Abstract
Microdialysis sampling is a commonly used technique for collecting solutes from the extracellular space of tissues in laboratory animals and humans. Large molecular weight solutes can be collected using high molecular weight cutoff (MWCO) membranes (100 kDa or greater). High MWCO membranes require addition of high molecular weight dextrans or albumin to the perfusion fluid to prevent fluid loss via ultrafiltration. While these perfusion fluid additives are commonly used during microdialysis sampling, the tissue response to the loss of these compounds across the membrane is poorly understood. Tissue reactions to implanted microdialysis sampling probes containing different microdialysis perfusion fluids were compared over a 7-day time period in rats. The base perfusion fluid was Ringer’s solution supplemented with either bovine serum albumin (BSA), rat serum albumin (RSA), Dextran-70, or Dextran-500. A significant inflammatory response to Dextran-70 was observed. No differences in the tissue response between BSA and RSA were observed. Among these agents, the BSA, RSA, and Dextran-500 produced a significantly reduced inflammatory response compared to the Dextran-70. This work demonstrates that use of Dextran-70 in microdialysis sampling perfusion fluids should be eliminated and replaced with Dextran-500 or other alternatives.
Keywords: Biocompatibility, Subcutaneous Tissue, Dextran, Foreign body response, Rat
1.0 Introduction
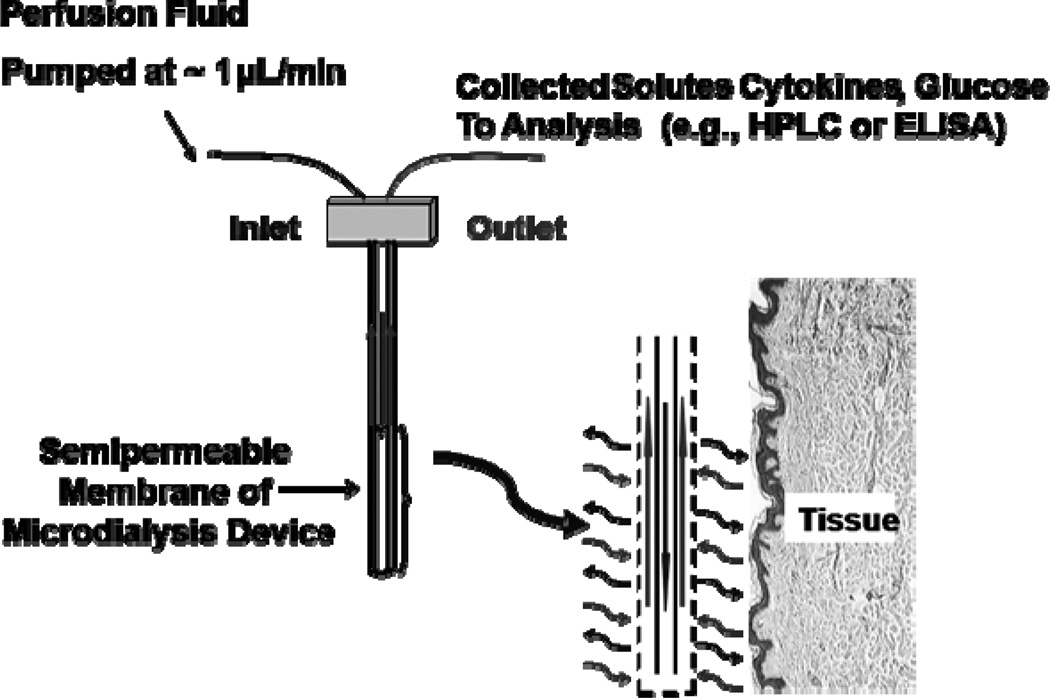
Microdialysis sampling is a widely used in vivo collection technique that has been used for more than 35 years for numerous life science applications (Muller, 2013; Robinson et al., 1991; Westerink and Cremers, 2007). This diffusion-based separation method uses an isotonic perfusion fluid that flows through inlet tubing into an inner cannula, the inner fiber lumen of a semi-permeable membrane, an outer cannula, and exits via an outlet tube where the dialysate is collected (Fig. 1). These devices are then implanted into tissue allowing collection of solutes from the extracellular fluid (ECF). The solute concentration gradient that exists between the perfusion fluid inside the probe and the surrounding ECF allows analytes smaller than the membrane molecular weight cutoff (MWCO) to diffuse into the membrane lumen to be collected and then quantified (Nandi and Lunte, 2009). The primary reasons for the success and variety of biomedical applications of microdialysis sampling include 1) it is minimally invasive allowing collections to be performed from targeted tissue sites in awake and freely-moving animals as well as in human subjects; 2) it provides analytically-clean samples that require either no or minimal sample preparation allowing a wide variety of chemical analysis schemes to be applied (Davies et al., 2000); 3) it reduces animal numbers since the animal in which the probe is implanted serves as its own control.
Figure 1.
Microdialysis probe schematic.
Microdialysis sampling was originally developed to collect small hydrophilic molecules such as the catecholamine and amino acid neurotransmitters. With the advent of commercially-available high MWCO membranes incorporated into microdialysis probes, it is now possible to collect peptides and proteins of biological significance including cytokines (Ao and Stenken, 2006; Clough, 2005; Nakamura et al., 1990). This has opened a wide range of possibilities for researchers investigating multiple disease states in different tissues that are believed to incur dysregulated cytokine function (Angst et al., 2008; Ao and Stenken, 2006; Clough et al., 2007; Garvin and Dabrosin, 2003; Helmy et al., 2011; Mellergard et al., 2008; Nielsen et al., 2009; Sjögren et al., 2012).
Prior to the use of high MWCO membranes during microdialysis sampling, it was common practice to use a saline solution, such as Ringer's or Ringer’s-Krebs, as a perfusion fluid since these solutions contain a balance of different ions (Na+, K+, Ca2+ and Cl−) similar to concentrations existing in the ECF (Benveniste and Huttemeier, 1990). When Ringer’s solution is the perfusion fluid through a high MWCO membrane, a significant reduction in expected fluid volumes can be observed due to a difference in hydrostatic pressure causing the perfusion fluid to leak through the membrane pores. This phenomenon is called ultrafiltration and would be expected for high MWCO ultrafiltration membranes, which is defined as any membrane with a MWCO of greater than 50 kDa.
Microdialysis sampling of large bioactive proteins including cytokines can be fraught with two major difficulties – ultrafiltration and non-specific adsorption to the device materials. Ultrafiltration is problematic because fluid is lost across the membrane into the tissue resulting in lower than expected sample volumes. This causes difficulties with chemical analysis techniques such as ELISA that have defined volume specifications. Additionally, the loss of fluid into the tissue space and its effect on tissue physiology is poorly understood. Non-specific adsorption is problematic especially with bioactive proteins since their concentrations are often in the pg/mL range. If non-specific adsorption is not reduced by inclusion of albumin as a blocking protein, proteins in such low concentrations may adsorb to the probe materials precluding their quantitation in dialysates. When using microdialysis membranes with 100 kDa or greater MWCO, colloids (high molecular weight dextrans, albumin, or a combination of the two) are added to the perfusion fluid to reduce ultrafiltration via an increased solution osmotic pressure within the membrane lumen (Hillman et al., 2005a; Rosdahl et al., 1997). Bovine serum albumin (BSA) and human serum albumin have also been used to reduce ultrafiltration and non-specific adsorption (Helmy et al., 2009; Trickler and Miller, 2003).
An unexplored area of research is whether these added colloids to the microdialysis perfusion fluid affect the surrounding tissue in deleterious ways. Fluid loss across the membrane may cause edema or other tissue damage. Albumin (~ 66 kDa), dextran-60, and dextran-70 can theoretically diffuse through the 100 kDa MWCO into the tissue. Bovine serum albumin is commonly used in rat studies since it can be procured at a significantly lower cost than rat serum albumin. However, there are considerable homology differences between these two albumins that could lead to the potential for immune response.
To date no studies have been performed to determine the effects of commonly used perfusion fluid reagents, i.e. Ringer's solution, Dextran-70, or BSA, on tissue surrounding implanted microdialysis probes. Likewise, it has not been determined if there are differences in the tissue reactions to perfused microdialysis probes vs. non-perfused (simply implanted) probes. These issues related to microdialysis sampling are critical to elucidate since microdialysis sampling is being widely used to collect cytokines and other bioactive proteins involved with inflammatory disease states in animals and human subjects. In this work, we demonstrate that a significant portion of the trauma caused at the site of a microdialysis probe appears to be due to the perfusion fluid agents rather than the implantation process.
2.0 Materials and Methods
2.1 Chemicals
The following chemicals were used in this study: bovine serum albumin (BSA) (Rockland Immunochemicals, Gilbertsville, PA); Dextran-70 and Dextran-500 (Sigma Aldrich, St Louis, MO); Ethylene oxide (Anderson Sterilizers, Inc, Haw River, NC); formalin, 10% and neutral buffered (BDH, VWR, West Chester, PA); HPLC grade water (Fisher Scientific, Waltham, MA); isoflurane (Abbott Laboratories, North Chicago, IL); povidone-iodine (Professional Disposables International Inc, Orangeburge, NY); and Rat Serum Albumin (RSA) (Sigma Aldrich, St Louis, MO). Ringer’s solution contained 150 mM NaCl, 5.4 mM KCl, 2.3 mM CaCl2, pH 7.4 and was prepared in HPLC-grade water. All other chemicals were reagent-grade or higher.
2.2 Microdialysis Sampling
All microdialysis sampling procedures were performed using CMA 20 microdialysis probes with polyethersulfone (PES) membranes, 100 kDa MWCO and 10 mm length (Harvard Apparatus, Holliston, MA). Prior to implantation, microdialysis probes were sterilized using ethylene oxide (Anderson Sterilizers, Inc, Haw River, NC). The probes are perfused using a BAS Bee pump with appropriate syringes (Bioanalytical Systems Inc., West Lafayette, IN). After completion of the surgical procedures to implant the microdialysis probe, the animal is placed into a CMA 120 freely moving animal collection system (CMA Microdialysis, Solna, Sweden1).
2.3 Surgical Procedures
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) in the weight range of 250–400 grams were used and housed in a climate controlled room at 72°F. Prior to surgical procedures, rats were allowed access to both food and water ad libitum. All animal experiments were approved by the University of Arkansas Institutional Animal Care and Usage Committee (IACUC) and were in compliance with the NIH standards for the ethical treatment of animals.
Rats were anesthetized in an induction chamber with 5% isoflurane in 0.8 L/min oxygen. The rat was then maintained on a nose cone via 2.5% isoflurane in 0.4 L/min oxygen during probe implantation. During the surgical procedure the body temperature was maintained using a CMA 150 temperature controller (CMA Microdialysis, Solna, Sweden). Surgical procedures were performed using aseptic technique. All surgical tools were autoclaved prior to use. The surgical site was shaved and then swabbed with povidone-iodine prior to any incisions.
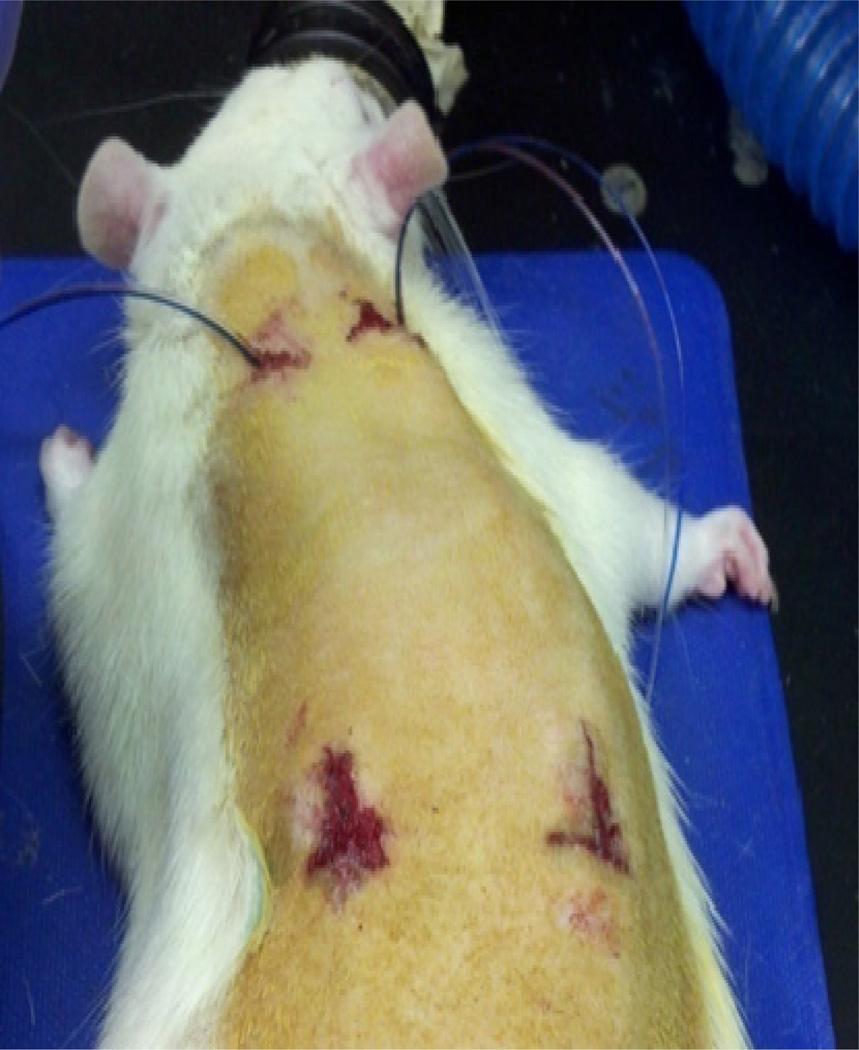
To implant the microdialysis probe, a '┴' shaped incision was made into the posterior dorsal subcutaneous tissue followed by a '-' shaped incision made near the base of the neck. Both incisions were about 0.5 cm in length. An autoclaved straw was then passed through the subcutaneous tissue from the posterior to anterior incisions. The tubing lines of the microdialysis probe were then run from the posterior to anterior end of the straw such that outlet lines were located on the anterior side of the rat. The straw was then removed from the animal. A plastic introducer that has split tubing (Harvard Apparatus, Holliston, MA) was then placed subcutaneously at the posterior incision site. The microdialysis probe was then placed in the introducer and the introducer was removed by pulling the tubing to the side and the fluid lines were pulled tight. The posterior incision was closed using Vetbond™ (3M, St Paul, MN). A second microdialysis probe was then implanted on the opposite side of the spine using the same procedure. The two probes were separated by at least 2.5 cm (Fig 2). Post collection, the animal was placed under light anesthesia and the lines were placed under the skin at the anterior incision in a subcutaneous pocket. The anterior incision was then closed using surgical staples and the animal was returned to housing.
Figure 2.
Microdialysis probe placement into the dorsal subcutaneous space of a rat.
2.4 Perfusion Fluid Comparisons
For all perfusion fluid comparisons, two probes were implanted into the rats. Three animals were used in each set. Unless otherwise specified, perfusions through the probes were performed every day for four days. At this point, the anterior incision with the pocket for tubing lines was closed using Vetbond™. On the seventh day post implantation, the animals were euthanized via CO2 inhalation and the probes, along with the tissue immediately surrounding the probe, were explanted by using scissors to remove a ~1 inch by ~2 inch piece of tissue which contained the probe. A sterile scalpel was then used to cut around the probe removing any excess tissue not in immediate contact with the probe. The tissue containing the probe was then held in a fashion which allowed for a scalpel blade to be used to cut the probe and its encapsulation tissue, which can be seen surrounding the probe (~ 1–2 mm), away from the remaining tissue. The probe and tissue were then stored in 10% neutral buffered formalin.
2.5 Probe Only vs. Ringer's Solution Filled Probe
One probe had no fluid passed through it while the other had Ringer's solution perfused through at a flow rate of 3 μL/min until fluid was observed exiting the outlet tubing at which point the flow was stopped. This allowed one probe to have the perfusion fluid in contact with the tissue to use as a comparison to either no fluid or perfused fluids. Fresh Ringer’s solution was infused through the implanted dialysis probe as described above each day for four days, including the day of implantation. On the fourth day, the anterior incision was permanently closed using Vetbond™ and the animals were returned to housing. Animals were sacrificed seven days post implantation and the probes were removed as described above.
2.6 Ringer's vs. Ringer's + Dextran-70
One probe was perfused using Ringer’s solution while the other was perfused using Ringer's solution with 6% (w/v) Dextran-70. After implantation of the probes, the animals were moved to a CMA 120 freely moving bowl which allows for microdialysis sampling to be performed in awake and freely-moving animals. Upon placement of the animal in the freely-moving animal system, a flush (3 μL/min) through the implanted probe was performed and reduced to 0.5 μL/min, in increments of 0.5 μL/min over 25 minutes. Infusions were then performed at 0.5 μL/min for 1 hour. At the end of the 1 hour infusion, a flush (3 μL/min) was performed using filter sterilized HPLC grade water.
2.7 RSA vs. BSA
The same procedure was followed as the Ringer's vs Ringer's with Dextran-70 except for the following differences. One probe was perfused with Ringer's solution with 3% (w/v) BSA and the other with 3% (w/v) Rat Serum Albumin (RSA). The 3% albumin solutions were necessary to prevent ultrafiltration.
2.8 Dextran-500 vs. Dextran-500 + BSA
The same procedure was followed as the Ringer's vs. Ringer's with Dextran-70 except for the following differences. One probe was perfused with Ringer's solution containing 6% (w/v) Dextran-500 (Sigma Aldrich St Louis, MO) and the other was perfused with Ringer's solution plus 6% (w/v) Dextran-500 and 0.1% (w/v) Bovine Serum Albumin (BSA).
2.9 Histological Analyses
Tissue was fixed in 10% neutral buffered formalin. It was subsequently embedded in paraffin blocks and cut using a microtome (~5 μm). The tissue sections were de-paraffinized, and then stained using standard protocol for both hematoxylin and eosin (H&E) and Masson's Trichrome by Mr. David Cross with the USDA Histology Lab, University of Arkansas. Tissue sections were then analyzed using a Zeiss Axioskop II plus microscope (Carl Zeiss Inc., Thornwood, NY) with Cannon EOS Digital Software for Rebel T2i camera.
3.0 Results
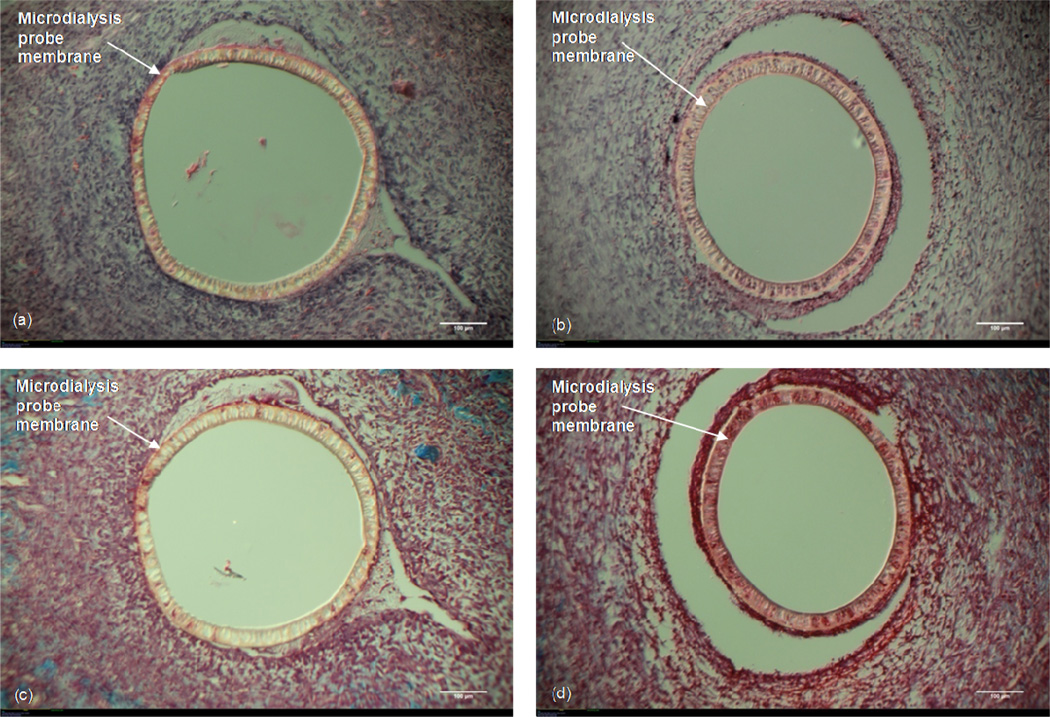
3.1 Probe vs. Ringer's Filled Probe
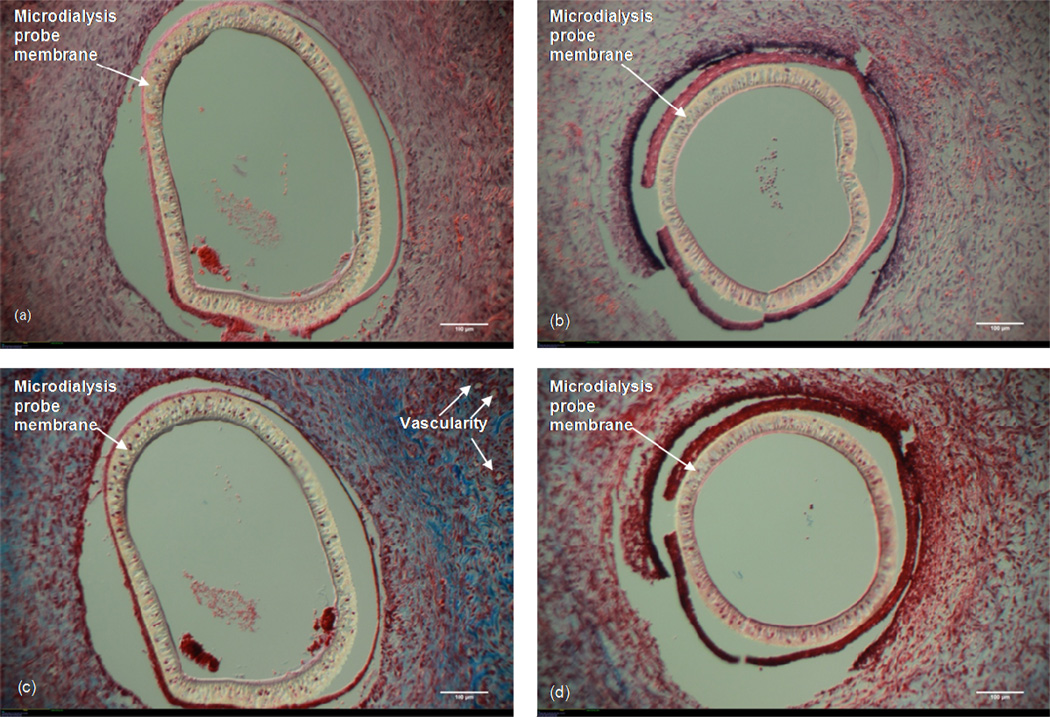
Figure 3 shows the (H&E) and Masson's Trichrome images for the probes that were either just implanted (no solution in the membrane fiber lumen) or allowed to have Ringer’s solution reside in the inner fiber lumen. Cells are seen interrogating both the probe (Fig 3a) and the Ringer’s fluid-filled probe (Fig 3b). No significant difference in the density of cells was observed surrounding either probe. The Masson's Trichrome stained sections show more collagen surrounding the probe only (Fig 3c) as compared to the Ringer’s fluid-filled probe (Fig 3d). There appears to be some vascularity surrounding the probe only (top right of Fig 3c) whereas no vascularity is seen surrounding the fluid-filled probe (Fig 3d). When these images are compared to those for the probes that were continuously perfused with Ringer's solution, the similarities in cell density, cellular organization, and collagen formation are striking. For this reason, all further treatment comparisons are made in relation to probes through which Ringer’s solution was perfused.
Figure 3. Probe vs. Ringer's Filled Probe.
10× Magnification. White size bar represents 100 μm. Top: H&E stain (a) Microdialysis probe implanted in the subcutaneous tissue (no perfusion fluid). (b) Microdialysis probe with Ringer's solution residing in the fiber lumen. Bottom: Masson's Trichrome stain. (c) Microdialysis probe implanted in the subcutaneous tissue (no perfusion fluid). (d) Microdialysis probe with Ringer's solution residing in the fiber lumen.
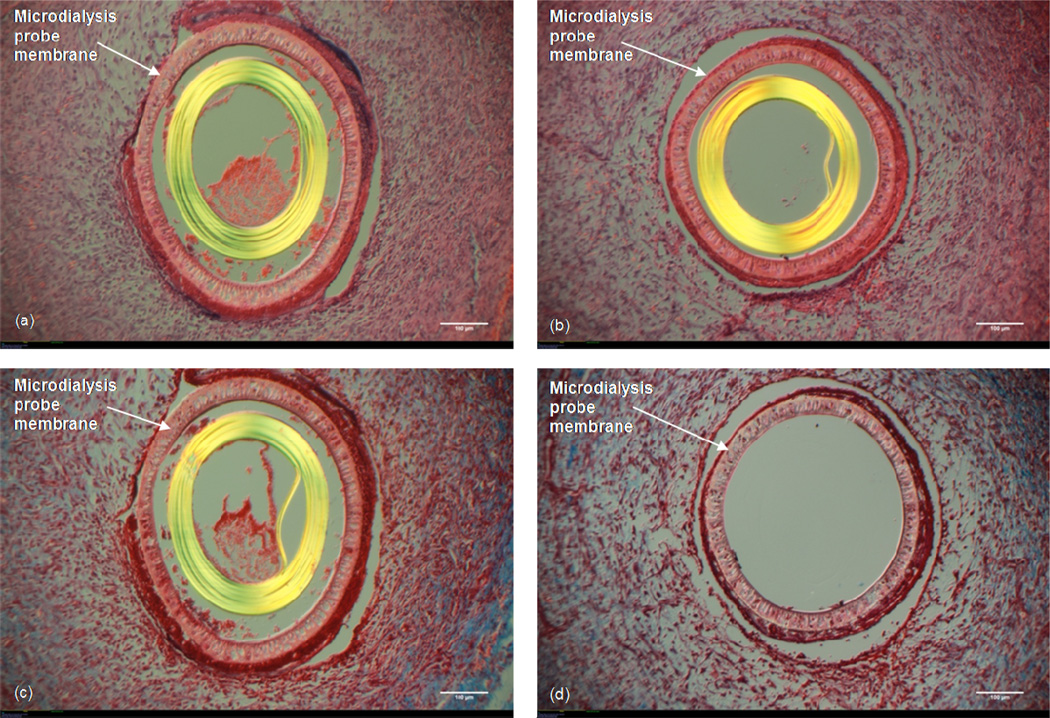
3.2 Ringer's vs. Ringer’s with 6% Dextran-70
With the H&E stain, the probe which had Ringer's solution perfused through shows cells near the membrane pores of the probe (Fig 4a). However, these cells, while well organized, are less densely packed around the probe as compared to the probe which had Ringer's with 6% Dextran-70 perfused (Fig 4b). The Masson’s Trichrome staining shows far less collagen found around the Ringer's-perfused probe (Fig 4c) as compared to the Ringer's with 6% Dextran-perfused probe (Fig 4d), and the collagen seen around the Ringer's + Dextran probe consists of thicker strands and is more densely packed than is seen around the Ringer's probe. Furthermore, there is a ~25–50 μm thick layer of densely packed cells immediately surrounding the probe membrane of the Ringer's with 6% Dextran probe which is lacking in the Ringer's-perfused probe site.
Figure 4. Ringer's vs. Ringer's + Dextran-70.
10× Magnification. White size bar represents 100 μm. Top: H&E stain (a) Microdialysis probe with Ringer’s solution perfused. (b) Microdialysis probe with Ringer's + 6% (w/v) Dextran-70 solution perfused. Bottom: Masson's Trichrome stain. (c) Microdialysis probe with Ringer’s solution perfused. (d) Microdialysis probe with Ringer's + 6% (w/v) Dextran-70 solution perfused.
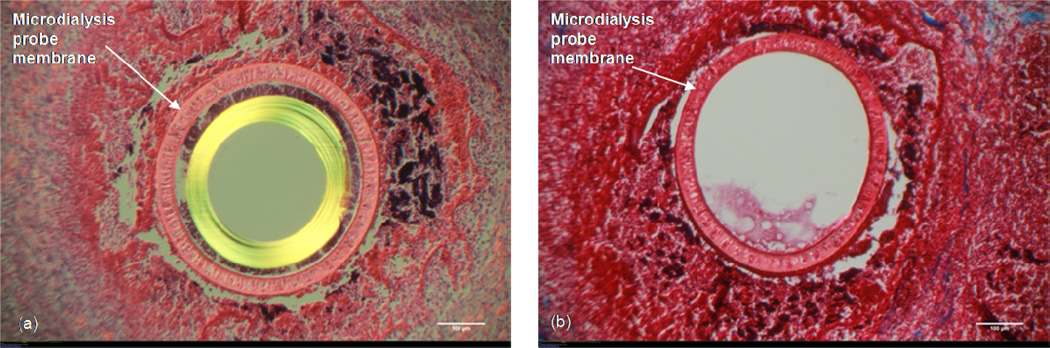
Occasionally, probes will fail and fluid flow will no longer be observed exiting the fluid line. Suspecting that the more pronounced inflammatory response observed for the Dextran-70 infused probes may be due to Dextran-70 diffusing out of the probe, we decided to let probes that failed remain in the tissue followed by histological analysis. Figure 5 shows both the H&E and the Masson’s Trichrome stain for a microdialysis probe implant that had failed. A significant amount of cellular material is located at the probe site with the failed probes indicating a nearly necrotic response (Figure 5a). The space is significantly inflamed with little collagen remaining as shown in Figure 5b.
Figure 5. Probe Failure with Dextran-70.
10× Magnification. White size bar represents 100 μm. H&E (Left) and Masson's Trichrome (Right) stains of an implanted microdialysis probe that failed.
3.3 RSA vs. BSA
Figures 6a and b show the H&E staining for the RSA- vs. BSA-infused probes. Cells are seen near the pores of the membrane (Fig 6 a,b). While these cells are well organized, they are not as densely packed as is seen with Dextran. The density of cells surrounding both probes closely resembles that for tissue surrounding the probes through which only Ringer’s was perfused. Additionally, the amount of collagen seen around each probe is also similar to that with only Ringer’s perfused (Figures 6c and d).
Figure 6. RSA vs. BSA.
10× Magnification. White size bar represents 100 μm. Top: H&E stain (a) Microdialysis probe perfused with Ringer's + 3% (w/v) BSA solution. (b) Microdialysis probe perfused with Ringer's + 3% (w/v) RSA. Bottom: Masson's Trichrome stain. (c) Microdialysis probe perfused with Ringer's + 3% (w/v) BSA solution. (d) Microdialysis probe perfused with Ringer's + 3% (w/v) RSA.
3.4 Ringer's + Dextran-500 vs. Ringer's + Dextran-500 + BSA
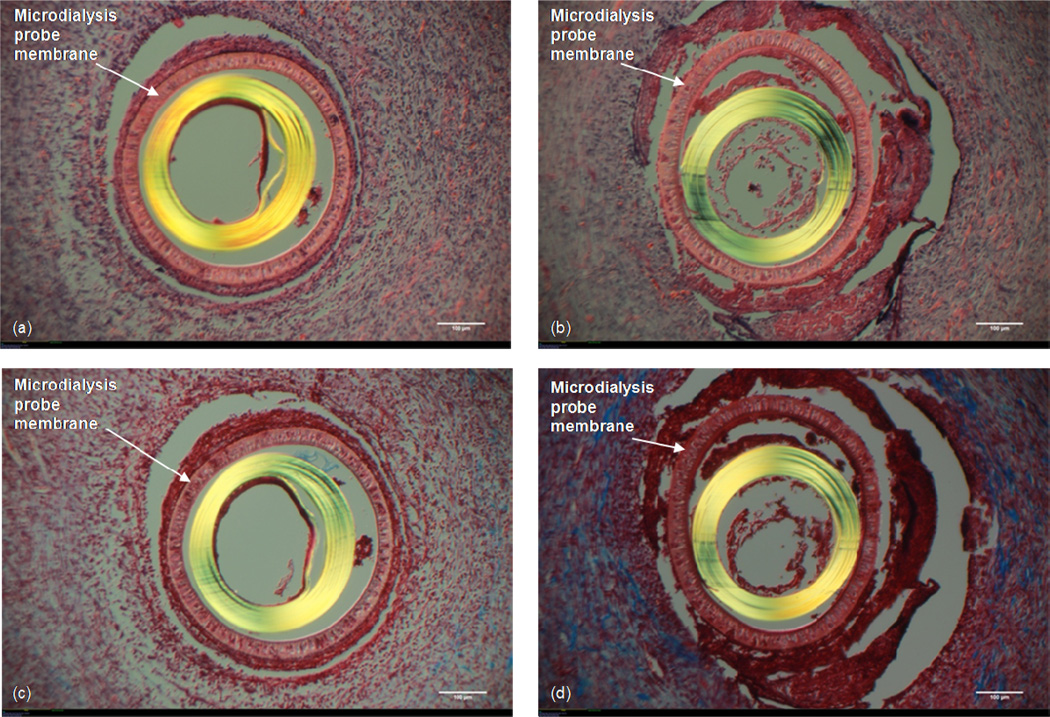
The H&E stains for probe explants through which Ringer’s and Dextran-500 vs. Ringer’s, Dextran-500, and BSA are shown in Figures 7a and b. Cells appear to be embedded within the membrane pores in the H&E stained tissue (Fig 7a,b). There appears to be no difference in the cellular density around the two probes. The density of cells seen around both probes seems to be the same as is seen in response to Ringer's only perfused probes and probes perfused with Ringer's and either BSA or RSA. Likewise, cells seen around these probes seem to be less densely packed than what is seen around the probes which had Dextran-70 infused. Also, there is no layer of extremely densely packed cells immediately surrounding the probe as is seen in the Dextran-70 infused probes. The collagen content observed around the two probes is also similar, suggesting there is no difference with respect to the tissue reaction to this infusion fluid between the two treatments (Fig 7 c,d). The prevalence of collagen seen around these probes is similar to the collagen seen around the Ringer's only probe as well as the Ringer's with either BSA or RSA.
Figure 7. Ringer's + Dextran-500 vs. Ringer's + Dextran-500 + BSA.
10× Magnification. White size bar represents 100 μm. Top: H&E stain (a) Microdialysis probe perfused with Ringer's + 6% (w/v) Dextran-500. (b) Microdialysis probe perfused with Ringer's + 6% (w/v) Dextran-500 + 0.1% (w/v) BSA solution. Bottom: Masson's Trichrome stain. (c) Microdialysis probe perfused with Ringer's + 6% (w/v) Dextran-500. (d) Microdialysis probe perfused with Ringer's + 6% (w/v) Dextran-500 + 0.1% (w/v) BSA solution.
4.0 Discussion
The inception of microdialysis sampling was based on an analogy that an implanted microdialysis sampling probe behaves like a benign capillary in the tissue allowing free diffusion of solutes across its porous semi-permeable membrane (Ungerstedt, 1991). Despite over 14,000 publications that have reported using microdialysis sampling, relatively few have investigated how the implant procedure or the perfusion fluids influence the tissue surrounding the implanted microdialysis device. The majority of the research literature that has described tissue reactions to microdialysis sampling has been focused on neural tissue (Benveniste and Diemer, 1987; Mitala et al., 2008). Ultrastructural studies have shown tissue disruptions to be seen as far as 1.4 mm away from the implant site in the brain (Clapp-Lilly et al., 1999). However, histological analyses of microdialysis probes implanted into other tissues have also revealed the expected presence of inflammatory cells surrounding the probe implant.
Out group has previously reported, microdialysis probe implants in the subcutaneous space that only had phosphate buffered saline solution passed through the probes showed a dense, avascular, cellular layer surrounded by a collagenous, vascular layer at 14 days post implantation (Mou et al., 2011). A long-term investigation of implanted dialysis fibers (not microdialysis probes and not infused) showed that at 3 weeks the fibers were encapsulated by a highly cellular disorganized capsule, at 6 weeks a highly cellular yet highly organized capsule, and at 12 weeks a mature fibrotic capsule was seen characterized by low cellularity and dense bands of collagen on the membrane surface (Clark et al., 2000). These results are consistent with Sanders' study which showed that a height threshold of 5.9 μm exists and is the most important factor in determining the formation of a fibrotic capsule around an implant (Sanders and Rochefort, 2003). The microdialysis probes used in this study had an outer diameter of 500 μm which exceeds the 5.9 μm reported as the threshold indicating that a fibrotic capsule would be expected. However, while the effects of implanting different types of polymeric membranes have been investigated, no investigations have looked at the effects of perfusion fluid components i.e. Dextran, BSA, and RSA with respect to their effect on tissue responses to the microdialysis probe implant.
Our work is the first to demonstrate that perfusion fluid additives used for microdialysis sampling lead to different inflammatory responses. While the insertion of a microdialysis probe is considered to be minimally invasive, it must be recognized that there is a certain amount of trauma associated with the implantation procedure. This can be seen by the densely packed cells surrounding an implanted microdialysis probe. These cells are responsible for the initial clearance of any cellular debris, the formation of the fibrotic capsule, and the eventual wound healing process. While the trauma due to probe implantation cannot be avoided, it is important to minimize the trauma due to the sampling procedure to gain a better understanding of the biochemical processes occurring at the implant site. While we have shown that there are differences in the cellular response seen surrounding a Ringer's perfused probe as compared to an implanted microdialysis probe where no perfusion occurs, they can be considered negligible. Given the nature of microdialysis sampling experiments in that the technique is used to sample from tissue, it is unavoidable to prevent a perfusion and thus the low-level inflammatory response to the perfusion fluid will be present.
The inclusion of high MWCO membranes into microdialysis probes has allowed for the collection of larger molecular weight solutes such as bioactive proteins. The tradeoff is that ultrafiltration, or loss of fluid across the membrane pores, can become a significant problem. To circumvent this problem, osmotic agents such as Dextran-60 (~ 60 kDa) or -70 (~70 kDa) were being added at high concentrations (1 to 4% w/v). These agents have since been widely used in microdialysis sampling with 100 kDa MWCO membranes (Abrahamsson et al., 2012; Dabrosin, 2003; Dostalova et al., 2009; Dostálová et al., 2003; Farnebo et al., 2009; Förster et al., 2013; Hillman et al., 2005b; Hillman et al., 2006; Mellergard et al., 2008; Mellergård et al., 2011; Rosenbloom et al., 2006; Sjoegren and Anderson, 2009; Sjögren et al., 2012; Sjögren et al., 2002; von Grote et al., 2011; Waelgaard et al., 2008; Zhong et al., 2007). Our results show that Dextran-70, when allowed to enter the extracellular space, causes an intense inflammatory response. While theoretically the use of Dextran-70 in perfusion fluids could result in the dextran leaving the probe, it has never been determined if its use results in an increased inflammatory response at the implant site. We found that Dextran-70 diffuses out of the probe resulting in increased inflammation at the probe site. This response cannot be seen as negligible and must be considered when collecting bioactive proteins such as the cytokines.
Due to the fact that Dextran-70 was shown to invoke an inflammatory response when perfused through the probe, alternative osmotic agents were tested. The use of BSA is commonly used in perfusion fluids at high concentrations (3.5–10% w/v) to reduce fluid loss (Trickler and Miller, 2003). Simultaneously, low concentrations of BSA (~0.1% w/v) have been used in perfusion fluids to prevent non-specific binding of proteins to the microdialysis outlet tubing. Theoretically, BSA (~66,000 Da) could exit the probe yet its effects on the tissue surrounding the implanted microdialysis probe have never been studied. Our results show that the tissue surrounding a BSA treated probe shows no appreciable difference to tissue seen surrounding a RSA treated probe or a Ringer's only perfused probe. This suggests that there is no significant inflammatory reaction seen in response to the BSA.
While the use of BSA as an osmotic reagent was shown to have no inflammatory effect on the tissue, it was shown to give lower fluid recoveries (data not shown) as compared to Dextran-70. Dextran-500 has been shown to give fluid recoveries of 100% when used at lower flow rates (Dahlin et al., 2010). In fact, we have found that Dextran-500, when used at 6% w/v, yields 100% fluid recovery in vitro (data not shown). Dextran-500, with or without 0.1% BSA, has also been shown to result in no additional inflammatory response as compared to Ringer's only perfused probes in vivo. While the data presented here shows Dextran-70 causes an inflammatory response in vivo, Dextran-500 appears to be a viable alternative as it retained the fluid recovery without causing an excessive inflammatory response.
5.0 Conclusions
While microdialysis sampling has gained popularity, there have been no studies reporting the effects of perfusion fluid components on inflammation surrounding the probe. In this study we investigated the effects of the actual perfusion of fluid through a microdialysis probe, Ringer's solution, Dextran-70, BSA, RSA, and Dextran-500 on the tissue surrounding the probe. What we found shows that the use of Dextran-70 in the perfusion fluid results in some of the Dextran diffusing into the ECM. The result of this Dextran diffusing out is highly inflamed tissue seen surrounding the probe. For this reason, we would suggest that Dextran-70, or any lower molecular weight Dextran, not be used in perfusion fluids during microdialysis sampling. Instead, we would suggest the use of Dextran-500 as this does not diffuse out of the probe and appears to not cause additional inflammation on top of the inflammation that is caused by the surgical implantation process.
Acknowledgement
We gratefully acknowledge NIH EB 014404 for funding of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CMA Microdialysis is now owned by Harvard Apparatus, Holliston, MA, USA
References
- Abrahamsson A, Morad V, Saarinen NM, Dabrosin C. Estradiol, tamoxifen, and flaxseed alter IL-1β and IL-1Ra levels in normal human breast tissue in vivo. J Clin Endocrinol Metab. 2012;97:E2044–E2054. doi: 10.1210/jc.2012-2288. [DOI] [PubMed] [Google Scholar]
- Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COXinhibitor: A microdialysis study. Pain. 2008;139:15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Ao X, Stenken JA. Microdialysis sampling of cytokines. Methods. 2006;38:331–341. doi: 10.1016/j.ymeth.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Diemer NH. Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol. 1987;74:234–238. doi: 10.1007/BF00688186. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Huttemeier PC. Microdialysis - theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J. Neurosci. Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Clark H, Barbari TA, Stump K, Rao G. Histologic evaluation of the inflammatory response around implanted hollow fiber membranes. J Biomed Mater Res. 2000;52:183–192. doi: 10.1002/1097-4636(200010)52:1<183::aid-jbm24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Clough GF. Microdialysis of large molecules. AAPS Journal. 2005;7:E686–E692. doi: 10.1208/aapsj070369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough GF, Jackson CL, Lee JJP, Jamal SC, Church MK. What can microdialysis tell us about the temporal and spatial generation of cytokines in allergen-induced responses in human skin in vivo? J Invest Dermatol. 2007;127:2799–2806. doi: 10.1038/sj.jid.5700930. [DOI] [PubMed] [Google Scholar]
- Dabrosin C. Variability of vascular endothelial growth factor in normal human breast tissue in vivo during the menstrual cycle. J Clin Endocrinol Metab. 2003;88:2695–2698. doi: 10.1210/jc.2002-021584. [DOI] [PubMed] [Google Scholar]
- Dahlin AP, Wetterhall M, Caldwell KD, Larsson A, Bergquist J, Hillered L, Hjort K. Methodological aspects on microdialysis protein sampling and quantification in biological fluids: an in vitro study on human ventricular CSF. Anal. Chem. 2010;82:4376–4385. doi: 10.1021/ac1007706. [DOI] [PubMed] [Google Scholar]
- Davies MI, Cooper JD, Desmond SS, Lunte CE, Lunte SM. Analytical considerations for microdialysis sampling. Adv Drug Deliv Rev. 2000;45:169–188. doi: 10.1016/s0169-409x(00)00114-9. [DOI] [PubMed] [Google Scholar]
- Dostalova I, Kavalkova P, Haluzikova D, Housova J, Matoulek M, Haluzik M. The use of microdialysis to characterize the endocrine production of human subcutaneous adipose tissue in vivo. Regul. Pept. 2009;155:156–162. doi: 10.1016/j.regpep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Dostálová I, Pacák K, Nedvı, amp x, dková J. Application of in vivo microdialysis to measure leptin concentrations in adipose tissue. Int J Biol Macromol. 2003;32:205–208. doi: 10.1016/s0141-8130(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Farnebo S, Karlander Lars E, Ingrid S, Sjoegren F, Folke S. Continuous assessment of concentrations of cytokines in experimental injuries of the extremity. Int. J. Clin. Exp. Med. 2009;2:354–362. [PMC free article] [PubMed] [Google Scholar]
- Förster Y, Gao W, Demmrich A, Hempel U, Hofbauer LC, Rammelt S. Monitoring of the first stages of bone healing with microdialysis. Acta Orthop. 2013;84:76–81. doi: 10.3109/17453674.2013.769080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin S, Dabrosin C. Tamoxifen inhibits secretion of vascular endothelial growth factor in breast cancer in vivo. Cancer Res. 2003;63:8742–8748. [PubMed] [Google Scholar]
- Helmy A, Carpenter Keri LH, Skepper Jeremy N, Kirkpatrick Peter J, Pickard John D, Hutchinson Peter J. Microdialysis of cytokines: methodological considerations, scanning electron microscopy, and determination of relative recovery. J Neurotrauma. 2009;26:549–561. doi: 10.1089/neu.2008.0719. [DOI] [PubMed] [Google Scholar]
- Helmy A, Carpenter KLH, Menon DK, Pickard JD, Hutchinson PJA. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal productionJCereb. Blood Flow Metab. 2011;31:658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J, Aneman O, Anderson C, Sjögren F, Säberg C, Mellergård P. A microdialysis technique for routine measurement of macromolecules in the injured human brain. Neurosurgery. 2005a;56:1264–1268. doi: 10.1227/01.neu.0000159711.93592.8d. discussion 1268-1270. [DOI] [PubMed] [Google Scholar]
- Hillman J, Åneman O, Anderson C, Sjögren F, Säberg C, Mellergård P. A Microdialysis Technique for Routine Measurement of Macromolecules in the Injured Human Brain. Neurosurgery. 2005b;56 doi: 10.1227/01.neu.0000159711.93592.8d. [DOI] [PubMed] [Google Scholar]
- Hillman J, Milos P, Yu ZQ, Sjögren F, Anderson C, Mellergård P. Intracerebral microdialysis in neurosurgical intensive care patients utilising catheters with different molecular cut-off (20 and 100 kD) Acta Neurochir. 2006;148:319–324. doi: 10.1007/s00701-005-0670-8. [DOI] [PubMed] [Google Scholar]
- Mellergard P, Aneman O, Sjogren F, Pettersson P, Hillman J. Changes in extracellular concentrations of some cytokines, chemokines, and neurotrophic factors after insertion of intracerebral microdialysis catheters in neurosurgical patients. Neurosurgery. 2008;62:151–157. doi: 10.1227/01.NEU.0000311072.33615.3A. discussion 157-158. [DOI] [PubMed] [Google Scholar]
- Mellergård P, Åneman O, Sjögren F, Säberg C, Hillman J. Differences in cerebral extracellular response of interleukin-1β, interleukin-6, and interleukin-10 after subarachnoid hemorrhage or severe head trauma in humans. Neurosurgery. 2011;68 doi: 10.1227/NEU.0b013e3181ef2a40. [DOI] [PubMed] [Google Scholar]
- Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, Weber SG, Michael AC. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. J. Neurosci. Methods. 2008;174:177–185. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou X, Lennartz MR, Loegering DJ, Stenken JA. Modulation of the foreign body reaction for implants in the subcutaneous space: microdialysis probes as localized drug delivery/sampling devices. J Diabetes Sci Technol. 2011;5:619–631. doi: 10.1177/193229681100500316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. Microdialysis in drug development. AAPS advances in pharmaceutical sciences series. 2013 [Google Scholar]
- Nakamura M, Itano T, Yamaguchi F, Mizobuchi M, Tokuda M, Matsui H, Etoh S, Hosokawa K, Ohmoto T, Hatase O. In vivo analysis of extracellular proteins in rat brains with a newly developed intracerebral microdialysis probe. Acta Med Okayama. 1990;44:1–8. doi: 10.18926/AMO/30465. [DOI] [PubMed] [Google Scholar]
- Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: A review. Anal Chim Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NB, Hojbjerre L, Sonne MP, Alibegovic AC, Vaag A, Dela F, Stallknecht B. Interstitial concentrations of adipokines in subcutaneous abdominal and femoral adipose tissue. Regul. Pept. 2009;155:39–45. doi: 10.1016/j.regpep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Justice JB Jr, editors. Microdialysis in the Neurosciences. Amsterdam: Elsevier; 1991. [Google Scholar]
- Rosdahl H, Ungerstedt U, Henriksson J. Microdialysis in human skeletal muscle and adipose tissue at low flow rates is possible if dextran-70 is added to prevent loss of perfusion fluid. Acta Physiol Scand. 1997;159:261–262. doi: 10.1046/j.1365-201X.1997.123358000.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AJ, Ferris R, Sipe DM, Riddler SA, Connolly NC, Abe K, Whiteside TL. In vitro and in vivo protein sampling by combined microdialysis and ultrafiltration. J Immunol Methods. 2006;309:55–68. doi: 10.1016/j.jim.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Sanders JE, Rochefort JR. Fibrous encapsulation of single polymer microfibers depends on their vertical dimension in subcutaneous tissue. J Biomed Mater Res A. 2003;67A:1181–1187. doi: 10.1002/jbm.a.20027. [DOI] [PubMed] [Google Scholar]
- Sjoegren F, Anderson CD. Sterile trauma to normal human dermis invariably induces IL 1β, IL6 and IL8 in an innate response to "danger". Acta Derm.-Venereol. 2009;89:459–465. doi: 10.2340/00015555-0683. [DOI] [PubMed] [Google Scholar]
- Sjögren F, Davidsson K, Sjöström M, Anderson C. Cutaneous microdialysis: Cytokine evidence for altered innate reactivity in the skin of psoriasis patients? AAPS J. 2012;14:187–195. doi: 10.1208/s12248-012-9331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren F, Svensson C, Anderson C. Technical prerequisites for in vivo microdialysis determination of interleukin-6 in human dermis. Br J Dermatol. 2002;146:375–382. [PubMed] [Google Scholar]
- Trickler WJ, Miller DW. Use of osmotic agents in microdialysis studies to improve the recovery of macromolecules. J Pharm Sci. 2003;92:1419–1427. doi: 10.1002/jps.10410. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Microdialysis--principles and applications for studies in animals and man. J Intern Med. 1991;230:365–373. doi: 10.1111/j.1365-2796.1991.tb00459.x. [DOI] [PubMed] [Google Scholar]
- von Grote EC, Venkatakrishnan V, Duo J, Stenken JA. Long-term subcutaneous microdialysis sampling and qRT-PCR of MCP-1, IL-6 and IL-10 in freely-moving rats. Mol Biosyst. 2011;7:150–161. doi: 10.1039/c0mb00059k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelgaard L, Thorgersen EB, Line P-D, Foss A, Mollnes TE, Tonnessen TI. Microdialysis monitoring of liver grafts by metabolic parameters, cytokine production, and complement activation. Transplantation. 2008;86:1096–1103. doi: 10.1097/TP.0b013e31818775ca. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Cremers TIFH. Handbook of Microdialysis Sampling: Methods, Applications, and Clinical Aspects. Amsterdam: Academic Press; 2007. [Google Scholar]
- Zhong H, Han B, Tourkova Irina L, Lokshin A, Rosenbloom A, Shurin Michael R, Shurin Galina V. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]