designed to facilitate quality improvement and clinical outcomes research. Moreover, in contrast to the prior clinical studies, the NSQIP methodology captures readmissions even to non-NSQIP hospitals. Our review of patients undergoing infra-inguinal LEB in the 2011 NSQIP demonstrated an overall readmission rate of 18% and a complication associated readmission rate of 8%. Independent risk factors for overall readmission were primarily patient related (Figure 1). However, return to the OR on index admission, the lone postoperative complication associated with readmission, was associated with the highest risk for readmission. While over half of patients in the NSQIP cohort were readmitted without a NSQIP defined post-discharge complication, our institutional data offered greater detail on readmission indication, confirming SSI as the most common reason for rehospitalization while also showing that one quarter of readmissions were either planned or unrelated to the surgical procedure. Finally, the NSQIP data emphasizes the importance of 30-day outcomes in the study of LEB, as one third of deaths occurred post-discharge and over 10% of patients suffered a post-discharge complication.
Our study’s overall readmission rate of 18% is generally in agreement with previously published reports for patients undergoing LEB on the national level in Medicare by Goodney16 (19%) and in single institution series by Jackson6 (15%) and McPhee17 (25%). However, while risk factors for readmission vary by study, there are many commonalities, and the differences may be attributable to study design and variables considered. Similar to McPhee who found tissue loss indication and preoperative CHF to predict readmission, our data also showed CLI, dialysis dependence, and cardiac comorbidity (including CHF) to be independent predictors of rehospitalization. Vogel et al, in a study of Medicare beneficiaries undergoing catheter based lower extremity revascularization for CLI, further confirmed these associations in showing gangrene, chronic renal failure and CHF to be associated with readmission. Additionally, obesity was found to be associated with readmission in our cohort but were not explicitly studied in previous reports.
While infrapopliteal target vessel was found to be a bivariate risk factor for readmission in both our study as well as McPhee’s study, the McPhee study also found distal inflow source (i.e. superficial femoral artery as compared to common femoral artery) to be an independent predictor of readmission. Though precise inflow source was not available for our analysis (i.e. common femoral artery vs. superficial femoral artery vs. popliteal artery), CPT coding did allow identification of those patients with a popliteal artery inflow source as compared to femoral. While we saw a trend toward increased readmission with these patients (21.9% vs. 18.0%; p=.069), it did not reach statistical significance.
Finally, pre-discharge complications including wound infection, graft failure and myocardial infarction predicted readmission in the McPhee study while only return to the OR on index admission showed such an association in our report, albeit an association with greatest risk for readmission (OR: 8; 95% CI: 7–10). Our study showed 28-fold risk increase (95% CI: 13–62) in reoperation for patients experiencing pre-discharge graft failure and a 5-fold risk increase (95% CI: 3–8) in reoperation for patients suffering a pre-discharge wound complication. In our view, this finding refines the previous data to suggest that those pre-discharge complications of sufficient severity to warrant a return to the OR have the highest risk for readmission and delineate a very high-risk group.
The aforementioned risk factors, including patients operated on for CLI, patients with poor baseline health and those patients requiring return to the OR on the index admission, constitute a group at increased risk for readmission. Yet, despite recognition of this high-risk status, these risk factors offer minimal opportunity in terms of actionable quality improvement targets. Though many of these risk factors are either not modifiable or minimally modifiable in the short term, we should nonetheless ensure that certain of these such as obesity, malnutrition, and cardiac function are optimized preoperatively in our patients. Indication for readmission in our institutional contribution to the NSQIP was primarily related unplanned (75%; N = 47/63). With 53% (N = 25/47) of unplanned readmissions related to wound complications, our institutional data reinforces the findings of McPhee and colleagues (63%) in showing wound complications to account for the largest proportion of unplanned readmissions. This figure exceeds the 25% of overall readmissions attributable to wound complications in the NSQIP. This may be related to specific aspects of the NSQIP definitions for wound infection. Interestingly, 9% (N=4/47) of unplanned readmissions in our institutional series were related to concern for a wound infection that was not ultimately categorized as a wound infection. This points to the need for improved monitoring with perhaps improved utilization of skilled outpatient nursing or electronic transmission of wound images as is being studied in select centers. There has been recent interest in utilizing outpatient nursing and electronic transmission of wound images in order to prevent confusion about medications and to spot developing symptoms/issues before they progress in severity. Some centers have employed nurse-conducted telephone calls post-discharge to see whether or not this would reduce readmission rates. We are currently advocating for the electronic transmission of wound images provided either by the patient or by the VNA/visiting nurse at select intervals, in order to catch wound infections at an early stage.
The high proportion of readmissions related to post-discharge surgical site infection points towards prevention of SSI as a means of decreasing unplanned readmissions. A 2011 report by Greenblatt and colleagues evaluated risk factors for 30-day SSI following LEB in the NSQIP from 2005 to 2008.24 While a number of baseline patient characteristics including female sex, dialysis dependence, and others predicted SSI in their study; operative time greater than 4 hours was the lone modifiable risk factor, conferring a 40% increased risk of SSI. Greenblatt and colleagues speculate that deficiencies in timely re-administration of prophylactic antibiotics, hypothermia and other factors may play a role in the development of SSI following long operations. Further emphasizing the importance of operative technique on SSI risk, a recent study from the Vascular Study Group of New England database by Tan et al found that perioperative blood transfusion was an independent risk factor for SSI with those patients receiving 3 or greater units of perioperative packed red cells at over three-fold increased risk for SSI.25 These studies suggest that intraoperative management including judicious use of blood transfusion may play a role in diminishing postoperative SSI and hospital readmissions.
As was the case in the report by McPhee and colleagues who saw a longer length of stay for readmitted patients as compared to non-readmitted patients (12.2 vs. 10.2 days; p<.001), patients readmitted following LEB in the NSQIP also had a longer length of post-operative stay than did non-readmitted patients (6.3 vs. 5.9 days; p=.033). However, different from the report by McPhee et al, our study showed length of postoperative stay longer than 6 days, the study mean, to independently predict readmission. Yet, despite this finding, the timing of post-discharge adverse events suggests that an additional day or two in hospital may be unlikely to prevent readmission. For example, as noted in the NSQIP morbidity/mortality section of the Results, post-discharge wound infections occurred, on average, over a week after leaving the hospital. This argues against the benefit of a marginal increase in length of stay to decrease the incidence of readmission.
Planned readmission rate was 1.5% (N = 7/465) which was in line with McPhee and colleagues who showed a 2.7% planned readmission rate. Notably, one quarter of readmissions were related planned (11%; N = 7/63) or unrelated (14%; N = 9/63). This underscores the difficulty in altering reimbursement patterns for readmissions following vascular surgery as a fourth of these readmissions are not true complication related readmissions and would thus incur an inappropriate penalty if reimbursement is based solely on overall readmission rate. In addition, many patients being readmitted with complications we felt to be related to the procedure did not have a corresponding NSQIP category for that complication, such as C. diff infection, hematoma, pain, atrial fibrillation, GI bleed. Adding these categories to NSQIP may help provide more detailed feedback to participating institutions about their procedure-related complications. Furthermore, we suggest that it would be beneficial for individual institutions to keep track of the specific indications for readmission, as this data may help reduce readmission and unearth issues that may provide insight into post-operative care. As the approach to the issue of readmission following LEB continues to evolve, care must be taken to ensure that robust mechanisms are in place to discern the relationship of a readmission to the index procedure, particularly in the case of contralateral limb pathology which cannot be distinguished from ipsilateral limb pathology based on ICD-9 codes alone.
The findings above must be interpreted in the context of the study design. Our study relies upon a prospectively collected clinical database and as such may only collect data that is available within the normal course of clinical care. Given this fact, certain data such as albumin level are not routinely checked and may be missing from patients in whom these measurements were not deemed indicated. To the extent that these decisions may be made in a non-differential fashion, we cannot make reliable determinations about the effect of certain parameters for which we have incomplete data. In this study, we found albumin level to be such a parameter and thus cannot make firm statements about the effect of albumin level on readmission. Additionally, the NSQIP database was conceived for broad applicability to a wide variety of surgical procedures and as such does not contain granular detail specifically related to vascular surgery. The next iteration of the NSQIP, which has already begun accruing data, has parameters specific to vascular surgery that may enrich future studies. It is for this reason that we relied on a retrospective review of our institutional data to further clarify indications for readmission in this population. Our single institution retrospective review is missing readmission to other institutions prior to 2010, and while this is a potential weakness of our study, only one readmission out of 18 after 2010 was to another institution indicating that the number of readmissions to outside institutions was likely a small minority. Though our institutional data were subjected to physician chart review to define the relationship of the readmission to the index procedure, this process carries a degree of subjectivity that we feel makes determination of reimbursement, or lack thereof, in this setting fraught with uncertainty.
CONCLUSIONS
This study of patients undergoing LEB, the largest review of clinical, non-administrative data related to this procedure, has further defined a population at increased risk for readmission. Patients with poor baseline health and, in particular, patients requiring return to the OR on their index admission represent a group that may benefit from improved follow up mechanisms particularly in light of a high incidence of post-discharge mortality. Moreover, we have reinforced the findings of prior reports which found post-discharge SSI to be associated with rehospitalization. Surgeon controlled risk factors such as operative time and perioperative blood transfusion may play a role in mitigating these SSI. Further, we hypothesize that early detection of SSI’s through improved monitoring may aid in early detection of SSI and possibly decrease the need for readmission. Though data on postoperative patient outreach to date has been mixed, innovation in this regard could play a large role in improving outcomes. Finally, we have shown that a significant number of readmissions are either planned or unrelated to the index procedure which suggests that reimbursement models assessing a penalty for readmission should be implemented with caution.
Footnotes
Presented at the Society for Vascular Surgery 2013 Annual Meeting in San Francisco, CA, May 30-June 1, 2013, Poster Session
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medicare Payment Advisory Commission (U.S.) Report to the Congress : reforming the delivery system. Washington, DC: Medicare Payment Advisory Commission; 2008. [Google Scholar]
- 2.Brown RE, Qadan M, Martin RC, 2nd, Polk HC., Jr The evolving importance of readmission data to the practicing surgeon. J Am Coll Surg. 2010;211:558–60. doi: 10.1016/j.jamcollsurg.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Ansari MZ, Collopy BT, Booth JL. Hospital characteristics associated with unplanned readmissions. Australian health review : a publication of the Australian Hospital Association. 1995;18:63–75. [PubMed] [Google Scholar]
- 4.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Annals of internal medicine. 1995;122:415–21. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Jackson BM, Nathan DP, Doctor L, Wang GJ, Woo EY, Fairman RM. Low rehospitalization rate for vascular surgery patients. J Vasc Surg. 2011;54:767–72. doi: 10.1016/j.jvs.2011.03.255. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. Journal of the National Medical Association. 2007;99:480–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Feinglass J, Pearce WH, Martin GJ, Gibbs J, Cowper D, Sorensen M, et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program. Surgery. 2001;130:21–9. doi: 10.1067/msy.2001.115359. [DOI] [PubMed] [Google Scholar]
- 9.Giles KA, Landon BE, Cotterill P, O’Malley AJ, Pomposelli FB, Schermerhorn ML. Thirty-day mortality and late survival with reinterventions and readmissions after open and endovascular aortic aneurysm repair in Medicare beneficiaries. Journal of vascular surgery. 2011;53:6–12. 3 e1. doi: 10.1016/j.jvs.2010.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt PJ, Poloniecki JD, Hofman D, Hinchliffe RJ, Loftus IM, Thompson MM. Re-interventions, readmissions and discharge destination: modern metrics for the assessment of the quality of care. Eur J Vasc Endovasc Surg. 2010;39:49–54. doi: 10.1016/j.ejvs.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Casey K, Hernandez-Boussard T, Mell MW, Lee JT. Differences in readmissions after open repair versus endovascular aneurysm repair. J Vasc Surg. 2013;57:89–95. doi: 10.1016/j.jvs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gioia LC, Filion KB, Haider S, Pilote L, Eisenberg MJ. Hospital readmissions following abdominal aortic aneurysm repair. Ann Vasc Surg. 2005;19:35–41. doi: 10.1007/s10016-004-0132-4. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt DY, Greenberg CC, Kind AJ, Havlena JA, Mell MW, Nelson MT, et al. Causes and implications of readmission after abdominal aortic aneurysm repair. Ann Surg. 2012;256:595–605. doi: 10.1097/SLA.0b013e31826b4bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel TR, Symons RG, Flum DR. Longitudinal outcomes after endovascular repair of abdominal aortic aneurysms. Vasc Endovascular Surg. 2008;42:412–9. doi: 10.1177/1538574408316143. [DOI] [PubMed] [Google Scholar]
- 15.Brooke BS, Goodney PP, Powell RJ, Fillinger MF, Travis LL, Goodman DC, et al. Early discharge does not increase readmission or mortality after high-risk vascular surgery. J Vasc Surg. 2013;57:734–40. doi: 10.1016/j.jvs.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg. 2003;238:161–7. doi: 10.1097/01.SLA.0000081094.66659.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPhee JT, Barshes NR, Ho KJ, Madenci A, Ozaki CK, Nguyen LL, et al. Predictive factors of 30-day unplanned readmission after lower extremity bypass. Journal of vascular surgery. 2013;57:955–62. doi: 10.1016/j.jvs.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 18.Vogel TR, Dombrovskiy VY, Carson JL, Graham AM. In-hospital and 30-day outcomes after tibioperoneal interventions in the US Medicare population with critical limb ischemia. J Vasc Surg. 2011;54:109–15. doi: 10.1016/j.jvs.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 19.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program Annals of surgery. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Advances in surgery. 2010;44:251–67. doi: 10.1016/j.yasu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Shiloach M, Frencher SK, Jr, Steeger JE, Rowell KS, Bartozkis K, Tomeh MG, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 22.User Guide for the 2011 Participant Use Data File. American College of Surgeons National Surgical Quality Improvement Program. 2012 [Google Scholar]
- 23.Hall BL, Hirbe M, Yan Y, Khuri SF, Henderson WG, Hamilton BH. Thyroid and parathyroid operations in veterans affairs and selected university medical centers: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1222–34. doi: 10.1016/j.jamcollsurg.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 24.Greenblatt DY, Rajamanickam V, Mell MW. Predictors of surgical site infection after open lower extremity revascularization. J Vasc Surg. 2011;54:433–9. doi: 10.1016/j.jvs.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Tan TW, Farber A, Hamburg NM, Eberhardt RT, Rybin D, Doros G, et al. Blood transfusion for lower extremity bypass is associated with increased wound infection and graft thrombosis. J Am Coll Surg. 2013;216:1005–14. e2. doi: 10.1016/j.jamcollsurg.2013.01.006. quiz 31–3. [DOI] [PMC free article] [PubMed] [Google Scholar]