Abstract
Background
Endovascular therapies are increasingly used for treatment of critical limb ischemia (CLI). Infrapopliteal (IP) occlusions are common in CLI, and successful limb salvage may require restoration of arterial flow in the distribution of a chronically occluded vessel. We sought to describe the procedural characteristics and outcomes of patients with IP occlusions who underwent endovascular intervention for treatment of CLI.
Methods
All patients with IP interventions for treatment of CLI from 2006 to 2012 were included. Angiographic and procedural data were compared between patients who underwent intervention for IP occlusions vs IP stenosis. Restenosis was determined by Doppler ultrasound imaging. Limb salvage was the primary end point of the study. Additional end points included primary patency, primary assisted patency, secondary patency, occlusion crossing success, procedural success, and amputation-free survival.
Results
A total of 187 patients with CLI underwent interventions for 356 IP lesions, and 77 patients (41%) had interventions for an IP occlusion. Patients with an intervention for IP occlusion were more likely to have zero to one vessel runoff (83% vs 56%; P < .001) compared with interventions for stenosis. Compared with IP stenoses, IP occlusions were longer (118 ± 86 vs 73 ± 67 mm; P < .001) and had a smaller vessel diameter (2.5 ± 0.8 vs 2.7 ± 0.5 mm; P =.02). Wire crossing was achieved in 83% of IP occlusions, and the overall procedural success for IP occlusions was 79%. The overall 1-year limb salvage rate was 84%. Limb salvage was highest in the stenosis group, slightly lower in the successful occlusion group, and lowest in the failed occlusion group (92% vs 75% vs 58%, respectively; P = .02). Unsuccessfully treated IP occlusions were associated with a significantly higher likelihood of major amputation (hazard ratio, 5.79; 95% confidence interval, 1.89–17.7) and major amputation or death (hazard ratio, 2.69; 95% confidence interval, 1.09–6.63).
Conclusions
Successful endovascular recanalization of IP occlusions can be achieved with guidewire and support catheter techniques in most patients. In patients selected for an endovascular-first approach for IP occlusions in CLI, this strategy can be successfully implemented with favorable rates of limb salvage.
Critical limb ischemia (CLI) is associated with high rates of limb loss and mortality. Within 6 months of presentation with CLI, ~25% of patients require major amputation.1 An estimated 250,000 major amputations are performed annually in the United States and Europe, resulting in a significant socioeconomic burden and severe reduction in quality of life indicators.2,3 Published rates of mortality for CLI approach 25% at 1 year and >50% at 5 years, exceeding rates observed in any other form of occlusive arterial disease.1,2
Although surgical revascularization has been the traditional treatment of choice for limb salvage in CLI, an increasing number of centers are adopting an endovascular-first approach to limb salvage. Initial studies suggested similar outcomes for these two strategies, especially among patients with a life expectancy of <2 years.4,5 More recent investigations have described procedural outcomes, techniques, and angiographic characteristics of lower extremity endovascular interventions.6–9
Infrapopliteal (IP) occlusive disease is a major independent contributor to morbidity and mortality among patients with CLI.10 Recent refinements in endovascular techniques have led to the development of new approaches for endovascular treatment of IP occlusive disease among patients with CLI.11 IP occlusions are common in this patient population and present significant challenges for endovascular intervention. Few data are available regarding procedural and limb salvage outcomes of endovascular treatment for IP occlusions.
We sought to describe our institutional experience with endovascular management of IP occlusions and to quantify the association of IP occlusion treatment with subsequent limb salvage rates among patients with CLI. We hypothesized that IP occlusions could be recanalized with a high rate of success using standard guidewire techniques and that successful treatment of IP occlusions would be associated with acceptable rates of limb salvage.
METHODS
This study was approved by the University of California Davis Medical Center Institutional Review Board.
Data source
The Peripheral Arterial Disease— University of California Davis Registry comprises all patients with a clinical diagnosis of peripheral arterial disease who underwent diagnostic peripheral angiography or therapeutic endovascular intervention at the University of California Davis Medical Center from 2006 to 2012. For this study, the subset of patients in the registry with CLI who underwent IP endovascular interventions (187 patients, representing 22% of the total registry) were analyzed. During the same time period (2006–2012), 80 patients were referred for surgical revascularization of critical IP disease, including 33 for rest pain, 26 for minor tissue loss, and 21 for major tissue loss.
CLI was defined as Rutherford category 4, 5, or 6 disease (defined as ischemic rest pain, minor tissue loss, or major tissue loss, respectively) with a reduced ankle-brachial index (ABI) to a level of <0.4, an ankle systolic pressure of <50 mm Hg, or a toe pressure of <40 mm Hg, based on a review of clinic notes, history, physical examination, noninvasive laboratory testing, and hospital discharge summaries. Rutherford categories were defined using published criteria.12
Procedural approach
The general procedural approach to IP interventions included ipsilateral anterograde or contralateral retrograde common femoral arterial access with a sheath advanced into the distal superficial femoral artery or popliteal artery to allow for better catheter/guide-wire control and reduced contrast administration. Our institutional practice has been to use retrograde “crossover” access in most cases to allow for visualization of the entire inflow of the contralateral leg; this approach permits treatment of disease at the iliac level if necessary. Additionally, in our experience, retrograde contralateral access with ultrasound guidance and a micropuncture technique is associated with lower rate of access site complications than the antegrade approach.
The stenosis or occlusion was then crossed using a standard hydrophilic-tipped guidewire, such as PT Graphix or PT2 (Boston Scientific, Quincy, Mass), and a low-profile catheter support, such as Quick Cross (Spectranetics, Colorado Springs, Colo). For lesions where this initial hardware could not successfully cross the occlusion, more supportive devices/wires were then used at the operator’s discretion; for example, Confianza or Miracle Bros 6 (Abbott Vascular, Abbott Park, Ill) or V18 Control (Boston Scientific). When deemed appropriate, devices designed specifically for chronic occlusions were used, including Outback (Cordis, Bridge-water, NJ) and Crosser (Bard, Tempe, Ariz), although these devices were used in only five cases.
In recent years, retrograde pedal or tibial access was occasionally performed after failed attempts at antegrade recanalization of occlusions. In these cases, the dorsalis pedis artery or distal posterior tibial artery was cannulated using fluoroscopic or ultrasound guidance and a 4F micro-puncture dilator was advanced retrograde into the vessel. A guidewire was then advanced through this dilator to the distal cap of the occlusion, and attempts were made to cross the lesion from below. Once the lesion was crossed from a retrograde approach, the wire was snared from above and externalized through the femoral arterial sheath. The procedure was then completed from an antegrade approach.
During the study period, all procedures were performed by three members of the institutional Vascular Center (three vascular surgeons and one interventional cardiologist).
Data collection and definitions
Baseline data were collected from a review of electronic medical record documentation and procedure notes. Office notes before and after the procedure, the admission history, and physical documentation were used to identify clinical presentation as well as postprocedural outcomes and medical management. This information was entered into a prespecified case report form with standardized data entry. Two authors (E.A., G.S.) reviewed all angiographic images to verify lesion location, presence of chronic occlusion, extent of calcification, and distal runoff. Quantitative angiography (Xcelera; Philips Medical Systems, Andover, Mass) was performed on all target lesions to evaluate the preintervention percentage of diameter stenosis, lesion length, and reference vessel diameter.
Procedural data included whether the lesion was a restenosis or reocclusion, the type of intervention, whether a stent was placed, and whether the intervention involved balloon angioplasty, cutting balloon angioplasty, excimer laser, excisional or rotational atherectomy, or cryoplasty. A nonocclusive stenosis was defined as ≤99% stenosis with antegrade flow on angiography. Occlusions were defined as complete arterial occlusion and absence of antegrade flow. Procedural success was defined as <30% stenosis at the conclusion of the procedure, without a major adverse event.13–15
During follow-up, deaths were identified through electronic documentation of clinic or inpatient notes as well as the Social Security Death Index. Major amputation was defined as any amputation above the level of the ankle joint. Lesion patency was monitored with serial ABI and toe-brachial index measures and duplex ultrasound (DUS) imaging at 0 to 30 days, 4 to 6 months, and 9 to 12 months. Loss of primary patency (PP) after the initial revascularization was defined as the presence of >50% stenosis during repeat angiography for progressive tissue loss or a surveillance DUS assessment indicating restenosis. For each vessel segment, the highest peak systolic velocity obtained was used as the sample measure. A peak systolic velocity ratio ≥2.0 was used to define ≥50% stenosis at each interval DUS examination.16,17
Outcomes
The primary study end point was limb salvage, defined as avoidance of a major amputation above the level of the ankle joint. Secondary end points included PP (<50% stenosis at any point during the follow-up period), primary assisted patency (PAP; reintervention for >50%–99% restenosis), secondary patency (SP; reintervention for reocclusion), occlusion crossing success, procedural success, and amputation-free survival.
Data analysis
Median values with interquartile ranges were used to describe continuous variables, and numeric values with percentages were used for categoric variables. Univariate analysis was used to identify differences between occlusions and stenosis in CLI patients with IP disease. Continuous variables were compared using the Wilcoxon rank sum test. Categoric values were compared by the χ2 or Fisher exact test. Kaplan-Meier analysis was used to compare outcomes of major amputation and mortality among the patient groups. All analyses were performed using Stata 11.2 software (StataCorp LP, College Station, Tex). A P value of <.05 was considered significant.
RESULTS
During the study period, 187 patients (119 male, 68 female) underwent peripheral artery angiography and IP intervention for management of CLI; of these, 77 (41%) had an endovascular intervention for an IP occlusion. The mean number of IP target vessels was 1.5 ±0.5 in the occlusion group and 1.3 ± 0.5 in the stenosis group (P = .5). Among the 77 patients in the IP occlusion group, 19 (25%) also had intervention in an IP stenosis vessel during the same procedure to maximize distal runoff. In the overall cohort, 56% of patients also had concomitant intervention in a femoropopliteal lesion during the same procedure. Patients undergoing intervention for an IP stenosis were more likely to undergo concomitant superficial femoral artery/popliteal intervention (65%) than patients with interventions for IP occlusions (47%; P = .01). Interestingly, we found that patients who underwent multilevel interventions actually had better limb salvage rates (92% at 1 year) than those who underwent IP interventions only (75% at 1 year).
Baseline characteristics and cardiovascular risk factors for the two groups are summarized in Table I. Compared with the stenosis group, IP occlusion patients were more likely to have a history of heart failure (35% vs 21%; P = .03) and were less likely to be taking statin medications (52% vs 71%; P = .008) before the procedure. Patients who underwent interventions for IP occlusions were more likely to have Rutherford class 5 (69% vs 54%) or 6 (16% vs 12%; P = .01) disease than patients in the stenosis group. The baseline toe-brachial index values were similar in both groups (0.26 ± 0.12 for IP occlusion vs 0.29 ± 013 for stenosis; P = .2), as were ABIs after exclusion of noncompressible readings (0.29 ± 0.19 for stenosis vs 0.35 ± 0.04 for IP occlusion; P = .2). No significant between-group differences were noted in other demographic characteristics, including age, body mass index, history of diabetes, smoking history, renal function, end-stage renal disease, or hemoglobin A1c. Scores according to the Prevention of Infrainguinal Vein Graft Failure III (PREVENT III) scoring system (4.0 ± 2.3 for stenosis vs 4.7 ± 2.5 for occlusions; range, 0–10; P = .05) and Finland National Vascular (FINNVASC) scoring system (2.3 ± 0.8 for stenosis vs 2.3 ± 0.9 for occlusions; range, 0–4; P = .9) also did not exhibit any statistically significant between-group differences.18
Table I.
Baseline patient characteristics
| Variablea | Occlusion (n = 77) | Stenosis (n = 110) | P |
|---|---|---|---|
| Age, years | 68 ± 12 | 69 ± 14 | .9 |
| Male | 50 (65) | 69 (62) | .8 |
| Body mass index, kg/m2 | 27.2 ± 6.3 | 27.0 ± 5.4 | .9 |
| Heart failure | 27 (35) | 23 (21) | .03 |
| Diabetes | 55 (71) | 77 (72) | .9 |
| End-stage renal disease | 19 (25) | 21 (19) | .4 |
| Prior or current smoker | 36 (47) | 65 (60) | .09 |
| GFR, mg/dL | 54 ± 33 | 60 ± 38 | .2 |
| Hypertension | 63 (82) | 97 (88) | .2 |
| Coronary artery disease | 33 (44) | 56 (51) | .4 |
| Stroke | 13 (17) | 29 (27) | .1 |
| Hemoglobin A1c, % | 7.5 ± 2.0 | 8.3 ± 2.5 | .2 |
| Statin use | 40 (52) | 78 (71) | .008 |
| Rutherford class | .01 | ||
| 4 | 11 (15) | 36 (35) | |
| 5 | 52 (69) | 56 (54) | |
| 6 | 12 (16) | 12 (12) | |
| ABIb | 0.29 ± 0.10 | 0.35 ± 0.04 | .2 |
| Toe-brachial index | 0.26 ± 0.12 | 0.29 ± 0.13 | .2 |
| PREVENT III score (0–10) | 4.0 ± 2.3 | 4.7 ± 2.5 | .05 |
| FINNVASC score (0–4) | 2.3 ± 0.8 | 2.3 ± 0.9 | .9 |
ABI, Ankle-brachial index; GFR, glomerular filtration rate; FINNVASC, Finland National Vascular scoring system; PREVENT III, Prevention of Infrainguinal Vein Graft Failure III scoring system.
Continuous data are shown as mean ± standard deviation and categoric data as number (%).
ABI after censoring of noncompressible ABIs.
Angiographic characteristics for each lesion are summarized in Table II. Of the 356 lesions treated during the study period, 126 (35%) were IP occlusions. IP occlusions were significantly longer (118 ± 86 vs 73 ± 67 mm; P < .001), were more likely to have moderate-severe calcification (23% vs 17%; P = .20), and were associated with worse preprocedural distal vessel runoff (83% vs 56% zero- or one-vessel runoff; P < .001). The most common target vessel in the IP occlusion group was the anterior tibial artery (45%), followed by the peroneal artery (28%).
Table II.
Angiographic characteristics
| Variablea | Occlusion (n = 126) | Stenosis (n = 230) | P |
|---|---|---|---|
| Length, mm | 118 ± 86 | 73 ± 67 | <.001 |
| Vessel diameter, mm | 2.5 ± 0.8 | 2.7 ± 0.5 | .02 |
| Preprocedure stenosis, % | 100 | 78 ± 23 | <.001 |
| Calcification | .2 | ||
| None-mild | 97 (77) | 191 (83) | |
| Moderate-severe | 29 (23) | 39 (17) | |
| Runoff | <.001 | ||
| 0–1 | 105 (83) | 129 (56) | |
| 2–3 | 21 (17) | 101 (44) | |
| Target vessel | .004 | ||
| Anterior tibial | 57 (45) | 68 (30) | |
| Tibioperoneal trunk | 25 (20) | 54 (23) | |
| Peroneal | 35 (28) | 64 (28) | |
| Posterior tibial | 9 (7) | 44 (19) |
Continuous data are shown as the mean ± standard deviation and categoric data as number (%).
Tables III and IV summarize the procedural characteristics and outcomes for each lesion. Wire-crossing success was 83% for IP occlusions, and the overall procedural success was 79%. Procedural success also translated into hemodynamic success, with 30-day postprocedural ABIs increased to 0.71 ± 0.20 for occlusion and 0.73 ± 0.18 for stenosis. All patients with procedural and angiographic success had an ABI improvement of >0.15 at 30 days post-procedure. Patients who underwent intervention for IP occlusions were more likely to be treated with laser atherectomy (23% vs 4%; P < .001) and stent deployment (8% vs 2%; P = .009) than the stenosis group. There were small but significantly higher rates of dissection (5% vs 1%; P = .006) and perforation (3% vs 0.4%; P = .05) among the IP occlusion group. None of these complications resulted in the need for urgent surgery. Fluoroscopy time was greater in the occlusion group (36 ± 18 vs 30 ± 16 minutes; P = .01). There was no difference in contrast use between groups (171 ± 93 vs 168 ± 96 mL; P = .9).
Table III.
Procedural characteristics
| Variablea | Occlusion (n = 126) | Stenosis (n = 230) | P |
|---|---|---|---|
| Cutting balloon | 8 (6) | 31 (13) | .04 |
| Cryotherapy | 8 (6) | 11 (5) | .5 |
| Atherectomy | <.001 | ||
| Laser | 28 (23) | 9 (4) | |
| Excisional | 1 (1) | 3 (1) | |
| Rotational | 0 | 5 (2) | |
| Stent placed | 10 (8) | 5 (2) | .009 |
| Femoral access | .1 | ||
| Anterograde | 11 (9) | 34 (15) | |
| Retrograde | 115 (91) | 198 (85) | |
| Fluoroscopy time, min | 36 ± 18 | 30 ± 16 | .01 |
| Total contrast, mL | 171 ± 93 | 168 ± 96 | .9 |
Categoric data are shown as number (%) and continuous data as mean ± standard deviation.
Table IV.
Complication and success rates
| Variablea | Occlusion (n = 126) | Stenosis (n = 230) | P |
|---|---|---|---|
| Dissection | 7 (6) | 2 (1) | .006 |
| Perforation | 4 (3) | 1 (0.4) | .05 |
| Wire crossing success | 104 (83) | 230 (100) | .001 |
| Procedural success | 99 (79) | 228 (99) | .001 |
| 30-day post-treatment ABI | 0.71 ± 0.20 | 0.73 ± 0.18 | .7 |
ABI, Ankle-brachial index.
Categoric data are shown as number (%) and continuous data as mean ± standard deviation.
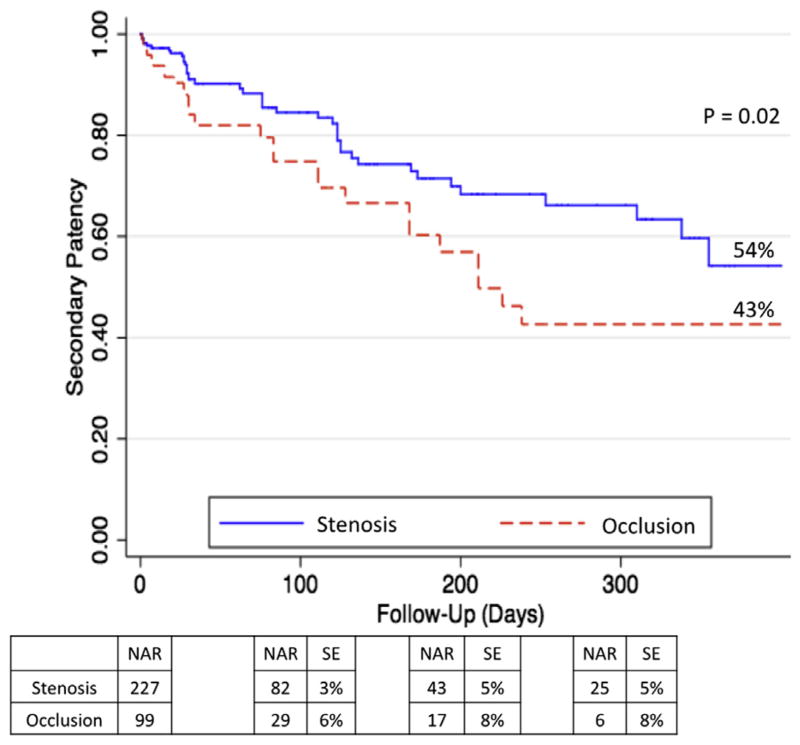
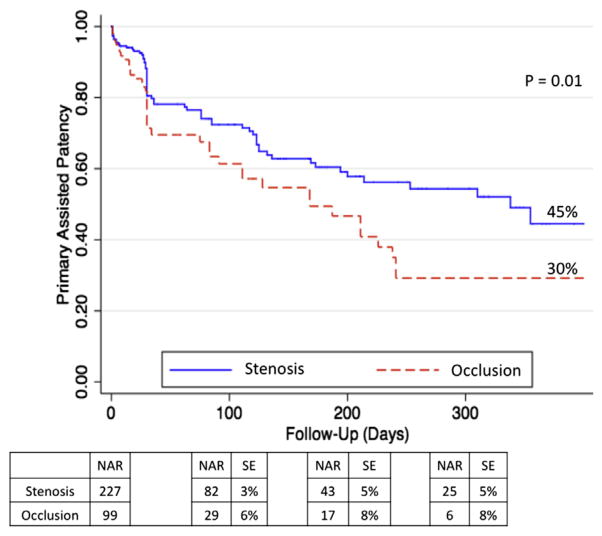
Kaplan-Meier curves for PP, PAP, and SP are shown in Figs 1–3, respectively. Rates of patency (Figs 1–3) were similar between the occlusion and stenosis groups until 200 days after the intervention, at which point the curves diverged. At 1 year, IP occlusions were associated with a significantly higher risk of subsequent restenosis (hazard ratio [HR], 1.8; 95% confidence interval [CI], 1.15–2.71) compared with IP stenosis lesions (Fig 1). The estimated PP at 1 year was 30% for IP occlusions and 42% for IP stenosis (P = .04). At the 1-year follow-up, PAP was 45% for IP stenosis lesions and 30% for IP occlusions (P = .01), and SP rates were 54% for IP stenosis vs 43% for IP occlusions (P = .02).
Fig 1.
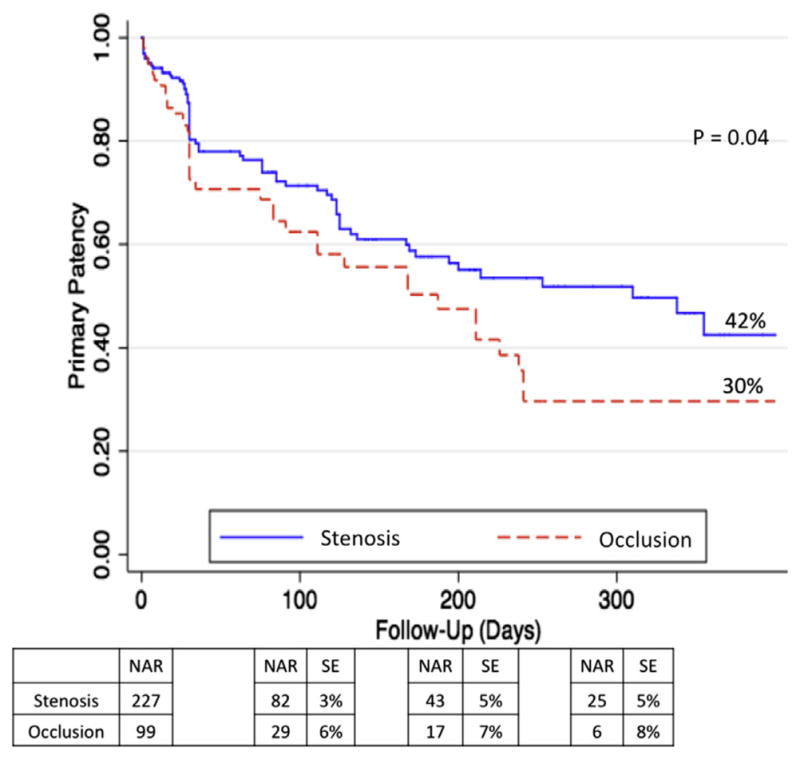
Primary patency (PP) at 1 year. Expressed in percentage. NAR, Number at risk; SE, standard error.
Fig 3.
Secondary patency (SP) at 1 year. Expressed in percentage. NAR, Number at risk; SE, standard error.
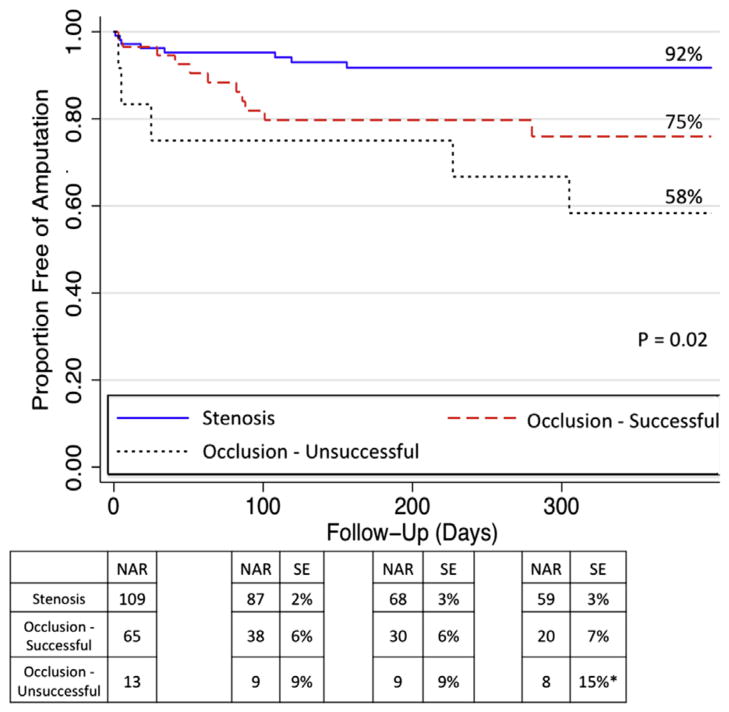
The 1-year limb salvage rate for the entire cohort was 84%. Limb salvage rates among patients who underwent intervention for IP occlusions vs stenosis are depicted in Fig 4. IP occlusions were further categorized into successful (n = 99) vs unsuccessful (n = 27) interventions. The Kaplan-Meier curves show the highest 1-year limb salvage for patients with an intervention for an IP stenosis (92%) and intermediate (75%) limb salvage rates among patients with successful IP occlusion interventions. Unsuccessful IP occlusion interventions were associated with a significantly lower 1-year limb salvage rate of 58% (P = .02). Compared with patients with IP stenosis, successful IP occlusion recanalizations remained associated with an increased risk of subsequent major amputation (HR, 2.88; 95% CI, 1.16–7.18). However, the hazard for amputation was greatest among patients with unsuccessful IP occlusion recanalizations (HR, 5.79; 95% CI, 1.89–17.7) compared with IP stenosis interventions.
Fig 4.
Limb salvage rates at 1 year. NAR, Number at risk; SE, standard error. *Standard error >10%.
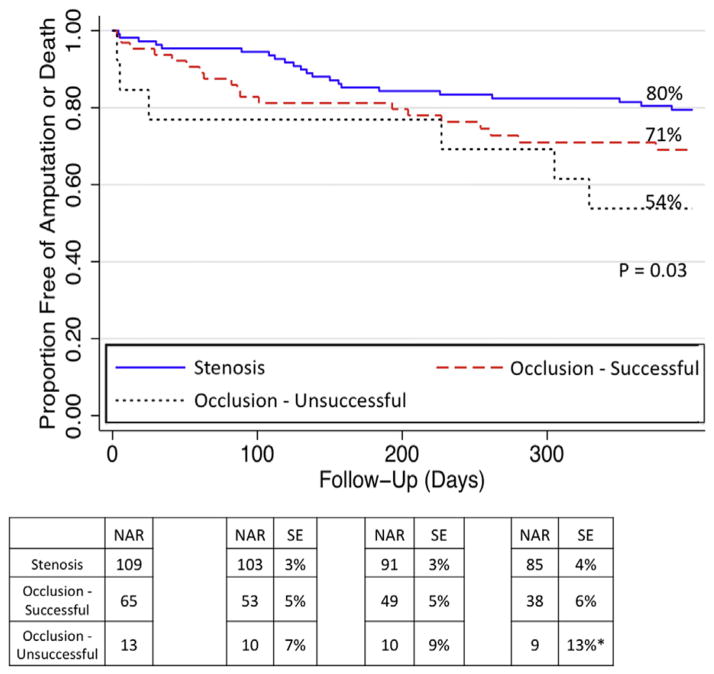
At 1 year, the estimated amputation-free survival (Fig 5) was 80% for IP stenosis interventions, 71% for successful IP occlusions, and 54% for failed IP occlusions. Patients with failed IP occlusion interventions had significantly higher rates of death or major amputation (HR, 2.69; 95% CI, 1.09–6.63; P = .03) at 1 year compared with the stenosis group.
Fig 5.
Amputation-free survival at 1 year. NAR, Number at risk; SE, standard error. *Standard error >10%.
Among the 27 unsuccessful IP occlusions, 13 (48%) were the anterior tibial artery, 10 (37%) were the posterior tibial artery, 3 (11%) were the peroneal artery, and 1 (4%) was the tibioperoneal trunk. Five (19%) of the failed occlusions were in patients with Rutherford 6 disease. Five (19%) of the occlusions were crossed with a guidewire, but despite multiple attempts at intervention, procedural success (<30% residual stenosis) was not achieved. Self-limited vessel injury (dissection or perforation) not requiring urgent surgical intervention was noted in seven (26%) of the failed IP occlusions. One patient (4%) with a failed IP occlusion intervention subsequently underwent surgical revascularization. Repeat endovascular therapy was attempted in four of the patients, of which two were successful; the repeat endovascular attempts were undertaken within 1 week to 3 months after the initial attempt.
A total of 22 amputations were performed despite attempted percutaneous revascularization; of these, 17 (77%) were initially successful (stenosis or occlusion) interventions that had progressive CLI. The median number of days from percutaneous intervention to amputation was 35. Before amputation, surgical revascularization was offered to two patients; one patient had progressive disease despite surgical revascularization necessitating amputation, and the other patient declined surgical revascularization. Surgical revascularization was not attempted in the other patients due to a variety of factors: 11 (55%) had inadequate distal targets for bypass, 10 (50%) had multiple cardiac and medical comorbidities making surgery prohibitively high-risk, 4 (20%) had uncontrolled lower extremity infection, and 4 (20%) had prior vein harvesting with no usable venous conduit. Among the 22 patients who subsequently required major amputation, review of postprocedural angiograms did not show any evidence of distal embolization, propagation of dissection, or other complications that might have limited the options for subsequent surgical bypass.
The characteristics of unsuccessful vs successful IP occlusion interventions are reported in Table V. There were no significant between-group differences in baseline patient characteristics. Specialized occlusion-crossing devices were used with greater frequency in the unsuccessful cohort (7% vs 3%), although this difference was not statistically different (P = .2). Use of retrograde access to achieve recanalization of an IP occlusion was similar between the two groups (4% vs 6%; P = .9). Among the seven retrograde access attempts, six (86%) were successful in crossing the lesion and thereby allowing for recanalization of the IP occlusion.
Table V.
Characteristics of unsuccessful vs successful infrapopliteal (IP) occlusion endovascular interventions
| Variablea | Occlusion
|
P | |
|---|---|---|---|
| Unsuccessful (n = 27) | Successful (n = 99) | ||
| Diabetes | 20 (74) | 75 (76) | .9 |
| End-stage renal disease | 9 (33) | 30 (31) | .8 |
| Prior or current smoker | 15 (56) | 50 (51) | .7 |
| Length, mm | 118 ± 82 | 116 ± 82 | .9 |
| Calcification | .2 | ||
| None-mild | 19 (76) | 77 (78) | |
| Moderate-severe | 8 (24) | 22 (22) | |
| Occlusion device used | 2 (7) | 3 (3) | .2 |
| Retrograde attempt | 1 (4) | 6 (6) | .9 |
| Fluoroscopy time, min | 42 ± 13 | 34 ± 18 | .05 |
| Total contrast, mL | 151 ± 60 | 174 ± 97 | .5 |
Categoric data are shown as number (%) and continuous data as mean ± standard deviation.
DISCUSSION
In this study, we report procedural and midterm outcomes of patients with CLI who underwent endovascular interventions for IP occlusions. Our data highlight three important findings: (1) use of a standard guidewire technique with support catheters is associated with high crossing success (83%) and high procedural success (79%) during recanalization of IP occlusions; (2) successful endovascular intervention of IP occlusions is associated with limb preservation and survival rates that are similar to interventions for IP stenosis; and (3) failure of attempted occlusion recanalization is associated with a significantly increased risk of subsequent major amputation.
The few dedicated analyses of IP interventions among patients with CLI have been limited by small sample size and lack of detailed data on IP occlusion characteristics.19–25 Odink et al19 performed a retrospective analysis of 90 patients with CLI and 161 IP lesions; 65% of the lesions were occlusions. These investigators reported an overall technical failure rate of 11%; however, they did not stratify their outcomes by occlusion status. Our study demonstrated a procedural failure rate of 1% in the stenosis group vs 21% in the IP occlusion group. This technical failure rate for IP occlusions is not unexpected given the chronicity of many of these occlusions, the long occlusion lengths, and presence of significant lesion calcification. Compared with other dedicated IP investigations with similar patient cohorts, our technical success/failure and complication rates are comparable to those achieved using dedicated occlusion crossing devices.10,26 Our findings suggest that the standard guidewire technique with use of a support catheter can be associated with a high success rate in recanalization of IP occlusions.
Restenosis after intervention of femoropopliteal and IP disease remains a consistent finding across many investigations, particularly if the treated lesion was an occlusion.27–30 IP lesions are associated with a particularly high rate of restenosis, partly due to small reference vessel diameter and long length of the treated lesions with extensive plaque burden. In a series of 68 ischemic limbs undergoing angioplasty for treatment of isolated IP disease, rates of restenosis approached 40% at 3 months and 82% at 1 year,27 as assessed by serial DUS imaging and angiography. However, the need for reintervention was only 48% at 1 year. In another investigation of 77 IP arteries of CLI patients with long-segment disease (average length, 184 mm), 65% of the intervened segments were occlusions.30 At 3 months, 70% of the intervened segments demonstrated reocclusion or >50% restenosis, as assessed by angiography. Despite this high rate of restenosis, clinical improvement with a marked reduction in ulcer size or a reduction in rest pain, or both, was seen in 76%.30 In another cohort of 73 patients with CLI undergoing an IP intervention, the 1-year PP was 60%, freedom from reintervention was 95%, and the limb salvage rate was 88%.31
Collectively, these investigations provide credence to the concept that restenosis is of less clinical importance in limb revascularization procedures because the goal of CLI intervention is to provide adequate arterial perfusion to meet the heightened metabolic demands of nonhealing tissue.5 Once this demand is met and tissue healing has occurred, restenosis or reocclusion may not be of clinical consequence if adequate tissue healing has already occurred. Consistent with these findings, we report SP rates of 54% in the IP stenosis cohort and 43% with the IP occlusion cohort after reintervention for restenosis or reocclusion. Despite these suboptimal patency rates, our overall limb salvage rate was 84%, which is comparable to other reported rates.31
Limb salvage rates were highest in the subset of CLI patients who underwent interventions for IP nonocclusive stenosis. IP occlusions were associated with increased rates of major amputation; however, we observed a gradient of effect, with a significantly higher rate of subsequent major amputation among patients with failed attempts at recanalization of IP occlusions. Although the decision to intervene on a given occlusion was made at the discretion of each operator, many of these IP occlusion interventions represented “last option” attempts at limb salvage. End-stage renal disease was present in 25% of the IP occlusion group, and 16% had Rutherford category 6 ischemia at the time of presentation. Our analysis of the amputation cohort supports this notion of “last option.” Surgical revascularization was not felt to be a viable approach for 91% of these patients due to absence of adequate venous conduit, lack of distal targets, or severe cardiac and noncardiac comorbidities yielding a prohibitive risk to surgery. Also supporting the “last option” notion is the finding that reintervention was attempted in <20% of this cohort. The data from the current study would suggest, however, that although patients with IP occlusions do represent a high-risk cohort, percutaneous recanalization should be attempted because rates of limb salvage are favorable if success is achieved. This finding has important clinical implications and supports the recent use of more extreme methods to achieve successful recanalization of IP occlusions, such as the use of tibial/pedal access, when traditional antegrade approaches fail. It also highlights the potential need for better IP total occlusion devices to optimize the chances of crossing success if standard guidewire and support catheter techniques are unsuccessful.32
In the present investigation, use of laser atherectomy was also more frequent for treatment of IP occlusions. Specific procedural aspects of laser atherectomy have previously been reported.33 In a retrospective single-center study of 443 patients with CLI from IP stenotic or occlusive lesions, or both,34 12-month PP and limb salvage rates were compared among patients receiving angioplasty alone (n = 79), angioplasty with stenting (n = 300), and laser excimer therapy (n = 64). At 12 months, the PP rates were not statistically different among the groups, at 69%, 76%, and 75%, respectively (P = not significant). However limb salvage rates were lower in the laser group (88%) vs angioplasty alone (97%) or angioplasty with stenting (99%) during the same time period. The authors concluded that the relatively inferior limb salvage rates in the laser-based intervention group was not unexpected because these patients tended to have longer and more heavily diseased lesions vs the focal lesions receiving angioplasty or stenting, or both.34
In a prospective multicenter registry, the Laser Angioplasty for Critical Limb Ischemia (LACI) trial35 enrolled 155 CLI patients (41% with IP stenosis or occlusions, or both) who were felt to be poor candidates for surgical revascularization. This registry demonstrated a procedural success rate of 86% and limb salvage rates of 93% at 6 months in patients with complex infrainguinal occlusive disease. In the present study, the overall small sample size of IP lesions undergoing laser debulking therapy precludes definitive conclusions. However, prior34,35 and current investigation demonstrates that laser debulking may serve as a useful adjunctive therapy in CLI patients with complex lesions or IP occlusions.
This study has several potential limitations. Because these data are observational, there was potential selection bias in choosing patients in whom operators were confident of procedural success. Some patients may have had such extensive disease that an attempt at endovascular intervention was impractical. Given the multiple treatment modalities, statistical power was insufficient to make any firm conclusions regarding the effectiveness of any particular therapy. Drug-coated balloons remain unavailable for use in the United States and represent an alternative treatment modality not evaluated in this series. Finally, although the scope of this report was to evaluate the outcomes in patients selected for an endovascular-first strategy, the lack of a surgical comparator cohort limits our ability to define the overall role of endovascular therapies as a treatment strategy for patients with CLI.
CONCLUSIONS
IP occlusion recanalization can be achieved with high procedural success rates and low complications rates using standard guidewire and support catheter techniques. In patients selected for an endovascular strategy, successful IP occlusion recanalization is associated with increased amputation-free survival compared with failed IP occlusion interventions. Further studies should be performed to compare endovascular recanalization with the outcomes of distal surgical bypass for patients with IP occlusions.
Fig 2.
Primary assisted patency (PAP) at 1 year. Expressed in percentage. NAR, Number at risk; SE, standard error.
Footnotes
Author conflict of interest: Dr Laird is a consultant or advisory board member for Bard Peripheral Vascular, Boston Scientific, Medtronic, Covidien, and Abbott Vascular, and receives research support from Atrium Medical and W. L. Gore. Dr Yeo receives research support from Abbott Vascular and Medtronic.
AUTHOR CONTRIBUTIONS
Conception and design: GS, EA, KY, WP, DD, JL
Analysis and interpretation: GS, EA, KY, JL
Data collection: GS, EA, SS, GW
Writing the article: GS, EA, JL
Critical revision of the article: GS, EA, KY, SS, GW, WP, DD, JL
Final approval of the article: GS, EA, KY, SS, GW, WP, DD, JL
Statistical analysis: GS, EA
Obtained funding: Not applicable
Overall responsibility: JL
References
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–64. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;25(1 Suppl):5–67A. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes GF. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicentre, randomized controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 5.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–81. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Kasapis C, Henke PK, Chetcuti SJ, Koenig GC, Rectenwald JE, Krishnamurthy VN, et al. Routine stent implantation vs percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30:44–55. doi: 10.1093/eurheartj/ehn514. [DOI] [PubMed] [Google Scholar]
- 7.Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwalder U, Beregi JP, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689–99. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 8.DeRubertis BG, Pierce M, Ryer EJ, Trocciola S, Kent KC, Faries PL. Reduced primary patency in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J Vasc Surg. 2008;47:101–8. doi: 10.1016/j.jvs.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Schanzer A, Conte MS. Critical limb ischemia. Curr Treat Options Cardiovasc Med. 2010;12:214–29. doi: 10.1007/s11936-010-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schamp KBC, Meerwaldt R, Reijnen MMPJ, Geelkerken RH, Zeebregts CJ. The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Ann Vasc Surg. 2012;26:1145–53. doi: 10.1016/j.avsg.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.DeRuvertis BG, Faries PL, McKinsey JF, Chaer RA, Pierce M, Kawowski J, et al. Shifting paradigms in the treatment of lower extremity vascular disease: a report of 1000 percutaneous revascularizations. Ann Surg. 2007;246:415–22. doi: 10.1097/SLA.0b013e31814699a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 13.Lo RC, Darling J, Bensley RP, Giles KA, Dahlberg SE, Hamdan AD, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg. 2013;57:1455–63. doi: 10.1016/j.jvs.2012.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–21. doi: 10.1161/CIRCULATIONAHA.113.001811. [DOI] [PubMed] [Google Scholar]
- 15.Diehm N, Baumgartner I, Jaff M, Do DD, Minar E, Schmidli J, et al. A call for uniform reporting standards in studies assessing endovascular treatment for chronic ischaemia of lower limb arteries. Eur Heart J. 2007;28:798–805. doi: 10.1093/eurheartj/ehl545. [DOI] [PubMed] [Google Scholar]
- 16.Scheinert D, Laird JR, Jr, Schröder M, Steinkamp H, Balzer JO, Biamino G. Excimer laser-assisted recanalization of long, chronic superficial femoral artery occlusions. J Endovasc Ther. 2001;8:156–66. doi: 10.1177/152660280100800210. [DOI] [PubMed] [Google Scholar]
- 17.Laird J, Jaff MR, Biamino G, McNamara T, Scheinert D, Zetterlund P, et al. Cryoplasty for the treatment of femoropopliteal arterial disease: results of a prospective, multicenter registry. J Vasc Interv Radiol. 2005;16:1067–73. doi: 10.1097/01.RVI.0000167866.86201.4E. [DOI] [PubMed] [Google Scholar]
- 18.Moxey PW, Brownrigg J, Kumar SS, Crate G, Holt PJ, Thompson MM, et al. The BASIL survival prediction model in patients with peripheral arterial disease undergoing revascularization in a university hospital setting and comparison with the FINNVASC and modified PREVENT scores. J Vasc Surg. 2013;57:1–8. doi: 10.1016/j.jvs.2012.04.074. [DOI] [PubMed] [Google Scholar]
- 19.Odink H, Berg A, Winkens B. Technical and clinical long term results of infrapopliteal percutaneous transluminal angioplasty for critical limb ischemia. J Vasc Interv Radiol. 2012;23:461–7. doi: 10.1016/j.jvir.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Ryu HM, Kim JS, Ko YG, Hong MK, Jang Y, Choi D. Clinical outcomes of infrapopliteal angioplasty in patients with critical limb ischemia. Korean Circ J. 2012;42:259–65. doi: 10.4070/kcj.2012.42.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soder HK, Manninen HI, Jaakkola P, Matsi PJ, Rasanen HT, Kaukanen E, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–31. doi: 10.1016/s1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- 22.Giles KA, Pomposelli FB, Spence TL, Hamdan AD, Blattman SB, Panossian H, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128–36. doi: 10.1016/j.jvs.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Vraux H, Bertoncello N. Subintimal angioplasty of tibial vessel occlusions in critical limb ischaemia: a good opportunity? Eur J Vasc Endovasc Surg. 2006;32:663–7. doi: 10.1016/j.ejvs.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55:1580–9. doi: 10.1016/j.jacc.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 25.Ingle H, Nasim A, Bolia A, Fishwick G, Naylor R, Bell PR, et al. Subintimal angioplasty of isolated infragenicular vessels in lower limb ischemia: long-term results. J Endovasc Ther. 2002;9:411–6. doi: 10.1177/152660280200900404. [DOI] [PubMed] [Google Scholar]
- 26.Dorros G, Jaff MR, Dorros AM, Mathiak LM, He T. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104:2057–62. doi: 10.1161/hc4201.097943. [DOI] [PubMed] [Google Scholar]
- 27.Iida O, Soga Y, Kawasaki D, Hirano K, Yamaoka T, Suzuki K, et al. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg. 2012;44:425–31. doi: 10.1016/j.ejvs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Kamabatidis D, Katsanos K, Siablis D. Infrapopliteal stents: overview and unresolved issues. J Endovasc Ther. 2009;16(Suppl 1):I153–62. doi: 10.1583/08-2593.1. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Ulrich M, Winkler B, Klaeffling C, Bausback Y, Braunlich S, et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal disease. Catheter Cardiovasc Interv. 2010;76:1047–54. doi: 10.1002/ccd.22658. [DOI] [PubMed] [Google Scholar]
- 31.Conrad MF, Crawford RS, Hackney LA, Paruchuri V, Abularrage CJ, Patel VI, et al. Endovascular management of patients with critical limb ischemia. J Vasc Surg. 2011;53:1020–5. doi: 10.1016/j.jvs.2010.10.088. [DOI] [PubMed] [Google Scholar]
- 32.Dorros G, Jaff MR, Murphy KJ, Mathiak L. The acute outcome of tibioperoneal vessel angioplasty in 417 cases with claudication and critical limb ischemia. Cathet Cardiovasc Diagn. 1998;45:251–6. doi: 10.1002/(sici)1097-0304(199811)45:3<251::aid-ccd7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation. 2007;116:2072–85. doi: 10.1161/CIRCULATIONAHA.107.715433. [DOI] [PubMed] [Google Scholar]
- 34.Bosiers M, Hart JP, Deloose K, Verbist J, Peeters P. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular. 2006;14:64–9. doi: 10.2310/6670.2006.00014. [DOI] [PubMed] [Google Scholar]
- 35.Laird JR, Zeller T, Gray BH, Scheinert D, Vranic M, Reiser C, et al. Limb salvage following laser-assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J Endovasc Ther. 2006;13:1–11. doi: 10.1583/05-1674.1. [DOI] [PubMed] [Google Scholar]