Abstract
In protozoan parasites, there is little information on the presence of covalent RNA modifications which comprise the epitranscriptome. Therefore, we determined if T. brucei tRNAAsp(GUC), tRNAGly(GCC), tRNAVal(AAC), and tRNATyr(GUA) contain 5-methylcytosines via RNA bisulfite sequencing. Most tRNAs examined have at least one 5-methylcytosine at the variable region-TψC junction. Only tRNAGly(GCC) displayed methylation of C40 in the anticodon stem, and there was partial methylation at this site. There is no evidence for methylation of C38 in the anticodon loop in the tRNAs analyzed. Analysis of tRNATyr(GUA) demonstrates that both unspliced and spliced molecules contain C48 methylation, indicating tRNA cytosine methylation can precede tRNA splicing. Overall, our data indicate that T. brucei tRNAs contain 5-methylcytosine residues in some, but potentially not all standard eukaryotic positions. The levels of cytosine methylation of different T. brucei tRNAs vary, suggesting the presence of a mechanism for methylation control.
Keywords: tRNA, 5-methylcytosine, sodium bisulfite sequencing, Dnmt2, NSun2
Epigenetic information is heritable genetic information that is not based on DNA sequence alone. One of the main types of epigenetic information is the presence of methyl groups. Both protein and DNA are methylated, and these modifications can impact numerous processes including gene expression, molecular structure, and DNA repair. RNA can be chemically modified after transcription, and this finding has led to the realization that, in addition to an epigenome, organisms possess an epitranscriptome [1]. RNA methylation events are at the heart of the epitranscriptome. RNA molecules can be methylated on both the sugar and base, and the focus of this work is RNA cytosine base methylation. There are many different types of RNAs that can be modified by cytosine methylation. In Escherichia coli and archaebacteria, 16S and 23S rRNA are modified by cytosine base methylation, and human 28S rRNA is modified as well [2, 3]. In archaebacteria and eukaryotes, but apparently not bacteria, tRNAs are methylated in a tRNA-specific manner and hotspots include the anticodon loop, anticodon stem, and the variable region-TψC junction [4]. Recent reports also indicate that there are 5-methylcytosine residues in mRNA from the archaeon Solfolobus solfataricus and 8,495 5-methylcytosine residues in the HeLa cell mRNA transcriptome [2, 5]. In HeLa cells, the presence of mRNA harboring 5-methylcytosine is dependent upon an enzyme that methylates tRNA (NSun2), suggesting a link between mRNA and tRNA methylation [5].
We were interested in determining if RNA methylation pathways exist in T. brucei for two reasons. First, in T. brucei, regulation of gene expression occurs at the posttranscriptional level where RNA is a key player and has been a focus of numerous investigations. Thus, RNA modifications may be especially important with respect to gene regulation in this organism. Second, it has been demonstrated that the eukaryotic enzyme DNA methyltransferase 2 (Dnmt2) has the ability to methylate RNA, in particular tRNA [6]. This may be relevant to protozoan parasites, as DNA methyltransferase enzymes have been identified in several organisms including Trypanosoma brucei and Entamoeba histolytica [7, 8]. However, a role for DNA methylation in protozoan parasites is relatively undefined. For example, in Entamoeba histolytica, blocking DNA methylation with the inhibitor 5-azacytidine affects the expression of only a small subset of genes (~2%) [9]. Additionally, some protozoan parasites such as Cryptosporidium parvum and Plasmodium falciparum contain DNA methyltransferase homologues but lack detectable DNA methylation [7, 10], which suggests that protozoal DNA methyltransferases may modify another target such as RNA.
With respect to RNA targets, we started with cytosine base modification of T. brucei tRNAs, as tRNAs are the best described methylated RNAs in other organisms. Eukaryotic tRNAs are methylated at several defined locations [4]. The majority of eukaryotic tRNAs that have been examined are methylated at the junction between the variable region and TψC stem at one or more positions via NSun2 (Trm4a/b in S. pombe) [4]. NSun2 is able to methylate cytosine 40 (C40) in the anticodon stem in some eukaryotic tRNAs as well [11]. C38 in the anticodon loop is methylated by Dnmt2 (pmt1 in S. pombe) in S. pombe, Drosophila melanogaster, mice, and humans [5-7, 12-14]. Dnmt2 was originally identified as a DNA methyltransferase homologue, but only has weak enzymatic activity toward DNA both in vitro and in vivo and is thought to function primarily as a tRNA methyltransferase [6, 7]. Other targets for cytosine modification also exist. For example, cytosines 60-62 are methylated in S. pombe tRNAAsp(GUC), and methylation is abrogated in trm4a/trm4b double-knockout strain [12]. It is currently unclear if cytosine base modifications exist in T. brucei RNA.
To determine if 5-methylcytosines exist in T. brucei tRNA, we used a sodium bisulfite sequencing approach. In this approach, RNA is treated with sodium bisulfite thereby converting cytosines to uracil. These residues are then present as thymines following cDNA synthesis and PCR amplification. Importantly, 5-methylcytosine is not converted to thymine by bisulfite treatment, and thus is read as a cytosine after DNA sequencing. This approach has been used heavily for the analysis of DNA methylation and has recently been modified for use with RNA molecules including tRNA and mRNA [2, 3, 5, 12-14]. Is it important to note that bases other than 5-methylcytosine can be resistant to bisulfite conversion [5]. The best described resistant base is 5-hydroxymethylcytosine, although the presence of 5-hydroxymethylcytosine in RNA has not been reported to our knowledge [2, 15]. For simplicity, we have used the term 5-methylcytosine to describe cytosines that are resistant to sodium bisulfite-mediated deamination, but cannot exclude the presence of other modified cytosine bases. For the sodium bisulfite sequencing reactions described below, we followed the protocol of Schaefer et al. [13]. RNA (1-2.5 μg) isolated from T. brucei strain 29-13 was treated with sodium bisulfite for 3-4 cycles, purified, and used as a template for cDNA synthesis. Stem loop primers were used for cDNA synthesis to lengthen the tRNA amplicons (Table I, Figure 1A), as these amplicons are short and can comigrate with PCR primer dimers. PCRs were performed using primers that bind to the deaminated versions of the cDNA (Table I, Figure 1B). PCR products for all tRNA targets were only detected when cDNA was made in the presence of reverse transcriptase, indicating sequencing results are from the tRNA itself and not the tRNA gene (Figure 1C). PCR products were inserted into pGEM-T Easy plasmids and sequenced. 20 clones or more were analyzed per experiment.
Table I.
Oligonucleotides used for reverse transcription and amplification of bisulfite treated small RNA
| Target | Expc | Oligonucleotides(s)d |
|---|---|---|
| tRNAAsp(GUC) | RT | 5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGCTTCCCA3′ |
| tRNAAsp(GUC) | PCR | 5′TTTTTGGTAGTATAGTGG3′, 5′CTGGTGTCGTGGAGT3′ |
| tRNAGly(GCC) | RT | 5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGTAATACACA3′ |
| tRNAGly(GCC) | PCR | 5′GTAGTTGGTTTAGTGGTAGA3′, 5′CTGGTGTCGTGGAGT3′ |
| tRNATyr(GUA), Ua | RT | 5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGTCCTTCC3′ |
| tRNATyr(GUA), Ua | PCR | 5′AATTGGTAGAGTATGTGATTGTAGAGTATG3′, 5′CTGGTGTCGTGGAGT3′ |
| tRNATyr(GUA), Sb | RT | 5′CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGGTCCTTCC3′ |
| tRNATyr(GUA), Sb | PCR | 5′GAGTATGTGATTGTAGATTATAGGGT3′, 5′CTGGTGTCGTGGAGT3′ |
| tRNAVal(AAC) | RT | 5′GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGGTAATACACCAAT3′ |
| tRNAVal(AAC) | PCR | 5′TGATGGTTTAGGTGGTTATGA3′, 5′GTGCAGGGTCCGAGGT3′ |
U, unspliced
S, spliced
RT, reverse transcription
stem loops in RT primers are underlined
Figure 1. PCR amplification of sodium bisulfite treated small RNAs.
Small RNAs were treated with sodium bisulfite and used as a template for cDNA synthesis. A) Primers for cDNA synthesis hybridize to the 3′ end of the tRNA and contain a stem loop. Each primer hybridizes to 7-12 nucleotides at the 3′ end of the tRNA. B) The forward PCR primer binds to the 5′ end of the tRNA. The reverse PCR primer binds to the stem loop. The cDNA primers and forward PCR primers were designed to be complementary to the deaminated cDNA assuming no modified cytosines in the priming region. C) PCRs either lacked template (H2O), contained mock cDNA made in the absence of reverse transcriptase (-RT), or contained cDNA made in the presence of reverse transcriptase (+RT). Reactions were separated by agarose gel electrophoresis and stained with GelRed.
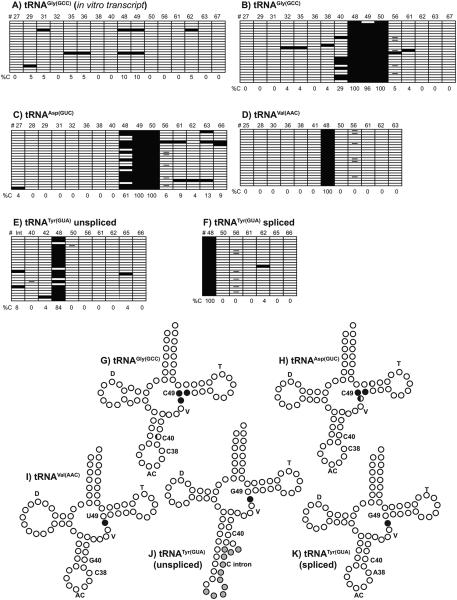
Successful sodium bisulfite sequencing is heavily dependent on robust deamination of non-modified cytosines to uracil, and a lack of cytosine deamination will result in a high rate of false positives. To determine the deamination rate of our procedure in regards to tRNA, we prepared in vitro synthesized tRNAGly(GCC) by transcription of a tRNAGly(GCC) PCR product with an engineered T7 promoter. The in vitro transcription reactions were performed in the absence of 5-methylcytosine to generate unmodified tRNAGly(GCC). The unmodified tRNA was analyzed by sodium bisulfite sequencing as described above. Only 9 of the 320 cytosines analyzed were resistant to bisulfite conversion (Figure 2A). Thus, the deamination rate for this reaction is 97.2%, and the level of false positives is low at <3%. Our RNA deamination rate is similar to rates recently reported by Edelheit et al. [2]. Most positions containing cytosines exhibited 100% deamination in our experiments, and all positions containing cytosines had 90% or greater deamination. Based on the data above, we considered specific cytosines with >10% bisulfite resistance to be 5-methylcytosines.
Figure 2. Sodium bisulfite sequencing analysis of procyclic form T. brucei tRNAs.
PCR products from bisulfite treated small RNAs were analyzed by DNA sequencing. A-F) Diagrams of modification patterns of in vitro synthesized tRNAGly(GCC) A) and native T. brucei tRNAs (B-F). Each horizontal row represents one sequencing reaction. Open boxes represent Ts, filled boxes represent Cs, and minus signs (-) represent missing sequencing information in individual clones. Numbers on the top of each diagram represent the position of individual Cs in the tRNA (#), and numbers at the bottom of each diagram represent the percent of sequences with Cs in a particular position (%C). G-K) Patterns of modification with respect to predicted tRNA secondary structures. D is the D-loop, T is the TψC loop, V is the variable region, and AC is the anticodon loop. For unspliced tRNATyr(GUA) (J), gray circles represent bases in the intron. Positions 38, 40, and 49 are labeled for reference. Open circles, cytosines with 10% or less modification (or non-cytosines); ¼ filled circles, cytosines with 11-40% modification; ½ filled circles, cytosines with 41-70% modification; and completely filled circles, cytosines with 71-100% modification.
To sequence native T. brucei tRNAs, we originally started with T. brucei total RNAs generated by Trizol extraction. However, our initial attempts with total RNA were largely unsuccessful, despite the presence of small RNAs in the sample (Supplemental Figure 1A). This was due to non-specific amplification using the AT-rich tRNA primers (data not shown). Thus, we enriched T. brucei total RNA for small RNAs using LiCl precipitation. Total RNA was incubated in the presence of LiCl, and large RNAs were collected via centrifugation. The supernatant was precipitated and analyzed by RNA bioanalysis (Supplemental Figure 1B and 1C). The supernatant was enriched for small RNAs less than 200 nucleotides, with a major peak ~75 nucleotides in size which is the typical size of a tRNA. Small RNA fractions were used for all downstream experiments.
We selected specific tRNAs for sodium bisulfite sequencing based on studies using sodium bisulfite sequencing in other organisms [5, 11-14]. The tRNAs analyzed in this study were tRNAAsp(GUC), tRNAGly(GCC), tRNAVal(AAC), and tRNATyr(GUA). The sequencing data are presented as a list of sequences in Figures 2B-F. In addition, the positions of 5-methylcytosines are mapped to predicted secondary structures of the tRNAs using tRNAScan-SE (Figures 2G-K) [16]. Based on our sodium bisulfite sequencing data, tRNAGly(GCC) showed robust methylation of three cytosines at the junction between the variable region and TψC stem, and partial methylation (29%) at C40 in the anticodon stem. There were no 5-methylcytosines present at C38 in the anticodon loop or at other positions in this tRNA. T. brucei tRNAAsp(GUC) showed robust methylation at the variable region-TψC junction at C47, C48, and C49. There was also weak evidence for the presence of 5-methylcytosine at C63 in this tRNA. There were no additional 5-methylcytosines found at other positions in T. brucei tRNAAsp(GUC), including C38 in the anticodon loop or C40 in the anticodon stem. For T. brucei tRNAVal(AAC), we observed robust methylation of C48 at the variable region-TψC junction, but not C50 in the TψC stem. There were no other 5-methylcytosines found in T. brucei tRNAVal(AAC), including C38 in the anticodon loop.
In T. brucei, only one tRNA requires splicing as part of the maturation pathway, that being tRNATyr(GUA) [17, 18]. Recent work by Rubio et al. demonstrated that the intron of tRNATyr(GUA) is modified by editing events (base changes), and the editing events are required for efficient intron removal [17]. We compared the cytosine methylation pattern of unspliced versus spliced tRNATyr(GUA). The unspliced tRNATyr(GUA) has one cytosine in the intron. An analysis of this C indicates that <10% of the cytosines are resistant to bisulfite, suggesting that the intron is not a major target of cytosine methylation and that intronic cytosine methylation is not a prerequisite for splicing. However, both unspliced and spliced tRNATyr(GUA) exhibit robust methylation at the junction between the variable region and TψC stem. The levels of methylation are 84% for the unspliced tRNATyr(GUA) and 100% for the spliced tRNATyr(GUA). These data indicate that tRNA cytosine methylation in the exons can precede splicing, and the simplest model is that methylation typically precedes splicing as almost every unspliced tRNATyr(GUA) sequence displays cytosine methylation.
One potentially interesting finding in our studies is the absence of detectable methylation at C38. Three of the four T. brucei tRNAs that were analyzed contain a cytosine at position 38 and have the potential to be methylated; tRNAAsp(GUC), tRNAGly(GCC), and tRNAVal(AAC). Although tRNAs from humans, mice, D. melanogaster, and S. pombe show evidence of C38 methylation in this position, it was not detectable in the three T. brucei tRNAs analyzed here. The lack of C38 methylation in T. brucei was not due to a technical issue, as we identified C38 methylation in 22/22 clones in tRNAAsp(GUC) analyzed from human placental RNA (data not shown). Thus, one simple model is that the cytosine 38 methylation pathway of T. brucei tRNAs is absent. Alternatively, it is possible that C38 tRNA methylation is present in another life cycle stage not examined in our studies, or under different growth conditions. We analyzed tRNAGly(GCC) and tRNAVal(AAC) for cytosine methylation in bloodstream form parasites (single marker strain) as examples of tRNAs with high and low methylation levels in procyclic form parasites (Supplemental Figure 2). We did not observe methylation of C38 in either tRNA in bloodstream forms, although methylation at the variable region-TψC junction was present in both tRNAs, and relatively high levels of C40 methylation were detected for tRNAGly(GCC). Yet, it is still possible that C38 methylation exists in other stages of the parasite’s life cycle not examined in our study. The vast majority of tRNAs do not harbor a cytosine at position 38, but it is not possible to rule out that T. brucei tRNAs other than those analyzed contain C38 methylation. T. brucei tRNAHis(GUG), tRNAGlu(CUC/UUC), and tRNALeu(UAG) all contain a predicted cytosine at position 38, but there is little evidence for C38 modification of these molecules in other organisms [12, 15]. In any case, further investigations will be required to determine if and when C38 is modified.
The lack of detectable C38 methylation may indicate that tRNA C38 methylation is an evolutionarily new modification compared to methylation at the junction between the variable region and TψC stem. C38 methylation is absent from bacteria and archaebacteria, but present in S. pombe, Drosophila, mice, and humans [5, 11-15]. It is important to note that T. brucei is an early branching eukaryote. One model for the evolution of tRNA methyltransferases is that bacterial DNA methyltransferases are ancestral to tRNA methyltransferases [7]. An analysis of the T. brucei genome indicates that there is one DNA methyltransferase homologue, TbDMT [8]. TbDMT contains the ten conserved motifs found in all enzymes that methylate DNA, and these ten motifs are also found in some enzymes that modify tRNA. Dnmt2 enzymes that modify tRNA have a conserved CFT motif adjacent to conserved motif IX. Interestingly, TbDMT is more homologous to bacterial DNA methyltransferases and the CFT motif is absent [8]. Thus, it is possible that TbDMT is not a member of the tRNA modification family, and this could explain the lack of detectable C38 methylation of T. brucei tRNAs. Several other protozoan parasites including Plasmodium spp., Cryptosporidium spp., and Entamoeba histolytica contain DNA methyltransferase homologs that do contain the CFT motif next to domain IX, and are homologous to Dnmt2 in contrast to bacterial DNA methyltransferases [7]. Thus, other protozoan parasites may have the ability to methylate C38 in tRNA, and the ability of the E. histolytica Dnmt2 homologue to methylate tRNAAsp in vitro is consistent with this model [19].
Three of the four T. brucei tRNAs analyzed here contain a cytosine at position 40 in the anticodon stem: tRNAGly(GCC), tRNAAsp(GUC), and tRNATyr(GUA). Of the three tRNAs, only one showed evidence of methylation, that being tRNAGly(GCC). Thus, T. brucei has the ability to methylate C40, yet not all tRNAs with a cytosine in this position are methylated. Furthermore, T. brucei tRNAGly(GCC) displays partial methylation at C40, meaning that not all tRNAGly(GCC) molecules are modified. Partial methylation of human tRNAGly(GCC) has been observed [11], although the significance is unknown. Partial modification of tRNAGly(GCC) suggests that there are two or more populations of tRNAGly(GCC) with potentially different functions and/or the levels of modification at this site are controlled.
In many eukaryotic tRNAs, there are one to three methylated cytosines at the junction between the variable region and tRNA TψC stem [4]. Our sodium bisulfite sequencing data indicate that all T. brucei tRNAs analyzed have between one to three 5-methylcytosines in this region, and thus the pathway to generate cytosine methylation at the variable region-TψC junction is present in T. brucei. BLAST searches for the presence of T. brucei NSun2 homologues are positive, consistent with a model for active cytosine methylation in this region (data not shown). Interestingly, the levels of methylation at this site are different for different tRNAs. For tRNAGly(GCC), all three Cs in the region are highly methylated. For tRNAAsp(GUC), two of the three cytosines are highly methylated, and one cytosine is partially methylated (61%). For tRNAVal(AAC) and the unspliced and spliced tRNATyr(GUA) tRNAs, one of the two cytosines present is methylated. Thus, all four T. brucei tRNAs analyzed demonstrate cytosine methylation near the variable region-TψC junction, but the levels of methylation are different for different tRNAs. Thus, the presence of a cytosine at a particular methylation hotspot is not sufficient to dictate modification.
It is interesting to note that the number of modified cytosines in a T. brucei tRNA region does not always match eukaryotic counterparts. For example, T. brucei tRNAAsp(GUC) seems to be hypermethylated at the variable region-TψC junction as the T. brucei tRNA has three modified cytosines, but only one to two modified cytosines are found in this position in S. pombe, Drosophila, mouse and human tRNAAsp(GUC) [5, 11-14]. In contrast, T. brucei tRNAVal(AAC) has one 5-methylcytosine near the variable region-TψC junction, whereas mouse tRNAVal(AAC) has two 5-methylcytosines [11]. Thus, while the variable region-TψC junction hotspot is apparently present in numerous organisms, the level of cytosine methylation is organism dependent.
Our data demonstrating cytosine modification at the variable region-TψC junction are consistent with a model in which modification of this region is evolutionarily ancient. Although methylation at the variable region-TψC junction has not been identified in bacteria, it is present in some archaebacteria, the early branching eukaryote T. brucei, unicellular eukaryotes such as S. pombe, and metazoans [2, 5, 11-15]. The function of cytosine methylation at the variable region-TψC junction is starting to emerge, and the modifications may stabilize tRNA against degradation [11]. In T. brucei, the levels of tRNAs do not obviously correlate with gene copy number, and it is possible that differential stability of tRNAs via different numbers of methylated cytosines could contribute to different steady-state tRNA levels [20]. In humans, the enzyme that modifies cytosine near the variable region-TψC junction, NSun2, also generates 5-methylcytosine in mRNA [5], and thus is it is possible that other types of RNA in T. brucei contain modified bases including mRNA. Future experiments will focus on determining which types of RNA are modified in protozoan parasites, and elucidating functions for these modifications.
Supplementary Material
Highlights.
The T. brucei tRNAs analyzed contain between 1-4 5-methylcytosines.
Cytosine residues near the variable region-TψC junction are frequently methylated.
T. brucei tRNAGly(GCC) displays cytosine methylation at C40 of the anticodon stem.
There is no evidence for 5-methylcytosine at C38 in the T. brucei anticodon loop.
Acknowledgements
The authors thank Daria Vorojeikina for technical support, Laurie K. Read for helpful discussions and Michelle Zanche for help with the RNA bioanalysis. The authors thank Robert D. Simon and Elizabeth Hutchison for critical reading of the manuscript. This work was funded by NIH grant R15AI074035 (K.T.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saletore Y, Meyer K, Korlach J, Vilfan ID, Jaffrey S, Mason CE. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol. 2012;13:175. doi: 10.1186/gb-2012-13-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013;9:e1003602. doi: 10.1371/journal.pgen.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motorin Y, Lyko F, Helm M. 5-methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Res. 2010;38:1415–30. doi: 10.1093/nar/gkp1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Towns WL, Begley TJ. Transfer RNA methytransferases and their corresponding modifications in budding yeast and humans: activities, predications, and potential roles in human health. DNA Cell Biol. 2012;31:434–54. doi: 10.1089/dna.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–33. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goll MG, Kirpekar F, Maggert KA, Yoder JA, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–8. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 7.Jurkowski TP, Jeltsch A. On the evolutionary origin of eukaryotic DNA methyltransferases and Dnmt2. PLoS One. 2011;6:e28104. doi: 10.1371/journal.pone.0028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Militello KT, Wang P, Jayakar SK, Pietrasik RL, Dupont CD, Dodd K, King AM, Valenti PR. African trypanosomes contain 5-methylcytosine in nuclear DNA. Eukaryot Cell. 2008;7:2012–6. doi: 10.1128/EC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali IK, Ehrenkaufer GM, Hackney JA, Singh U. Growth of the protozoan parasite Entamoeba histolytica in 5-azacytidine has limited effects on parasite gene expression. BMC Genomics. 2007;8:7. doi: 10.1186/1471-2164-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gissot M, Choi SW, Thompson RF, Greally JM, Kim K. Toxoplasma gondii and Cryptosporidium parvum lack detectable DNA cytosine methylation. Eukaryot Cell. 2008;7:537–40. doi: 10.1128/EC.00448-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuorto F, Liebers R, Musch T, Schaefer M, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19:900–5. doi: 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- 12.Becker M, Muller S, Nellen W, Jurkowski TP, Jeltsch A, Ehrenhofer-Murray AE. Pmt1, a Dnmt2 homolog in Schizosaccharomyces pombe, mediates tRNA methylation in response to nutrient signaling. Nucleic Acids Res. 2012;40:11648–58. doi: 10.1093/nar/gks956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer M, Pollex T, Hanna K, Lyko F. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res. 2009;37:e12. doi: 10.1093/nar/gkn954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–62. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio MA, Paris Z, Gaston KW, Fleming IM, Sample P, Trotta CR, Alfonzo JD. Unusual noncanonical intron editing is important for tRNA splicing in Trypanosoma brucei. Mol Cell. 2013;52:184–192. doi: 10.1016/j.molcel.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider A, McNally KP, Agabian N. Splicing and 3′-processing of the tyrosine tRNA of Trypanosoma brucei. J Biol Chem. 1993;268:21868–74. [PubMed] [Google Scholar]
- 19.Tovy A, Hofmann B, Helm M, Ankri S. In vitro tRNA methylation assay with the Entamoeba histolytica DNA and tRNA methyltransferase Dnmt2 (Ehmeth) enzyme. J Vis Exp. 2010;44:2390. doi: 10.3791/2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan TH, Pach R, Crausaz A, Ivens A, Schneider A. tRNAs in Trypanosoma brucei: genomic organization, expression, and mitochondrial import. Mol Cell Biol. 2002;22:3707–17. doi: 10.1128/MCB.22.11.3707-3716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.