INTRODUCTION
Attention Deficit Hyperactivity Disorder (ADHD) affects millions of children and adults in the US and is a significant independent risk factor for smoking (Kessler et al., 2006; Lambert & Hartsough, 1998; Milberger et al., 1997a; Milberger et al., 1997b; Molina & Pelham, 2003; Pomerleau et al., 1995; Visser et al., 2007). Individuals with ADHD start smoking at an earlier age and become more dependent (Milberger et al., 1997a; Wilens et al., 2008). Moreover, individuals with ADHD or high levels of ADHD symptoms are also more likely to progress from smoking experimentation to regular use (Rohde et al., 2004). For example, we found that high levels of ADHD symptoms in a population-based sample were associated with progression from experimentation or no use in the teenage years to daily smoking in the early 20s (Fuemmeler et al., 2007). Given the extraordinary morbidity and mortality associated with cigarette smoking (CDC, 2002), the high rate of smoking observed among those with ADHD is therefore a significant public health issue. A clearer understanding of the factors that increase risk for smoking in those with ADHD would be an important step towards preventing and treating smoking in this high-risk group.
The association between ADHD and smoking is complex and involves multiple stages (McClernon & Kollins, 2008), although the precise mechanisms conferring risk have not been thoroughly elucidated. Convergent evidence suggests that dopamine-mediated disruptions in reinforcement processes are involved in key aspects of smoking behavior among individuals with ADHD. The overall goal of the present review is to consider the evidence for the role of dopamine and reinforcement processes in increased risk for smoking and related outcomes in patients with ADHD. The review will be organized as follows. First, we will review both historical and current perspectives on the role of dopamine functioning in ADHD. Second, we will consider evidence implicating disrupted reinforcement processes in ADHD. We will then discuss the few studies that have explicitly linked dopaminergic dysfunction to altered reinforcement processes in ADHD. The relevance for this association to understanding smoking risk in individuals with ADHD will then be evaluated. We will conclude with suggestions for future research in this area.
DOPAMINE AND ADHD
Historical Perspectives on Catecholamine Function in ADHD
For decades, researchers and clinicians have speculated about the role of disrupted neurotransmission and subsequent reinforcement processes as key features of ADHD. Paul Wender, an early pioneer in the study of ADHD and its treatment, wrote in 1973 that Minimal Brain Dysfunction (MBD; a nosological precursor to ADHD) was “characterized by…a diminished sensitivity to positive and negative reinforcement,” and “…that these deficits are secondary to disorders of monoamine metabolism and that such disorders may occur on a genetic basis”(Wender, 1973). This reasoning was supported by several clinical and scientific observations: 1) MBD and related problems were likely to run in families; 2) stimulant drugs were effective for improving behavior problems in children with MBD and related difficulties (Bradley, 1937); and 3) that these same drugs facilitated monoamine neurotransmission in animals (Schildkraut & Kety, 1967; Wender et al., 1971). In the 40 years since Wender’s prescient speculation, significant progress has been made that provides support for his hypotheses. We will briefly consider the evidence for the genetic basis of ADHD (with emphasis on genes associated with dopamine neurotransmission) and the direct measurement of dopamine neurotransmission in individuals with ADHD.
Genetic Studies of ADHD – Links to Dopamine Function
As Wender noted, it has long been observed that problems associated with ADHD run in families. Family, twin, and adoption studies all provide strong support for the genetic basis of the disorder, with heritability estimates from twin studies as high as 0.7-0.8 (Faraone et al., 2005). Since the mid-1990s, several hundred candidate gene studies have been conducted to isolate specific variants conferring risk for the disorder. Although these studies have often been characterized by small effect size and failures to replicate, several gene variants have consistently been shown to increase risk for ADHD. Perhaps not surprisingly, most of these candidate genes are involved in catecholamine function generally, and dopamine function specifically. In one meta-analysis, seven candidate genes were identified that demonstrated significant pooled odds ratios for conferring risk for ADHD across at least 3 separate studies. Of these, 5 of the genetic variants were explicitly involved in dopamine neurotransmission: 2 variants of the dopamine D4 receptor gene (DRD4), the dopamine D5 receptor gene (DRD5), the dopamine transporter gene (DAT) and the dopamine beta-hydroxylase gene (DBH) (Faraone et al., 2005). More recently, a meta-analysis specifically focused on dopamine receptor genes (D1-D5), found associations between variants of the DRD4 gene, the DRD5 gene, and the dopamine D2 (DRD2) gene and risk for ADHD, although due to heterogeneity, findings for the DRD2 gene were deemed to be invalid. (Wu et al., 2012). Although genome-wide linkage and association studies of ADHD have generally not found evidence for the involvement of regions relevant to dopamine neurotransmission (Faraone & Mick, 2010), the results of candidate gene studies provide some support for dopamine-related genes as contributors of risk, albeit small, for the development of ADHD.
Functional Role of Dopamine Gene Variants Implicated in ADHD
Although the functional role of specific dopamine gene variants implicated in ADHD has been partially characterized in nonhuman species and in vitro, work in humans more limited. The most common variant of the DRD4 gene associated with ADHD is a VNTR polymorphism in exon III of the gene, specifically a 7-repeat polymorphism. This variant has been shown to cause a blunted dopaminergic response as compared to other (eg., 4-repeat, 2-repeat) variants (Asghari et al., 1995; Van Tol et al., 1992). In humans, the 7-repeat variant of DRD4 has been linked to differences in ventral striatal activity during reward tasks (Forbes et al., 2009; Nikolova et al., 2011).
The DRD5 gene is expressed widely in CNS and has a significantly higher affinity for dopamine than the DRD1 gene, despite strong similarity in membrane structure (Wu et al., 2012). In general, the DRD5 gene is thought to modulate hypothalamic function and aspects of motor control (Apostolakis et al., 1996; Rivkees & Lachowicz, 1997; Sibley, 1999). The specific functional role of the variant that has been associated with ADHD – a 148-bp allele located in18.5 kb at the end of the 5’ flank – in not known (Wu et al., 2012).
Variations in the dopamine transporter gene (DAT1/SLC6A3) have been implicated in striatal dopamine function in both human and in vitro studies, though findings are somewhat mixed (Heinz et al., 2000; van de Giessen et al., 2009; VanNess et al., 2005). The 10-repeat variant of a VNTR polymorphism in the 3’ untranslated region of the gene is most often implicated in the presentation of ADHD, and some studies have reported that 9-repeat carriers of this variant express higher levels of striatal dopamine, while other studies have reported that 10-repeat carriers express more DAT (Heinz et al., 2000; VanNess et al., 2005). Animal models that either knock down or knock out the DAT transporter altogether present with many ADHD-like phenotypes, including increased activity levels that are normalized to wild type levels with stimulant administration (Gainetdinov et al., 1999; Giros et al., 1996; Zhuang et al., 2001).
The DBH gene is involved in the enzymatic pathway that controls the conversion of dopamine to norepinephrine. Several mutations of this gene have been shown to result in DBH deficiency, a relatively rare condition in which low levels of norepinephrine cause difficulty regulating blood pressure and other autonomic nervous system problems (Senard & Rouet, 2006). The specific variant of the DBH gene most widely studied in ADHD is a TaqI polymorphism in the 5th intron (Daly et al., 1999; Faraone et al., 2005). Although this variant has been reported to confer risk for other psychiatric/behavioral conditions (e.g., smoking, schizophrenia) (Freire et al., 2006; Wei et al., 1998), the specific function of this polymorphism has not been reported.
Assessing dopamine dysfunction in ADHD
In addition to progress in the identification of dopamine relevant genetic factors associated with ADHD risk, technological advances in the past 25 years have allowed for more specific inferences to be made regarding how dopamine and other aspects of brain function are related to the clinical features of the disorder. Early attempts to quantify the role of dopamine and other neurotransmitters in ADHD focused on peripheral measures, such as the excretion of urinary metabolites, and results were mixed (Khan & Dekirmenjian, 1981; Shekim et al., 1982; Wender et al., 1971). Since a number of factors can contribute to the levels of such excreted metabolites, these findings are difficult to interpret vis-à-vis the role of these neurotransmitters in the central nervous system.
Wender lamented 40 years ago that “Currently available techniques do not permit direct assessment of neurotransmitter function in the central nervous system” (Wender, 1973). In the ensuing years, however, a number of neuroimaging techniques have been developed to more directly evaluate brain structure and function. Early studies using magnetic resonance imaging (MRI) reported consistent morphological differences between ADHD patients and control subjects in brain regions known to be richly innervated by dopamine containing neurons (eg., caudate nucleus; (Castellanos et al., 1994; Hynd et al., 1993)). Studies using functional MRI also reported differences in activation between ADHD and control subjects in dopamine-rich regions, and showed that stimulant drugs altered the patterns of activation in predictable ways (Rubia et al., 1999; Vaidya et al., 1998).
While MRI and fMRI studies allow for inferences regarding physiological activation in dopaminergic brain regions, Positron Emission Tomography (PET) imaging directly measures dopamine activity by measuring the extent to which the neurotransmitter is displaced by radio-labeled tracers in the working brain. The first study to use PET in patients with ADHD was conducted by Zametkin and colleagues and demonstrated deficits in overall cerebral glucose metabolism in adults with ADHD compared to non-ADHD individuals (Zametkin et al., 1990). One of the first studies to explicitly examine dopamine activity using PET imaging used the radiotracer [fluorine-18]fluorodopa, which allows for the assessment of presynaptic dopamine receptor density, in adults with and without ADHD. Results showed significantly reduced presynaptic dopamine storage in the prefrontal cortex that was significantly correlated with a clinical measure of ADHD symptoms/function (Ernst et al., 1998). Similar findings were subsequently reported in children with ADHD, as well as identifying similar patterns of dopamine storage deficiencies in midbrain regions (Ernst et al., 1999).
A number of subsequent studies have examined the density of dopamine transporter (DAT) in midbrain regions. As noted previously, the DAT gene has been implicated in ADHD and the transporter is known to be a central site of action for methylphenidate. Initial studies were mixed, with some reporting decreased DAT density in ADHD patients compared to controls (Volkow et al., 2007), some reporting increased DAT density (Spencer et al., 2007), and some reporting no differences (van Dyck et al., 2002). Other studies demonstrated increases in dopamine D2/D3 receptor activity in striatal regions in adolescents with ADHD (Lou et al., 2004).
Some of these inconsistencies across PET studies of DAT density could have been related to methodological factors (eg., small sample sizes, use of different radiotracers) or patient characteristics (eg., varying histories of medication use). One of the more recently published PET studies of dopamine receptor density in ADHD sought to address some of these difficulties by enrolling a relatively large sample of adults with (n=53) and without (n=44) ADHD, who had no previous history of medication treatment (Volkow et al., 2009). Using [11C]cocaine and [11C]raclopride to image DAT and D2/D3, respectively, this study reported significant decreases in receptor density for both subtypes in brain regions associated with reward and motivation – nucleus accumbens, caudate, midbrain). Moreover, DAT and D2/D3 density was negatively correlated with severity of ADHD symptoms (Volkow et al., 2009).
Collectively, both genetic and neuroimaging studies in the past 20-30 years have supported early speculation regarding the importance of dopamine activity in the presentation of ADHD. Genetic factors associated with dopamine receptor regulation are consistently associated with ADHD and neuroimaging studies have shown that brain structure and function in dopamine-relevant regions are altered in patients with ADHD.
ADHD AND DISRUPTED REINFORCEMENT PROCESSES
Although historically, ADHD was conceptualized primarily as a disorder associated with disrupted cognition and executive functioning, (Barkley, 1997), most recent models of the disorder have acknowledged the critical role of motivational and reinforcement processes in the presentation of the disorder (Luman et al., 2010; Johansen et al., 2009; Nigg & Casey, 2005; Sagvolden et al., 1998; Sagvolden et al., 2005; Sonuga-Barke, 2005; Sonuga-Barke, 2002; Tripp & Wickens, 2008; Williams & Dayan, 2005). Although defined in different ways, these models are consistent in theorizing that the behavior of individuals with ADHD is not influenced by motivationally-relevant stimuli in the same manner as non-ADHD individuals. In both theoretical models and empirical studies, the construct of motivation has generally been defined on the basis of how externally imposed consequences differentially influence behavior in those with and without ADHD. Work in this area has generated several consistent and important findings (Luman et al., 2010; Luman et al., 2005).
Immediately delivered rewards improve the behavior of individuals with ADHD
A number of studies have examined the effects of contingent monetary incentives on the performance of individuals with ADHD (Luman et al. 2005). These studies have been conducted in both pediatric and adult populations, and have examined a range of outcomes. In the majority of studies, immediate and externally-delivered reward improved the performance of ADHD patients, often to a greater extent than non-diagnosed controls (Carlson et al., 2000; Carlson & Tamm, 2000; Dovis et al., 2012; Konrad et al., 2000; Marx et al., 2013; McInerney & Kerns, 2003; Strand et al., 2012). For example, in one recent study, the effects of reward were studied across a range of cognitive tasks in adults with and without ADHD. On measures of attention (reaction time variability on a Continuous Performance Test), impulsivity (Commission errors on a Continuous Performance Test) and time perception, the relative effects of reward on performance were greater for adults with ADHD compared to controls (Dovis et al., 2012). Similarly, in a study with children diagnosed with ADHD, immediately delivered rewards improved performance to a similar degree as a moderate dose of MPH, though performance was still not “normalized” compared to a control group of non-diagnosed children performing the task without medication or reward (Strand et al., 2012). Also of relevance, a number of studies that have examined the effects of immediate incentives on behavior/performance have also found that these consequences increase self-ratings of task motivation (Carlson et al., 2000; Carlson & Tamm, 2000; Dovis et al., 2012; McInerney & Kerns, 2003; Scheres et al., 2001).
A vast clinical literature also supports the use of immediate and contingent reinforcement as the most effective non-pharmacological approach to modifying the behavior of individuals with ADHD. Recently developed and validated non-pharmacological treatments specifically for adults with ADHD also focus on teaching patients ways to increase motivation or self-deliver rewards for adaptive behavior. (Safren et al., 2010; Solanto et al., 2010) For example, 2 of the 3 core modules of Solanto et al.’s meta-cognitive therapy for adult ADHD involve training patients how to “provide contingent self-reward” and “sustain motivation toward distant goals by visualizing long-term rewards.”(Solanto et al., 2010)
Delayed Rewards are Ineffective and Reinforcement Learning is Disrupted in ADHD
Patients with ADHD prefer immediate versus delayed rewards, even if such a choice reduces the overall size of the reward.(Bitsakou et al., 2009; Marco et al., 2009; Rapport et al., 1986; Sonuga-Barke & Taylor, 1992; Sonuga-Barke et al., 1992; Tripp & Alsop, 2001) Individuals with ADHD also exhibit steeper temporal discounting functions, suggesting that the value of delayed rewards diminishes faster as the amount of time until its receipt increases.(Costa Dias et al., 2013; Demurie et al., 2012; Scheres et al., 2010; Scheres et al., 2013) The relative lack of efficacy of delayed reinforcers for those with ADHD is most often interpreted as evidence of impulsivity and it has been demonstrated that methylphenidate, a front line stimulant medication used to treat the disorder, decreases temporal discounting in children with ADHD. (Shiels et al., 2009) Individuals with ADHD also have more difficulty learning to adaptively allocate behavior in order to maximize reinforcement and there is evidence that stimulant drugs improve learning under these conditions (Kollins et al., 1997; Luman et al., 2009a; Luman et al., 2009b; Murray & Kollins, 2000; Tripp & Alsop, 1999). This phenomenon has been studied with a number of different experimental paradigms. A series of elegant experiments using multiple fixed-interval extinction (FI-EXT) schedules of reinforcement demonstrated a number of ways in which the behavior of children with ADHD differed from non-diagnosed children (Sagvolden et al., 1998; Sagvolden, 2000). Importantly, this same experimental arrangement was used to validate behavioral differences between a rat model of ADHD and “control” animals (Sagvolden et al., 1992; Sagvolden, 2000).
Overall, there is clear evidence from the literature supporting a role for altered reinforcement processes in individuals with ADHD. Although some models of the disorder propose that these deficits may be relevant for only a subset of individuals who meet criteria for the clinical diagnosis (Sonuga-Barke, 2003; Sonuga-Barke, 2005), the implications of altered reinforcement processes and learning are evident in day-to-day observations of patients with ADHD. Individuals with ADHD are often observed to persist in maladaptive behaviors despite negative consequences, and are commonly reported to be bored easily – suggesting the reinforcing efficacy of stimuli wanes more quickly for patients than for their non-ADHD peers. Finally, the treatment literature is clear in that salient and immediately delivered consequences are effective for promoting adaptive behavior in individuals with ADHD.
LINKING DOPAMINE DYSFUNCTION AND DISRUPTED REINFORCEMENT
It is well known that dopamine neurotransmission is integrally involved with behavioral reinforcement (Aggarwal & Wickens, 2011; Wickens et al., 2007; Wise, 2006), and decades of work have dissected the neurobiological and neuropharmacological mechanisms involved with reward and reinforcement processes. “Incentive salience” is commonly used to indicate the motivational tag assigned to a specific stimulus, driving the appropriate behavior to obtain a reward (Berridge & Robinson, 1998; Berridge, 2007; Robinson & Berridge, 1993). Incentive salience, thought to elicit the the ‘wanting’ component of the reward response, is associated with mesolimbic dopamine release. Dopaminergic projections from the VTA to the nucleus accumbens are associated with the behavioral response to cues that predict an upcoming reward (Kosobud et al., 1994; Yun et al., 2004). In classic reinforcement studies utilizing Pavlovian conditioning, dopamine release at the time of the conditioned stimulus attributes a motivational salience to the CS (de Borchgrave et al., 2002; Shaham et al., 2003; Bindra, 1978). Recent optogenetic work has demonstrated that phasic dopaminergic activity is sufficient to drive behavioral conditioning (Tsai et al., 2009). However, it has been proposed that tonic levels of dopamine mediate the overall vigor of the motivated response (Niv et al., 2007), and along these lines it has been shown that the hippocampal disinhibition of dopamine neurons both increases spontaneous firing and amplifies phasic responses to the external stimulation (Lodge & Grace, 2005; Lodge & Grace, 2006). Thus, both tonic and phasic dopamine release are critical for the attribution of incentive salience.
Projections from the prefrontal cortex also play an important role in regulating the mesolimbic pathway during reinforcement and motivation. Early work in anesthetized animals established a physiological link between prefrontal inputs to VTA and dopamine release in the nucleus acumbens (Murase et al., 1993; Karreman & Moghaddam, 1996; Karreman et al., 1996), and more recent work has implicated these circuits in motivated behavior and reward learning: In humans working to obtain reward, information about expected reward activated VTA only via its effects on the prefrontal cortex (Ballard et al., 2011), a finding echoed in physiological work in behaving animals (Takahashi et al., 2011) . Glutamatergic projections from the prefrontal cortex to the VTA are necessary for Pavlovian reward learning (Parker et al., 2011) . Together with hippocampally-mediated VTA disinhibition, (Floresco et al., 2001; Grace et al., 2007), prefrontal inputs are also critical for setting tonic dopamine levels in the nucleus accumbens (Taber et al., 1995). Blocking prefrontal glutamatergic transmission to VTA decreases dopaminergic tone (Karreman & Moghaddam, 1996; Karreman et al., 1996) Consequently, prefrontal hypoactivity has the potential to result in overall reduced responsivity to reward-related cues, with potential implications for a broad range of motivated behavior. Schizophrenia patients, another population that exhibits prefrontal dysfunction, are also notably lacking in motivational drive (Barch, 2005; Grace, 1991; Knable & Weinberger, 1997; Weinberger et al., 1986). Prefrontal hypoactivity to natural reinforcers may account for decreased incentive salience in addiction (Volkow et al., 2002a; Volkow et al., 2002b). Together these lines of evidence strongly imply that prefrontal dysfunction will dysregulate dopaminergic function and thus disrupt reinforcement and motivation.
As noted previously, both altered dopamine function and disrupted behavioral reinforcement processes have been implicated in the pathophysiology of ADHD. However, in spite of these demonstrations and despite progress in characterizing the neural mechanisms of behavioral reinforcement, surprisingly little work has been conducted to link observed problems in dopamine neurotransmission with altered reinforcement processes in individuals with ADHD. One study reported that DAT and D2/D3 receptor density in midbrain region was correlated with scores on a trait measure of motivation, an indirect assessment of reinforcement functioning (Volkow et al., 2011). A related study examined the effects of the stimulant drug methylphenidate on extracellular dopamine release during both an academic task and a neutral task. (Volkow et al., 2004). This study found that methylphenidate increased dopamine release, but only in the presence of the academic task, and also reported that the combination of methylphenidate administration and academic task performance increased ratings of motivation and interest. The authors interpreted these findings as evidence for dopamine-mediated increases in reinforcement salience following methylphenidate administration (Volkow et al., 2004).
A series of other studies have explored brain activation in dopamine-rich regions -- specifically the ventral striatum and related areas – during a task designed to assess reinforcement/reward processing. Though not without exception, these studies have generally reported that individuals with ADHD exhibit hyporesponsivity compared to non-diagnosed individuals during relevant tasks. Most studies have examined brain activation during the Monetary Incentive Delay task (MID)(Knutson et al., 2001), in which brain activation is measured during both reward anticipation and following delivery. Studies with both adolescents and adults have reported VTA hypoactivation in ADHD patients during reward anticipation(Carmona et al., 2012; Edel et al., 2013; Hoogman et al., 2013; Scheres et al., 2007; Strohle et al., 2008), but see also (Paloyelis et al., 2012; Stoy et al., 2011). In addition to decreased activation during reward anticipation, other studies have showed striatal hypo-responsiveness in ADHD patients compared to controls during delayed reward processing (Plichta et al., 2009); and altered nucleus accumbens functional connectivity that was associated with temporal discounting in patients with ADHD (Costa Dias et al., 2013)
Though challenging to characterize real-time dopamine function in patients with ADHD, more work is needed to link such dysfunction to behavioral deficits in reinforcement processes, including learning. Such work could focus on the extent to which differences in dopamine functioning are related to deficits in clinically relevant endpoints, such as learning and memory. Some studies have shown a significant association between measures of dopamine function and broad band clinical ratings of ADHD symptoms or trait measures of motivation (Volkow et al., 2009; Volkow et al., 2011), but such studies should be extended to include additional measures.
DOPAMINE, REINFORCEMENT, AND SMOKING RISK IN ADHD
The relationship among dopamine function, reinforcement processes, and risk for smoking among individuals with ADHD is complex, but convergent findings from a number of sources highlight the potential importance of dopamine-mediated reinforcement processes in conferring risk for cigarette smoking and nicotine addiction in patients with ADHD. As reviewed thus far, individuals with ADHD exhibit decreased dopaminergic activity in striatal brain regions that are correlated with symptom severity and impairment (Volkow et al., 2009; Volkow et al., 2011), and also exhibit disruptions in reinforcement processes that are known to be influenced by dopamine activity in the same regions (Luman et al., 2010; Luman et al., 2005; Wickens et al., 2007; Wise, 2006).
Similar brain mechanisms have been implicated in the development and maintenance of nicotine addiction. Preclinical studies have demonstrated that acute nicotine administration facilitates dopamine signaling in similar areas of the striatum, including the nucleus accumbens, and these actions underlie the rewarding/reinforcing effects of the drug (De Biasi & Dani, 2011). In contrast, chronic nicotine exposure and subsequent acute withdrawal both result in substantial reductions in tonic dopamine activity and associated reward-related brain function (Epping-Jordan et al., 1998; Perez et al., 2012; Zhang et al., 2012). Moreover, nicotine withdrawal following chronic exposure results in enhanced sensitivity of DA release to phasic stimulation, such as with acute nicotine administration (Zhang et al., 2012). Moreover, nicotine has been hypothesized to function behaviorally to enhance the reinforcing effects of of non-drug stimuli (Chaudhri et al., 2006; Perkins & Karelitz, 2013).
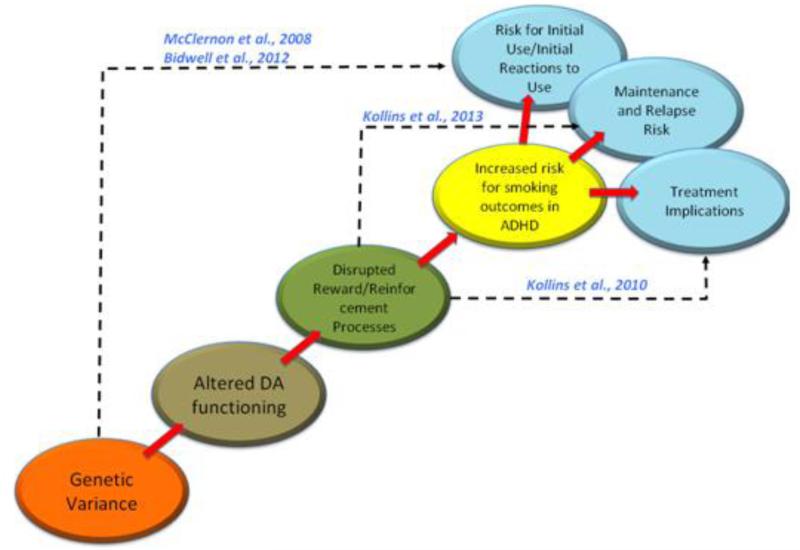
Figure 1 illustrates a simplified pathway linking genetic variation, alterations in dopamine function and related reinforcement processes, and their association with a range of potential smoking outcomes in individuals with ADHD. To this point, the present review has highlighted the extant literature with respect to how genetic factors influence dopamine function and subsequent behavioral reinforcement processes. We will turn attention now to how some of the steps along this trajectory have been explicitly studied with respect to smoking risk in ADHD. Though no studies to date have systematically evaluated this pathway in a single study, results from several investigations support the relevance of different parts of this pathway.
Figure 1.
Proposed translational trajectory for understanding links among genetic varience, dopamine neurotransmission, altered reinforcement processes, and adverse smoking outcomes in individuals with ADHD. Dotted lines and citations refer to work that has been conducted to link various levels of analysis. These studies are described in more detail in the manuscript.
Genetic Variation interacts with ADHD to Predict Smoking Outcomes
Two studies have reported that the association between genetic variation and ADHD symptoms specifically predicts smoking outcomes. These studies used data derived from the National Longitudinal Study of Adolescent Health (Add Health), a population-based survey of more than 15,000 young adults (Harris et al., 2006). One study reported that levels of self-reported ADHD symptoms in the presence of specific variants of the DRD2 and Monoamine Oxidase A (MAO-A) genes predicted risk of lifetime regular smoking, defined as ever having smoked daily for 30 consecutive days (McClernon et al., 2008a). Specifically, individuals with high levels of hyperactive-impulsive symptoms who were also homozygous for the DRD2 A2 allele were significantly more likely to be regular smokers. Moreover, among females, high levels of both inattention and hyperactive-impulsive symptoms, combined with carrying the active allele of the MAO-A gene conferred significant risk for regular smoking (McClernon et al., 2008a).
Another study from the same dataset reported that ADHD symptoms in the presence of gene variants predicted initial reactions to smoking experiences (Bidwell et al., 2012). This study found that individuals with high levels of hyperactive-impulsive symptoms and who were homozygous for the DRD2 A2 allele or who carried a variant of the SLC6A4 (serotonin transporter) gene reported significantly greater pleasant initial reactions to cigarette smoking. Elevated levels of inattention symptoms and variants of the MAO-A and CYP2A6 gene were also significantly related to reports of unpleasant initial reactions to cigarette smoking (Bidwell et al., 2012). These findings suggest that individuals with high levels of ADHD symptoms and specific genotypes may experience initial experiences with smoking in a qualitatively different manner, and this could have substantial implications for predicting risk.
Abstinence-Induced Smoking Reinforcement is Different in Individuals with ADHD
It has been established previously that the reinforcing effects of psychoactive drugs may be different in individuals with and without ADHD (Kollins et al., 2009). A recent study found that following biochemically verified 24-hour abstinence, the reinforcing effects of cigarette smoking are significantly greater in adult regular smokers with ADHD compared to smokers without the disorder (Kollins et al., 2013). Smokers with ADHD in this study worked significantly harder for cigarette puffs compared to money following abstinence and were also more likely to report high subjective ratings of withdrawal symptoms. Combined with other studies showing increased severity of smoking withdrawal in ADHD smokers (McClernon et al., 2008b; McClernon et al., 2011), these findings help explain why individuals with ADHD may have more difficulty quitting smoking compared to those without the disorder (Covey et al., 2008; Humfleet et al., 2005; Pomerleau et al., 1995).
Findings from this study along with other literature reviewed heretofore suggest that individuals with ADHD exhibit alterations in striatal dopamine signaling that are potentially further modified following chronic nicotine exposure. Following acute withdrawal, this hypodopaminergic state is further exacerbated, while increasing the sensitivity of the dopamine system to phasic stimulation, such as exposure to cigarette puffs or cues for smoking (i.e., sensory stimuli). This provides some neurobiological explanation for the findings and highlights the need for additional work to clarify the role of altered dopamine signaling in smoking withdrawal among smokers with ADHD.
Reinforcement-Based Interventions Reduce Smoking in Individuals with ADHD
As noted above, it is well-established that providing immediate and clearly defined rewards contingent on some target behavior can be effective for reducing some of the cognitive and behavioral deficits observed in patients with ADHD. Similar principles of behavioral reinforcement are central to contingency management approaches for reducing smoking and other substance use problems (Ledgerwood, 2008; Petry, 2010; Prendergast et al., 2006). Such treatments typically involve the administration of some kind of reward (eg., money, vouchers, chances for prize drawings, etc.) contingent upon biochemically verified abstinence from drug use. Only study examined a standard contingency management approach for reducing smoking in individuals with and without ADHD (Kollins et al., 2010). Though this study included non-treatment seeking smokers who were generally unmotivated for long-term quitting, results showed that contingency management substantially reduced daily smoking over the course of 2 weeks. Moreover, smokers with ADHD actually maintained slightly higher rates of abstinence compared to non-ADHD smokers (63% vs. 50%), though this difference was not statistically significant. Moreover, rates of abstinence were achieved in spite of higher levels of self-reported withdrawal symptoms (McClernon et al., 2011). These results underscore the potential importance of reinforcement processes in development of novel interventions for smokers with ADHD since immediate and salient (ie., money) consequences for non-smoking contributed to abstinence in spite of worse withdrawal symptoms.
CONCLUSIONS AND FUTURE DIRECTIONS
It is evident that ADHD can be characterized, in part, by both disruptions in dopamine function and altered behavioral reinforcement processes. These constructs are also likely to give rise to a range of adverse outcomes associated with cigarette smoking and nicotine dependence in individuals with ADHD. To date, however, relatively few studies have been conducted that mechanistically link levels of analysis to understand how these various genetic, neurobiological, and behavioral processes can be leveraged to develop better approaches to prevention and treatment for smoking among individuals with ADHD. As shown in Figure 1, several studies have started this translational work by linking 1 or 2 levels, but future work needs to fill in the gaps. From a clinical perspective, very few studies have investigated how standard treatment approaches for smoking cessation fare in individuals with ADHD. Two randomized trials of stimulant drug treatment as an adjunct to nicotine replacement therapy reported that drug (extended release formulations of methylphenidate or amphetamine) were no more effective than placebo for maintaining smoking abstinence, though in both trials, smokers with ADHD reduced overall smoking rates (Kollins et al., 2012; Winhusen et al., 2010). The development of additional novel interventions could be informed by a better understanding of how dopamine and reinforcement processes are altered during regular smoking and abstinence in individuals with ADHD. In addition, given the findings that ADHD symptoms interact with genotype to predict initial responses to smoking, it may be possible to identify a priori, those children or young adolescents who are most prone to smoking and target prevention efforts more directly. Overall, the rates of smoking among individuals with ADHD remain significantly higher than the general population and these individuals find it harder to quit once they do start. There is great potential for reducing the public health impact of this comorbidity by continued work to understand the basic mechanisms that underlying such risk.
Highlights.
ADHD is a risk factor for adverse outcomes related to cigarette smoking
ADHD is associated with altered dopamine neurotransmission
ADHD is associated with disrupted reinforcement processes
Links among dopamine function and reinforcement can help elucidate mechanisms underlying smoking risk in ADHD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal M, Wickens JR. A role for phasic dopamine neuron firing in habit learning. Neuron. 2011;72:892–894. doi: 10.1016/j.neuron.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Apostolakis EM, Garai J, Clark JH, O'Malley BW. In vivo regulation of central nervous system progesterone receptors: cocaine induces steroid-dependent behavior through dopamine transporter modulation of D5 receptors in rats. Mol Endocrinol. 1996;10:1595–1604. doi: 10.1210/mend.10.12.8961269. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31:10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Garrett ME, McClernon FJ, Fuemmeler BF, Williams RB, Ashley-Koch AE, Kollins SH. A preliminary analysis of interactions between genotype, retrospective ADHD symptoms, and initial reactions to smoking in a sample of young adults. Nicotine Tob Res. 2012;14:229–233. doi: 10.1093/ntr/ntr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: A perceptual-motivational alternative to response reinforcements. Behavioral and Brain Sciences. 1978;1:41–52. [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay Aversion in Attention Deficit/Hyperactivity Disorder: an empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94:577–585. [Google Scholar]
- Carlson CL, Mann M, Alexander DK. Effects of reward and response cost on the performance and motivation of children with ADHD. Cognitive Therapy & Research. 2000;24:87–98. [Google Scholar]
- Carlson CL, Tamm L. Responsiveness of children with attention deficit-hyperactivity disorder to reward and response cost: differential impact on performance and motivation. J Consult Clin Psychol. 2000;68:73–83. doi: 10.1037/0022-006X.68.1.73. [DOI] [PubMed] [Google Scholar]
- Carmona S, et al. Response inhibition and reward anticipation in medication-naive adults with attention-deficit/hyperactivity disorder: a within-subject case-control neuroimaging study. Hum Brain Mapp. 2012;33:2350–2361. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Eckburg P, Marsh WL, Vaituzis AC, Kaysen D, Hamburger SD, Rapoport JL. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am J Psychiatry. 1994;151:1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- CDC Annual smoking-attributable mortality, years of potential life lost, and economic costs -- United States 1995-1999. Mortality and Morbidity Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking cessation and inattention or hyperactivity/impulsivity: a post hoc analysis. Nicotine Tob Res. 2008;10:1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Borchgrave R, Rawlins JN, Dickinson A, Balleine BW. Effects of cytotoxic nucleus accumbens lesions on instrumental conditioning in rats. Exp Brain Res. 2002;144:50–68. doi: 10.1007/s00221-002-1031-y. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Temporal discounting of monetary rewards in children and adolescents with ADHD and autism spectrum disorders. Dev Sci. 2012;15:791–800. doi: 10.1111/j.1467-7687.2012.01178.x. [DOI] [PubMed] [Google Scholar]
- Dovis S, Van der Oord S, Wiers RW, Prins PJ. Can motivation normalize working memory and task persistence in children with attention-deficit/hyperactivity disorder? The effects of money and computer-gaming. J Abnorm Child Psychol. 2012;40:669–681. doi: 10.1007/s10802-011-9601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel MA, Enzi B, Witthaus H, Tegenthoff M, Peters S, Juckel G, Lissek S. Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. J Psychiatr Res. 2013;47:350–356. doi: 10.1016/j.jpsychires.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Jons PH, Cohen RM. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209–1215. doi: 10.1176/ajp.156.8.1209. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MT, Marques FZ, Hutz MH, Bau CH. Polymorphisms in the DBH and DRD2 gene regions and smoking behavior. Eur Arch Psychiatry Clin Neurosci. 2006;256:93–97. doi: 10.1007/s00406-005-0610-x. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32:1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. The National Longitudinal Study of Adolescent Health: Research Design. 2006 [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Onnink M, Cools R, Aarts E, Kan C, Arias Vasquez A, Buitelaar J, Franke B. The dopamine transporter haplotype and reward-related striatal responses in adult ADHD. Eur Neuropsychopharmacol. 2013;23:469–478. doi: 10.1016/j.euroneuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Munoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob Res. 2005;7:453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Hern KL, Novey ES, Eliopulos D, Marshall R, Gonzalez JJ, Voeller KK. Attention deficit-hyperactivity disorder and asymmetry of the caudate nucleus. J Child Neurol. 1993;8:339–347. doi: 10.1177/088307389300800409. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Killeen PR, Russell VA, Tripp G, Wickens JR, Tannock R, Williams J, Sagvolden T. Origins of altered reinforcement effects in ADHD. Behav Brain Funct. 2009;5:7. doi: 10.1186/1744-9081-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BH, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Dekirmenjian H. Urinary excretion of catecholamine metabolites in hyperkinetic child syndrome. Am J Psychiatry. 1981;138:108–110. doi: 10.1176/ajp.138.1.108. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, Lane SD, Shapiro SK. Experimental analysis of childhood psychopathology: A laboratory matching analysis of children diagnosed with Attention Deficit Hyperactivity Disorder (ADHD) The Psychological Record. 1997;47:25–44. [Google Scholar]
- Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;204:73–83. doi: 10.1007/s00213-008-1439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, English JS, Itchon-Ramos N, Chrisman AK, Dew R, O'Brien B, McClernon FJ. A Pilot Study of Lis-Dexamfetamine Dimesylate (LDX/SPD489) to Facilitate Smoking Cessation in Nicotine-Dependent Adults With ADHD. J Atten Disord. 2012 doi: 10.1177/1087054712440320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, English JS, Roley ME, O'Brien B, Blair J, Lane SD, McClernon FJ. Effects of smoking abstinence on smoking-reinforced responding, withdrawal, and cognition in adults with and without attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2013;227:19–30. doi: 10.1007/s00213-012-2937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Van Voorhees EE. Monetary incentives promote smoking abstinence in adults with attention deficit hyperactivity disorder (ADHD) Exp Clin Psychopharmacol. 2010;18:221–228. doi: 10.1037/a0019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Lack of inhibition: a motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychol. 2000;6:286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Kosobud AE, Harris GC, Chapin JK. Behavioral associations of neuronal activity in the ventral tegmental area of the rat. J Neurosci. 1994;14:7117–7129. doi: 10.1523/JNEUROSCI.14-11-07117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM. Contingency management for smoking cessation: where do we go from here? Curr Drug Abuse Rev. 2008;1:340–349. doi: 10.2174/1874473710801030340. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314:201–206. doi: 10.1124/jpet.105.084913. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lou HC, Rosa P, Pryds O, Karrebaek H, Lunding J, Cumming P, Gjedde A. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46:179–183. doi: 10.1017/s0012162204000313. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci Biobehav Rev. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Luman M, Van Meel CS, Oosterlaan J, Sergeant JA, Geurts HM. Does reward frequency or magnitude drive reinforcement-learning in attention-deficit/hyperactivity disorder? Psychiatry Res. 2009a;168:222–229. doi: 10.1016/j.psychres.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Luman M, van Noesel SJ, Papanikolau A, Van Oostenbruggen-Scheffer J, Veugelers D, Sergeant JA, Oosterlaan J. Inhibition, reinforcement sensitivity and temporal information processing in ADHD and ADHD+ODD: evidence of a separate entity? J Abnorm Child Psychol. 2009b;37:1123–1135. doi: 10.1007/s10802-009-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Marco R, et al. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Marx I, Hopcke C, Berger C, Wandschneider R, Herpertz SC. The impact of financial reward contingencies on cognitive function profiles in adult ADHD. PLoS One. 2013;8:e67002. doi: 10.1371/journal.pone.0067002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Fuemmeler BF, Kollins SH, Kail ME, Ashley-Koch AE. Interactions between genotype and retrospective ADHD symptoms predict lifetime smoking risk in a sample of young adults. Nicotine Tob Res. 2008a;10:117–127. doi: 10.1080/14622200701704913. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141:131–147. doi: 10.1196/annals.1441.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl) 2008b;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Van Voorhees EE, English J, Hallyburton M, Holdaway A, Kollins SH. Smoking withdrawal symptoms are more severe among smokers with ADHD and independent of ADHD symptom change: results from a 12-day contingency-managed abstinence trial. Nicotine Tob Res. 2011;13:784–792. doi: 10.1093/ntr/ntr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney RJ, Kerns KA. Time reproduction in children with ADHD: motivation matters. Child Neuropsychol. 2003;9:91–108. doi: 10.1076/chin.9.2.91.14506. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997a;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict. 1997b;6:318–329. [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Murray LK, Kollins SH. Effects of methylphenidate on sensitivity to reinforcement in children diagnosed with attention deficit hyperactivity disorder: an application of the matching law. J Appl Behav Anal. 2000;33:573–591. doi: 10.1901/jaba.2000.33-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:722–732. doi: 10.1016/j.jaac.2012.05.006. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, Palmiter RD. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez X, Ly J, McIntosh JM, Quik M. Chronic Nicotine Exposure Depresses Dopamine Release in Nonhuman Primate Nucleus Accumbens. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology (Berl) 2013;228:479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM. Contingency management treatments: controversies and challenges. Addiction. 2010;105:1507–1509. doi: 10.1111/j.1360-0443.2009.02879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Tucker SB, DuPaul GJ, Merlo M, Stoner G. Hyperactivity and frustration: the influence of control over and size of rewards in delaying gratification. J Abnorm Child Psychol. 1986;14:191–204. doi: 10.1007/BF00915440. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Lachowicz JE. Functional D1 and D5 dopamine receptors are expressed in the suprachiasmatic, supraoptic, and paraventricular nuclei of primates. Synapse. 1997;26:1–10. doi: 10.1002/(SICI)1098-2396(199705)26:1<1::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohde P, Kahler CW, Lewinsohn PM, Brown RA. Psychiatric disorders, familial factors, and cigarette smoking: II. Associations with progression to daily smoking. Nicotine Tob Res. 2004;6:119–132. doi: 10.1080/14622200310001656948. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, Otto MW. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010;304:875–880. doi: 10.1001/jama.2010.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav Brain Res. 1998;94:61–71. [PubMed] [Google Scholar]
- Sagvolden T, Metzger MA, Schiorbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. discussion 419-68. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Lee Thoeny A. Steep temporal reward discounting in ADHD-Combined type: Acting upon feelings. Psychiatry Res. 2013 doi: 10.1016/j.psychres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. 2010;67:641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Scheres A, Oosterlaan J, Sergeant JA. Response inhibition in children with DSM-IV subtypes of AD/HD and related disruptive disorders: the role of reward. Child Neuropsychol. 2001;7:172–189. doi: 10.1076/chin.7.3.172.8746. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ, Kety SS. Biogenic amines and emotion. Science. 1967;156:21–37. doi: 10.1126/science.156.3771.21. [DOI] [PubMed] [Google Scholar]
- Senard JM, Rouet P. Dopamine beta-hydroxylase deficiency. Orphanet J Rare Dis. 2006;1:7. doi: 10.1186/1750-1172-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shekim WO, Javaid J, Dekirmenjian H, Chapel JL, Davis JM. Effects of d-amphetamine on urinary metabolites of dopamine and norepinephrine in hyperactive boys. Am J Psychiatry. 1982;139:485–488. doi: 10.1176/ajp.139.4.485. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LWJ, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WEJ, Waxmonsky JG, Gangloff BP. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol. 1999;39:313–341. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Marks DJ, Wasserstein J, Mitchell K, Abikoff H, Alvir JM, Kofman MD. Efficacy of meta-cognitive therapy for adult ADHD. Am J Psychiatry. 2010;167:958–968. doi: 10.1176/appi.ajp.2009.09081123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E. The effect of delay on hyperactive and non-hyperactive children's response times: a research note. J Child Psychol Psychiatry. 1992;33:1091–1096. doi: 10.1111/j.1469-7610.1992.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Heptinstall E. Hyperactivity and delay aversion--II. The effect of self versus externally imposed stimulus presentation periods on memory. J Child Psychol Psychiatry. 1992;33:399–409. doi: 10.1111/j.1469-7610.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, Meltzer PC, Martin J, Rauch S, Fischman AJ. Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane. Biol Psychiatry. 2007;62:1059–1061. doi: 10.1016/j.biopsych.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Strohle A. Reward processing in male adults with childhood ADHD--a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology (Berl) 2011;215:467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- Strand MT, Hawk LWJ, Bubnik M, Shiels K, Pelham WEJ, Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. J Abnorm Child Psychol. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Wilson RC, Toreson K, O'Donnell P, Niv Y, Schoenbaum G. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat Neurosci. 2011;14:1590–1597. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2001;42:691–698. [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J Child Psychol Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Quinlan DM, Cretella LM, Staley JK, Malison RT, Baldwin RM, Seibyl JP, Innis RB. Unaltered dopamine transporter availability in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2002;159:309–312. doi: 10.1176/appi.ajp.159.2.309. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119:Suppl 1, S99–106. doi: 10.1542/peds.2006-2089O. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002a;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002b;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Ramchand CN, Hemmings GP. TaqI polymorphic sites at the human dopamine beta-hydroxylase gene possibly associated with biochemical alterations of the catecholamine pathway in schizophrenia. Psychiatr Genet. 1998;8:19–24. doi: 10.1097/00041444-199800810-00003. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wender PH. Some speculations concerning a possible biochemical basis of minimal brain dysfunction. Ann N Y Acad Sci. 1973;205:18–28. doi: 10.1111/j.1749-6632.1973.tb43159.x. [DOI] [PubMed] [Google Scholar]
- Wender PH, Epstein RS, Kopin IJ, Gordon EK. Urinary monoamine metabolites in children with minimal brain dysfunction. Am J Psychiatry. 1971;127:1411–1415. doi: 10.1176/ajp.127.10.1411. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Parcell T, Westerberg D, Biederman J. Concordance between cigarette smoking and the modified Fagerstrom Tolerance Questionnaire in controlled studies of ADHD. Am J Addict. 2008;17:491–496. doi: 10.1080/10550490802409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Dayan P. Dopamine, learning, and impulsivity: a biological account of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:160–79. doi: 10.1089/cap.2005.15.160. discussion 157-9. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, et al. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:1680–1688. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xiao H, Sun H, Zou L, Zhu LQ. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012;45:605–620. doi: 10.1007/s12035-012-8278-5. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry. 2012;71:184–191. doi: 10.1016/j.biopsych.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]