Abstract
Background
Major depression is an independent predictor of increased mortality in patients presenting with acute coronary syndromes (ACS). There have been several mechanisms proposed to explain the link between depression and ischemic heart disease. Both abnormal platelet physiology and inflammation have been suggested as potential confounding variables.
Objective
We set out to examine platelet activation, inflammation, and levels of depression in hospitalized patients presenting with ACS.
Methods
We enrolled 28 patients with ACS and assessed levels of depression by PHQ-9. Platelet activation was assessed by the measurement of platelet microparticle levels and platelet aggregation to adenosine diphosphate and serotonin. Inflammatory markers were assessed by the measurement of TNF alpha, IL-6, and CRP.
Results
We found that ACS patients with moderate depressive symptoms who had higher TNF alpha, IL-6, and CRP levels had higher levels of platelet microparticles. We also found that ACS patients with PHQ-9 ≥ 10 had higher platelet aggregation to ADP.
Conclusion
Our results suggest that patients hospitalized for the treatment of an ACS who have moderate depression have increased platelet aggregation. These patients also have a positive association between elevated inflammatory markers and platelet activation, thus suggesting a pro-inflammatory component in ACS patients with depressive symptoms that may alter platelet function. These results are intriguing in that a potential pathway to explain the connection between depression, inflammation, and increased cardiovascular thrombosis might be found when both platelet activation and inflammation are measured.
The prevalence of major depression in the general population is approximately 5%; however, among those with acute coronary syndromes (ACSs), the prevalence is approximately 17.6%.1 Major depression is an independent predictor of increased mortality following a myocardial infarction.2 Even minimal symptoms of depression are associated with significantly increased 4-month mortality after myocardial infarction.3 Several studies have attempted to investigate potential mechanisms to explain the connection between depression and increased mortality in individuals with cardiovascular disease. Among the most commonly cited etiologies are platelet functional abnormalities4,5 and systemic inflammation.6 Increased platelet reactivity is central to the pathophysiology of atherosclerosis, thrombosis, and acute coronary events.4,5 Several studies have demonstrated increased platelet activation in individuals with depression when compared with healthy controls.6–10 Fewer studies have investigated increased platelet activation in individuals with depression who have coronary artery disease (CAD).
Platelet-derived microparticles (platelet microparticles, PMP) are fragments of platelet membranes that have been shown to participate in arterial thrombosis and correlate with platelet activation.11 To our knowledge, there have not been any studies examining PMP levels and depression.
We examined platelet activation, systemic inflammation, and levels of depression within 48 hours after hospitalization for an ACS. We investigated whether there exists a proinflammatory component that may alter platelet function in patients with ACSs who had depressive symptoms. We hypothesized that depressive symptoms are associated with platelet function in patients with ACSs and that there may be a proinflammatory component that may modify platelet response to activation in patients with ACS.
METHODS
Participants
All patients hospitalized with an ACS at a single urban academic medical center between January and December 2007 were screened and if eligible were enrolled within the first 48 hours of hospitalization. The inclusion criteria required meeting at least 2 of the following 3 criteria for a diagnosis of an ACS: (1) typical cardiac symptoms, (2) elevated Troponin I levels, and (3) characteristic changes on the electrocardiogram indicative of an ischemic cardiac event. The exclusion criteria included (1) receipt of a platelet glycoprotein IIb/IIIa receptor blocker such as eptifibatide or abciximab, (2) symptoms occurring in patients actively using cocaine by self-report and toxicity screen, and (3) onset of symptoms more than 48 hours before recruitment. Although the inclusion criteria were met by some individuals, enrollment was not pursued owing to mechanical ventilation, transfer of location, language barrier, and cognitive impairment assessed by personal interview or dementia diagnosis or both. As controls, we used admitted patients with ACSs who had no or minimal depressive symptoms.
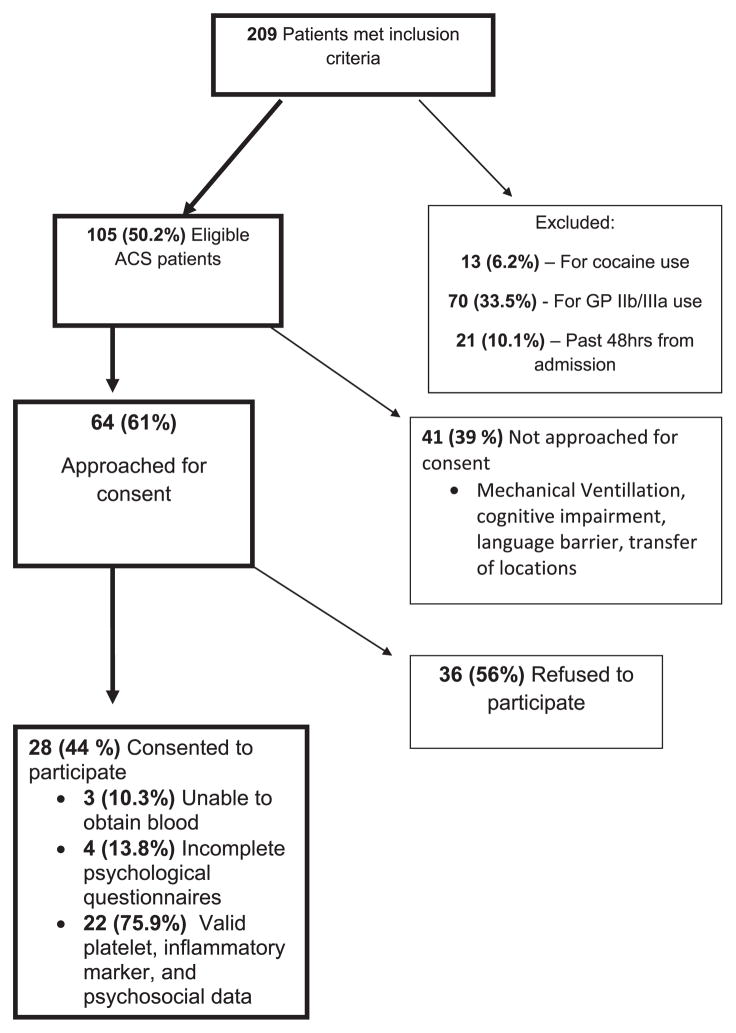
All potentially eligible patient medical records were reviewed by a cardiologist (R. C. Z. or M. S. W.) before consent. The recruitment flow process has been given in Figure 1. The study was approved by the Johns Hopkins Institutional Review Board and all patients provided written informed consent. All patients had platelet functional testing followed by completion of verbally administered psychologic questionnaires.
FIGURE 1.
Recruitment Flow Chart Glycoprotein IIb/IIIa (GP IIb/IIIa) Such as Eptifibatide, or Abciximab. Cocaine Use Defined by Self Report and Toxicity Screen. Cognitive Impairment Assessed by Personal Interview and/or Dementia Diagnosis.
DEPRESSION
Depression symptom severity was measured using the 9-item patient health questionnaire (PHQ-9) as well as the Beck Depression Inventory Second Edition.12,13 We report results based on PHQ-9 assessment as this is the instrument recommended by the American heart association for screening.14 The PHQ-9 has also been advocated by some over the Beck Depression Inventory Second Edition because it is shorter and simpler, which may be particularly important to patients hospitalized for an acute cardiac event who may be easily fatigued.15 Scores on the PHQ-9 range from 0–27 and are scored as follows: no (0), minimal (1–5), mild (6–9), moderate (10–14), moderately severe (15–19), or severe (≥ 20) depression.16 A score < 10 indicates a low probability of clinically significant depression and a total score ≥ 10 indicates a high probability of depression.13,14 For purposes of comparison in our study, we used a PHQ-9 cut off of < 10 to correlate with minimal depressive symptoms of short duration (a PHQ score of 0 represented no depressive symptoms) and ≥ 10 to correlate with at least moderate depressive symptoms.13 The PHQ-9 has been validated as a useful tool in the assessment of depressive symptoms in patients with cardiovascular disease and is the instrument recommended by the American Heart Association for screening.14
ASSESSMENT OF PLATELET FUNCTION
We assessed platelet function within the first 48 hours of presentation by measuring platelet activation and aggregation. Platelet activation was assessed by measuring PMP levels. Platelet aggregation was assessed by measuring the response to serotonin (5-HT) and adenosine diphosphate (ADP) at various doses. Blood samples were taken from all the patients; however, if there was evidence of hemolysis after centrifugation, certain platelet functional assays were omitted.
Platelet Activation
Platelet Microparticles
The blood samples were collected in citrate, theophylline, adenosine, and dipyridamole Vacutainers and centrifuged twice for preparation of platelet-free plasma. Fluorescein isothiocyanate-labeled mouse IgG (Sigma Aldrich) was used for nonspecific binding. Microparticle labeling was performed using both anti-CD41a PerCP (Sigma Aldrich) and anti-CD42b fluorescein isothiocyanate (Sigma Aldrich). Incubation was carried out at room temperature with subsequent addition of carboxylate-modified polystyrene fluorescent red latex beads (Sigma Aldrich) for microparticle gating. Following incubation, 7.32-μm large beads (Bang laboratories, Indianapolis, IN) were used for purposes of microparticle calculations. The results are expressed as total microparticle number. PMPs were chosen as an outcome measure as they area product of activated platelets and have been shown to provide a transport and delivery system for bioactive molecules that participate in hemostasis, thrombosis, and inflammation.11
Platelet Aggregation
Consent was obtained from the eligible patients on the morning before collection of samples. Samples of whole blood were obtained by venipuncture from patients in a fasting state and were collected in Vacutainer tubes with 3.8% sodium citrate (Becton Dickinson, Stamford, CT). The first 5 mL was discarded. All assays were performed at room temperature within 2 hours of drawing blood. Platelet-rich plasma was obtained by centrifugation of whole blood samples. Platelet aggregation was measured by optical aggregometry and performed on a 560CA aggregometer (Chrono-Log, Havertown, PA) as previously described.17 Platelet aggregation was initiated by the addition of increasing doses of 5-HT (final concentrations 0.3, 3, and 30 μM) and 5 μM and 20 μM ADP. The maximal amplitude of aggregation in response to 5-HT and the EC50 of ADP, the concentration of ADP necessary to reach 50% of maximal aggregation amplitude, was calculated for each patient. Lower EC50 indicates higher platelet activation and higher EC50 indicates lower platelet activation.
INFLAMMATORY MARKER ASSESSMENT
Blood samples were collected in vacuum tubes containing dry reagents (ethylenediaminetetraacetic acid). The tubes were immediately kept on ice until separation of plasma could be carried out. The tubes containing the samples were centrifuged to form serum that was stored at −80°C until analysis. The plasma samples from all enrolled patients were assayed at the same time. All samples were labeled and processed in duplicates for quality assurance. The assays were performed at Johns Hopkins University in the laboratory of Dr Gayle Page.
Tumor necrosis factor-α (TNF-α) levels were measured by an ultrasensitive, solid-phase sandwich enzyme-linked immunosorbent assay using a monoclonal antibody specific for TNFα (Quantikine HS Human TNFα Immunoassay; R&D Systems, Minneapolis, MN). The detection range is 15.6–1000 pg/mL per the manufacturer. Values from normal volunteers are < 15.6 pg/mL per the manufacturer.
Interleukin-6 (IL-6) was measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN). A monoclonal anti-IL6 antibody was coated on the plastic support and a polyclonal anti-IL6 antibody was used as the sandwich antibody. The amount of bound IL-6 was determined by a color reaction. The detection range is 3.12–300 pg/mL. Normal value for IL-6 from healthy volunteers is < 3.12 g/mL per the manufacturer.
High-sensitivity C-reactive protein (CRP) levels were measured by ultrasensitive enzyme-linked immunosorbent assay (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN). A monoclonal anti-CRP antibody was coated on the plastic support and a polyclonal anti-CRP antibody was used as the sandwich antibody. The amount of CRP bound was determined by a color reaction. The detection range is 0.78–50 ng/mL. Expected values for CRP in normal, healthy individuals are ≤3 mg/L per the manufacturer.
STATISTICAL ANALYSIS
Distributions of demographic and clinical characteristics of the study sample are described as mean and standard deviation for continuous variables and as frequency (%) for categorical variables. The median and range of platelet function measures were reported for subgroups of patients with ACSs who had minimal depressive symptoms vs those who had moderate depressive symptoms defined by PHQ-9 scores. Patients with moderate depressive symptoms were defined as those with PHQ-9 scores ≥ 10. The 2-sample Wilcoxon rank sum test was used to compare the distributions of platelet function measures between the patient groups. Unadjusted linear regression was used to assess potential differences in the associations between PMPs and inflammatory markers by level of depressive symptoms. An interaction term of inflammatory marker level by depressive symptom group indicator was used to test the statistical significance of such association modification. All tests were 2-sided, with p ≤ 0.05 considered as statistically significant.
RESULTS
Participants
There were a total of 28 patients enrolled, of whom 22 had all platelet testing, inflammatory testing, and psychologic testing carried out. The demographic and clinical characteristics of patients with ACSs are presented in Table 1. The prevalence of depression was 8 (29%) of 28 in this cohort of patients with ACSs. The mean age of the participants was 65 years. Men made up 71% of the sample, and the participants were more likely to be white (82%). Most patients had hypertension and dyslipidemia, about one-third had diabetes mellitus, approximately half had prior myocardial infarction, and 10% had a history of depression. Close to a quarter of the participants were active smokers. The mean body mass index was 28.8 kg/m2. Almost all patients received aspirin, heparin, and β-blockers; close to two-thirds received angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; and about half were treated with clopidogrel. Antidepressant medications were used by 10% of enrolled participants. The mean PHQ-9 score was 7.51 with a range of 0–24. There were 27.6% of patients who scored PHQ-9 ≥ 10. When comparing the PHQ-9 < 10 and ≥ 10 scores of the 2 groups, there were a few significant differences. There were more women with at least moderate depressive symptoms (PHQ-9 ≥ 10). Patients with at least moderate depressive symptoms had lower rates of hypertension and dyslipidemia, higher rates of depression history, and had decreased clopidogrel use.
TABLE 1.
Baseline Characteristics of ACS Sample (n = 28)
| All (n = 28) | PHQ-9 < 10 (n = 20) | PHQ-9 ≥ 10 (n = 8) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (y) | 65.0 (SD = 13.8) | 65 (SD = 15.6) | 65 (SD = 9.7) | 0.92 |
| Men | 71% (n = 20) | 85% (17/20) | 15% (3/8) | |
| Women | 29% (n=8) | 15% (3/20) | 63% (5/8) | 0.02 |
| White | 82% (n = 23) | 80% (16/20) | 88% (7/8) | |
| African American | 18% (n = 5) | 20% (4/20) | 12% (1/8) | 0.39 |
| Medical history | ||||
| History of hypertension | 86% (n = 24) | 95% (19/20) | 63% (5/8) | 0.05 |
| History of dyslipidemia | 79% (n = 22) | 82% (18/22) | 50% (4/8) | 0.03 |
| History of diabetes | 35% (n = 10) | 32% (6/19) | 43% (3/7) | 0.12 |
| Prior myocardial infarction | 48% (n = 13) | 50% (10/20) | 38% (3/8) | 0.28 |
| History of depression | 10% (n = 3) | 0 | 43% (3/7) | 0.02 |
| Lifestyle | ||||
| Current smoker | 24% (n = 7) | 20% (4/20) | 38% (3/8) | 0.23 |
| Mean body mass index (BMI), kg/m2 | 28.8 (SD = 5.2) | 29.4 (SD = 5.4) | 25.3 (SD = 3.2) | 0.06 |
| Laboratory Values | ||||
| Mean troponin I levels, ng/mL | 7.4 (SD = 12.5) | 8.8 (SD = 14.3) | 4.3 (SD = 7.2) | 0.52 |
| In-hospital medications | ||||
| Aspirin | 93% (n = 27) | 95% (19/20) | 88% (7/8) | 0.42 |
| Clopidogrel | 52% (n = 15) | 70% (14/20) | 13% (1/8) | < 0.01 |
| Heparin | 97% (n = 28) | 100% (20/20) | 88% (7/8) | 0.29 |
| Warfarin | 3% (n = 1) | 5% (1/20) | 0 | 0.71 |
| Statin | 79% (n = 23) | 85% (17/20) | 75% (6/8) | 0.32 |
| β-Blocker | 97% (n = 28) | 100% (20/20) | 88% (7/8) | 0.28 |
| ACE/ARB inhibitor | 62% (n = 18) | 70% (14/20) | 50% (4/8) | 0.26 |
| Ca2+ channel blocker | 24% (n = 7) | 35% (7/20) | 0 | 0.07 |
| Nitroglycerin | 76% (n = 22) | 85% (17/20) | 63% (5/8) | 0.17 |
| Any antidepressant | 10% (n = 3) | 5% (1/20) | 25% (2/8) | 0.17 |
ACE =angiotensin-converting enzyme; ARB =angiotensin receptor blockers; PHQ =patient health questionnaire; SD =standard deviation.
Inflammatory Markers and Platelet Response
TNF-α as a Modifier in PMP Formation
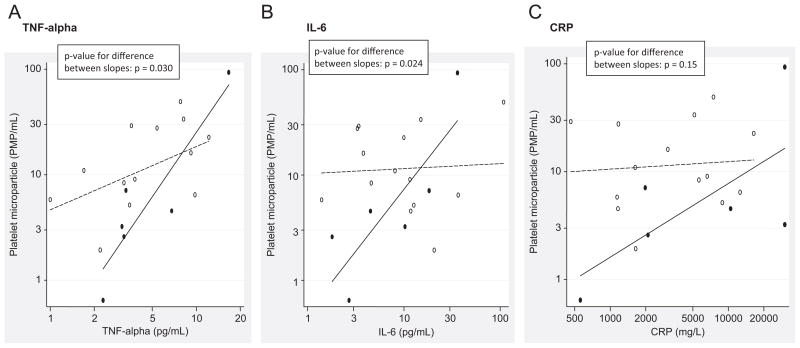
Elevated levels of TNF-α were significantly associated with increased platelet-derived microparticle formation in patients with ACSs who had moderate depressive symptoms as assessed by PHQ-9 (PHQ-9 ≥ 10; 2.2% higher microparticle count per 1% higher TNF-α and p < 0.0001). In contrast, TNF-α levels were not significantly associated with platelet-derived microparticle formation in patients with ACSs who had minimal depressive symptoms (0.6% higher microparticle count per 1% higher TNF-α and p = 0.076) (Figure 2A). The association of platelet-derived microparticles with TNF-α differs significantly between those with depressive symptoms vs those without depressive symptoms (difference between slopes; p = 0.030).
FIGURE 2.
The Association of Platelet Derived Microparticles with TNF-alpha, IL-6, and CRP among those with Depressive Symptoms, PHQ ≥ 10 (Solid Line, Closed Circles) Vs. those without Depressive Symptoms, PHQ <10 (Dashed Line, Open Circles). Panel A, TNF-alpha (Pg/mL) Values Plotted Vs. Platelet Microparticle/ml. Panel B, IL-6 (Pg/mL) Values Plotted Vs. Platelet Microparticle/ml. Panel C, CRP (Mg/L) Values Plotted Vs. Platelet Microparticle Number.
IL-6 as a Modifier in PMP Formation
Elevated levels of IL-6 were significantly associated with increased platelet-derived microparticle formation in patients with ACSs who had moderate depressive symptoms as assessed by PHQ-9 (PHQ-9 ≥ 10; 1.2% higher microparticle count per 1% higher IL-6 and p < 0.007). There was no association between IL-6 and PMP formation in ACS patients with minimal or no depressive symptoms (0.05% higher microparticle count per 1% higher IL-6 and p = 0.85) (Figure 2B). Similar to TNF-α results, the association of platelet-derived microparticles with IL-6 differs significantly between those with depressive symptoms vs those without depressive symptoms (difference between slopes; p = 0.024).
CRP as Modifier in PMP Formation
Elevated levels of CRP were significantly associated with increased platelet-derived microparticle formation in patients with ACSs who had moderate depressive symptoms as assessed by PHQ-9 (PHQ-9 ≥ 10; 0.68% higher microparticle count per 1% higher CRP and p = 0.033). There was no association between CRP and PMP formation in patients with ACSs who had minimal or no depressive symptoms (0.07% higher microparticle count per 1% higher CRP and p = 0.80) (Figure 2C). Unlike TNF-α and IL-6, the association of platelet-derived microparticles with CRP did not reach statistical significance between those with depressive symptoms vs those without depressive symptoms (difference between slopes; p = 0.15).
Inflammatory Markers and Depressive Symptoms
PHQ-9 scores were not associated with the levels of inflammatory markers (Table 2).
TABLE 2.
Results of Platelet Functional Testing in Acute Coronary Syndrome Patients With Depressive Symptoms (PHQ-9 ≥ 10) and Without Depressive Symptoms (PHQ-9 < 10)
| PHQ-9 < 10 (n = 15) | PHQ-9 ≥ 10 (n = 7) | p | |
|---|---|---|---|
| Inflammatory markers | |||
| TNF-α (pg/mL) | 4.9 (1.0, 12.2) (n = 15) | 3.3 (2.3, 16.7) (n = 7) | 0.97 |
| IL-6 (pg/mL) | 10.0 (1.4, 108.9) (n = 15) | 10.3 (1.8, 111.6) (n = 7) | 0.92 |
| hs-CRP (ng/mL) | 3077.5 (463.5, 16,406.5) (n = 15) | 10,459.0 (559.4, 30,000.0) (n = 7) | 0.19 |
| Functional test | n = 20 | n = 8 | |
|
|
|||
| ADP EC50 μM | 6.94 (1.39, 16.21) (n = 17) | 1.87 (1.25, 3.73) (n = 7) | 0.004 |
| ADP EC50 μM* | 3.55 (2.35–6.94) (n = 6) | 1.87 (1.25, 3.73) (n = 7) | 0.046 |
| 30 μmol 5HT Percentage aggregation (amplitude) | 14 (4, 28) (n = 16) | 17 (0, 24) (n = 7) | 0.87 |
| 3 μmol 5HT Percentage aggregation (amplitude) | 11 (0, 24) (n = 15) | 14 (0, 28) (n = 5) | 0.97 |
| 0.3 μmol 5HT Percentage aggregation (amplitude) | 5 (1, 17) (n = 14) | 10 (2, 16) (n = 5) | 0.23 |
| PMP | 13.56 (0.65, 49.07) (n = 18) | 3.87 (0.65, 93.62) (n = 6) | 0.13 |
ADP = adenosine diphosphate; hs-CRP = high-sensitivity C-reactive protein; IL = interleukin; PHQ = patient health questionnaire; PMP = platelet microparticles; TNF = tumor necrosis factor. Adenosine Diphosphate (ADP) EC50 – concentration of ADP needed to obtain 50% maximal aggregation (μM). Serotonin (5HT). Data presented as Median [Min, Max]. N=number. Platelet-derived microparticles (PMP).
Denotes comparison of patients not on Clopidogrel.
Bold values ar those p values that were significant those p values that were significant
Correlation of Psychologic Scores With Platelet Function
Platelet Aggregation to ADP
The median concentration of ADP needed to achieve 50% maximal platelet aggregation was 3.7-fold lower in patients with at least moderate depressive symptoms, as assessed by PHQ-9 (Table 2). The median and range of the EC50 to ADP was 1.87 μM (1.25–3.73 μM) vs 6.94 μM (1.39–16.21 μM), p < 0.01, for patients with ACSs who had PHQ-9 ≥ 10 compared with those who had PHQ-9 < 10. We also analyzed the subset of these patients who were not on clopidogrel therapy and found that the EC50 to ADP was 1.87 μM (1.25–3.73 μM) vs 3.55 μM (2.35–6.94), p = 0.046 for patients with ACSs who had PHQ-9 ≥ 10 compared with patients with ACSs who had PHQ-9 < 10.
Platelet Aggregation to Serotonin
PHQ-9 scores were not associated with platelet aggregation amplitude in response to serotonin (Table 2).
Platelet Microparticles
PHQ-9 scores were not directly associated with levels of microparticles (Table 2).
DISCUSSION
Inflammation and Depression in ACS
In the present study, we found interesting modifiers of platelet response. We found that patients with moderate depressive symptoms who had higher TNF α, IL-6, and CRP levels had higher formation of PMP, thus suggesting a proinflammatory component that may alter platelet function in patients with ACSs who had depressive symptoms.
PMP are markers of platelet activation and are highly procoagulant.18 Research has shown that PMP may play an important role in the transport and delivery of bioactive molecules and signals throughout the body and contain cytokines.11,19 They have been found to be increased in patients with ACSs; however, they have not been measured in depressed states.20,21 Although we did not find a direct association between PMP and depression, we believe that this may be owing to the association of depression with inflammation in patients with ACSs. Proinflammatory markers such as TNF α, IL-6, and CRP have all been shown to be increased in patients with cardiovascular disease.22,23 Similarly IL-1, IL-6, and CRP have all been positively associated with depression in a meta-analysis.24 It has been hypothesized that increased inflammation leads to increased platelet activation in patients with ACSs.25 To our knowledge, this is the first demonstration of an association between inflammatory markers and platelet activation as measured by PMP levels in a cohort of patients with ACSs who had moderate depressive symptoms. We hypothesize that neurohormones such as serotonin or brain-derived neurotrophic factor in individuals with depression may potentiate the platelet-activating properties of inflammatory cytokines. Given that individuals with depression have been found to have elevated whole blood serotonin and plasma brain-derived neurotrophic factor levels, this is a hypothesis that warrants additional investigation.26,27
Furthermore, we observed that the association of platelet-derived microparticles with TNF-α and IL-6 levels differed significantly between those with depressive symptoms vs those without depressive symptoms; however, this contrast was not significant for CRP. One possible explanation for the weaker finding of CRP on PMP levels is that CRP is not a cytokine but an acute phase reactant that is more distant from the initiation of inflammation leading to increased PMP levels. In contrast, both TNF α and IL-6 are cytokines that are upstream modifiers of inflammation. Our results are intriguing, necessitating further study in this area.
Platelet Activation and Depression in ACSs
Patients with ACSs who had moderate depression, as defined by PHQ-9 ≥ 10, demonstrated increased platelet aggregation in response to ADP. Although our cohort of patients with ACSs had significantly less antiplatelet therapy in the form of the ADP receptor blocker clopidogrel, when we analyzed the results of ADP aggregation among only those patients who were not on clopidogrel therapy, patients with ACSs who had moderate depression showed increased platelet aggregation. Our results suggest that patients hospitalized for the treatment of an ACS who have depression have enhanced platelet aggregation. Our findings are consistent with other clinical studies of platelet function and depression. A review of 15 clinical trials have shown that patients with depression and either risk factors for ischemic heart disease or clinically evident ischemic heart disease have circulating platelets that demonstrate increased platelet “stickiness.”28
Although there are several studies that have examined platelet function in individuals with coronary artery disease who have depression, there are only a handful of studies that have examined platelet function immediately at the time of presentation with an ACS8–10,29–31 and to our knowledge no other study has combined the examination of PMP levels and inflammation and its association with ACSs and depression.
Study Limitations
Our study is limited by the inability to control for various factors owing to a small sample size and therefore there may be confounding variables yet unrecognized. In studying this cohort of patients, sample size is often a difficult issue. One contributing factor is likely apathy by those with depression to be involved with research trials. Although we could not assess the depression scores of those who refused participation, there is concern that a substantial refusal rate (56%) may be explained by such a factor. Given the importance of such a topic and the fact that conditions such as timing of platelet assessment, co-morbidities, and use of antiplatelet medications are all potentially confounding variables when examining platelet function in ACS and depression, we continued to pursue enrollment. Other limitations contributing to a small sample size include the exclusion of patients based on antiplatelet medications such as glycoprotein IIb/IIIa. The use of these agents indicates that patients are much more unstable from a cardiovascular perspective. Hence, our cohort was not likely the sickest population of patients with ACSs. For this reason, this makes our findings all the more important. We had a largely white and male patient sample and used self-reported scales for the assessment depression instead of a formal interview, all of which can limit the generalizability of such a study.
However, the assessment of depression and platelet function within 48 hours of presentation of an ACS is unique and can provide helpful insights into biobehavioral mechanisms and therefore, we believe that despite these limitations, useful information can be gleaned from such a study. In prior studies, the potential reasons for discrepancies in platelet function included the methods used to assess platelet aggregation and doses of agonist used. In our study, we examined platelet function within 48 hours of presentation, with blood samples of each individual being collected before noon. Timing of functional assays is also important, and in our study all samples were processed within 2–4 hours of collection.
CONCLUSION
In conclusion, we found evidence of increased platelet aggregation, and furthermore, we found an association between increased levels of inflammatory markers and PMP levels in patients with ACSs who had moderate symptoms of depression. There are several interesting results that suggest further studies are needed. In particular, the finding of correlations between proinflammatory markers and PMP levels warrants further study. The immediate assessment of platelet function in patients with ACSs and depression should be one criterion to be given serious consideration when examining platelet function in these individuals.
Acknowledgments
This study was supported by the Johns Hopkins Center for Mind-Body Research through the National Center for Complementary and Alternative Medicine (Grant no. R24AT004641) and the NIH CTSA Grant UL1 RR 025005. This study was also in part supported by NIH RO1 HL096694.
Footnotes
Disclosure: The authors disclosed no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Amin AA, Jones AM, Nugent K, Rumsfeld JS, Spertus JA. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928–934. doi: 10.1016/j.ahj.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. J Am Med Assoc. 1993;270:1819–1825. [PubMed] [Google Scholar]
- 3.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose JA. Plaque disruption and the acute coronary syndromes of unstable angina and myocardial infarction: if the substrate is similar, why is the clinical presentation different? J Am Coll Cardiol. 1992;19:1653–1658. doi: 10.1016/0735-1097(92)90632-w. [DOI] [PubMed] [Google Scholar]
- 5.Cohen M. Treatment of unstable angina: the role of platelet inhibitors and anticoagulants. J Invasive Cardiol. 1999;11:147–159. [PubMed] [Google Scholar]
- 6.Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–1317. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 7.Shimbo D, Child J, Davidson K, et al. Exaggerated serotonin-mediated platelet reactivity as a possible link in depression and acute coronary syndromes. Am J Cardiol. 2002;89:331–333. doi: 10.1016/s0002-9149(01)02236-6. [DOI] [PubMed] [Google Scholar]
- 8.Markovitz JH, Shuster JL, Chitwood WS, May RS, Tolbert LC. Platelet activation in depression and effects of sertraline treatment: an open-label study. Am J Psychiatry. 2000;157:1006–1008. doi: 10.1176/appi.ajp.157.6.1006. [DOI] [PubMed] [Google Scholar]
- 9.Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol. 2000;20:137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry. 1997;42:290–295. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 11.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 15.Hammash MH, Hall LA, Lennie TA, et al. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2012;12:446–453. doi: 10.1177/1474515112468068. [DOI] [PubMed] [Google Scholar]
- 16.Cameron IM, Cardy A, Crawford JR, et al. Measuring depression severity in general practice: discriminatory performance of the PHQ-9, HADS-D, and BDI-II. Br J Gen Pract. 2011;61:e419–e426. doi: 10.3399/bjgp11X583209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MS, Kickler TS, Vaidya D, Ng’alla LS, Bush DE. Evaluation of platelet function in aspirin treated patients with CAD. J Thromb Thrombolysis. 2006;21:241–247. doi: 10.1007/s11239-006-6968-4. [DOI] [PubMed] [Google Scholar]
- 18.Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 19.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 20.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 21.Skeppholm M, Mobarrez F, Malmqvist K, Wallen H. Platelet-derived microparticles during and after acute coronary syndrome. Thromb Haemost. 2012;107:1122–1129. doi: 10.1160/TH11-11-0779. [DOI] [PubMed] [Google Scholar]
- 22.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. New Engl J Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 23.Sukhija R, Fahdi I, Garza L, et al. Inflammatory markers, angiographic severity of coronary artery disease, and patient outcome. Am J Cardiol. 2007;99:879–884. doi: 10.1016/j.amjcard.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 24.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 25.Bergandi L, Cordero M, Anselmino M, et al. Altered nitric oxide/cGMP platelet signaling pathway in platelets from patients with acute coronary syndromes. Clin Res Cardiol. 2010;99:557–564. doi: 10.1007/s00392-010-0157-3. [DOI] [PubMed] [Google Scholar]
- 26.Wulsin LR, Musselman D, Otte C, Bruce E, Ali S, Whooley MA. Depression and whole blood serotonin in patients with coronary heart disease from the Heart and Soul Study. Psychosom Med. 2009;71:260–265. doi: 10.1097/PSY.0b013e31819cc761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serra-Millas M, Lopez-Vilchez I, Navarro V, et al. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology. 2011;216:1–8. doi: 10.1007/s00213-011-2180-0. [DOI] [PubMed] [Google Scholar]
- 28.Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67(1 Suppl):S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Sevilla JA, Garcia-Vallejo P, Guimon J. Enhanced alpha 2-adrenoceptor-mediated platelet aggregation in patients with major depressive disorder. Eur J Pharmacol. 1983;94:359–360. doi: 10.1016/0014-2999(83)90430-2. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Sevilla JA, Padro D, Giralt MT, Guimon J, Areso P. Alpha 2-adrenoceptor-mediated inhibition of platelet adenylate cyclase and induction of aggregation in major depression. Effect of long-term cyclic antidepressant drug treatment. Arch Gen Psychiatry. 1990;47:125–132. doi: 10.1001/archpsyc.1990.01810140025005. [DOI] [PubMed] [Google Scholar]
- 31.Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry. 2000;57:875–882. doi: 10.1001/archpsyc.57.9.875. [DOI] [PubMed] [Google Scholar]