Summary
Isoniazid (INH), a first-line drug for tuberculosis control, frequently causes liver injury. Multiple previous reports suggest that CYP3A is involved in INH metabolism, bioactivation and hepatotoxicity, although direct evidence is unavailable. In the current study, wild-type and Cyp3a-null mice were used to determine the potential role of Cyp3a in INH metabolism in vivo. Compared to wild-type mice, there were no significant differences in the pharmacokinetic profiles of INH and acetyl-isoniazid in Cyp3a-null mice after an oral administration of 50 mg/kg INH. With the same treatment, distribution of INH and its major metabolites was similar in the liver of wild-type and Cyp3a-null mice. A reactive metabolite of INH was trapped by N-α-acetyl-L-lysine in mouse liver microsomes, but Cyp3a does not contribute to this bioactivation pathway. In addition, no liver injury was observed in wild-type and Cyp3a-null mice treated with 60 or 120 mg/kg INH. In summary, Cyp3a has no effect on systemic pharmacokinetics of INH in mice. Further studies are needed to determine whether and how exactly CYP3A is involved in INH bioactivation and hepatotoxicity.
Keywords: Isoniazid, Metabolism, Cytochrome P450 3A, Hepatotoxicity, Mice
Introduction
Isoniazid (INH) is highly effective for the treatment of tuberculosis. However, INH frequently causes liver injury and even liver failure. 1) Metabolism and bioactivation of INH, with possible involvement of arylamine N-acetyltransferase and CYP2E1, is thought to be critical in INH-induced liver injury. 2–4) The formation of acetyl-isoniazid (AcINH) by arylamine N-acetyltransferase is the predominant metabolic pathway of INH. AcINH can be further metabolized to produce acetylhydrazine (AcHZ) that has been proposed as the cause of INH hepatotoxicity via a CYP2E1-mediated bioactivation pathway. 5) Thus, rapid acetylators are thought to have a higher risk of INH hepatotoxicity than slow acetylators. 6) However, this theory is not supported by the data from clinical practice, and oppositely, slow acetylators seem to have a higher risk of INH hepatotoxicity than rapid acetylators 3, 7).
CYP3A, the most abundant CYP in liver, is responsible for metabolism of more than 50% of clinically used drugs. 8) Recent studies suggest that CYP3A4 may contribute to INH metabolism, bioactivation and hepatotoxicity. In a cell-culture system containing CYP3A4 supersomes and INH, cell viability was dramatically decreased and the toxicity was reversed by a CYP3A inhibitor. 9) Similar results were noted in a cell line with low expression of superoxide dismutase 2 and high expression of CYP3A4, in which INH cytotoxicity was significantly increased. 10) In addition, anti-CYP3A4 antibody was found in tuberculosis patients who had INH-associated hepatotoxicity. 11) However, the exact role of CYP3A in INH metabolism, bioactivation and toxicity remains unknown. The current work was designed to address this question using a Cyp3a-null mouse model.
Methods
Chemicals
INH, hydrazine (HZ), AcHZ, midazolam, 4-nitrocatechol, NADPH, p-nitrophenol, and N-α-acetyl-L-lysine (NAL) were obtained from Sigma-Aldrich (St. Louis, MO). AcINH and 1′-hydroxymidazolam were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada).
Animals
Cyp3a-null mice are lacking all functional Cyp3a genes, 12) which were provided by Taconic Farms, Inc. (Hudson, NY). Male wild-type (WT) and Cyp3a-null mice, 2–4 months old, were maintained under a standard 12-h light/12-h dark cycle with water and chow provided ad libitum. Handling was in accordance with animal study protocols provided by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Cyp2e1 and Cyp3a expression and activity in Cyp3a-null mice
Liver microsomes were prepared and used for profiling CYP450 expression and activity. Cyp2e1 and Cyp3a expression were analyzed by Western-blot. Midazolam and p-nitrophenol were used as probes for Cyp3a and Cyp2e1 activities, respectively. An ultra-performance liquid chromatography coupled to a hybrid quadrupole orthogonal time-of-flight mass spectrometry (UPLC-QTOFMS) was used for metabolite analysis.
INH pharmacokinetics
WT and Cyp3a-null mice were treated with 50 mg/kg INH by oral gavage. Blood samples were collected at 0.083, 0.25, 0.50, 1, 2, 4, and 8 h after administration. Serum was separated for the analysis of INH and AcINH by UPLC-QTOFMS. Pharmacokinetic parameters were calculated by using the non-compartmental analysis module of WinNonlin (Version 5.2, Pharsight, Mountain View, CA).
Distribution of INH and its metabolites in liver
Liver tissues were collected at 30 min after INH treatment. Liver tissues were homogenized in water (100 mg liver in 300 μl H2O) and then 300 μl of methanol was added to 100 μl of homogenate. INH and its metabolites were extracted from the mixture and analyzed by UPLC-QTOFMS.
Metabolism and bioactivation of INH in vitro
Incubations were carried out in 1 × phosphate-buffered saline (pH 7.4), containing mouse liver microsomes (0.2 mg), 50 μM INH and 2 mM NAL in a final volume of 190 μl. The reactions were initiated by adding 10 μl of 20 mM NADPH, continued at 37°C for 40 min, and terminated by adding 200 μl of ice-cold acetonitrile. NAL was used as a trapping reagent for reactive metabolites of INH. 13) Lopinavir (LPV) was used as a positive control for the CYP3A-mediated bioactivation assay. 14)
Analysis of metabolites by UPLC-QTOFMS
INH and its metabolites were analyzed using a previously described method. 15)
CYP3A in INH-induced hepatotoxicity in mice
WT and Cyp3a-null mice were treated with INH (60 or 120 mg/kg, p.o.). At 18 h after INH treatment, all mice were sacrificed and blood samples were collected for evaluation of liver functions. Serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities were determined using assay kits from Pointe Scientific INC (Canton, MI).
Statistical analysis
All values are expressed as the mean ± S.D. Group differences were determined by two-tailed student’s t test.
Results and Discussion
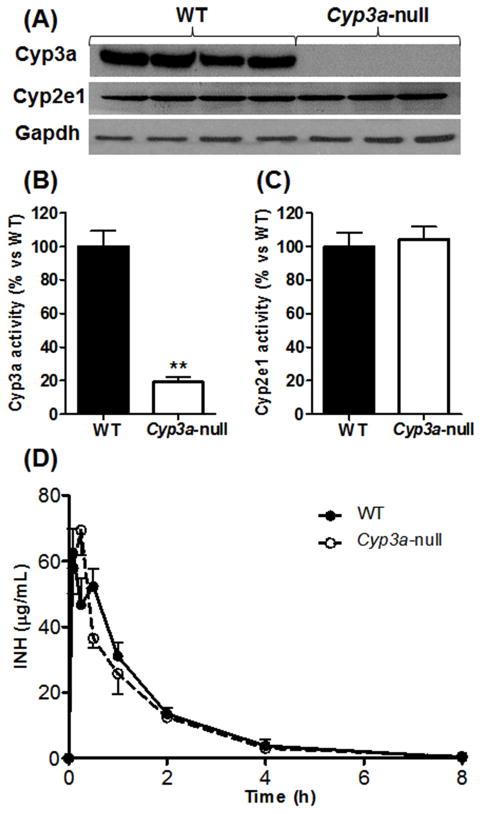
As expected, Cyp3a was not detected in Cyp3a-null mice (Fig. 1A). Because CYP2E1 is thought to be involved in INH metabolism, 2) Cyp2e1 expression in Cyp3a-null mice was also analyzed. There was no difference in Cyp2e1 expression between WT and Cyp3a-null mice (Fig. 1A). In addition, the activities of Cyp3a and Cyp2e1 were determined. Compared to WT mice, catalytic activity of Cyp3a was significantly decreased in Cyp3a-null mice (Fig. 1B). Nevertheless, there was no significant difference in Cyp2e1 activity between WT and Cyp3a-null mice (Fig. 1C). These data suggest that the Cyp3a-null mouse model is an ideal tool to determine the role of Cyp3a in INH metabolism. After oral administration of INH, the absorption and elimination of INH were rapid in both WT and Cyp3a-null mice, and there was no significant difference in the pharmacokinetic parameters between these two genotypes (Fig. 1D, Supplemental Table 1). AcINH is the dominant metabolite of INH and is thought to be related to INH hepatotoxicity. 16) Cyp3a deficiency has no effect on AcINH metabolism either, as revealed by pharmacokinetic data of WT and Cyp3a-null mice (Supplemental Fig. 1, Supplemental Table 1). These results indicate that Cyp3a is not critical in systemic pharmacokinetics of INH.
Figure 1. Cyp3a expression, activity and its effect on INH pharmacokinetics in Cyp3a-null mice.
(A) Western-blot analysis of Cyp3a and Cyp2e1 expression. Gapdh was used as loading control. (B and C) Activity assays of CYP450s. Midazolam and p-nitrophenol were used as probes for CYP3A (B) and CYP2E1 (C), respectively. Data are expressed as means ± S.D., n = 3. The data in WT mice were set as 100%. **P < 0.01 compared with WT mice. (D) Serum concentration-time curve of INH. WT and Cyp3a-null mice were treated with a single oral dose of 50 mg/kg INH. Blood samples were obtained at 0.083, 0.25, 0.5, 1, 2, 4, and 8 h after administration. INH was analyzed by UPLC-QTOFMS. Data are expressed as means ± S.D., n = 4 at each time point.
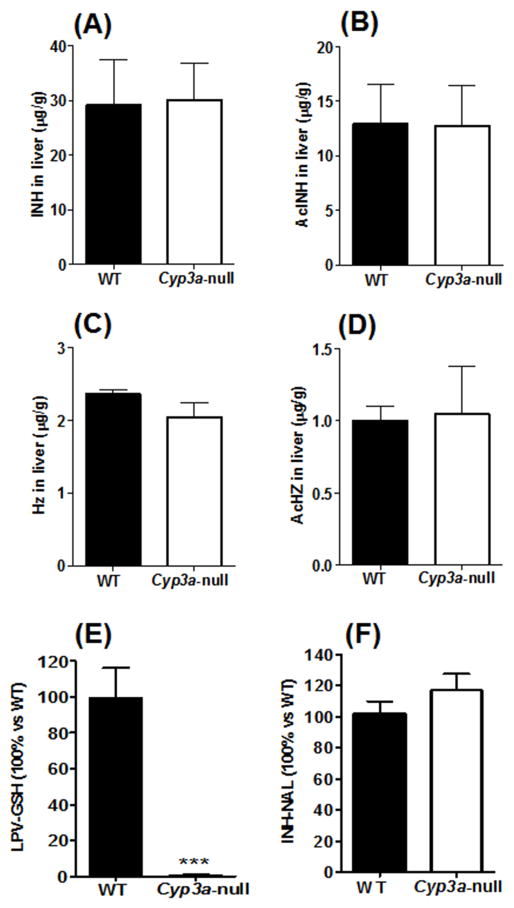
Consistent with the pharmacokinetic data, there were no significant differences in distribution of INH or AcINH in the liver of WT and Cyp3a-null mice after INH treatment (Fig. 2A and 2B). HZ and AcHZ are the further metabolites of INH and they have been proposed to be the causes of INH-induced hepatotoxicity. 17–19) In the current study, deletion of the Cyp3a gene in mice did not change the distribution of AcHZ and HZ in the liver (Fig. 2C and 2D). These results suggest that Cyp3a has no effect on disposition of INH and its metabolites in liver.
Figure 2. Effects of Cyp3a on hepatic distribution and bioactivation of INH.
(A–D) Concentrations of INH and its metabolites in liver. WT and Cyp3a-null mice were treated orally with 50 mg/kg INH and liver tissues were collected at 30 min after administration. INH (A), AcINH (B), HZ (C) and AcHZ (D) were analyzed by UPLC-QTOFMS. Data are expressed as means ± S.D., n = 4. (E–F) INH bioactivation in liver microsomes of WT and Cyp3a-null mice. (E) LPV bioactivation trapped by glutathione (GSH). (F) INH bioactivation trapped by NAL. Data are expressed as means ± S.D., n = 3. The data in WT mice were set as 100%. ***P < 0.001 compared to WT mice.
Products of INH bioactivation can be trapped by NAL. 13) The INH bioactivation pathway was evaluated in liver microsomes of WT and Cyp3a-null mice. CYP3A-mediated bioactivation of LPV, 14) an HIV protease inhibitor, was used as a positive control. Compared to WT mice, bioactivation of LPV was significantly decreased in liver microsomes of Cyp3a-null mice (Fig. 2E). However, no difference of INH bioactivation was found between WT and Cyp3a-null mice (Fig. 2F). In addition, there was no significant difference in the formation of INH N-oxide in liver microsomes of WT and Cyp3a-null mice (data not shown). In the toxicity study, INH treatment had no effect on serum ALT and ALP activities (Supplemental Fig. 2), indicating no liver injury happened.
CYP450-mediated bioactivation of INH and/or its metabolites, via CYP2E1, CYP2C or CYP3A4, has been proposed as a key step in INH-induced liver injury. 2, 4, 13, 20) However, this theory is not supported from recent pharmacogenetic studies, as polymorphisms of CYP2E1, CYP2C9, 2C19, or 3A4 are not associated with anti- tuberculosis drug-induced liver injury. 21–23) Further studies are suggested to determine whether and how exactly CYP450, including CYP3A, is involved in INH bioactivation and hepatotoxicity.
In summary, the current study did not find evidences for the involvement of Cyp3a in INH bioactivation and hepatotoxicity in mice. Cyp3a has no effect on systemic pharmacokinetics of INH in mice.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [DK090305]. We thank Dr. Martha Montello for editing the manuscript.
References
- 1.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 2.Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–930. doi: 10.1053/jhep.2003.50144. [DOI] [PubMed] [Google Scholar]
- 3.Ohno M, Yamaguchi I, Yamamoto I, Fukuda T, Yokota S, Maekura R, Ito M, Yamamoto Y, Ogura T, Maeda K, Komuta K, Igarashi T, Azuma J. Slow N-acetyltransferase 2 genotype affects the incidence of isoniazid and rifampicin-induced hepatotoxicity. Int J Tuberc Lung Dis. 2000;4:256–261. [PubMed] [Google Scholar]
- 4.Timbrell JA, Mitchell JR, Snodgrass WR, Nelson SD. Isoniazid hepatoxicity: the relationship between covalent binding and metabolism in vivo. J Pharmacol Exp Ther. 1980;213:364–369. [PubMed] [Google Scholar]
- 5.Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, Nicod L, Desmeules J, Hochstrasser D. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol. 2006;62:423–429. doi: 10.1007/s00228-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Suou T, Hirayama C. Elevated serum aminotransferase induced by isoniazid in relation to isoniazid acetylator phenotype. Hepatology. 1986;6:295–298. doi: 10.1002/hep.1840060223. [DOI] [PubMed] [Google Scholar]
- 7.Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, Chang FY, Lee SD. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology. 2002;35:883–889. doi: 10.1053/jhep.2002.32102. [DOI] [PubMed] [Google Scholar]
- 8.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Vignati L, Turlizzi E, Monaci S, Grossi P, Kanter R, Monshouwer M. An in vitro approach to detect metabolite toxicity due to CYP3A4-dependent bioactivation of xenobiotics. Toxicology. 2005;216:154–167. doi: 10.1016/j.tox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa Y, Hosomi H, Fukami T, Nakajima M, Yokoi T. Establishment of knockdown of superoxide dismutase 2 and expression of CYP3A4 cell system to evaluate drug-induced cytotoxicity. Toxicol In Vitro. 2009;23:1179–1187. doi: 10.1016/j.tiv.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Metushi IG, Sanders C, Lee WM, Uetrecht J. Detection of anti-isoniazid and anti-cyp antibodies in patients with isoniazid-induced liver failure. Hepatology. 2013 [Google Scholar]
- 12.van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, van der Valk MA, van Tellingen O, van der Hoorn JW, Rosing H, Beijnen JH, Schinkel AH. Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest. 2007;117:3583–3592. doi: 10.1172/JCI33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metushi IG, Nakagawa T, Uetrecht J. Direct oxidation and covalent binding of isoniazid to rodent liver and human hepatic microsomes: humans are more like mice than rats. Chem Res Toxicol. 2012;25:2567–2576. doi: 10.1021/tx300341r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Lu J, Ma X. CYP3A4-mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab Dispos. 2012;40:18–24. doi: 10.1124/dmd.111.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Miao Y, Zhang L, Neuenswander SA, Douglas JT, Ma X. Metabolomic analysis reveals novel isoniazid metabolites and hydrazones in human urine. Drug Metab Pharmacokinet. 2011;26:569–576. doi: 10.2133/dmpk.DMPK-11-RG-055. [DOI] [PubMed] [Google Scholar]
- 16.Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Curr Drug Metab. 2007;8:839–851. doi: 10.2174/138920007782798216. [DOI] [PubMed] [Google Scholar]
- 17.Nelson SD, Mitchell JR, Timbrell JA, Snodgrass WR, Corcoran GB., 3rd Isoniazid and iproniazid: activation of metabolites to toxic intermediates in man and rat. Science. 1976;193:901–903. doi: 10.1126/science.7838. [DOI] [PubMed] [Google Scholar]
- 18.Sarich TC, Youssefi M, Zhou T, Adams SP, Wall RA, Wright JM. Role of hydrazine in the mechanism of isoniazid hepatotoxicity in rabbits. Arch Toxicol. 1996;70:835–840. doi: 10.1007/s002040050347. [DOI] [PubMed] [Google Scholar]
- 19.Lee KK, Fujimoto K, Zhang C, Schwall CT, Alder NN, Pinkert CA, Krueger W, Rasmussen T, Boelsterli UA. Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes. Free Radic Biol Med. 2013;65C:584–594. doi: 10.1016/j.freeradbiomed.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA, Snodgrass WR, Nelson SD. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med. 1976;84:181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- 21.Cho HJ, Koh WJ, Ryu YJ, Ki CS, Nam MH, Kim JW, Lee SY. Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis (Edinb) 2007;87:551–556. doi: 10.1016/j.tube.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Gupta VH, Amarapurkar DN, Singh M, Sasi P, Joshi JM, Baijal R, Ramegowda PH, Amarapurkar AD, Joshi K, Wangikar PP. Association of N-acetyltransferase 2 and cytochrome P450 2E1 gene polymorphisms with antituberculosis drug-induced hepatotoxicity in Western India. J Gastroenterol Hepatol. 2013;28:1368–1374. doi: 10.1111/jgh.12194. [DOI] [PubMed] [Google Scholar]
- 23.Tang SW, Lv XZ, Chen R, Wu SS, Yang ZR, Chen DF, Zhan SY. Lack of association between genetic polymorphisms of CYP3A4, CYP2C9 and CYP2C19 and antituberculosis drug-induced liver injury in a community-based Chinese population. Clin Exp Pharmacol Physiol. 2013;40:326–332. doi: 10.1111/1440-1681.12074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.