Summary
Follistatin-like protein 1 (FSTL-1) is overexpressed in a number of inflammatory conditions characterized by elevated IL-1β. Here we found that FSTL-1 serum concentration was increased three-fold in patients with bacterial sepsis and four-fold following administration of endotoxin (LPS) to mice. To test the contribution of FSTL-1 to IL-1β secretion, wild-type and FSTL-1-deficient mice were injected with LPS. While LPS induced IL-1β in the sera of wild-type mice, it was low or undetectable in FSTL-1-deficient mice. Monocytes/macrophages, a key source of IL-1β, do not normally express FSTL-1. However, FSTL-1 was found in tissue macrophages after injection of LPS into mouse footpads, demonstrating that macrophages are capable of taking up FSTL-1 at sites of inflammation. In vitro, intracellular FSTL-1 localized to the mitochondria. FSTL-1 activated the mitochondrial electron transport chain, increased the production of ATP (a key activator of the NLRP3 inflammasome) and IL-1β secretion. FSTL-1 also enhanced transcription of the NLRP3 and pro-caspase-1 genes, two components of the NLRP3 inflammasome. Adenovirus-mediated overexpression of FSTL-1 in mouse paws led to activation of the inflammasome complex and local secretion of IL-1β and IL-1β-related proinflammatory cytokines. These results suggest that FSTL-1 may act on the NLRP3 inflammasome to promote IL-1β secretion from monocytes/macrophages.
Keywords: Follistatin-like protein 1, inflammasome, monocytes, macrophages, cytokines
Introduction
The cross-talk between hematopoietic and non-hematopoietic cells plays an important role in the coordination of the innate and adaptive immune response to various pathogens. Within the inflammatory microenvironment, a wide range of soluble factors released by non-hematopoietic cells may directly regulate immune cell function. Follistatin-like protein 1 (FSTL-1) is a secreted glycoprotein produced mainly by cells of the mesenchymal lineage such as cardiomyocytes, chondrocytes, fibroblasts, and endotheliocytes [1–6]. It is not produced by cells of the hematopoietic lineage [2]. FSTL-1 was initially cloned from a mouse osteoblast cell line as a TGFβ-inducible gene [7]. Based on sequence homology, FSTL-1 belongs to a family of secreted proteins acidic and rich in cysteine (SPARC/BM-40/osteonectin) containing both follistatin-like and EF-hand calcium-binding domains [8]. However, unlike other family members, the EF hand calcium binding domains of FSTL-1 are non-functional [8]. Moreover, in contrast to follistatin, FSTL-1 does not bind activin [3], suggesting that FSTL-1 exhibits unique properties.
FSTL-1 function is still unclear, but it appears to have two distinct activities. In cells that normally express FSTL-1, it promotes cell survival and metabolism. For example, FSTL-1 inhibits apoptosis in cardiomyocytes [1] and endothelial cells [4], promotes endothelial cell function and blood vessel growth through Akt and endothelial nitric oxide synthase dependent mechanisms [9], and enhances metastatic potential in prostate tumors [10]. FSTL-1 null mice have a perinatal lethal phenotype and die at birth demonstrating hypoplasia of numerous tissues [5, 6].
We propose that FSTL-1 has a second functional role as a pro-inflammatory protein. This hypothesis is based on the observation that FSTL-1 is an important mediator in the pathogenesis of arthritis and other systemic autoimmune diseases. In our previous studies, we discovered that FSTL-1 gene was overexpressed in the synovial tissue in humans with rheumatoid arthritis [11] and in the joints of mice with collagen-induced arthritis (CIA) [12]. We demonstrated that neutralization of endogenous FSTL-1 by specific antibody attenuates CIA [11], while adenovirus-mediated overexpression of FSTL-1 in mouse paws induces synovitis [13]. We also found that increasing serum levels of FSTL-1 correlated positively with the severity of CIA in FSTL-1 hypomorphic mice [14]. FSTL-1 gene expression is induced by proinflammatory cytokines (IL-1β, TNFα) [2] and TLR4 signaling [11]. FSTL-1 increases gene expression of proinflammatory cytokines and chemokines by activated macrophages in vitro [11, 13, 14], further supporting a potential role of FSTL-1 in inflammation.
Our previous studies [13] suggest that FSTL-1 enhances the inflammatory response through up-regulation of IL-1β synthesis by monocytes and macrophages. We have demonstrated marked elevations of FSTL-1 level in the sera of children with diseases characterized by increased IL-1β, including systemic juvenile idiopathic arthritis and Kawasaki disease [2, 15]. IL-1β is a powerful mediator of fever and inflammatory response to infections or sterile insults [16]. IL-1β is synthesized by immune cells, mainly monocytes and macrophages, as an inactive precursor form (p35) [17]. The precursor (pro-IL-1β) molecule must be cleaved into active IL-1β (p17), which is then secreted from the cell. Cleavage of pro-IL-1β is triggered by the enzyme caspase-1 (also known as IL-1-converting enzyme) and therefore the biological activity of IL-1β is directly dependent on the activity of caspase-1. The activation of caspase-1, in turn, is mediated by the inflammasome, which functions in various cells of immune system [18]. Several different inflammasome complexes have been identified, of which the NLRP3 (nucleotide-binding domain leucine-rich repeat containing (NLR) family, pyrin domain containing 3) inflammasome has been the most intensively studied because of its central role in inflammatory diseases [19]. The active NLRP3 inflammasome is a multiprotein oligomer consisting of NLRP3, the adaptor protein ASC (apoptosis-associated speck-like protein), and pro-caspase-1, which allows autoproteolytic processing to generate mature (active) caspase-1 [20].
The current study was designed to identify mechanisms by which FSTL-1 promotes IL-1β secretion from monocytes/macrophages and to determine the relationship between FSTL-1 and the NLRP3 inflammasome.
Results
FSTL-1 is induced in vivo in response to endotoxin and regulates IL-1β secretion
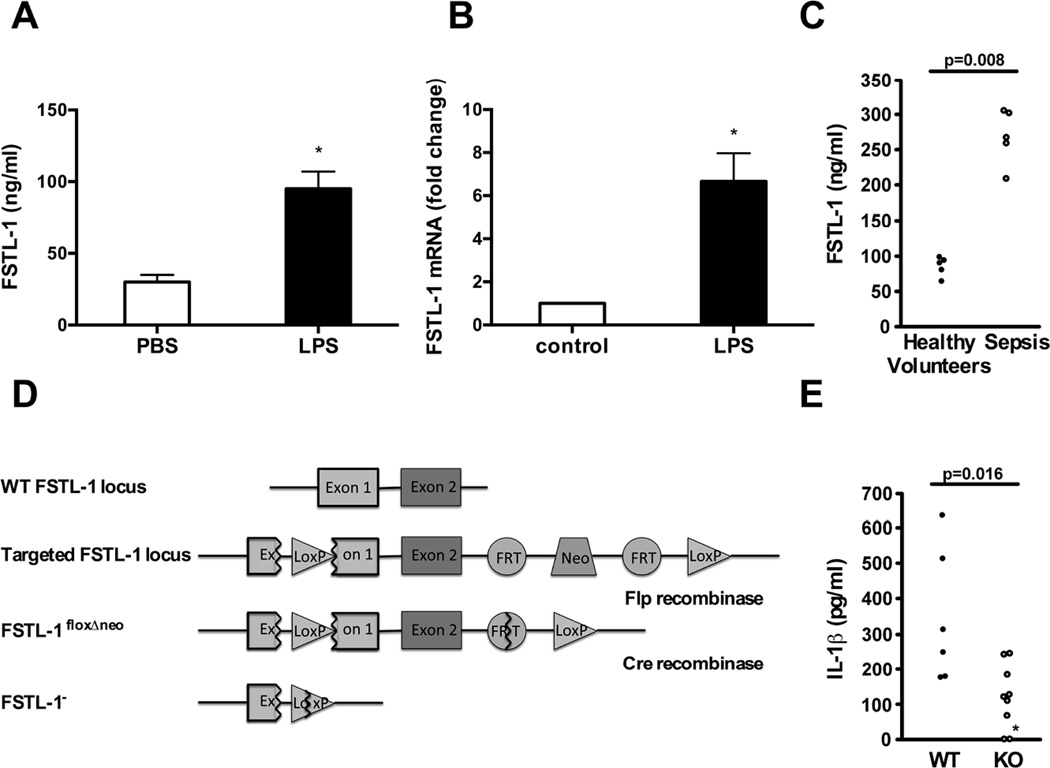
Intraperitoneal administration of LPS (endotoxin) increased FSTL-1 serum concentration (Fig. 1A) and injection of LPS in the rear footpads induced local FSTL-1 gene expression in mice (Fig. 1B). Since endotoxin is a mediator of bacterially-induced septic shock, we measured FSTL-1 in the sera of patients with bacterial sepsis and observed a three-fold increase (p=0.008) compared to healthy controls (Fig. 1C). This suggests that FSTL-1 might mediate pro-inflammatory events in sepsis. IL-1β is a particularly powerful mediator of fever and inflammation and it is induced by endotoxin [16]. To test the contribution of FSTL-1 to IL-1β secretion in vivo, wild-type and FSTL-1-deficient mice (Fig. 1D) were injected with LPS. While LPS induced IL-1β in the sera of wild-type mice, IL-1β was low or undetectable in FSTL-1-deficient mice (Fig. 1E).
Figure 1. FSTL-1 is involved in the modulation of the inflammatory response in vivo.
(A) DBA/1 mice were injected i.p. with 50 µg of LPS and serum was analyzed by FSTL-1 ELISA. The results are expressed as the mean+SEM, n=4 mice/group from one experiment representative of three performed.*p < 0.05 versus PBS, Mann-Whitney test. (B) DBA/1 mice were injected in the hind footpads with 2.5 µg of LPS and euthanized on day 3 after treatment. RNA from paw tissue was assayed by QRT-PCR for FSTL-1. The graph represents a fold change in mRNA level compared with control. The results are expressed as the mean+SEM, n=4 mice/group from one experiment representative of three performed.*p < 0.01 versus control, Mann-Whitney test. (C) Serum samples from healthy volunteers and patients with sepsis were assayed for FSTL-1 by ELISA. Each circle represents an individual subject. Statistical significance determined by Mann-Whitney test. (D) Schematic representation of the gene knockout strategy. (E) Wild type (WT) and FSTL-1 null (KO) mice were injected i.p. with 200 µg of LPS for 3 h and sera were analyzed by ELISA. Each circle represents an individual mouse. The data shown are from one representative experiment of two performed. *IL-1β level was below the 150 pg/mL detection limit. IL-1β in the sera of unstimulated mice was below the level of detection (data not shown). Statistical significance determined by Mann-Whitney test.
FSTL-1 uptake by monocytes/macrophages occurs in vitro and in vivo
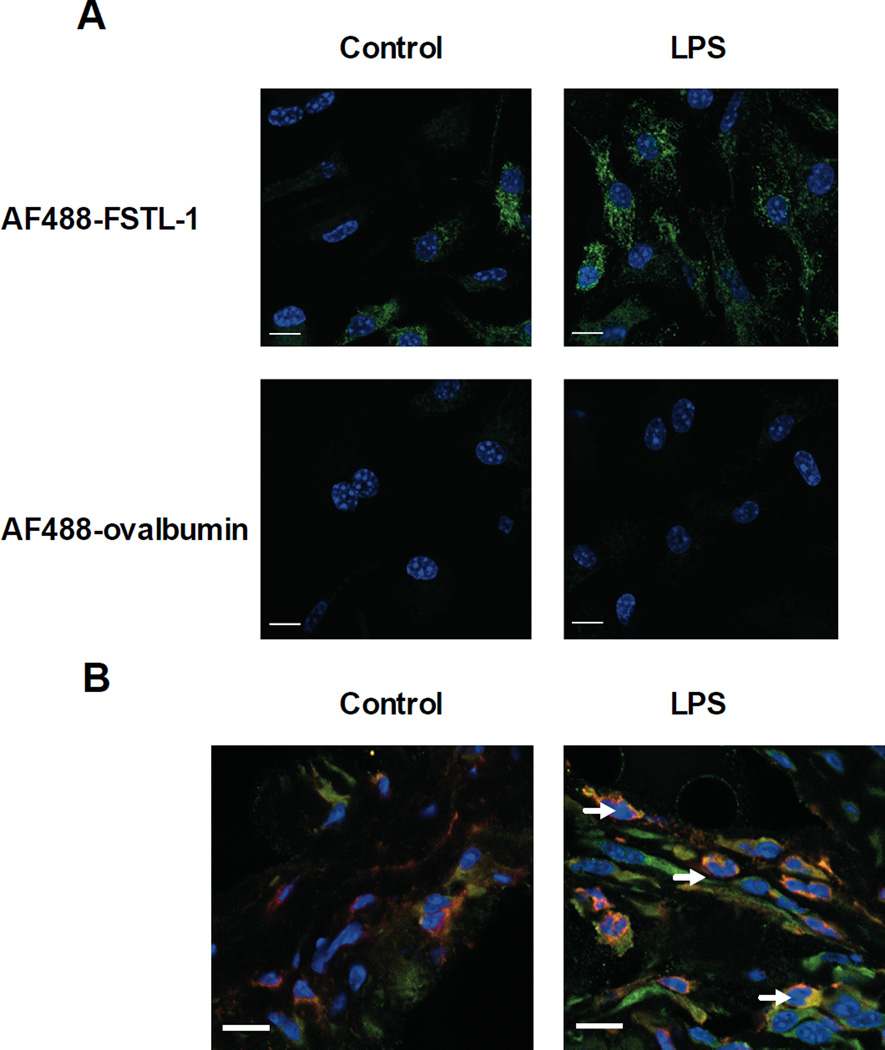
Monocytes are a key source for IL-1β secretion in sepsis [16]. Since FSTL-1 is not expressed in monocytes, we reasoned that these cells must be responding to FSTL-1 secreted by mesenchymal cells. To determine whether FSTL-1 was able to bind to monocytes/macrophages, BMDM (bone marrow-derived macrophages) were incubated with AlexaFluor (AF) 488-labeled FSTL-1 or ovalbumin (control protein) and subjected to confocal microscopy. A weak fluorescent signal was observed in resting BMDM (Fig. 2A). Virtually all cells stimulated with LPS stained with AF488-FSTL-1. No untreated or LPS-treated cells stained with the control protein AF488-ovalbumin (Fig. 2A). To evaluate whether FSTL-1 gains entry into monocytes/macrophages at the site of inflammation, we examined synovial tissues from paws of mice before and after injection of LPS. Macrophages in the synovial lining of untreated mice did not stain positively for FSTL-1. However, after local injection of LPS intracellular FSTL-1 was found in CD11b-positive cells in the synovial tissue (Fig. 2B), demonstrating that macrophages are capable of taking up FSTL-1 at the site of inflammation in vivo. Our finding that monocytes/macrophages specifically bind and take up FSTL-1 indicates that these cells may serve as the target cells for the stimulatory effect of FSTL-1 on IL-1β production.
Figure 2. Immunolocalization of FSTL-1 in BMDM and mouse paws.
(A) Mouse BMDM were incubated with AF488-FSTL-1 or AF488-ovalbumin (green) for 7 h with or without LPS. Nuclei (blue) were co-stained with Hoechst. (B) Mice were injected in the hind footpads with 2.5 µg of LPS and euthanized on day 3 after treatment. Paws from control and LPS-injected mice were cryosectioned (4 µm) for immunohistochemistry. Anti-FSTL-1 (green); anti-CD11b (red); dual-labeled cells (yellow) are indicated by arrows. Nuclei are visualized by Hoechst fluorescence (blue). (A, B) Slides were imaged using a 63× oil-immersion objective. Scale bar 10 µm. The data shown are from one representative experiment of three performed.
FSTL-1 promotes IL-1β secretion from activated monocytes and macrophages
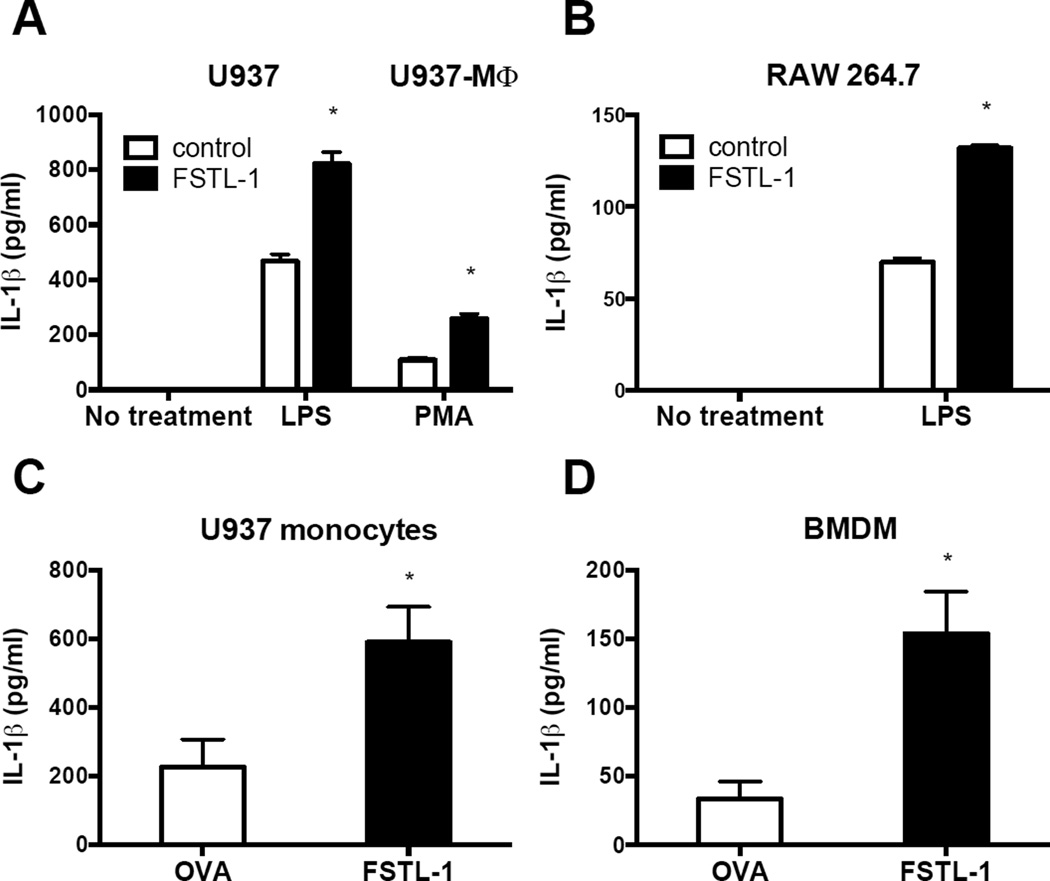
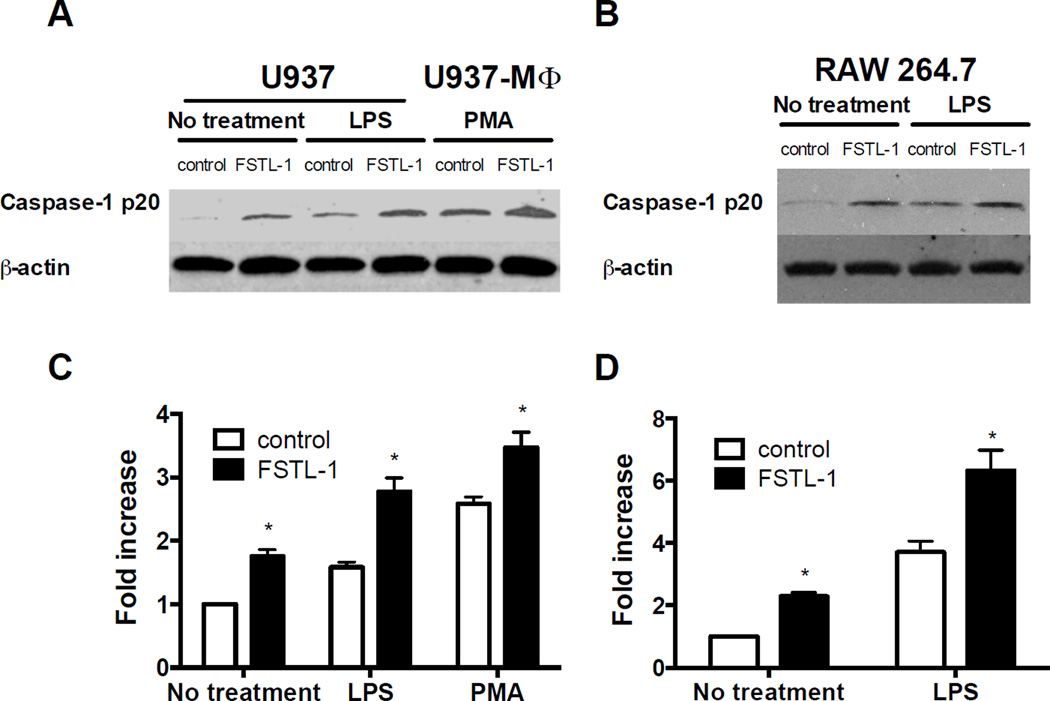
Addition of soluble recombinant FSTL-1 did not result in increased IL-1β secretion from monocyte/macrophages (data not shown). We therefore stably transfected the human monocyte cell line U937 and the mouse macrophage cell line RAW264.7 with a plasmid encoding human and mouse FSTL-1, respectively. To induce differentiation of U937 monocytes into macrophages (U937 macrophages), the cells were stimulated with PMA. Cells were incubated in the absence or presence of LPS, and cell culture supernatants were assayed for secreted (mature) IL-1β. As shown in Fig. 3A and B, no detectable IL-1β was produced by cells without stimulation. Upon LPS or PMA stimulation, we observed a substantial induction of IL-1β secretion from U937 monocytes, U937 macrophages and mouse macrophages (Fig. 3A and B). A significant increase in secretion of IL-1β was observed in FSTL-1-transfected cells (Fig. 3A and B). We observed a similar increase in IL-1β production by activated U937 cells and BMDM when FSTL-1 was delivered into cells using a protein transfection reagent (Fig. 3C and D). These results indicate that FSTL-1 can promote IL-1β secretion from monocytes and macrophages under priming conditions.
Figure 3. FSTL-1 promotes IL-1β secretion from monocytes and macrophages.
(A) Human U937 cells stably transfected with plasmid encoding FSTL-1 or a control plasmid were treated with LPS (200 ng/mL) or differentiated into U937-MΦ with PMA (0.5 mM). (B) FSTL-1-transfected mouse macrophage RAW264.7 cells were stimulated with or without 1 µg/mL LPS for 24 h. (C) U937 monocytes were transfected with FSTL-1 protein, or ovalbumin. Cells were stimulated with PMA (10 ng/mL) and LPS (200 ng/mL). (D) BMDM transfected with FSTL-1 protein (or ovalbumin) were stimulated with LPS (1 ng/mL) for 6 h. ATP (0.5 mM) was added for the final 30 min. Supernatants were assayed for human (A, C) or mouse (B, D) IL-1β by ELISA. (A, B, C, D) The results are expressed as the mean+SEM of triplicate samples from one experiment representative of three performed. *P <0.05 versus the controls, two-tailed Student’s test.
FSTL-1 localizes to the mitochondria of macrophages and affects mitochondrial function
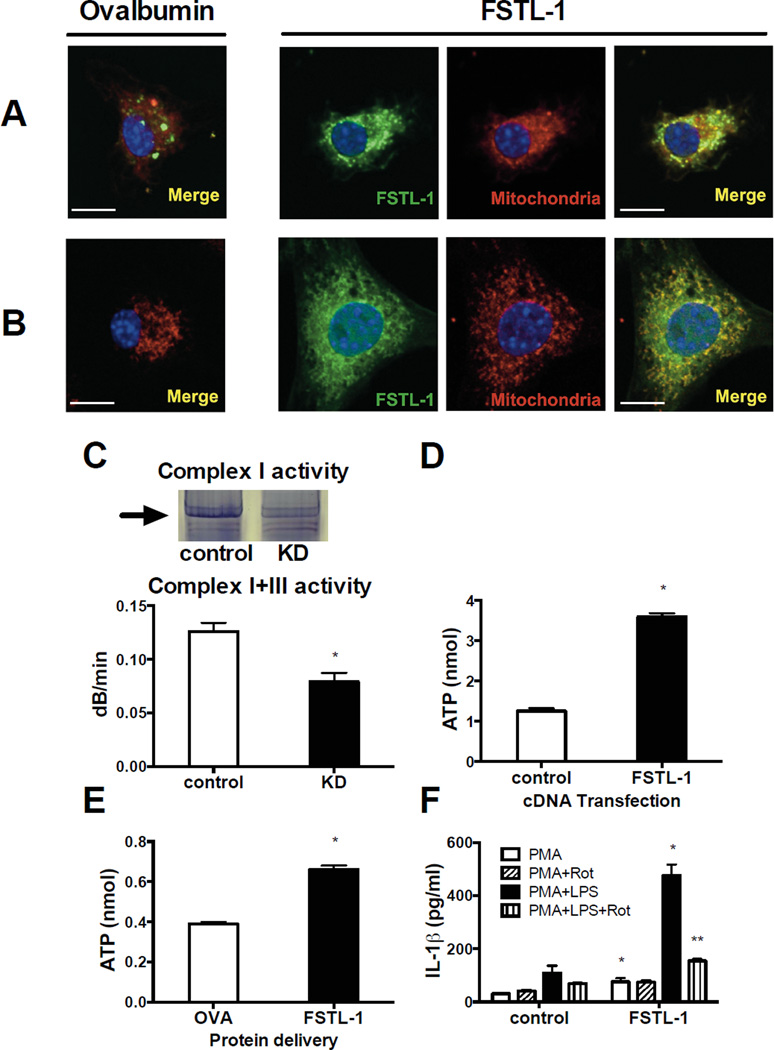
We next investigated the subcellular localization of FSTL-1 following delivery of AF488-labelled FSTL-1 directly into BMDM using the cationic lipid-based reagent Pro-Ject. A strong signal was observed that co-localized to mitochondria (overlap coefficient 0.88) (Fig. 4A). A similar co-localization of FSTL-1 with mitochondria was evident (overlap coefficient 0.82) in cells permeabilized with Triton X100 and stained with AF488-FSTL-1 (Fig. 4B). These results imply that under certain conditions FSTL-1 is capable of binding mitochondria.
Figure 4. FSTL-1 localizes to the mitochondria, increases mitochondrial respiratory complex activity and ATP synthesis and induces IL-1β secretion.
(A) Mouse BMDM were transfected with AF488-FSTL-1 or AF488-ovalbumin (green) and incubated for 7 h. (B) Cells were fixed with PFA, permeabilized with Triton X100 and stained with AF488-labeled proteins. (A, B) Slides were imaged using a 63× oil-immersion objective. Mitochondria (red) and nuclei (blue) were co-stained with Mitotracker Red and Hoechst 33342, respectively. Co-localization of FSTL-1 and mitochondria are shown by the yellow color (merged). Scale bar 10 µm. The data shown are from one representative experiment of three performed. (C) Complex I and III enzymatic activity is decreased in mitochondrial extracts from ST2 knockdown (KD) cells: in-gel assay (upper panel), spectrophotometric assay (lower panel). (D) ATP level in intact U937 cells transfected with plasmid encoding human FSTL-1, or a control plasmid. (E) ATP level in U937 cells transfected with FSTL-1 protein. Ovalbumin was used as a control protein. (F) PMA-differentiated U937 transfectants were pre-treated with rotenone (Rot) for 2 h and incubated for an additional 24 h with or without LPS. Supernatants were assayed for IL-1β by ELISA. (C, D, E, F) The results are expressed as the mean+SEM of triplicate samples from one experiment representative of three performed. *P<0.05 versus the controls, **P<0.05 versus cells without rotenone treatment, two-tailed Student’s test.
The mitochondrial targeting suggested that FSTL-1 plays a role in cellular energy production. To test this, we assayed enzymatic activity of complex I and III of the mitochondrial electron transport chain (ETC) in ST2 stromal cells in which FSTL-1 expression was suppressed using lentiviral shRNA. Inhibition of FSTL-1 expression led to a significant reduction in the activity of complex I and III (Fig. 4C). Moreover, transfection of U937 cells, which do not normally express FSTL-1, with FSTL-1 cDNA increased ATP production (Fig. 4D). Similarly, intracellular delivery of the FSTL-1 protein induced ATP production (Fig. 4E). Exocytosis of ATP, followed by activation of P2 receptors, is one of the major pathways of macrophage activation, leading to release of cytokines [21]. To determine whether reduction in ATP production may affect FSTL-1-induced IL-1β secretion, U937 cells expressing FSTL-1 were incubated with rotenone, an inhibitor of the ETC complex I. Rotenone blocked the increase in IL-1β secretion upon stimulation with PMA and LPS (Fig. 4F). We also found that pre-treatment of U937 macrophages expressing FSTL-1 with the @P2X7 inhibitor oxidized ATP suppressed IL-1β release in response to LPS (Supporting information Fig. 1).
FSTL-1 activates caspase-1 and increases expression of NLRP3 inflammasome components in monocytes and macrophages
The capacity of FSTL-1 to affect mitochondrial function and increase ATP production may be an important factor in activation of caspase-1 by the NLRP3 inflammasome. We measured the cleaved caspase-1 (p20) protein level and the caspase-1 activity to test if FSTL-1 can affect caspase-1 activation. As shown in Fig. 5A and B, transfection with FSTL-1 led to an increase in cleaved (active) caspase-1 in U937 and RAW264.7 cells. Addition of LPS or PMA induced activation of caspase-1 in control cells and cells transfected with FSTL-1. The levels of cleaved caspase-1 in FSTL-1-transfected U937 and RAW264.7 were enhanced compared to levels in controls (Fig. 5A and B). In addition, the activity of caspase-1 in U937 and RAW264.7 cells transfected with FSTL-1 was higher than in control cells under all conditions (Fig. 5C and D). These results indicate that FSTL-1 can activate caspase-1 in human monocytes/macrophages and mouse macrophages with or without priming.
Figure 5. FSTL-1 promotes caspase-1 activation in monocytes and macrophages.
Human U937 cells stably transfected with plasmid encoding FSTL1 or a control plasmid were treated with LPS (200 ng/mL) or differentiated into U937-MΦ with PMA (0.5 mM). FSTL1-transfected mouse macrophage RAW264.7 cells were stimulated with or without 1 µg/mL LPS. Cells were collected at 6 h and processed for Western blot analysis of caspase-1 (A, B) or caspase-1 activity assay (C, D). (A, B) The data shown are from one representative experiment of three performed. (C, D) The results are expressed as the mean + SD of triplicate samples from one experiment representative of three performed, *P<0.05 versus control cells, two-tailed Student’s test.
As the NLRP3 inflammasome is a major regulator of caspase-1 activation and IL-1β processing, we hypothesized that FSTL-1 can regulate NLRP3 inflammasome expression. First, gene expression of the NLRP3 inflammasome components, NLRP3, pro-caspase-1 and ASC was examined in resting or activated monocytes and macrophages. FSTL-1 increased the expression of caspase-1 and NLRP3 mRNA in U937 and RAW264.7 cells stimulated with or without LPS or PMA, while the levels of ASC message remained unchanged (Fig. 6A and B). We next examined the expression of NLRP3 and pro-caspase-1 at the protein level. NLRP3 and pro-caspase-1 levels were higher in FSTL-1-transfected cells than in control cells (Fig. 6C and D). Thus, in addition to driving the production of ATP, a known activator of the NLRP3 inflammasome, FSTL-1 may also promote inflammasome activation by increasing the availability of NLRP3 and pro-caspase-1 in monocytes and macrophages.
Figure 6. FSTL-1 increases the NLRP3 inflammasome expression in monocytes and macrophages.
Cells were stimulated as described in the legend to Figure 5, collected at 6 h and processed for real-time PCR (A, B) or Western blot analysis (C, D) of NLRP3 inflammasome components. (A, B) The results are expressed as the mean + SEM of triplicate samples from one experiment representative of three performed, *P<0.05 versus control cells, two-tailed Student’s test. (C, D) The data shown are from one representative experiment of three performed.
FSTL-1 regulates NLRP3 inflammasome activity in vivo
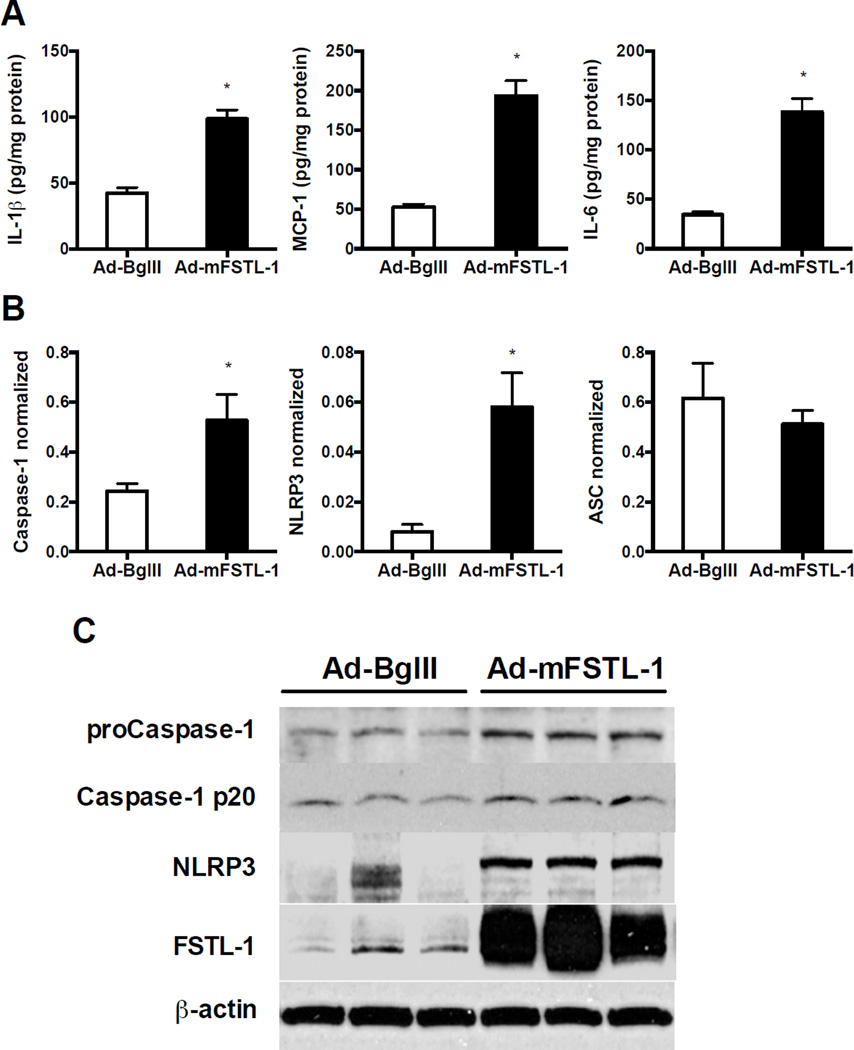
The above results demonstrate that FSTL-1 increases NLRP3 inflammasome activity and IL-1β secretion in vitro. In order to determine whether the same is true in vivo, an adenovirus encoding either mouse FSTL-1 (Ad-mFSTL-1) or a backbone virus lacking a transgene (Ad-BglII) was injected intradermally into paws of unimmunized DBA/1 mice. We have previously reported that injection of Ad-mFSTL-1 into mouse paws resulted in severe inflammation (paw swelling, erythema, mononuclear infiltration of the synovium and surrounding tissue) [13]. Protein homogenates from the paws injected with Ad-mFSTL-1 showed a significant increase not only in IL-1β protein, but also in MCP-1 and IL-6, as compared to that in control paws (Fig. 7A). To determine if FSTL-1 can regulate NLRP3 inflammasome activity in vivo, we examined the expression of the NLRP3 inflammasome components at the mRNA and protein level in the mouse paws injected with Ad-mFSTL-1 or Ad-BglII. Ad-mFSTL-1 significantly increased caspase-1 and NLRP3 gene expression in the mouse paws (Fig. 7B). No significant difference was observed in ASC gene expression (Fig. 7B). As shown in Fig. 7C, injection of Ad-mFSTL-1 resulted in overexpression of FSTL-1 protein within the paw tissue. The protein levels of pro-caspase-1 and NLRP3 were elevated in mouse paws injected with Ad-mFSTL-1 compared to control paws (Fig. 7C). Taken together, these data demonstrate that FSTL-1 enhances activation of the inflammasome complex and secretion of IL-1β and IL-1β-related proinflammatory cytokines in vivo.
Figure 7. FSTL-1 enhances secretion of IL-1β, MCP-1, IL-6 and increases expression of NLRP3 inflammasome components in mouse paws.
Paws of healthy male DBA/1 mice were injected with Ad-mFSTL-1 or with a control virus, Ad-BglII. Mice were sacrificed on day 8. (A) Paw homogenates were assayed for IL-1β, MCP-1 and IL-6 by corresponding ELISA. (B) Total RNA was isolated from paws and gene expression was analyzed by real time PCR. Expression levels of a particular gene were normalized to 18S rRNA. (A, B) The results are expressed as the mean+SEM, n=4 mice/group from one experiment representative of two performed.*p < 0.05 versus control, Mann-Whitney test. (C) Total protein was assayed for pro-caspase-1, cleaved caspase-1 (p20), NLRP3 and FSTL-1 by Western blotting. The data shown are from one representative experiment of two performed.
Discussion
In the present study, we used FSTL-1-deficient mice to investigate the role of FSTL-1 in the regulation of IL-1β production. Our results provide the first in vivo demonstration of the importance of FSTL-1 in inducing circulating IL-1β following administration of LPS. We also show that FSTL-1 can enhance IL-1β release from activated monocytes and macrophages in vitro through regulation of ATP production and activity of the NLRP3 inflammasome.
Cells of the mesenchymal lineage are capable of FSTL-1 production, and FSTL-1 expression can be induced by IL-1β [2]. It is possible that during the early stage of inflammation, IL-1β, activated in response to any injurious challenge, up-regulates FSTL-1 expression, which may, in turn, result in an increase in IL-1β release from monocytes/macrophages, forming a positive feedback loop. Since IL-1β is one of the key control points for the development and severity of inflammation, FSTL-1 may contribute to IL-1β mediated inflammatory responses.
We also found that monocytes/macrophages capable of making IL-1β take up FSTL-1 in vivo after challenge with LPS. Internalization of FSTL-1 may be receptor-mediated. Recently, disco-interacting protein 2 homolog A (DIP2A) has been shown to be one potential receptor for FSTL-1 [22, 23]. FSTL-1 can also bind to CD14, TLR4 and to proteins of the TGFβ superfamily, such as activin, TGFβ, bone morphogenetic protein 2/4, their receptors and follistatin [5, 23, 24]. In addition, high affinity binding between FSTL-1 and the α1 subunit of Na+, K+-ATPase has been shown [25]. However, we failed to observe binding of FSTL-1 to HEK293 cells overexpressing DIP2A (data not shown) and TLR4-deficient macrophages retain their full capacity to take up FSTL-1 (Supporting information Fig. 2). The inflammatory environment in vivo appears critical in regulating the uptake of FSTL-1, and it remains to be demonstrated if any other putative receptors are responsible for FSTL-1 uptake in this type of environment in vivo.
Under normal physiological conditions, FSTL-1 acts as a secreted protein. In the current study, we have discovered that after direct intracellular delivery or following permeabilization FSTL-1 localizes to the mitochondria of macrophages. Similarly, several other secreted factors such as CCN proteins, epidermal growth factor, fibroblast growth factor, and protein hormones appear to have dual roles in cellular regulation, acting at the cell surface by engaging membrane receptors and also acting intracellularly by directly regulating gene transcription [26]. Internalization of cell surface epidermal growth factor receptor and routing to mitochondria (where it can affect mitochondiral activity) has also been demonstrated [27], indicating the existence of potential pathways allowing FSTL-1 access to this intracellular compartment. Mitochondrial localization of FSTL-1 suggests that FSTL-1 may act to modulate cellular bioenergetics. Indeed, knockdown of FSTL-1 expression in mesenchymal cells leads to dysregulation of the electron transport chain, while overexpression of FSTL-1 in monocytes results in an increase in total cellular ATP pool. The known effect of FSTL-1 on enhancing cell growth, survival and metabolic activity in mesenchymal cells (in which it is normally expressed) is consistent with our observation that FSTL-1 can increase mitochondrial ATP production. Extracellular ATP can trigger NLRP3 inflammasome activation [28]. Release of ATP has been observed from multiple cell types during hypoxia, ischemia/reperfusion, inflammation, and cell death [29, 30]. It has been reported that endogenous ATP from monocytes can activate the NLRP3 inflammasome and induce IL-1β secretion [31]. Thus, FSTL-1 may regulate NLRP3 inflammasome activation in monocytes undergoing stress conditions through enhanced endogenous ATP production. Inflammasome activation is triggered by ROS derived from mitochondria [32]. Treatment of macrophages with ATP results in rapid production of ROS and an increase of inflammasome activity [33]. Oxidized mitochondrial DNA released into the cytosol is responsible for NLRP3 inflammasome activation [34]. In cells stimulated with inflammasome activators, NLRP3 and ASC relocalize to the mitochondria and mitochondria-associated endoplasmic reticulum membranes [32]. Based on these observations, FSTL-1 may have the opportunity to directly interact with NLRP3 and caspase-1 and promote assembly of inflammasome components.
In addition to potentially modulating the activity of the inflammasome, we found that FSTL-1 activated caspase-1 expression at both mRNA and protein level. It has been reported that caspase-1 gene expression is up-regulated in response to various cytokines, such as IFNγ or TNFα [35, 36]. Here, we show for the first time that FSTL-1 activates the gene expression of caspase-1 in monocytes/macrophages. The mechanism of activation of caspase-1 gene expression by FSTL-1 is currently under investigation.
The inflammasome is a large, multiprotein complex, which is composed of oligomers of a specific NLR, procaspase-1, and ASC. Although more than 20 NLRs have been identified in humans, only NLRP1, NLRP3, and NLRC4 have been demonstrated to activate caspase-1 [19]. Our data demonstrate that NLRP3 gene and protein expression is induced by FSTL-1 under basal conditions and upon priming. These results are consistent with reports indicating that the NLRP3 gene can be regulated by a wide variety of stimuli, including molecules derived from infectious agents as well as various host-derived stress-associated molecules [28, 37]. The expression of NLRP3 has been recently identified as a critical checkpoint for the NLRP3 inflammasome activation [20]. In monocytes and macrophages, NLRP3 gene expression is regulated mainly through the NF-κB pathway [38], and the level of NLRP3 strongly correlates with the amount of IL-1β processing [39]. Yet, the mechanism by which FSTL-1 activates NLRP3 expression remains unclear.
Since the expression of both NLRP3 and pro-caspase 1 appear to be regulated by FSTL-1, and since FSTL-1 also has the ability to regulate the production of ATP, FSTL-1 is in a position to regulate both of the major signals responsible for IL-1β production. Thus, in the absence of appropriate regulation, FSTL-1 might be capable of promoting inappropriate/pathological activation of the inflammasome, which could lead to a hyperactive inflammasome state [40]. This hypothesis is supported by our recent observation that serum FSTL-1 is elevated in patients with the most severe form of the cryopyrin-associated periodic syndromes, neonatal onset multisystem inflammatory disease [41]. FSTL-1 levels remained elevated despite treatment with anakinra (anti-IL-1 therapy), suggesting that FSTL-1 is not just a marker of the disease.
Recently, a critical role for the NLRP3 inflammasome in the pathogenesis of arthritis has been reported [42]. We have previously shown that adenovirus-mediated overexpression of FSTL-1 in mouse paws led to severe inflammation characterized by infiltration of neutrophils and mononuclear cells as well as cartilage destruction [13]. In the present study, in addition to our data obtained in vitro, we showed that overexpression of FSTL-1 in mouse paws by gene transfer resulted in higher expression of most of the NLRP3 inflammasome proteins, activation of caspase-1 and increased production of IL-1β and IL-1β-related cytokines. Although we cannot rule out the possibility that this difference may be due to the inflammatory milieu that regulates the expression of these inflammatory mediators, the results of current work, demonstrating direct effects of FSTL-1 on the expression of the inflammasome in monocytes, highlight a possible mechanism for arthritogenic action of FSTL-1.
The present study suggests that FSTL-1 might play a critical role in IL-1β production and inflammasome activation in vivo. This conclusion is based on two key observations: (1) FSTL-1 knockout mice produce less IL-1β in response to injection of LPS; (2) injection of Ad-mFSTL-1 into mouse paws induces inflammasome proteins and IL-1β. We have not yet been able to identify the in vivo mechanism responsible for FSTL-1 cell entry. Incubation of BMDM with FSTL-1 secreting cells or simple addition of soluble recombinant FSTL-1 to monocyte/macrophage cultures did not lead to FSTL-1-mediated increase in IL-1β secretion by monocyte/macrophages (data not shown), suggesting that some key factor(s) is/are lacking in our in vitro cell culture conditions. However, we were able to cause FSTL-1 to act in vitro in a similar manner to what we observed in vivo both by transfecting FSTL-1 cDNA and by protein transfection. While both of these two transfection methods are not physiologic, our results suggest the likelihood that conditions also exist in the in vivo inflammatory environment that allow FSTL-1 to gain entry to the intracellular compartment. The mechanism by which this might occur remains to be elucidated and is currently under investigation.
In summary, our findings suggest a mechanism for the proinflammatory effects of FSTL-1 through regulation of the NLRP3 inflammasome activity. We propose that FSTL-1 participates in the response to infectious organisms or various other noxious stimuli by gaining access to the intracellular space of cells that do not normally express FSTL-1, such as monocytes/macrophages, through a yet unidentified mechanism. FSTL-1 then traffics to the mitochondria, where it may enhance ATP production. FSTL-1 also increases NLRP3 and procaspase-1 expression. Together, these promote IL-1β release from activated monocytes and macrophages. These findings may provide a basis for targeting FSTL-1 as a therapeutic approach for various inflammatory conditions.
Materials and Methods
Patient samples
Sera were obtained from critically ill patients with new onset (<48 hours) sepsis defined by international consensus criteria [43]. All patients (mean age: 57 years; 3 females, 2 males; all Caucasians) were admitted to intensive care and samples were obtained after informed consent was obtained (either from the patient or their surrogate). Control samples were obtained from 5 healthy volunteers. Collection and use of samples was approved by the Institutional Review Board at the University of Pittsburgh. Human FSTL-1 ELISA was performed as described [14].
Generation of FSTL-1-deficient Mice
The study was approved by the Children’s Hospital of Pittsburgh’s Animal Research and Care Committee. The targeting vector and the FSTL-1WT/flox mice were generated by Ozgene. The construct contained two loxP sequences inserted in exon 1 and intron 2 of the FSTL-1 gene, and two frt sites flanking the neomycin resistance selection cassette (Fig. 1D). The construct was electroporated into W9.5 embryonic stem cells. Transfected cells were then microinjected into C57BL/6 blastocysts to generate chimeras that were used for breeding with C57BL/6 mice. To remove the Neo-cassette (Δneo), the FSTL-1WT/flox mice were bred with homozygous FlpE-“deletor” C57BL/6 mice (Ozgene). To generate tamoxifen-inducible conditionally FSTL-1 deficient (FSTL-1−/−) mice (global disruption of the FSTL-1 gene), the FSTL-1WT/floxΔneo mice were bred with heterozygous Cre-“deletor” B6.Cg-Tg(CAG-cre/ESR1*)5Amc/J (The Jackson Laboratory). Offspring was genotyped before weaning by tail DNA PCR to confirm the presence of the Cre recombinase allele. To induce Cre activity, the mice were injected i.p. with tamoxifen (Sigma) in corn oil (1 mg/300 µL/mouse). The absence of FSTL-1 in serum two weeks after treatment was confirmed by mouse FSTL-1 ELISA [14].
LPS administration
DBA/1 wild-type mice were injected i.p. with 50 µg of LPS, after 7 h serum was analyzed by FSTL-1 ELISA. In some mice, 2.5 µg of LPS were injected in the hind footpads of DBA/1 mice. On day 3, animals were euthanized, the hind paws were collected and used for further analysis.
FSTL-1 knockout and control (no tamoxifen) mice were injected i.p. with 200 µg of LPS, after 3 h serum was harvested and assayed for IL-1β by ELISA.
Adenoviral vectors and overexpression of human FSTL-1
A recombinant, E1a-E3-deleted replication defective adenovirus type 5 vector encoding the human FSTL-1 gene (Ad-hFSTL-1) and the control vector lacking an insert (Ad-BglII) were generated, grown in 293 cells and purified as described [44]. To overexpress FSTL-1, A549 cells were infected with Ad-hFSTL-1 adenovirus (1000 viral particles/cell). The cells were cultured in serum-free medium for 4 days; the supernatant was collected and used for further FSTL-1 purification.
Induction and assessment of spontaneous synovitis
DBA/1 mice were treated by i.d. injection with 108 particles of adenoviral vectors in 50 µL of PBS. Evaluation of paw swelling and histologic analysis was carried out as previously described [13].
Purification of human FSTL-1 from cell culture supernatants
Human FSTL-1 purification was performed on a Q anion-exchange column (HiTrap Q XL 5mL, GE Healthcare) using AKTA prime FPLC system (GE Healthcare). The supernatant was concentrated by ultrafiltration using an Amicon Ultra 10K centrifugal filter device (Millipore). Equal volume of 20mM Tris buffer pH8.0 (buffer A) was added to the supernatant followed by filtration through a 0.22 µm syringe filter. The supernatant was loaded on the Q column and the column was washed sequentially with buffer A, buffer B (20mM Tris pH8.0, 150mM NaCl), buffer C (20mM MES pH6.0), buffer D (20mM MES pH6.0, 150mM NaCl), buffer E (20mM Citrate buffer pH6.0) until the baseline stabilized. A linear gradient from buffer E to buffer F (20mM Citrate buffer pH3.0) was used to elute the FSTL-1 protein from the column. Fractions containing human FSTL-1 were pooled and dialyzed against PBS. Purity and identity of the isolated protein was confirmed by SDS-PAGE and MS/MS analysis, respectively.
Protein labeling
Purified FSTL-1 and ovalbumin (Sigma) were labeled using Alexa Fluor 488 protein labeling kit (Invitrogen) according to the supplier’s instructions. Labeled proteins were stored at 4°C in the dark until use.
Preparation of murine bone marrow-derived macrophages (BMDM)
BM cells were obtained from femurs of C57BL/6 mice (Jackson Laboratories). Macrophages were generated from BM cells by cultivating them for 7 days in RPMI 1640 supplemented with 10% FBS and 30% L929 cell-conditioned medium as a source of M-CSF. Retrovirus-transformed BMDM derived from wild type and TLR4-null mice were a generous gift from Dr. Jerrold Weiss (University of Iowa, Iowa City, IA, USA) [45].
Confocal microscopy
The hind paws from LPS-injected and control mice were frozen in liquid nitrogen-cooled isopentane. Four-micron sections were prepared with the Cryojane Tape Transfer system (Instrumedics). Frozen sections were fixed with 2% paraformaldehyde (PFA) and permeabilized with 0.1% Triton X100 in BSA buffer (0.5% BSA and 0.15% glycine in PBS). The slides were then blocked with 5% normal donkey serum (Sigma-Aldrich) in BSA buffer, followed by incubation with polyclonal goat anti-mouse FSTL-1 antibody (R&D Systems). Bound antibody was visualized using AF488-conjugated donkey anti-goat IgG (Invitrogen). For identification of macrophages, slides were also stained with rat anti-mouse CD11b antibody (BD PharMingen), and signal was visualized with AF594-conjugated donkey anti-rat IgG (Invitrogen).
To study FSTL-1 binding and intracellular trafficking in live cells under normal conditions, BMDM grown on glass coverslips were incubated for 7 h with AF488-labeled FSTL-1 or ovalbumin. To deliver fluorescently labeled proteins into the cell cytosol, the proteins were transfected using the Pro-Ject transfection reagent (Thermo) according to the manufacturer’s instructions, and the cells were incubated with the labeled proteins for additional 7 h. Fifteen min before the end of incubation period, all samples received 250 nM MitoTracker Red (Invitrogen) for mitochondrial labeling, then washed and fixed in PFA. To study FSTL-1 binding to permeabilized cells, BMDM were incubated with MitoTracker Red, fixed in PFA, permeabilized with 0.1% Triton X100 in PBS-0.2% BSA, and stained with AF488-FSTL-1 or AF488-ovalbumin. Nuclei were counterstained with Hoechst 33342 dye (Invitrogen), and the coverslips were mounted onto slides.
The slides were imaged using a Zeiss 710 confocal microscope under oil-immersion objective (63×). Co-localization of proteins was quantified using Zeiss Zen software, which calculates overlap coefficient based on Pearson’s correlation coefficient. The values for the overlap coefficient range from 0 to 1. An overlap coefficient with a value of 1 represents perfectly co-localized pixels.
Lentiviral transduction of ST2 cells
ST2 stromal cells were transduced with a lentivirus encoding mouse FSTL-1 small hairpin RNA (shRNA) (Santa Cruz Biotechnology) or a control lentivirus carrying scrambled shRNA as described [14].
Preparation of Mitochondria from ST2 cells and ETC enzyme activity assays
Cells were resuspended in a buffer containing 50 mM Potassium phosphate pH8.0, 1 mM EDTA, 2.5% glycerol, 250 mM sucrose, protease inhibitor mixture (Sigma), followed by homogenization with a cooled ball-bearing homogenizer (cell cracker) from European Molecular Biology Laboratory (50 strokes with a 10 µm clearance) [46]. Mitochondria were isolated and purified by differential centrifugation [47]. In situ gel staining for ETC complex I activity and ETC complex I plus III activity assays were performed essentially as described [47].
Cell transfection and stimulation
Human monocytic U937 cells (ATCC) and mouse macrophage RAW264.7 cells (ATCC) were transfected with the plasmid pRcCMV-hFSTL-1 (encoding human FSTL-1) or pRcCMV-mFSTL-1 (encoding mouse FSTL-1), respectively. Control cells were transfected with the plasmid pRcCMV (without a transgene) using the Amaxa Nucleofector System (Lonza) as described [14].
To study the effect of rotenone on IL-1β release from U937 transfectants, cells were differentiated into macrophages by incubating them overnight with PMA (10 ng/mL). The next day the cells were washed, pre-treated with rotenone for 2 h and incubated for additional 24 h with or without LPS.
For caspase-1 study, U937 cells and RAW264.7 cells were incubated with 200 ng/mL or 1 µg/mL LPS, respectively, for the time indicated in the legends to figures. Differentiation of U937 cells into macrophages (U937-MΦ) was performed as previously described [37]. Briefly, 0.5 mM PMA was added for 3 h the day before the experiment, then cells were washed twice with medium, plated in 24-well plates at 250000 cells/well, and incubated overnight at 37°C. The next day the medium was changed to fresh medium without PMA, and cells were incubated for another time indicated in the legends to figures.
ATP assay
ATP determination in cell lysates was performed by using an ATP colorimetric assay kit (BioVision) as suggested by the supplier.
Intracellular protein delivery and stimulation of U937 cells and BMDM
U937 cells were plated at 106/ mL in a 48-well plate. The cells were transfected with FSTL-1 or ovalbumin (final concentration 4 µg/mL) using the Pro-Ject transfection reagent (Thermo Scientific) according to the manufacturer’s instructions followed by stimulation with PMA (10 ng/mL) and LPS (200 ng/mL). Supernatants were collected for analysis 48 h posttransfection and analyzed by ELISA for human IL-1β.
BMDM were plated at 2.5×105/ mL in a 24-well plate and transfected with FSTL-1 or ovalbumin (4 µg/ml). After 4 h of transfection, complete RPMI 1640 medium was added to each well and the cells were incubated overnight at 37°C. The next day BMDM were stimulated with LPS (1 ng/mL). After 6 h of LPS stimulation, 0.5 mM ATP was added for 30 min. Supernatants were collected and analyzed by ELISA for mouse IL-1β.
Western blotting
Cells were lyzed with Cell Lysis Buffer (Sigma) supplemented with protease inhibitor cocktail (Sigma) and PMSF following the manufacturer’s protocol. Mouse paws were frozen in liquid nitrogen, pulverized on dry ice using a mortar and pestle, ground paw tissues were stored at −80°C until analysis. Paw lysates were obtained using Tissue Protein Extraction Reagent (Thermo) according to the manufacturer’s instructions. Samples were analyzed for protein content by BCA assay (Sigma), the same amount of protein in each sample was mixed with loading buffer containing 2-mercaptoethanol, and boiled for 10 min prior to electrophoresis. Proteins were separated by a 10–20% Tris-glycine SDS-polyacrylamide gradient gel (Invitrogen) and transblotted onto a nitrocellulose membrane followed by blocking with 5% non-fat dry milk in TBS-0.1% Tween-20. The anti-caspase-1 rabbit polyclonal antibody, detecting pro-caspase-1 and the caspase-1 p20 subunit (Cell Signaling Technology), the anti-NLRP3 antibody (Santa Cruz), the goat anti-mouse FSTL1 antibody (Abcam) or the goat anti-human FSTL-1 antibody (R&D Systems) were applied, followed by a HRP-conjugated anti-rabbit secondary IgG (Thermo) or HRP-anti-goat antibody (Thermo). To control for equal protein loading, membranes were reprobed with anti-actin antibody (Cell Signaling). Immunoreactivity was visualized using the SuperSignal West Femto Chemiluminescent Substrate (Thermo), membranes were scanned by LAS-3000 imaging system (Fuji Film) and images were analyzed by Multi Gauge software.
Quantitative real-time PCR
Total cDNA was produced from cells or frozen paw tissue using the RNeasy Mini Kit (Qiagen) and the SuperScript II Reverse Transcriptase Kit (Invitrogen). PCR was performed in a LightCycler (Mx3000P; Stratagene) using the Brilliant SYBR Green QPCR Master Mix (Agilent) as described [11]. The copy number (number of transcripts) of amplified product was calculated from a standard curve obtained by plotting known input concentrations of plasmid DNA. Expression levels of each gene were normalized to 18S ribosomal RNA (See Supporting Information Table 1 for primer sequences).
ELISAs
Human and mouse IL-1β, mouse IL-6, MCP-1 were determined using commercial ELISA reagents (BD Biosciences)
Measurement of caspase-1 activity
The activity of caspase-1 was determined in a colorimetric assay (R&D Systems) with a substrate (WEHD-pNA) specific for this enzyme. The results are expressed as fold increase in caspase activity of treated cells over that of untreated control (pRcCMV) cells.
Statistical analysis
The parametric Student’s t-test or non-parametric Mann-Whitney U test was used to assess the significance of differences between groups. Data are presented as mean+SEM unless otherwise specified, p values <0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Jerrold Weiss (University of Iowa, Iowa City, IA, USA) for wild type and TLR4-deficient immortalized BMDM cell lines.
This work was supported by NIH grants RO1 AI073556, RO1 AR056959 (to R. Hirsch).
Abbreviations used in this paper
- FSTL-1
Follistatin-like protein 1
- CIA
collagen-induced arthritis
- BMDM
bone marrow-derived macrophages
- ETC
electron transport chain
- NLRP3
nod-like receptor family, pyrin domain containing 3
- AF
Alexa Fluor
Footnotes
Conflict of interest:
The University of Pittsburgh has filed a patent application for the use of FSTL-1 as a disease target listing A. Marinov and R. Hirsch as inventors.
References
- 1.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, Hirsch R. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660–668. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Shen H, Xu M, Liu O, Zhao L, Liu S, Guo Z, Du J. FRP inhibits ox-LDL-induced endothelial cell apoptosis through an Akt-NF-{kappa}B-Bcl-2 pathway and inhibits endothelial cell apoptosis in an apoE-knockout mouse model. Am. J. Physiol. Endocrinol. Metab. 2010;299:E351–E363. doi: 10.1152/ajpendo.00005.2010. [DOI] [PubMed] [Google Scholar]
- 5.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, Qiao L, Geng H, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7058–7063. doi: 10.1073/pnas.1007293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sylva M, Li VS, Buffing AA, van Es JH, van den Born M, van der Velden S, Gunst Q, Koolstra JH, et al. The BMP antagonist follistatin-like 1 is required for skeletal and lung organogenesis. PLoS ONE. 2011;6:e22616. doi: 10.1371/journal.pone.0022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur. J. Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 8.Hambrock HO, Kaufmann B, Muller S, Hanisch FG, Nose K, Paulsson M, Maurer P, Hartmann U. Structural characterization of TSC-36/Flik: analysis of two charge isoforms. J. Biol. Chem. 2004;279:11727–11735. doi: 10.1074/jbc.M309318200. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, Gretz N, Alken P, et al. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25:183–191. [PubMed] [Google Scholar]
- 11.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J. Immunol. 2009;182:234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton S, Sowders D, Aronow B, Witte DP, Brunner HI, Giannini EH, Hirsch R. DNA microarray analysis reveals novel gene expression profiles in collagen-induced arthritis. Clin. Immunol. 2002;105:155–168. doi: 10.1006/clim.2002.5227. [DOI] [PubMed] [Google Scholar]
- 13.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J. Immunol. 2006;177:4758–4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 14.Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–1088. doi: 10.1002/art.33422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelik M, Wilson DC, Cloonan YK, Shulman ST, Hirsch R. Plasma Follistatin-Like Protein 1 is Elevated in Kawasaki Disease and May Predict Coronary Artery Aneurysm Formation. J. Pediatr. 2012;161:116–119. doi: 10.1016/j.jpeds.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 17.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 18.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 20.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS ONE. 2013;8:e59778. doi: 10.1371/journal.pone.0059778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J. Biol. Chem. 2010;285:7127–7134. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Murakami K, Ozaki S, Imura Y, Tong XP, Watanabe T, Sawaki T, Kawanami T, et al. DIP2 disco-interacting protein 2 homolog A (Drosophila) is a candidate receptor for follistatin-related protein/follistatin-like 1--analysis of their binding with TGF-beta superfamily proteins. FEBS J. 2010;277:4278–4289. doi: 10.1111/j.1742-4658.2010.07816.x. [DOI] [PubMed] [Google Scholar]
- 24.Murakami K, Tanaka M, Usui T, Kawabata D, Shiomi A, Iguchi-Hashimoto M, Shimizu M, Yukawa N, et al. Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett. 2012;586:319–324. doi: 10.1016/j.febslet.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, Zhang KH, Lu YJ, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+,K+-ATPase. Neuron. 2011;69:974–987. doi: 10.1016/j.neuron.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Planque N. Nuclear trafficking of secreted factors and cell-surface receptors: new pathways to regulate cell proliferation and differentiation, and involvement in cancers. Cell Commun. Signal. 2006;4:7. doi: 10.1186/1478-811X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demory ML, Boerner JL, Davidson R, Faust W, Miyake T, Lee I, Huttemann M, Douglas R, et al. Epidermal growth factor receptor translocation to the mitochondria: regulation and effect. J. Biol. Chem. 2009;284:36592–36604. doi: 10.1074/jbc.M109.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 33.Martinon F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010;40:616–619. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 34.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain N, Gupta S, Sudhakar C, Radha V, Swarup G. Role of p73 in regulating human caspase-1 gene transcription induced by interferon-{gamma} and cisplatin. J. Biol. Chem. 2005;280:36664–36673. doi: 10.1074/jbc.M413261200. [DOI] [PubMed] [Google Scholar]
- 36.Jain N, Sudhakar C, Swarup G. Tumor necrosis factor-alpha-induced caspase-1 gene expression. Role of p73. FEBS J. 2007;274:4396–4407. doi: 10.1111/j.1742-4658.2007.05969.x. [DOI] [PubMed] [Google Scholar]
- 37.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 38.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J. Immunol. 2007;179:1274–1281. doi: 10.4049/jimmunol.179.2.1274. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman HM. Therapy of autoinflammatory syndromes. J. Allergy Clin. Immunol. 2009;124:1129–1138. doi: 10.1016/j.jaci.2009.11.001. quiz 1139–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorelik M, Bushnell D, Goldbach-Mansky RT, Hoffman HM, Hirsch R. Elevated Serum Follistatin-Like Protein 1 Suggests an Interleukin-1 Independent Pathway for Inflammation in Patients with Cryopyrin Associated Periodic Syndromes. Arthritis Rheum. 2012;64:S1125–S1126. [Google Scholar]
- 42.Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, Eisenbarth SC, Flavell RA. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 44.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teghanemt A, Weiss JP, Gioannini TL. Radioiodination of an endotoxin.MD-2 complex generates a novel sensitive, high-affinity ligand for TLR4. Innate Immun. 2013;19:545–560. doi: 10.1177/1753425913475688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balch WE, Rothman JE. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch. Biochem. Biophys. 1985;240:413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Mohsen AW, Mihalik SJ, Goetzman ES, Vockley J. Evidence for physical association of mitochondrial fatty acid oxidation and oxidative phosphorylation complexes. J. Biol. Chem. 2010;285:29834–29841. doi: 10.1074/jbc.M110.139493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.