Abstract
Objectives
The goal of this study was to use a status epilepticus steady-state chemical model in rats using the convulsant, 3-mercaptopropionic acid (3-MPA), and to compare the changes in striatal neurotransmission on a slow (5 minute) and fast (60 second) timescale. In vivo microdialysis was combined with electrophysiological methods in order to provide a complete evaluation of the dynamics of the results obtained.
Objective
To compare the effects of a steady-state chemical model pof status epilepticus on striatal amino-acid and amine neurotransmitters contents, as measured via in vivo microdialysis combined with electrophysiological methods. Measurements were performed on samples collected every 60 seconds and every 5 minutes. “Fast” (60s) and “slow” (5 min.) sampling timescales were selected, to gain more insight into the dynamics of GABA synthesis inhibition and of its effects on other neurotransmitters and on cortical electrical activity.
Methods
3-MPA was administered in the form of an intra-venous load(60 mg/kg) followed by a constant infusion (50 mg/kg/min) for min. Microdialysis samples were collected from the striatum at intervals of 5 minutes and 60 seconds and analyzed for biogenic amine and amino acid neurotransmitters. ECoG activity was monitored via screws placed over the cortex.
Results
In the 5 minute samples, glutamate (Glu) increased and γ-aminobutyric acid (GABA) decreased monotonically while changes in dopamine (DA) concentration were bimodal. In the sixty second samples, Glu changes were bimodal, a feature that was not apparent with the five minute samples. ECoG activity was indicative of status epilepticus.
Conclusions
This study describes the combination of in vivo microdialysis with electrophysiology to monitor the effect of 3-MPA on neurotransmission in the brain. This led to a better understanding of the chemical changes in the striatum due to the applied 3-MPA chemical model of status epilepticus.
Keywords: Epilepsy, Chemical Seizure Models, 3-mercaptopropionic acid, dopamine, glutamate, HPLC, status epilepticus steady-state chemical model, microdialysis sampling, catecholamines, neurotransmitter amino acids
1. INTRODUCTION
Epileptic seizures are known to result from imbalances within the neurotransmitter systems in the brain. Historically, epileptic seizures have been viewed as hyperexcitable events (Siegel et al., 1999; Nyitrai et al., 2006; Starr, 1996). This collection of research all point to the excitatory and inhibitory amino acid neurotransmitters, specifically glutamate (Glu) and γ-aminobutyric acid (GABA), and the biogenic amine neurotransmitters, in particular dopamine (DA) (Starr, 1996), as the main components of interest in gaining a more thorough neurochemical understanding of epilepsy. The interactions of Glu, GABA, DA, and all other important transmitters can be described as complex at best. Much work has been accomplished in order to understand the relationships between the amino acid and biogenic amine neurotransmitters in many different areas of the brain, including the striatum (Morari et al., 1998; Adams et al., 2002; Bert et al., 2002; Konradi, 1998; Shimizu et al., 1990; Takahata and Moghaddam, 2000), hippocampus (Clinckers et al., 2005), nucleus accumbens (Youngren et al., 1993), and ventral tegmental area (Chen and Rice, 2002; Karreman et al., 1996), but many of the observations are still unexplained.
A comprehensive understanding of the inter-relation between neurotransmitter systems and various epileptic seizure models is invaluable to the advancement of treatments for epilepsy and the development of new antiepileptic drugs (AEDs). The ability to better understand the neurophysiology of epilepsy and epileptic seizures would allow for better animal seizure models to be developed which mimic more closely human epilepsy. Work recently completed in this laboratory produced a steady-state 3-MPA dosing model in which the concentration of 3-MPA was held at a steady concentration allowing for further evaluation of the neurotransmitter changes associated with the model (Crick et al., 2007). A good working knowledge of the neurotransmitter systems in relation to 3-MPA is non-existent. The ability to obtain data, regarding the changes in neurotransmission in different brain regions during the 3-MPA chemical seizure model, is critical in strengthening the developed model for its further use in the understanding of generalized seizures in the laboratory and clinical settings.
Fast neurochemical events have been monitored to transpire on the timescale of 0.5 – 5 milliseconds (Siegel et al., 1994; Vyklicky et al., 1991). Many authors, beginning in the late 1970’s with R. N. Adams (Adams, 1976; Wightman et al., 1978), have attempted to track these changes, primarily focusing on dopamine (DA), by electrochemical means (Budygin et al., 2000; Budygin et al., 2001; Greco et al., 2006; Robinson et al., 2003). Other authors have also attempted to understand the role of certain ions, such as potassium and chloride, within neurological disorders (Gorji et al., 2006; Obrenovitch and Zilkha, 1995). While these typical carbon fiber (CF) electrodes perform very accurately at monitoring neurochemical changes, they are limited in functionality due to their inability to monitor multiple analytes simultaneously. This continues to be one area where microdialysis prevails. Microdialysis has recently been reported for monitoring neurochemical events at temporal resolutions ranging from 6 – 30 seconds (Bert et al., 2002; Parrot et al., 2003; Bert et al., 1996; Lada et al., 1997; Tucci et al., 1997).
We describe in this paper, the analysis of neurochemical changes using both High Performance Liquid Chromatography (LC) and Capillary Electrophoresis (CE) separation techniques and microdialysis. We also detail observations in the differences between the different microdialysis sampling frequencies during the 3-MPA induced seizure activity (model of convulsive status elipticus). Also discussed is the correlation between observed neurotransmitter activity and brain 3-MPA concentration. This is the first report, to our knowledge, that multiple changes in neurotransmitters have been correlated to 3-MPA induced seizure activity.
2. METHODS
2.1. Chemicals/Reagents
Monobasic sodium phosphate, disodium ethylenediamine tetraacetate (Na2EDTA), 85% o-phosphoric acid, acetonitrile, methanol, hydrochloric acid (HCl), sodium hydroxide (NaOH), and 0.3 μm alumina powder were obtained from Fisher Scientific (Pittsburgh, PA). Ammonium acetate, 1-octanesulfonic acid [sodium salt] (SOS), sodium tetraborate decahydrate, boric acid, lithium tetraborate, lithium dodecyl sulfate (LDS), tetradecyltrimethylammonium bromide (TTAB), β-alanine (β-Ala), sodium cyanide, 4-hydroxybenzoic acid (4-HBA), L-glutamic acid (Glu), L-aspartic acid (Asp), L-arginine (Arg), γ-amino-n-butyric acid (GABA), DL-2-aminoadipic acid (AAP), sodium cyanide, 3,4-dihydroxybenzylamine (DHBA), 3,4-dihydroxyphenethylamine hydrochloride (DA), L-arterenol (NE), homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxytryptamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA) were obtained from Sigma-Aldrich (St. Louis, MO). Naphthalene-2,3-dicarboxaldehyde (NDA) was obtained from Invitrogen (Carlsbad, CA). All solutions were prepared in 18.2 MΩ distilled, deionized water (Labconco, Kansas City, MO) and filtered through 0.22 μm pore size membrane filters prior to use unless otherwise noted.
2.2. Animals
Male Wistar rats weighing 300 – 450 grams (Charles River Laboratories, Wilmington, MA) were used. The animals were kept on 12 hour light-dark cycles until the beginning of the experiment. Free access to food and water were allowed. The research described in this report was conducted in compliance with all applicable federal statutes and regulations related to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, NIH publication 86-23, 1996 edition.
2.3. Surgical Procedure
2.3.1. Brain implantation of cortical electrodes, microdialysis guide cannula and probe
On the day of the experiment, rats were pre-anaesthetized with isoflurane. A subcutaneous injection of 67.5 mg/kg ketamine: 3.4 mg/kg xylazine: 0.67 mg/kg acepromazine was then administered for full anesthesia. Supplemental doses of 100 mg/mL ketamine were given at a rate of 0.2 mL/hr to maintain the same plane of anesthesia. The anaesthetized rat was placed on a stereotaxic instrument (Harvard Appartus, Holliston, MA, USA) and then connected to a Homeothermic Blanket Control Unit (Harvard Apparatus, Holliston, MA, USA) where the body temperature was maintained at 37.0 ± 0.3 °C. A midline incision was made on the scalp and the skull was exposed. Four electrodes (1 mm O.D. stainless steel screws (Ace Hardware, Lawrence, KS, USA)) were placed over the cortex for recording of electrical activity. Two of the four electrodes were placed over the right hemisphere 4.2 mm anterior and 5.8 mm posterior and −1.4 mm lateral with respect to bregma; of the remainder electrodes, one was used as a ground electrode on the right hemisphere 5.8 mm posterior and +1.4 mm lateral with respect to bregma, and the other as reference (nasion).
Microdialysis intracerebral guide cannulas (CMA Microdialysis Inc., North Chemlsford, MA, USA) were implanted into the brain with the following coordinates: posterior 0.2 mm, lateral +3.2 mm, ventral 3.5 mm (striatum) and anterior 5.6 mm, lateral +4.8 mm, ventral 3.5mm (hippocampus) with respect to the bregma (Paxinos and Watson, 1986). The guide cannulas were fixed to the skull surface with Duralay dental cement (Worth, IL, USA). A CMA/12 microdialysis probe with a 4 mm membrane (CMA Microdialysis Inc., North Chemlsford, MA, USA) was then placed through the guide cannula into both the striatum and hippocampus.
2.3.2. Femoral vein cannulation
A 25 mm length of MRE-033 tubing was inserted in the femoral vein and affixed properly by suturing. The skin incision was closed with tissue staples.
2.4. Experimental Design
The animals were allowed to recover (under anesthesia at a rate of 0.2 mL of 100 mg/mL ketamine administered intramuscular (i.m.) every 1.5 hours) for a period of 6 hours. During this time, the environment surrounding the microdialysis probe is allowed to “recover” from the trauma suffered during the microdialysis probe implantation. It is well noted that during the time period following the implantation of a brain microdialysis probe that gliosis occurs (Plock and Kroft, 2005; Robinson and Justice, 1991). Also during this time period, the brain tissue function becomes disturbed due to the excess neurochemical release of numerous cellular storage compartments as well as increased glucose metabolism and decreased blood flow (Robinson and Justice, 1991). The time lapse between the implantation of the brain probe and the commencement of the experiment can last from 30 minutes to 24 hours or longer depending upon the analyte(s) of interest for the study. However, it is necessary that the appropriate length of time has passed in order to be able to monitor a steady basal level of the analyte(s).
Following the implantation of the electrodes and the brain microdialysis probes, the rat was connected to a CMA/100 microinjection pump (CMA Microdialysis Inc., North Chelmsford, MA, USA). Both microdialysis probes were first perfused with a solution of 2.65 g/mL 3-MPA at a rate of 1 μL/min for 3 hours to allow for microdialysis probe calibration. Then both probes were flushed with artificial cerebrospinal fluid (aCSF) for microdialysis [145 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 1.2 mM CaCl2, 0.45 mM NaH2PO4, and 2.33 mM Na2HPO4, pH 7.4], at a rate of 1 μL/min for 3 hours to allow for cleansing and stabilization of the probe environment. NANOpure water (18.2 Ω, Labconco, USA) was used in preparing all solutions.
The dosing of 3-MPA for these experiments followed the developed protocol in our previous research (Crick et al., 2007). Briefly, a loading dose of 60 mg/kg 3-MPA was administered via intravenous (i.v.) injection. Immediately following the loading dose, a constant i.v. infusion of 3-MPA at a rate of 50 mg/kg/min was started and was continued for a time period of 50 minutes before removal.
Following the administration of 3-MPA, microdialysate samples were collected on either 60 second or 5 minute intervals from the striatum until the termination of the experiment. The microdialysis samples were analyzed the day of the experiment.
2.5. Electrocorticographical (ECoG) Recording
ECoG recordings were obtained beginning 30 minutes prior to dosing with 3-MPA (baseline). The signals were acquired through a Biomedical amplifier (SA Instruments, San Diego, CA, USA) using a 0.1 Hz high pass filter, a 6000 Hz low pass filter, a sampling rate of 15 kHz and a NIDAQ PCI 6731 data acquisition card (National Instruments, Austin, TX). The data were stored in the computer hard drive and analyzed, using custom built software (Osorio et al., 1998; Osorio et al., 2002). The total number of seizures, their duration and intensity were determined using an algorithm developed by Osorio et al. (Osorio et al., 1998; Osorio et al., 2002). Seizures are detected by filtering the ictal component from the raw ECoG using a wavelet spectral filter that enhances frequencies between 8–42 Hz, with a peak at 25 Hz and computing in real-time the median power of the filtered foreground (2 seconds), and dividing it by the median power of the filtered background (30 minutes). The resulting ratio is an estimate of the instantaneous seizure content of the ECoG. For this study, seizures were defined as any automated detection whose threshold reached a value of 22 for at least 1.8s (Osorio et al, 1998).
2.6. Microdialysis Sample Analysis
Upon collection of the microdialysis sample, care was taken in regards to the sample storage if the sample could not be analyzed immediately. Two different LC instruments were employed to accomplish all of the required analyses (LC-EC and LC-Fluorescence). The first step after sample collection was to split the sample into 2.5 L aliquots into two separate vials. Then a volume of an analytical internal standard (IS) was added to each vial. This IS was prepared in 0.1 M perchloric acid. The purpose of the perchloric acid was to severely slow the rate of autooxidation of the analytes present in the sample matrix, aCSF. It has been shown that the addition of perchloric acid to an aCSF matrix containing neurotransmitters, especially the catecholamines, which are very low in concentration, will help to assist in the stability of the sample (Kankaanpaa et al., 2001; Thorre et al., 1997, Zhang et al., 2001). If the microdialysis samples could not be analyzed immediately, they were placed into a −20 °C laboratory freezer which also helps to control the sample degradation.
2.6.1. Amino Acid Neurotransmitters by HPLC
2.5 μL of the microdialysis sample was taken and derivatized using an adapted NDA/CN− scheme from Robert et al.1998. Briefly, to a 2.5 μL dialysate sample, 1.5 μL of the internal standard (α-aminoadipic acid prepared in aCSF), 1 μL of 500 mM borate: 87 mM CN− (100:20 v:v), and 0.5 μL of 3 mM NDA was added. The sample was then mixed by vortex and allowed to react at room temperature for one minute prior to the injection of 5 μL of sample.
The derivatized microdialysis sample was directly injected onto a Synergi 4μ Hydro-RP column (150 × 2.0 mm, Phenomenex, Torrance, CA). A binary LC-Fluorescence was used to analyze the samples. The liquid chromatographic system consisted of two Shimadzu LC-10ADVP pumps, a Shimadzu 20 μL SUS micro-mixer, and a Rheodyne 9725i PEEK sample injector valve connected to a Phenomenex C18 guard column. The binary gradient system was controlled by a Shimadzu SCL-10AVP system controller. Mobile phase A consisted of 50 mM ammonium acetate and 0.5 mM Na2EDTA with the pH adjusted to 6.8 using concentrated glacial acetic acid. Tetrahydrofuran (THF) was added making the final composition 95% acetate buffer : 5% methanol (v:v). Mobile phase B was 100% methanol. This method was adapted from Shah et al. (2002).
The Shimadzu RF-10AXL fluorescence detector was operated at an excitation wavelength of 442 nm and an emission wavelength of 490 nm (Robert et al., 1998; Brajter-Toth and Chambers, 2002). The data were collected at 2.5 Hz and processed using version 7.3 EZStart Chromatography Software (Shimadzu Scientific Instruments, Columbia, MD).
2.6.2. Biogenic Amine Neurotransmitters by HPLC
To the remaining 2.5 μL of microdialysis sample, 0.3 μL of internal standard (DHBA prepared in 0.1 M perchloric acid) was added. The sample was then mixed by vortex and 2.5 μL was injected immediately into the HPLC.
The microdialysis sample was injected onto a Zorbax C18 Eclipse-XDB (3.5 μm) column (75 × 2.1 mm, Agilent, Palo Alto, CA). An LC-EC system was used to analyze the samples. The LC system consisted of a Shimadzu LC-20AD pump, and a Rheodyne 9725i PEEK sample injector valve connected to a Phenomenex C18 guard column. The mobile phase was adapted from Agilent, Inc. [45] and consisted of 140 mM monobasic sodium phosphate, 0.75 mM SOS, and 20 mM Na2EDTA with the pH adjusted to 3.5 using 85% o-phosphoric acid. Methanol was then added making the final composition 91% phosphate buffer : 9% methanol (v:v).
The dual-electrode electrochemical detector consisted of one gold-mercury (Au/Hg) amalgam electrode and one glassy carbon (GC) electrode. The potentials were controlled by two LC-4C potentiostats (Bioanalytical Systems, West Lafayette, IN) with W1 operating the Au/Hg electrode and W2 operating the GC electrode. The data were collected at 10 Hz and processed using a Chrom&Spec Chromatography Data System (Ampersand International, Beachwood, OH). A typical two channel chromatogram for the dual detection of 3-MPA and the biogenic amines was collected holding W1 at +100 mV versus Ag/AgCl and W2 at +750 mV versus Ag/AgCl.
2.6.3. Amino Acid and Biogenic Amine Neurotransmitters by CE
Separations were performed on a Beckman P/ACE MDQ (Beckman Coulter, Palo Alto, CA) with and external ZETALIF LIF detector (Picometrics, France). The LIF detector was comprised of an OmniChrome He/Cd laser (Melles Griot, Carlsbad, CA) with an excitation wavelength of 442 nm and an emission filter at 490 nm. Fused silica separation capillaries with an i.d. of 50 μm and o.d. of 360 μm (Polymicro Technologies, Phoenix, AZ) and a length of 75 cm were used. An active window was created by burning off a 3 mm length of the polyimide coating from the fused silica capillary 15 cm from the outlet, and then cleaning the outside of the capillary with copious amounts of methanol. The data were acquired using a 32 Karat Gold software package (Beckman Coulter, Palo Alto, CA) at 4 Hz.
The background electrolyte (BGE) for the CE-LIF analyses was adapted from Siri et al. (2006) and was comprised of 22.5 mM lithium tetraborate, 20 mM LDS, pH 9.2 (unadjusted). Prior to use, the BGE was filtered through a 0.22 μm syringe membrane filter (Millipore, Bedford, MA).
Daily, before use, the separation capillary was “activated” by conditioning with 100% methanol (10 min), water (3 min), 0.1 M HCl (10 min), water (3 min), 0.1 M NaOH (15 min), water (3 min), and BGE (10 min) sequentially at a pressure of 20 psi. Prior to the first use of the BGE, an Ohm’s Plot was conducted in order to determine the linear range where Joule heating was effectively dissipated. The separation voltage for these experiments was 21 kV. Between injections, the capillary was flushed with 100% methanol (2 min), 0.1 M NaOH (3 min), water (1 min), and BGE (3 min) sequentially at 20 psi to rinse the capillary of any remaining analytes and to minimize the adsorption of the LDS to the wall of the capillary.
3. RESULTS
3.1. Five minute microdialysis sample collection
3.1.1. Amino Acid Neurotransmitters
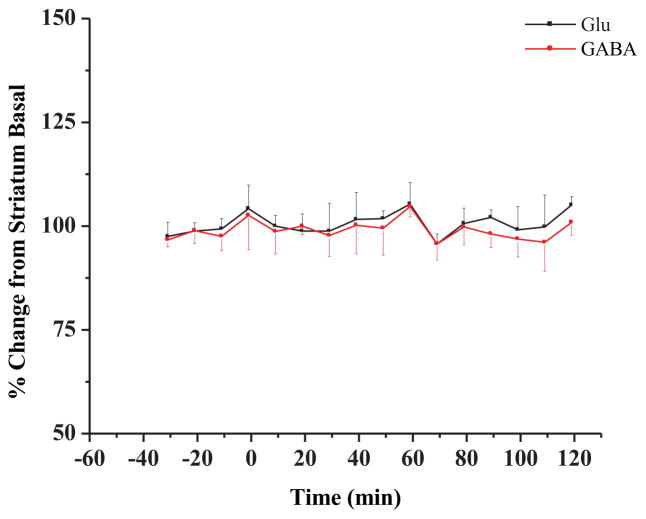
Control experiments consisting of “sham” infusions of saline did not cause significant changes in Glu or GABA (Figure 1)
Figure 1.
Glutamate (Glu) and GABA time profiles in the striatum during saline dosing (n = 2 rats). Saline was administered at t = 0 min. as described in the Methods Section.
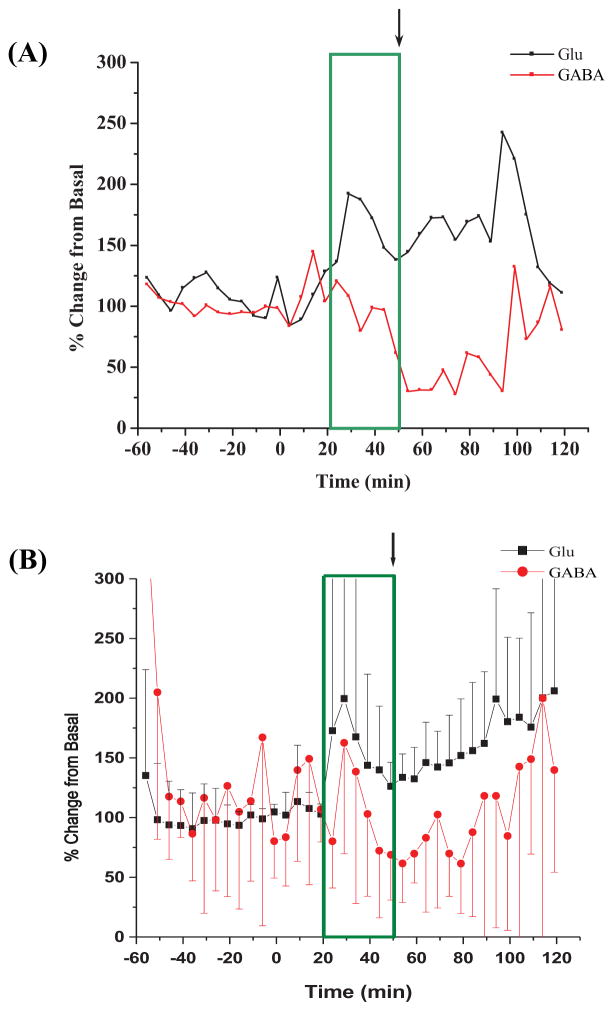
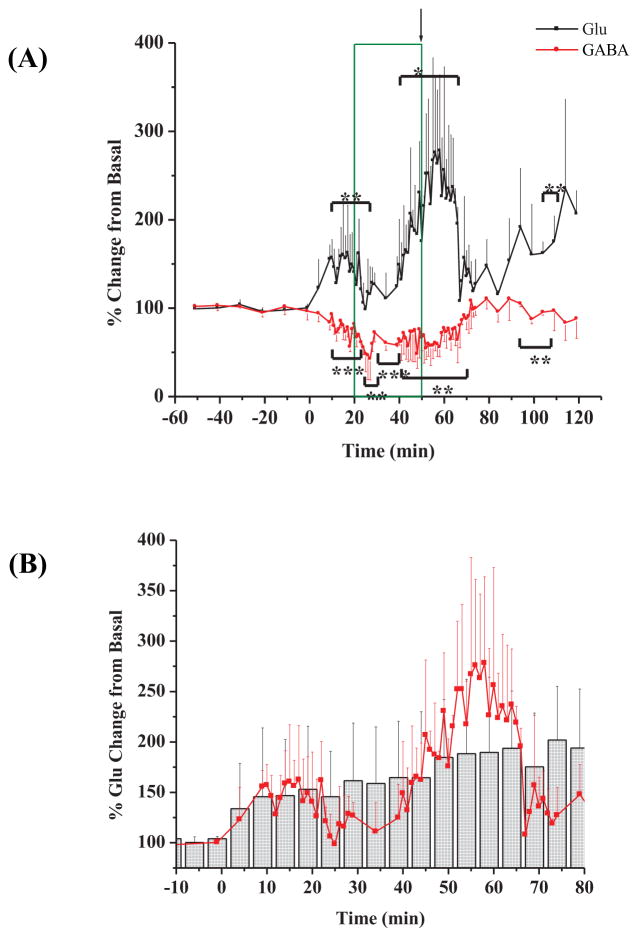
Figure 2(A) shows a representative plot of the percent change from basal for both Glu and GABA in samples collected every 5 min., over the time course of one experiment. The 30 minute period (t = 20 – 50 minutes; boxed in on the plots) represents the time that 3-MPA was at a steady-state concentration in the striatum. The average Glu and GABA deviations from basal levels after the administration of 3-MPA are shown in Figure 2(B). After the cessation of the 3-MPA infusion, Glu remained increased over the basal values. GABA decreased following cessation of 3-MPA infusion. Table 1 shows the ECoG data collected from the collective experiments such as: a) mean latency to onset of the first seizure defined as the first ictal electrical change compared to baseline; b) mean total number of seizures detected oveal all experiments; c) mean seizure and, d) mean maximal seizure intensity (Rmax).
Figure 2.
Time profiles for amino-acid neurotransmitter concentration (as percent changes of baseline) in the striatum during constant systemic infusion of 3-MPA. 3-MPA was administered at t = 0 min. as described in the Methods Section. The box marks the time (20 – 52 min.) during which a steady-state concentration of 3-MPA was achieved in each sampled region. The arrow marks the time at which the infusion was stopped. (A) Glu and GABA deviations vs. time in one examplary experiment; (B) Glu and GABA deviations vs. time in all experiments (n = 10 rats).
Table 1.
ECoG data for convulsive status epilepticus model.
| 60 mg/kg bolus + 50 mg/kg/min infusion (n = 12 rats) | |
|---|---|
| Latency to Seizure Onset (s) | 363.2 ± 148.8 |
| Number of Seizures Detected | 592 ± 187 |
| Average of the Average Seizure Duration (s) | 0.87 ± 1.78 |
| Rmax | 71.8 ± 22.2 |
3.1.2. Biogenic Amine Neurotransmitters
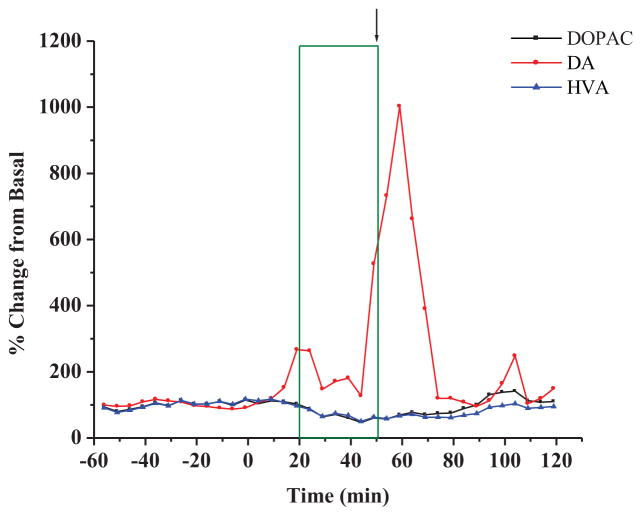
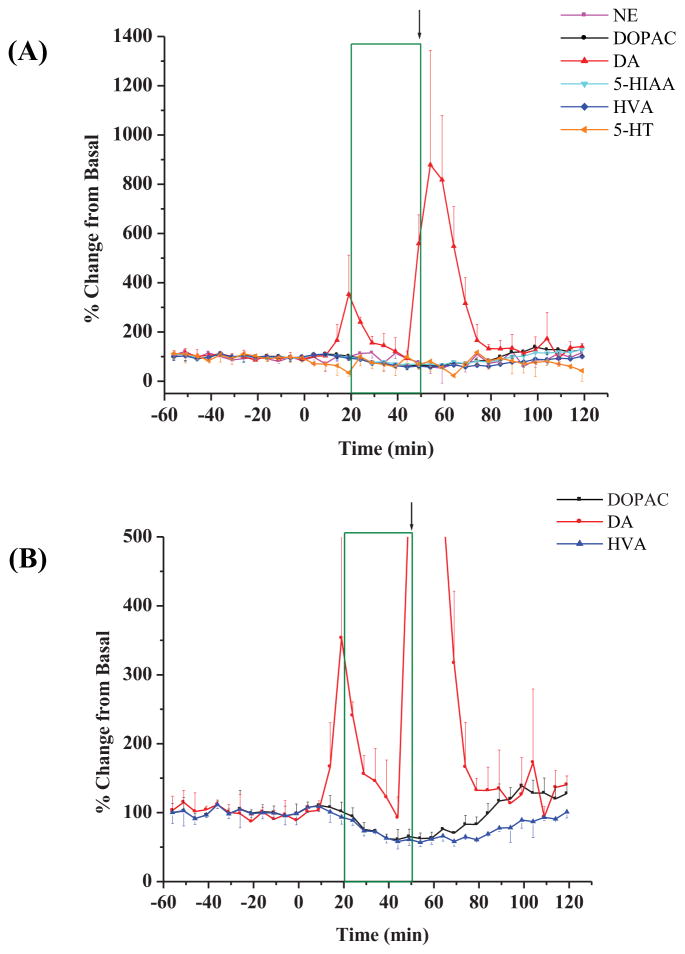
The biogenic amines were analyzed using a 5 minute sampling interval. Figure 3 shows an example of the experimental results which match the striatal experimental results shown in Figure 2(A) for changes in the levels of biogenic amines after the administration of the 3-MPA seizure model. A collection of six experiments from the striatum are shown in Figure 4(A). Upon closer inspection of the basal monoamine levels, it was observed that the only other significant changes from the basal levels were for the metabolites of DA as shown in Figure 4(B). Both DOPAC and HVA decreased a maximum of approximately 40% from basal levels, a finding similar to that report by Segovia and Mora (1998) that used Glu to excite NMDA and DA receptors. Other biogenic amines, including NE, 5-HT and 5-HIAA were not significantly different from the recorded basal values, due to the sparse 5-HT projections into the striatum and the limited (5 minute) temporal resolution of the microdialysis technique.
Figure 3.
Examplary experiment displaying concentration (as percent changes of baseline) in biogenic amines in the striatum during constant infusionof 3-MPA administered systemically at t= 0 min. All other details as stated in Figure 2.
Figure 4.
Changes (as percentages of baseline) in biogenic amine concentrations in striatum during constant infusion of 3-MPA. (A) Data compiled from striatal experiments (n = 6 rats); (B) Changes in DA, DOPAC, and HVA concentrations shown at a smaller scale (y-axis). 3-MPA was administered at t = 0 min. All other details as stated in Figure 2.
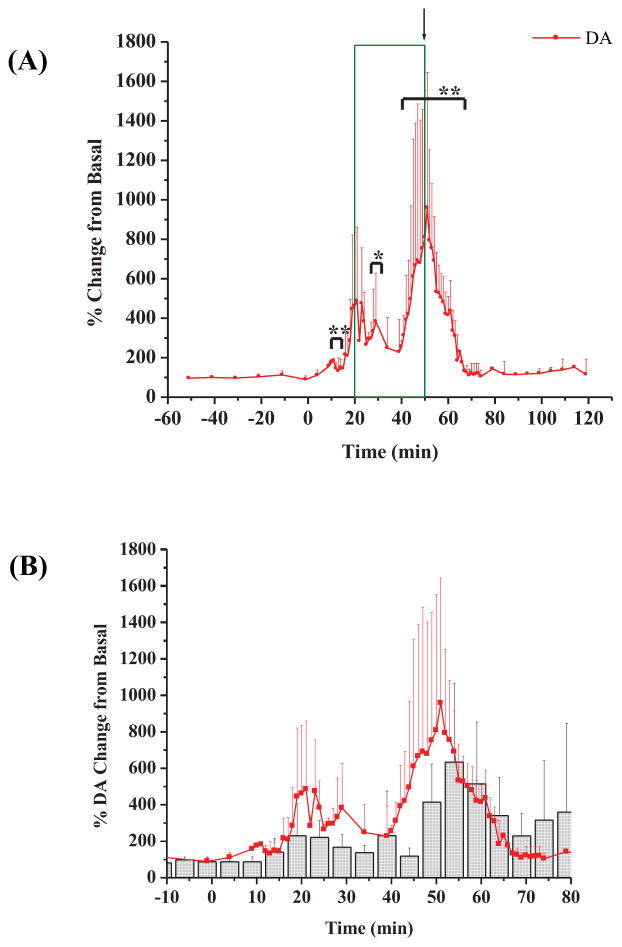
Due to the pronounced and reproducible biphasic increase in DA activity observed within the striatum, further analysis of the data was performed in order to decipher a cause for its occurrence. Table 2 illustrates the median time for each of the phasic spikes in DA activity. The Δt values which represent the spacing between the biphasic DA spiking within the striatum were also very reproducible.
Table 2.
Time trends within biphasic spiking of dopamine activity.
| Median Time (t) for 1st DA Spike (min) | Median Time (t) for 2nd DA Spike (min) | t (min) | |
|---|---|---|---|
| Rat 1 | 19 | 59 | 40 |
| Rat 2 | 19 | 59 | 40 |
| Rat 3 | 19 | 54 | 35 |
| Rat 4 | 24 | 54 | 30 |
| Rat 5 | 39 | 89 | 50 |
| Rat 6 | 14 | 54 | 40 |
| Avg ± SD | 22 ± 9 | 62 ± 14 | 39 ± 7 |
3.2. Sixty second microdialysis sample collection
For this set of experiments, the sampling frequency was increased to 60 seconds over the time periods from 10 – 30 minutes (to encompass the first phasic activity of DA) and 40 – 75 minutes (to encompass the second phasic activity of DA) as previously observed in Figure 4.
Figure 5(A) displays the amino acid neurotransmitter data from a collection of 3 experiments using this 60 second microdialysis sampling. A very interesting observation arises upon the investigation of the Glu data. A biphasic activity was now observed with Glu. Between the time frames of 5 – 25 minutes Glu significantly spiked (p < 0.05 and again at 40–65 minutes, but not significantly (p < 0.10, respectively). There was also a sustained increase in Glu during the final 30 minutes of the experiment. The activity of GABA was consistent with what was previously observed (see Figure 2). GABA significantly decreased to varying degrees between 10 – 70 minutes as was expected (p < 0.05 and p < 0.01). This 60 second sampling frequency proved to be advantageous in the case of Glu when compared against the 5 minute sampling of Glu. Figure 5(B) portrays the average Glu data from this investigation superimposed onto the average Glu data from the 5 minute temporal resolution.
Figure 5.
Glu and GABA data from 60 second microdialysis sampling. (A) Percent change in Glu and GABA over the time course of the experiment (n=3 rats); (B) 60 second microdialysis sampling superimposed onto 5 minute microdialysis sampling for DA. Red circles represent 60 second samples and gray histograms represent the 5 minute samples. Plot is magnified to show areas of significance. [* = p < 0.10; ** = p < 0.05; *** = p < 0.01] 3-MPA was administered at t = 0 min.
The biogenic amine neurotransmitter data are shown in Figure 6(A) (for clarity purposes, only the DA data are plotted). Noticeable upon first inspection of the data was that, again, the biphasic activity of DA was still present. These biphasic changes were significant (p < 0.10 and p < 0.05) when compared to the basal values and better defined on the 60 second microdialysis sampling. Figure 6(B) overlays the 60 second average DA data with the average DA data from the 5 minute temporal resolution experiments.
Figure 6.
DA data from 60 second microdialysis sampling. (A) Percent change in DA over the time course of the experiment (n=3 rats); (B) 60 second microdialysis samples superimposed onto 5 minute microdialysis samples for DA. Red circles represent 60 second samples and gray histograms represent the 5 minute samples. Plot is magnified to show areas of significance. [* = p < 0.10; ** = p < 0.05]. 3-MPA was administered at t = 0 minutes.
4. DISCUSSION
4.1. Amino Acid Neurotransmitters
The results obtained from the amino acid neurotransmitters were not expected. It was thought that Glu and GABA would return to basal levels soon after the cessation of the 3-MPA i.v. infusion, particularly since seizure activity ceased spontaneously. The results from one example experiment as well as the overall average from 10 experiments show that this was not the case. Actually, Glu continued to gradually increase and GABA remained below baseline concentrations for quite a period of time following the discontinuation of the 3-MPA i.v. infusion. One possible explanation for this sustained increase in Glu over the entirety of the experiment lies with the Glu receptors themselves. The sources of Glu are synaptic and astrocytic/glial release (Clinckers et al., 2005; Timmerman and Westerink, 1997). Very high densities of Glu transporters are located on the astrocytes which surround the synapse (Pfreiger and Barres, 1996). The Glu transporters on the astrocytes, along with ones on the glial cells, are responsible for the reuptake of Glu from the extracellular space (Siegel et al., 1999). Regardless of the cause of excess Glu release/spillover into the synaptic cleft, it is believed that upon excess excitation, there is not full reuptake of Glu into these cells by the reuptake receptors or transporters (Siegel et al., 1999; Clinckers et al., 2005), possibly due to Glu receptor desensitization. If the time in which Glu resides in the synaptic cleft is greater than the time in which the AMPA receptor is open, the receptor may become desensitized (Trussell et al., 1993; Tran et al., 1999; Vyklicky et al., 1991), entering a permanently “closed” state, thereby not allowing the reuptake of Glu into glia. AMPA receptor desensitization can be selectively blocked with CTZ (Trussell et al., 1993; Vyklicky et al., 1991). The use of CTZ to “resensitize” the AMPA receptors was explored in the 3-MPA seizure model in order to try to explain the increase in Glu well past the spontaneous termination of status. Results from these studies are discussed below.
4.2. Biogenic Amine Neurotransmitters
The first interesting piece of information extracted from this plot was an unexpected bimodal change in DA, as others have reported monophasic changes with DA with epileptic seizures (Bert et al., 2002).
These data could be explained by looking into a mechanism which correlates the DA activity with the aforementioned Glu activity. It is well known that the striatum is a key component of the motor system. The striatum is comprised of glutamatergic afferent neurons which descend from the cortex via the corticostriatal pathway and dopaminergic afferent neurons that project from the substantia nigra pars compacta via the nigro-striatal pathway (Castaneda et al., 2004). Bouyer et al. (1984) discovered that the glutamatergic and dopaminergic projections form appositional contacts thereby suggesting the possibility of cross-communication between these terminals. Many authors believe that glutamatergic neuronal activity works to modulate the dopaminergic neuronal activity (Shimizu et al., 1990; Takahata and Moghaddam, 2000; Youngren et al., 1993; Aultman and Moghaddam, 2001; Jedema and Moghaddam, 1994; Kretschmer, 1999; Nieoullon et al., 1983; Pistis et al., 2002) while others believe the opposite is true (Konradi, 1998; Bamford et al., 2004). This modulatory relationship has been strengthened with the discovery that DA synapses contain DA receptors as well as both AMPA and NMDA receptors. The opposite also holds true, Glu synapses contain AMPA, NMDA and DA receptors (Chen and Rice, 2002).
4.3. Sixty second Microdialysis Studies
It was shown that having the higher sampling frequency did help to detect an increased number of spiking events with the Glu data. The biphasic activity in Glu was not detected until the microdialysis collections were reduced from 5 minutes to 60 seconds.
It has been debated heavily over the past two decades about the relationship which Glu and DA share in terms of their neuronal activity. Authors have argued for the glutamatergic modulation of dopaminergic neuronal activity (Aultman and Moghaddam, 2001; Jedema and Moghaddan, 1994; Kretschmer, 1999; Nieoullon et al., 1983; Pistis et al., 2002; Shimizu et al., 1990; Takahata and Moghaddam, 2000; Youngren et al., 1993) while others have argued for the dopaminergic modulation of the glutamatergic neuronal activity (Bamford et al., 2004; Konradi, 1998). Other authors additionally argue the role of Glu and DA to be a dual excitatory-inhibitory one. For example, Leviel et al. (1990) showed that the perfusion of Glu at low concentrations (10−8 M) caused an increase in the release of DA while the perfusion of Glu at higher concentrations (10−4 M) proved to cause a decrease in the amount of DA observed. Using the 60 second microdialysis sampling and further analysis of the collected data, two different hypotheses have been developed to help explain the findings believed to link the activity of Glu and DA within the striatum.
The time trends relating the onset of changes in Glu and/or DA as well as the time between the phasic activities could relay some knowledge of a correlation between the two. Table 3(A) displays the time trends observed from the 60 second microdialysis sampling for Glu. This was possible as a direct result of the faster temporal resolution achieved with 60 second sampling. Table 3(B) shows the same type of time trend data DA. It was observed that the first phasic increase in Glu occured before DA (p < 0.01), and the second phasic increase in Glu occured at approximately the same time as the second phasic increase in DA. It is hypothesized that the first phasic increase in Glu actually causes the first phasic increase in DA. Several authors have reported that Glu acts as a non-specific ionotropic receptor agonist, activating both NMDA and AMPA receptors when released into the synaptic cleft (Kretschmer, 1999; Chen and Rice, 2002; David et al., 2005; Desce et al., 1992). The activation of NMDA and AMPA receptors caused a subsequent activation in locomotor activity, and furthermore an increase in the release of DA. This was further validated by the observed convulsions in the animal and the latency to seizure onset. The electrical seizure onset times consistently preceded the observed release in the neurotransmitters. For example, the first seizure activity in the animal was observed consistently to occur on average 9.6 minutes before the first phasic activity in Glu. DA has its first phasic response exactly 5 minutes after the Glu rise.
Table 3.
Time trends for excessive glutamate and dopamine using 60 second microdialysis sampling.
| (A) | |||
|---|---|---|---|
| Median Time (t) for 1st Glu Spike (min) | Median Time (t) for 2nd Glu Spike (min) | t (min) | |
| Rat 1 | 15 | 55 | 40 |
| Rat 2 | 17 | 56 | 39 |
| Rat 3 | 18 | 60 | 42 |
| Avg ± SD | 17 ± 2 | 57 ± 3 | 40 ± 2 |
| (B) | |||
|---|---|---|---|
| Median Time (t) for 1st DA Spike (min) | Median Time (t) for 2nd DA Spike (min) | t (min) | |
| Rat 1 | 20 | 51 | 31 |
| Rat 2 | 23 | 53 | 30 |
| Rat 3 | 24 | 57 | 33 |
| Avg ± SD | 22 ± 2 | 54 ± 3 | 31 ± 2 |
The second phasic activity in both Glu and DA was hypothesized to arise from the modulatory effect DA has over Glu. This was due to the start of the second phasic release in DA which consistently occurred before the start of the second phasic release for Glu. As mentioned above, excess Glu release activates both NMDA and AMPA receptors. Konradi (1998) revealed that upon activation of an NMDA receptor, the DA D1 receptor acts to facilitate the further release of Glu. The other major DA receptor, the D2 receptor, acts to facilitate the release of Glu when the AMPA receptor is activated.
Another question which arises is why, or how, does the DA stop being excessively released in each phase? The answer to this may lie with the GABAA and/or GABAB receptors. DA neurons are also known to have, in addition to AMPA and NMDA receptors, GABAA and GABAB receptors located on their spines (Chen and Rice, 2002). Upon the activation of either GABA receptor, the membranes become hyperpolarized and the DA activity is decreased. There was a noted correlation in the times in which GABA made a return towards basal values as well as when DA falls back to basal values. When the corresponding DA data was observed for this time frame, it was shown to fall abruptly back to its basal level. These data would now indicate a possible role of the GABA receptors in the overall mechanism of action implemented due to the 3-MPA seizure model.
An alternative way to investigate the Glu and DA data is a way in which the 3-MPA seizure model is considered as a model which produces hypoxic and/or ischemic events. The striatum is an area of the brain very susceptible to hypoxia and ischemia (Smith et al., 1986). This is mainly due to the deep, centralized location of the striatum within the brain, as well as the numerous dopaminergic neurons located within the striatum. These factors also allow for the striatum to be a site for acidification, occurring when glucose becomes utilized for anaerobic glycolysis instead of oxidative metabolism (Remblier et al., 1999). It is believed that lactic acid production increases as a result of hypoxia and/or ischemic events (Siesjo, 1988). The lactic acid accumulation in the tissue is linearly accompanied by an intracellular, as well as an extracellular lowering of the pH (La Manna et al., 1992; Much and Hansen, 1984). It has been shown that upon lactic acid buildup in the striatum, DA is excessively released (Globus et al., 1988). This excess release of DA can be cytotoxic, resulting from both enzymatic and non-enzymatic oxidation processes which generate reactive oxygen species (ROS) (Graham, 1978; Hasting, 1995).
Research involving both Glu and DA has concluded that the formation of free radicals due to ischemia can facilitate the release of Glu, and that this increased release of Glu can further facilitate the release of DA (Pelligrini-Giampietro et al., 1989; Pelligrini-Giampietro et al., 1988). Research by Remblier et al. (1998, 1999) describes a study involving the addition of lactic acid to the perfusion medium which aided in the formation of lactacidosis within the striatum. The outcome of this work led to an observed biphasic increase in the DA activity, but only a monophasic increase for the Glu activity within the striatum. It was concluded that the first phasic release of DA was due to the excessive release of Glu (Glu excitotoxicity) as well as ROS formation. The second phasic release in DA (not accompanied by Glu release) was thought to be purely due to ROS formation. These data could help to explain the some of the trends observed with the data collected from the 60 second microdialysis samples using the 3-MPA seizure model. This theory agreed that the excessive Glu release observed modulates the first phasic release of DA observed. However, the second phasic Glu response was not validated using this theory. A further investigation into ROS production using the 3-MPA seizure model would be of great interest. Oxygen deprivation and a depletion of energy stores have a great affect on cellular metabolism, eventually leading to depolarization of the neuron, and the release of an excessive amount of Glu. The subsequent activation of the Glu ionotropic receptors cause the uptake of extra Ca2+, which is known to trigger cascading second-messenger systems, eventually leading to cell death (Siegel et al., 1999; Pelligrini-Giampietro et al., 1989).
5. CONCLUSION
The 3-MPA chemical seizure model previously developed (Crick et al., 2007) was applied to obtain a more detailed analysis of amino acid and biogenic amine neurotransmission as they were associated with the seizures elicited by 3-MPA. Within the striatum, large and significant changes were observed for both Glu and GABA which were sustained over a period of time. The reason behind this sustained release was believed to be Glu receptor desensitization; however, the initial rise may be caused by the decrease in inhibitory tone leading to seizures and thus to more Glu release.
Increasing the microdialysis sampling temporal resolution to the 60 second time scale proved to be valuable in assessing the neurochemical changes. The 60 second temporal resolution was sufficient in this research, showing an increase in the amount of information attainable on the neurotransmitter systems versus 5 minute resolution. A biphasic activity in Glu, matching the biphasic activity in DA, was observed when the 60 second temporal resolution was implemented that had been missed using 5 minute microdialysis sampling.
The changes which were observed in the neurotransmitters, especially Glu and DA, are hypothesized to be part of a mechanism which relates the neuronal activities of the two. It was possible that Glu was regulating the initial release of DA via the activation of DA D2 receptors located on the AMPA receptors. It was also hypothesized that the changes observed in Glu and DA functions could be related to ischemic events within the striatum. The second phasic release of DA could be related to either hypoxic or ischemic events which occurred as a result of the 3-MPA seizure model. Further experimentation should be performed in order to evaluate whether one or both of these hypothetical situations were occurring.
Table 4.
ECoG data from 60 second microdialysis sampling compared with that from 5 minute sampling (Table 1).
| 60 mg/kg bolus + 50 mg/kg/min infusion: 5 minute samplinga | 60 mg/kg bolus + 50 mg/kg/min infusion: 60 second samplingb | |
|---|---|---|
| Latency to Seizure Onset (s) | 363.2 ± 148.8 | 438.0 ± 165.5 |
| Number of Seizures Detected | 592 ± 187 | 830 ± 62 |
| Average of the Average Seizure Duration (s) | 0.87 ± 1.78 | 0.6 ± 0.2 |
| Rmax | 71.8 ± 22.2 | 25.4 ± 1.5 |
: n = 12 rats
: n = 3 rats
Acknowledgments
The funding for this project was provided by the NIH (R01 HL069014) and the Alliance for Epilepsy Research (Kansas City, Kansas).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams BA, Bradberry CW, Moghaddam B. NMDA antagonist effects on striatal dopamine release: Microdialysis studies in awake monkeys. Synapse. 2002;43:12–18. doi: 10.1002/syn.1114. [DOI] [PubMed] [Google Scholar]
- Adams RN. Probing brain chemistry with electroanalytical techniques. Anal Chem. 1976;48:1128A–1137A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodents working memory using a clinically relevant task. Psychopharmacology. 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Bert L, Robert F, Denoroy L, Stoppini L, Renaud B. Enhanced temporal resolution for the microdialysis monitoring of catecholamines and excitatory amino acids using capillary electrophoresis with laser-induced fluorescence detection: Analytical developments and in vitro validations. J Chromatogr A. 1996;755:99–111. doi: 10.1016/s0021-9673(96)00595-x. [DOI] [PubMed] [Google Scholar]
- Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF, Renaud B. In vivo temporal sequence of rat striatal glutamate, aspartate and dopamine efflux during apomorphine, nomifensine, NMDA and PDC in situ administration. Neuropharmacology. 2002;43:825–835. doi: 10.1016/s0028-3908(02)00170-3. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Brajter-Toth A, Chambers JQ, editors. Electroanalytical Methods for Biological Materials. Marcel Dekker, Inc; New York: 2002. [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PEM, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tegmental area. J Neurochem. 2002;81:158–169. doi: 10.1046/j.1471-4159.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- Clinckers R, Gheuens S, Smolders I, Meurs A, Ebinger G, Michotte Y. In vivo modulatory action of extracellular glutamate on the anticonvulsant effects of hippocampal dopamine and serotonin. Epilepsia. 2005;46:828–836. doi: 10.1111/j.1528-1167.2005.57004.x. [DOI] [PubMed] [Google Scholar]
- Crick EW, Osorio I, Bhavaraju NC, Linz TH, Lunte CE. An investigation into the pharmacokinetics of 3-mercaptopropionic acid and development of a steady-state chemical seizure model using in vivo microdialysis and electrophysiological monitoring. Epilepsy Res. 2007;74:116–125. doi: 10.1016/j.eplepsyres.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Deng L, Chen G. Cyclothiazide potentially inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses. Proc Natl Acad Sci USA. 2003;100:13025–13029. doi: 10.1073/pnas.2133370100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desce JM, Godeheu G, Galli T, Artaud F, Cheramy A, Glowinski J. L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and NMDA receptors. Neuroscience. 1992;47:333–339. doi: 10.1016/0306-4522(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Globus MYT, Busto R, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of ischemia on the in vivo release of striatal dopamine, glutamate and γ-aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988;51:1455–1464. doi: 10.1111/j.1471-4159.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Gorji A, Stemmer N, Rambeeck B, Jurgens UH, May TW, Pannek HW, Behne F, Ebner A, Straub H, Speckmann EJ. Neocortical microenvironment in patients with intractable epilepsy: potassium and chloride concentrations. Epilepsia. 2006;47:297–310. doi: 10.1111/j.1528-1167.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Greco PG, Meisel RL, Heidenreich BA, Garris PA. Voltammetric measurement of electrically evoked dopamine levels in the striatum of the anesthetized Syrian hamster. J Neurosci Methods. 2006;152:55–64. doi: 10.1016/j.jneumeth.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hasting TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem. 1995;64:919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Figueroa L. Glutamate receptor desensitization block potentiates the stimulated GABA release through external Ca2+-independent mechanisms from Granule cells of olfactory bulb. Neurochem Res. 2001;26:1177–1185. doi: 10.1023/a:1013930803677. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Moghaddam B. Glutamatergic control of dopamine release during stress in the rat prefrontal cortex. J Neurochem. 1994;63:785–788. doi: 10.1046/j.1471-4159.1994.63020785.x. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa A, Meririnne E, Ariniemi K, Seppala T. Oxalic acid stabilizes dopamine, serotonin, and their metabolites in automated liquid chromatography with electrochemical detection. J Chromatogr B. 2001;753:413–419. doi: 10.1016/s0378-4347(00)00553-3. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BHC, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer BD. Modulation of the mesolimbic dopamine system by glutamate: Role of NMDA receptors. J Neurochem. 1999;73:839–848. doi: 10.1046/j.1471-4159.1999.0730839.x. [DOI] [PubMed] [Google Scholar]
- Konradi C. Dopamine modulation of responses mediated by excitatory amino acids in the neostriatum. In: Goldstein DS, Eisenhofer G, McCarty R, editors. Catecholamines: Bridging Basic Science with Clinical Medicine. Academic Press; San Diego: 1998. pp. 724–729. [DOI] [PubMed] [Google Scholar]
- La Manna JC, Giffith JK, Cordisco BR, Lin CW, Lust WD. Intracellular pH in rat brain in vivo and in brain slices. Can J Physiol Pharmacol. 1992;70:5269–5277. doi: 10.1139/y92-272. [DOI] [PubMed] [Google Scholar]
- Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis on-line with capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1997;69:4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- Leviel V, Gobert A, Guibert B. The glutamate-mediated release of dopamine in the rat striatum: further characterization of the dual excitatory-inhibitory function. Neuroscience. 1990;39:305–312. doi: 10.1016/0306-4522(90)90269-a. [DOI] [PubMed] [Google Scholar]
- Much WA, Hansen AJ. Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. J Cereb Blood Flow Metab. 1984;4:17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Kerkerian L, Dusticier N. Presynaptic controls in the neostriatum: Reciprocal interactions between the nigro-striatal dopaminergic neurons and the cortico-striatal glutamatergic pathway. Exp Brain Res Supp. 1983;7:54–65. [Google Scholar]
- Nyitrai G, Kekesi KA, Juhasz G. Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr Top Med Chem. 2006;6:935–940. doi: 10.2174/156802606777323674. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Zilkha E. High extracellular potassium, not extracellular glutamate, is required for the propagation of spreading depression. J Neurophysiol. 1995;73:2107–2114. doi: 10.1152/jn.1995.73.5.2107. [DOI] [PubMed] [Google Scholar]
- Osorio I, Frei MG, Wilkinson SB. Real-time automated detection and quantitative analysis of seizures and short-term prediction of clinical onset. Epilepsia. 1998;39:615–627. doi: 10.1111/j.1528-1157.1998.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Osorio I, Frei MG, Giftakis J, Peters T, Ingram J, Turnbull M, Herzog M, Rise MT, Schaffner S, Wennberg RA, Walczak TS, Risinger MW, Ajmone-Marsan C. Performance reassessment of a real-time seizure-detection algorithm on long ECoG series. Epilepsia. 2002;43:1522–1535. doi: 10.1046/j.1528-1157.2002.11102.x. [DOI] [PubMed] [Google Scholar]
- Parrot S, Bert L, Mouly-Badina L, Sauvinet V, Colussi-Mas J, Lambas-Senas L, Robert F, Bouilloux JP, Suaud-Chagny MF, Denoroy L, Renaud B. Microdialysis monitoring of catecholamines and excitatory amino acids in the rat and mouse brain: recent developments based on capillary electrophoresis with laser-induced fluorescence detection - a mini-review. Cell Mol Neurobiol. 2003;23:793–804. doi: 10.1023/A:1025009221285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrini-Giampietro DE, Cherici G, Alesiani M, Carla V, Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free-radical formation. J Neurochem. 1988;51:1960–1963. doi: 10.1111/j.1471-4159.1988.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Pelligrini-Giampietro DE, Lombardi G, Cherici G, Carla V, Moroni F. Excitatory amino acid release during in vitro and in vivo models of ischemia: are free radicals involved? In: Biggio G, et al., editors. Excitatory Amino Acids and Brain Ischemia: Pharmacological and Clinical Aspects. Pergamon Press; Oxford: 1989. pp. 45–56. [Google Scholar]
- Pfrieger FW, Barres BA. New views on synapse-glia interactions. Curr Opin Neurobiol. 1996;6:615–621. doi: 10.1016/s0959-4388(96)80093-6. [DOI] [PubMed] [Google Scholar]
- Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P. Δ9-Tetradydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- Plock N, Kloft C. Microdialysis - theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Remblier C, Jolimay N, Wahl A, Pariat C, Piriou A, Huguet F. Extracellular dopamine and catabolites in rat striatum during lactic acid perfusion as determined by in vivo microdialysis. Brain Res. 1998;804:224–230. doi: 10.1016/s0006-8993(98)00695-7. [DOI] [PubMed] [Google Scholar]
- Remblier C, Pontcharraud P, Tallineau C, Piriou A, Huguet F. Lactic acid-induced increase of extracellular dopamine measured by microdialysis in rat striatum: evidence for glutamatergic and oxidative mechanisms. Brain Res. 1999;837:22–28. doi: 10.1016/s0006-8993(99)01699-6. [DOI] [PubMed] [Google Scholar]
- Robert F, Bert L, Parrot S, Denoroy L, Stoppini L, Renaud B. Coupling on-line brain microdialysis, precolumn derivatization and capillary electrophoresis for routine minute sampling of o-phosphoethanolamine and excitatory amino acids. J Chromatogr A. 1998;817:195–203. doi: 10.1016/s0021-9673(98)00321-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Justice JB., Jr . In: Microdialysis in the Neurosciences. Huston JP, editor. Amsterdam: Elsevier; 1991. [Google Scholar]
- Robinson DL, Venton JL, Heien MLA, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Segova G, Mora F. Role of nitic oxide in modulating the release of dopamine, glutamate, and GABA in striatum of the freely moving rat. Brain Res Bull. 1998;45:275–279. doi: 10.1016/s0361-9230(97)00402-4. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Crespi F, Heidbreder C. Amino acid neurotransmitters: separation approaches and diagnostic value. J Chromatogr B. 2002;781:151–163. doi: 10.1016/s1570-0232(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Duan S, Hori T, Oomura Y. Glutamate modulates dopamine release in the striatum as measured by brain microdialysis. Brain Res Bull. 1990;25:99–102. doi: 10.1016/0361-9230(90)90258-2. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 6. Lippincott Williams & Wilkins; Philadelphia: 1999. [Google Scholar]
- Siesjo BK. Acidosis and ischemic brain damage. Neurochem Pathol. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]
- Siri N, Lacroix M, Garrigues JC, Poinsot V, Couderc F. HPLC-fluorescence detection and MEKC-LIF detection for the study of amino acids and catecholamines labelled with naphthalene-2,3-dicarboxyaldehyde. Electrophoresis. 2006;27:4446–4455. doi: 10.1002/elps.200600165. [DOI] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjo BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab. 1986;6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Starr MS. The role of dopamine in epilepsy. Synapse. 1996;22:159–194. doi: 10.1002/(SICI)1098-2396(199602)22:2<159::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Target-specific glutamatergic regulation of dopamine neurons in the ventral tegmental area. J Neurochem. 2000;75:1775–1778. doi: 10.1046/j.1471-4159.2000.0751775.x. [DOI] [PubMed] [Google Scholar]
- Thorre K, Pravda M, Sarre S, Ebinger G, Michotte Y. New antioxidant mixture for long term stability of serotonin, dopamine, and their metabolites in automated microbore liquid chromatography with dual electrochemical detection. J Chromatogr B. 1997;694:297–303. doi: 10.1016/s0378-4347(97)00126-6. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tran MN, Higgs MH, Lukasiewicz PD. AMPA receptor kinetics limit retinal amacrine cell excitatory synaptic responses. Vis Neurosci. 1999;16:835–842. doi: 10.1017/s0952523899165039. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Tucci S, Rada P, Sepulveda MJ, Hernandez L. Glutamate measured by 6-s resolution brain microdialysis: capillary electrophoretic and laser-induced fluorescence detection application. J Chromatogr B. 1997;694:343–349. doi: 10.1016/s0378-4347(96)00488-4. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Patneau DK, Mayer M. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/Kainate receptors. Neuron. 1991;7:971–984. doi: 10.1016/0896-6273(91)90342-w. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Strope E, Plotsky P, Adams RN. In vivo voltammetry: monitoring of dopamine metabolites in CSF following release by electrical stimulation. Brain Res. 1978;159:55–68. doi: 10.1016/0006-8993(78)90109-9. [DOI] [PubMed] [Google Scholar]
- Youngren KD, Daly DA, Moghaddam B. Distinct actions of endogenous excitatory amino acids on the outflow of dopamine in the nucleus accumbens. J Pharmacol Exp Ther. 1993;264:289–293. [PubMed] [Google Scholar]
- Zhang X, Fuller RR, Dahlgren RL, Potgieter K, Gillette R, Sweedler JV. Neurotransmitter sampling and storage for capillary electrophoresis analysis. Fresenius J Anal Chem. 2001;369:206–211. doi: 10.1007/s002160000654. [DOI] [PubMed] [Google Scholar]