Abstract
Hypothesis
Decreased insulin sensitivity (IS) exists in type 1 diabetes. Serum uric acid (SUA), whose concentration is related to renal clearance, predicts vascular complications in type 1 diabetes. SUA is also inversely associated with IS in non-diabetics, but has not been examined in type 1 diabetes. We hypothesized SUA would be associated with reduced IS in adolescents and adults with type 1 diabetes.
Methods
The cross-sectional and longitudinal associations of SUA with IS was investigated in 254 adolescents with type 1 diabetes and 70 without in the Determinants of Macrovascular Disease in Adolescents with Type 1 Diabetes Study, and in 471 adults with type 1 diabetes and 571 without in the Coronary Artery Calcification in Type 1 diabetes (CACTI) study.
Results
SUA was lower in subjects with type 1 diabetes (p<0.0001), but still remained inversely associated with IS after multivariable adjustments- in adolescents (β±SE: −1.99±0.62, p=0.001, R2=2%) and adults (β±SE:−0.91±0.33, p=0.006, R2=6%) with type 1 diabetes, though less strongly than in non-diabetic controls (adolescents: β±SE: −2.70±1.19, p=0.03, R2=15%, adults: β±SE:−5.99±0.75, p<0.0001, R2=39%).
Conclusion
We demonstrated a significantly weaker relationship between SUA and reduced IS in subjects with type 1 diabetes than non-diabetic controls.
Keywords: insulin sensitivity, type 1 diabetes, serum uric acid, adolescents and adults
Introduction
Reduced insulin sensitivity is well documented in both adolescents and adults with type 1 diabetes, and is thought to contribute both to the initiation and progression of macro- and microvascular complications (1, 2). The exact mechanism of reduced insulin sensitivity in type 1 diabetes is poorly understood, but is not completely explained by body mass index (BMI), visceral fat, physical activity, hyperglycemia or systemic inflammation (3, 4). Significantly higher prevalence of metabolic syndrome (38% in men and 40% in women) has also been reported in subjects with type 1 diabetes than non-diabetic subjects (5), although the definition of metabolic syndrome in type 1 diabetes may not be directly comparable to non-diabetic subjects. Several factors have been implicated including prolonged exposure to supraphysiologic levels of exogenous insulin, loss of the insulin sensitizing effect of plasma palmitoleic acid and the genetic and environmental factors that lead to type 2 diabetes (4, 6–9).
Hyperuricemia was historically viewed as a consequence of the effects of insulin to increase renal uric acid reabsorption (10). However, more recent studies have reported that SUA can also predict the development of hyperinsulinemia, metabolic syndrome and type 2 diabetes (11–14) likely via its intracellular effects on the liver, adipocyte and endothelial cell. Subjects with type 1 diabetes have reduced SUA concentrations (15, 16), but there is still robust evidence linking SUA to vascular complications in type 1 diabetes (17–19). SUA and insulin sensitivity remain attractive therapeutic targets due to the availability of generic medications like allopurinol and metformin. Little, if any data, however, exist on a possible association between SUA and insulin sensitivity in type 1 diabetes, and whether they are independent pathways in vascular pathogenesis, or as strongly associated as what is observed in subjects without type 1 diabetes. For that reason, further data are needed on the relationship between SUA and insulin sensitivity in type 1 diabetes.
SUA levels tend to increase with age, and the relationship between SUA and insulin sensitivity might change over time as patients become older. Accordingly, we explored the association between SUA and insulin sensitivity among adolescent and adults with type 1 diabetes. We hypothesized that SUA would be associated with insulin sensitivity cross-sectionally, and that baseline SUA would predict worsening of insulin sensitivity over 2 and 6 years respectively in adolescents and adults with and without type 1 diabetes.
Materials and Methods
The Determinants of Macrovascular Disease in Adolescents with type 1 diabetes study cohort
The Determinants of Macrovascular Disease in Adolescents was initiated to investigate atherosclerotic disease risk in youth with and without type 1 diabetes. The study enrolled subjects 12–19 years old, with and without type 1 diabetes. Study participants with type 1 diabetes were diagnosed by islet cell antibody or by provider clinical diagnosis, had diabetes duration >5 years at entry into the study, and received care at the Barbara Davis Center for Childhood Diabetes, Aurora, Colorado. Control subjects were recruited from friends of the study subjects as well as from campus and community advertisements. No siblings or first-degree relatives of patients with type 1 diabetes were included. Subjects were excluded for diabetes of any other type and for any history of abnormal cardiac anatomy or arrhythmia that would preclude the subject from vascular function measurements. All subjects with data available for SUA and estimated insulin sensitivity (eIS) were included in this analysis, which included 254 adolescents with type 1 diabetes and 70 controls at baseline and 193 adolescents with type 1 diabetes and 50 controls at 2-year follow-up. The study was approved by the Colorado Multiple Institution Review Board, and informed consent and assent (for subjects <18 years) was obtained from all subjects.
After subjects had been laying supine for a minimum of 5 minutes, blood pressure measurements were obtained using a Dynapulse 5200A (Pulse Metric, San Diego, California), and 3 measurements were averaged. Height was measured to the nearest 0.1 cm with shoes removed using a wall-mounted stadiometer, and weight was measured to the nearest 0.1 kg using a Detecto scale (Detecto, Webb City, Missouri). Waist circumference was measured at the navel on bare skin using the Figure Finder Tape Measure by Novel Products (Rockton, Illinois), which provides consistent and repeatable oz of tension and accuracy to 3/32 inch.
All subjects fasted overnight (≥8 hours). HbA1c was measured on the DCA Advantage by Siemens (Princeton, New Jersey). Total cholesterol, high-density lipoprotein cholesterol (HDL-c), and triglycerides were performed using an Olympus AU400e Chemistry (Olympus, Brea, California). Low-density lipoprotein cholesterol was calculated using the Friedwald formula. High-sensitivity C-reactive protein was measured using a multiplex assay platform Siemens (formally Dade Behring) BNII Nephelometer. Serum creatinine was measured using an Olympus AU400e, and urinary creatinine was measured by the Jaffe method using Roche Diagnostics reagent on the Hitachi 917 autoanalyzer. Serum uric acid levels were measured on stored baseline samples via the Clinical Analyzer utilizing a uricase-based commercial kit. Urinary uric acid was analyzed without dilution for uric acid using the ACE autoanalyzer (Alfa Wassermann, West Caldwell, NJ) employing the uricase method with final endpoint analysis. Fractional excretion of uric acid calculated by: FeUA = [(urinary uric acid)*(serum creatinine)*(100%)]/[(serum uric acid)*(urinary creatinine)]. All uric acid measures were performed in the laboratory of Dr. Richard Johnson.
Insulin sensitivity for adolescents was calculated by Insulin Sensitivity Score (eIS) model derived from SEARCH Clamp Study: logeIS = 4.64725 − 0.02032 (waist [cm]) − 0.09779 (HbA1c [%]) − 0.00235 (TG [mg/dL]) (R2 = 0.74 in the initial SEARCH model) (20).
The Coronary Artery Calcification in Type 1 diabetes (CACTI) Cohort
The CACTI Study enrolled subjects 19–56 years old, with and without type 1 diabetes, who were asymptomatic for cardiovascular disease (CVD) at the baseline visit in 2000–02 and then were re-examined 3 and 6 years later, as previously described (21). Subjects with serum creatinine >2mg/dl were excluded at baseline, unless they were participants in the pilot study. Data on baseline SUA and insulin sensitivity at baseline were available for 471 patients with type 1 diabetes and 571 controls, and follow-up SUA and insulin sensitivity at 6 years were available for 420 patients with type 1 diabetes and 513 controls. The study was approved by the Colorado Multiple Institutional Review Board and all participants provided informed consent.
We measured height and weight, and calculated body mass index (BMI) in kg/m2. Resting systolic (SBP) and fifth-phase diastolic blood pressure (DBP) were measured three times while the patient was seated, and the second and third measurements were averaged. Hypertension was defined as current anti-hypertensive therapy or untreated hypertension (BP 140/90 mmHg) at the time of the study visit. Anti-hypertension medication use was determined by a medication inventory as previously described (21) and use of an ACE inhibitor (ACEi) or an angiotensin receptor blocker (ARB) were combined for these analyses.
Serum uric acid levels were measured on stored baseline samples via the Clinical Analyzer utilizing a uricase-based commercial kit. These samples had been thawed twice in the past. The results were reported in milligrams per deciliter. Total plasma cholesterol and triglyceride levels were measured using standard enzymatic methods, HDL cholesterol was separated using dextran sulfate and LDL cholesterol was calculated using the Friedewald formula. High performance liquid chromatography was used to measure HbA1c (HPLC, BioRad variant). Serum creatinine was measured according to package insert instructions using a Roche Mira Plus II analyzer until 2006 and then an Olympus AU400e (r=0.9999 between methodologies) traceable to the National Institutes of Standards and Technology Standard Reference Material in the University of Colorado Clinical Translational Research (CTRC) Lab and standardized longitudinally as previously described (22).
Two timed overnight urine samples were collected in duplicate and urine creatinine and albumin were measured (RIA, Diagnostic Products) and averaged. Insulin sensitivity was calculated by Insulin Sensitivity Index (ISI) model derived from CACTI clamp studies (23). The equation to calculate ISI explained 63% of the variance in the glucose disposal rate (GDR). The equation to calculate the ISI is: exp(4.1075 − 0.01299*waist (cm) − 1.05819*insulin dose (daily units per kg) − 0.00354*triglycerides (mg/dl) − 0.00802*diastolic blood pressure (mm Hg)) (24).
Statistical Analysis
Analyses were performed in SAS (version 9.3 for Windows; SAS Institute, Cary, NC). Differences between type 1 diabetes patients and normal controls were assessed using Chi-Square for categorical variables and t-test for continuous variables. Variables with skewed distributions (e.g. SUA and serum creatinine) were log-transformed and then back transformed to compute geometric means. Linear regression and ANCOVA were employed to fit three models with baseline LnSUA as a continuous predictor and then quartiles of SUA as categorical predictors of baseline insulin sensitivity and change in insulin sensitivity: unadjusted model, model 1 adjusted for age, sex, BMI and LnSCr, model 2 adjusted for model 1 + disease duration, SBP, HbA1c, LDL-c, HDL-c, fasting blood glucose and current smoking status. In the adolescent cohort, we further adjusted for FeUA in an attempt to control for the proposed uricosuric effect observed in subjects with type 1 diabetes.
In adolescent and adult cohorts, we had 2 and 6-year follow-up data, respectively, to explore longitudinal relationships. We defined worsening of insulin sensitivity as a decrease from baseline insulin sensitivity over time. Logistic regression was used to see if baseline SUA predicted worsening of insulin sensitivity in type 1 diabetes and controls. For linear regression, data are presented as β ± SE. For ANCOVA, data are presented as least square mean ± SE. For logistic regression, data are presented as OR (95% CI). All analyses were stratified by type 1 diabetes status, and significance was based on an α-level of 0.05.
Results
The Determinants of Macrovascular Disease in Adolescents with type 1 diabetes study
Type 1 diabetes patients had higher HbA1c, higher SBP, slightly lower serum creatinine, lower SUA, lower insulin sensitivity, and higher LDL compared to controls (Table 1). FeUA did not differ by diabetes status (p=0.91, Table 1) and, as expected, was negatively correlated with Ln SUA (r= −0.23, p<0.0001).
Table 1.
Characteristics of Adolescent Type 1 diabetes Patients and Controls
| Means ± SD and p-values for comparisons by group |
|||
|---|---|---|---|
| Variable | Type 1 diabetes (N = 254) |
Controls (N = 70) |
P-Value |
| Age (years) | 15.4 ± 2.2 | 15.4 ± 2.2 | 0.85 |
| Sex (Male) | 49.6% | 42.9% | 0.32 |
| Currently Smoking (%) | 7.1% | 7.1% | 0.99 |
| Duration (years) | 8.7 ± 3.0 | -- | -- |
| BMI Z-Score (kg/m2) | 0.6 ± 0.8 | 0.2 ± 1.1 | 0.002 |
| HbA1c (%) | 9.0 ± 1.6 | 5.3 ± 0.3 | < 0.0001 |
| HbA1c (mmol/mol) | 75 ± 15 | 34 ± 1 | < 0.0001 |
| SCR (mg/dL) | 0.65 ± 0.14 | 0.70 ± 0.15 | 0.01 |
| SBP (mm Hg) | 113 ± 8 | 108 ± 9 | < 0.0001 |
| Uric Acid (mg/dL) | 4.6 ± 0.8 | 5.0 ± 1.0 | < 0.0001 |
| FeUA | 4.6 ± 4.3 | 4.6 ± 4.3 | 0.91 |
| eIS (mg/kg−1min−1) | 7.8 ± 2.4 | 11.7 ± 2.8 | < 0.0001 |
| HDL (mg/dL) | 51 ± 10 | 49 ± 10 | 0.17 |
| LDL (mg/dL) | 89 ± 27 | 82 ± 22 | 0.03 |
| Blood glucose (mg/dL) | 183.4 ± 79.3 | 82.0 ± 7.2 | <0.0001 |
Cross-sectionally in type 1 diabetes, Ln SUA was negatively correlated with insulin sensitivity in unadjusted analyses (β ± SE: −1.93 ± 0.82, p= 0.02), and explained only 2% of the variability of insulin sensitivity. In comparison, BMI accounted for 28% and HbA1c 37% of the variability of insulin sensitivity respectively. After adjustments for FeUA, age, sex, BMI, serum creatinine, type 1 diabetes duration, SBP, HbA1c, HDL, LDL, and current smoking, Ln SUA remained negatively associated with eIS (β ± SE: −1.99 ± 0.62, p= 0.001, Table 2). Moreover, in the same multivariable-adjusted model the 4th quartile of SUA was associated with lower eIS than the 1st quartile of SUA (p = 0.04, Table 2).
Table 2.
Relationship between baseline serum uric acid and insulin sensitivity in adolescents
| Type 1 diabetes | Ln SUA |
Quartile 1 of SUA |
Quartile 2 of SUA |
Quartile 3 of SUA |
Quartile 4 of SUA |
|
β*
± SE P-Value |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
|
| eIS (unadjusted) | −1.93 ± 0.82 P = 0.02 |
8.52 ± 0.28** | 7.75 ± 0.28 | 7.41 ± 0.35 | 7.16 ± 0.35 |
| eIS (adjusted for FeUA) | −2.29 ± 0.89 P = 0.01 |
8.57 ± 0.29** | 7.78 ± 0.30 | 7.48 ± 0.31 | 7.09 ± 0.36 |
| eIS (adjusted for model 1: age, sex, BMI, LnSCr) | −2.50 ± 0.84 P = 0.003 |
8.33 ± 0.24** | 7.66 ± 0.24 | 7.67 ± 0.25 | 7.35 ± 0.30 |
| eIS (adjusted for model 2: model 1, SBP, diabetes duration, LDL-c, HDL-c, glucose and current smoking) | −1.99 ± 0.62 P = 0.001 |
8.26 ± 0.18** | 7.73 ± 0.18 | 7.74 ± 0.19 | 7.42 ± 0.22 |
| Controls | Ln SUA |
Quartile 1 of SUA |
Quartile 2 of SUA |
Quartile 3 of SUA |
Quartile 4 of SUA |
|
β*
± SE P-Value |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
|
| eIS (unadjusted) | −5.80 ± 1.70 P = 0.001 |
14.01 ± 0.88** | 12.58 ± 0.64** | 12.68 ± 0.64** | 10.18 ± 0.44 |
| eIS (adjusted for FeUA) | −5.70 ± 1.83 P = 0.003 |
13.92 ± 0.91** | 12.73 ± 0.66** | 12.47 ± 0.69** | 10.23 ± 0.46 |
| eIS (adjusted for model 1: age, sex, BMI, LnSCr) | −2.77 ± 1.09 P = 0.01 |
11.86 ± 0.51 | 12.34 ± 0.34** | 11.84 ± 0.35 | 11.14 ± 0.24 |
| eIS (adjusted for model 2: model 1, SBP, LDL-c, HDL-c, glucose, and current smoking) | −2.70 ± 1.19 P = 0.03 |
12.02 ± 0.54 | 12.40 ± 0.36 | 11.60 ± 0.37 | 11.24 ± 0.26 |
β-coefficient represents the difference in estimated insulin sensitivity (eIS) for every 1-unit difference, and where appropriate for every natural log difference in the independent variable.
P < 0.05 compared with the 4th Quartile.
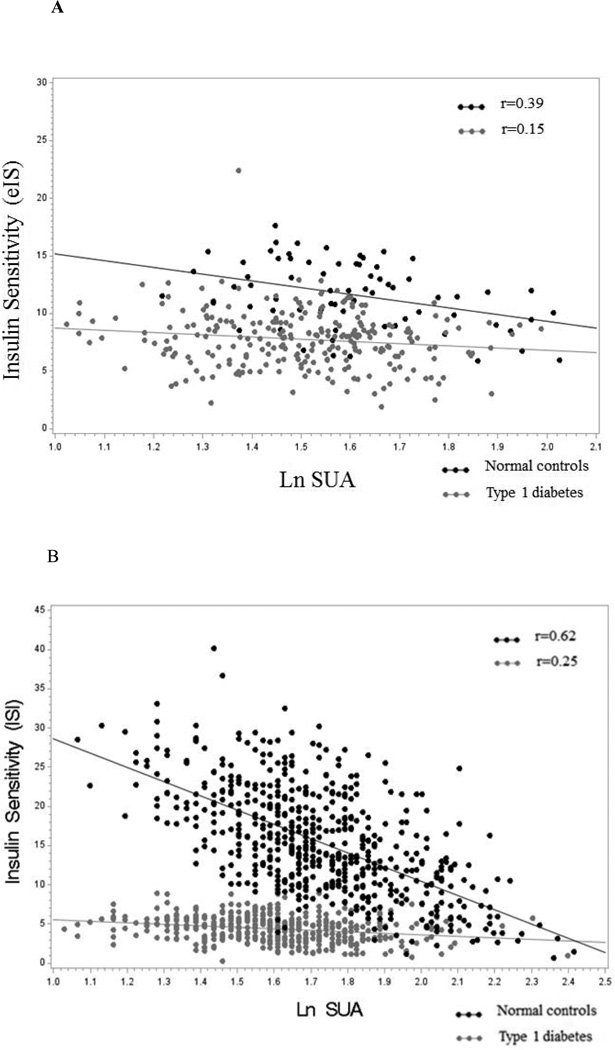
Cross-sectionally in controls, Ln SUA was negatively correlated with insulin sensitivity in unadjusted analyses (β ± SE: −5.80 ± 1.70, p= 0.001) and explained 15% of the variability in insulin sensitivity (Figure 1A). In comparison, HbA1c accounted for 4% and BMI 72% of the variability of insulin sensitivity, respectively. After multivariable adjustments the association between Ln SUA and eIS remained significant (β±SE: −2.70±1.19, p=0.03, Table 3). There was also an association between quartiles of SUA and eIS (p=0.048) (Table 2).
Figure 1.
Relationship of baseline LnUA with eIS in adolescent with and without type 1 diabetes
Relationship of baseline LnUA with ISI in adults with and without type 1 diabetes
Table 3.
Characteristics of Adult Type 1 diabetes Patients and Controls
| Visit 1 | |||
|---|---|---|---|
| Variable | Type 1 diabetes (N = 471) |
Controls (N = 571) |
p-value |
| Age (years) | 37± 9 | 40 ± 9 | < 0.0001 |
| Sex (Males) | 47.1% | 50.4% | -- |
| NHW (%) | 93% | 80.7% | 0.81 |
| Duration (years) | 23.0 ± 8.9 | -- | <0.0001 |
| BMI (kg/m2) | 26.1 ± 4.2 | 26.0 ± 4.7 | <0.0001 |
| HbA1c (%) | 7.9 ± 1.2 | 5.5 ± 0.4 | 0.95 |
| HbA1c (mmol/mol) | 63 ± 11 | 37 ± 2 | 0.02 |
| SCR (mg/dL) | 1.2 ± 0.2 | 1.2 ± 0.2 | <0.0001 |
| SBP (mmHg) | 116 ± 13 | 114 ± 12 | <0.0001 |
| Uric Acid (mg/dL) baseline | 5.0 ± 1.1 | 5.7 ± 1.3 | 0.04 |
| Uric Acid (mg/dL) 6 Years | 5.3 ± 1.2 | 6.1 ± 1.4 | <0.0001 |
| Change in Uric Acid (mg/dL) | 0.3 ± 1.0 | 0.4 ± 1.0 | <0.0001 |
| ISI baseline (mg/kg−1min−1) | 4.4 ± 1.6 | 15.5 ± 6.6 | <0.0001 |
| ISI 6 Years (mg/kg−1min−1) | 4.7 ± 1.8 | 15.7 ± 6.6 | <0.0001 |
| HDL (mg/dL) | 55 ± 16 | 51 ± 15 | <0.0001 |
| LDL (mg/dL) | 99 ± 28 | 116 ± 34 | <0.0001 |
| Blood glucose (mg/dL) | 184.1 ± 90.6 | 90.5 ± 10.1 | <0.0001 |
Longitudinally, SUA at baseline was not associated with increased odds of worsening of eIS over 2 years in subjects with type 1 diabetes (OR = 0.14, 95% CI: 0.01–1.69, p=0.12) or controls (OR = >999, 95% CI: 0.28–>999, p=0.08).
The Coronary Artery Calcification in Type 1 diabetes (CACTI) Cohort (Adults)
There were several differences between those with type 1 diabetes and controls; type 1 diabetes patients were younger, had higher HbA1c, lower SUA at baseline and 6 years, lower insulin sensitivity at baseline and 6 years, higher HDL-c and lower LDL-c (Table 2).
Cross-sectionally in type 1 diabetes, Ln SUA was negatively correlated with ISI in unadjusted analyses (β±SE: −1.90±0.34, p<0.0001) and explained only 6% of the variability of ISI (Figure 1B). In comparison, HbA1c accounted for 8% and BMI for 28% of the variability of ISI, respectively. After adjustments for age, sex, BMI, serum creatinine, type 1 diabetes duration, SBP, HbA1c, HDL, LDL, and current smoking, Ln SUA remained negatively associated with ISI (β ± SE: −0.91 ± 0.33, p=0.006, Table 4). Moreover, in a fully adjusted model the 4th quartile was associated with lower insulin sensitivity than the 1st quartile of SUA (p=0.04) (Table 4).
Table 4.
Relationship between baseline serum uric acid and insulin sensitivity in adults
| Type 1 diabetes | Ln SUA |
Quartile 1 of SUA |
Quartile 2 of SUA |
Quartile 3 of SUA |
Quartile 4 of SUA |
|
β*
± SE P-Value |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
|
| eIS (unadjusted) | −1.90 ± 0.34 P < 0.0001 |
4.53 ± 0.12 | 4.49 ± 0.14 | 4.15 ± 0.16 | 4.13 ± 0.22 |
| eIS (adjusted for model 1: age, sex, BMI, LnSCr) | −0.86 ± 0.35 P = 0.0155 |
4.45 ± 0.09** | 4.55 ± 0.11** | 4.25 ± 0.13 | 3.88 ± 0.19 |
| eIS (adjusted for model 2: model 1,SBP, duration, LDL-c, HDL-c, glucose and current smoking) | −0.91 ± 0.33 P = 0.0063 |
4.33 ± 0.11** | 4.31 ± 0.18 | 4.08 ± 0.13 | 3.79 ± 0.18 |
| Controls | Ln SUA | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|
β*
± SE P-Value |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
Least square Mean ± SE |
|
| eIS (unadjusted) | −18.19 ± 0.96 P < 0.0001 |
19.34 ± 0.62† | 17.78 ± 0.51† | 16.38 ± 0.48† | 11.56 ± 0.42 |
| eIS (adjusted for model 1: age, sex, BMI, LnSCr) | −10.96 ± 1.02 P < 0.0001 |
17.91 ± 0.44† | 17.04 ± 0.37† | 15.38 ± 0.34** | 13.58 ± 0.31 |
| eIS (adjusted for model 2: model 1, SBP, LDL-c, HDL-c, glucose and current smoking) | −5.99 ± 0.75 P < 0.0001 |
16.65 ± 0.36† | 16.17 ± 0.31† | 15.39 ± 0.29** | 14.37 ± 0.28 |
β-coefficient represents the difference in estimated insulin sensitivity (ISI) for every 1-unit difference, and where appropriate for every natural log difference in the independent variable.
P < 0.05 compared with the 4th Quartile.
P < 0.0001 compared with the 4th Quartile.
Cross-sectionally in controls, Ln SUA was negatively correlated with ISI in unadjusted analyses (β±SE: −18.19±0.96, p<0.0001), and explained 39% of the variability of ISI (Figure 1B). In comparison, HbA1c accounted for 33% and BMI 41% of the variability of ISI, respectively. After multivariable adjustments, Ln SUA remained negatively associated with ISI (β ± SE: −5.99 ± 0.75, p< 0.0001, Table 4). The 4th quartile of SUA was also associated with lower ISI than the 1st, 2nd and 3rd quartiles. In a fully adjusted model the 4th quartile was still significantly associated with lower ISI than the 1st, 2nd and 3rd quartiles of SUA (p<0.0001) (Table 4).
Longitudinally, a one mg/dL increase in SUA from baseline to 6 years was associated with increased odds of worsening ISI in controls (OR = 1.59, 95% CI: 1.30–1.93, p < 0.0001), while an increase in SUA was not associated with worse ISI in type 1 diabetes (OR = 1.05, 95% CI: 0.86–1.27, p = 0.65).
Conclusions
SUA and insulin sensitivity are both implicated in vascular complications in type 1 diabetes. The association between SUA and reduced insulin sensitivity is well-recognized in type 2 diabetes and metabolic syndrome, but has not previously been investigated in type 1 diabetes. In this study, we report lower levels of SUA in adolescents and adults with type 1 diabetes, and a weak inverse relationship between SUA and insulin sensitivity in both adolescents and adult subjects with type 1 diabetes. In contrast, in non-diabetic adolescents and adults SUA accounted for a significantly greater amount of the variability of insulin sensitivity, and a longitudinal association was also observed between SUA and worsening insulin sensitivity in adult subjects. These data suggest that even though subjects with type 1 diabetes have lower insulin sensitivity than their non-diabetic counterparts, the association observed between SUA and reduced insulin sensitivity in non-diabetic subjects is less strong in those with type 1 diabetes. Furthermore, the associations between SUA and reduced insulin sensitivity were stronger among adults than adolescent subjects with and without type 1 diabetes, suggesting the relationship between SUA and insulin sensitivity may strengthen over time as patients become older. Few, if any, studies have examined the association between SUA and reduced insulin sensitivity across a wide age span (12–56 years) in subjects with and without type 1 diabetes, which is a strength of our study.
The exact mechanism linking SUA and reduced insulin sensitivity in non-diabetic subjects remains unclear. Elevated levels of SUA have been observed with reduced insulin sensitivity and were historically attributed to hyperinsulinemia, as insulin reduces renal excretion of SUA (10). However, increased SUA often precedes the development of hyperinsulinemia. The strongest evidence of a role for SUA in the development of worsening insulin sensitivity comes from studies in animal models showing that decreasing SUA may prevent or even reverse features of reduced insulin sensitivity (25, 26). A number of mechanisms have been suggested by which SUA could affect insulin sensitivity. Elevated SUA has been found to be associated with oxidative stress, systemic inflammation and endothelial dysfunction induced by decreased endothelial nitric oxide production, all of which play roles in the development of impaired insulin sensitivity (27, 28). There is also supporting evidence that fructose-induced elevation of SUA may have a pathogenic role in reduced insulin sensitivity (29, 30). Nakagawa et al. showed that in rats receiving a high-fructose diet, the lowering of SUA with either allopurinol or banzobromarone prevented features of metabolic syndrome and insulin resistance (29). There is to our knowledge, however, no mechanistic data exploring the association between SUA and insulin sensitivity in type 1 diabetes.
The reduced concentrations of SUA observed in subjects with type 1 diabetes, as well as the weaker association between SUA and insulin sensitivity may be due to the effect of intermittent glycosuria that occurs in diabetes. SUA levels have been shown to fall in subjects once they become diabetic, as glycosuria may lead to proximal tubular dysfunction and uricosuria (31). For that reason SUA may actually decrease in people with diabetes, especially if they are poorly controlled. We included measurements of concurrent fasting blood glucose and HbA1c in both cohorts and FeUA in the adolescent cohort in an attempt to control for the potential effect of hyperglycemia on urinary excretion of uric acid. Adjusting for these variables did not change the relationships between SUA and insulin sensitivity in our cohorts. Furthermore, FeUA was the same in adolescents subjects with and without T1D (4.6 vs. 4.6, p=0.9), despite significantly different HbA1c (9.0 vs. 5.3, p<0.0001). For these reasons the uricosuric effect is unlikely to explain the differences observed in adolescents with type 1 diabetes and normal controls. We were unable to test this hypothesis in the adult cohort. Another possible explanation for the weaker association observed between SUA and reduced insulin sensitivity in subjects with type 1 diabetes is that the phenotype of reduced insulin sensitivity in type 1 diabetes might differ from that of type 2 diabetes/metabolic syndrome. The hypothesis of different phenotypes is supported by the observation of insulin resistance in type 1 diabetes, despite elevated levels of adiponectin, an adipocyte-secreted protein important in determination of insulin sensitivity (32), which stands in contrast to type 2 diabetes where there is a well-recognized inverse relationship between adiponectin and insulin resistance (33). Furthermore, insulin resistance has been documented in type 1 diabetes subjects with normal BMI and HbA1c (34) and cannot be explained by plasma lipids, visceral fat or systemic inflammation (3, 4).
The weak association we report between SUA and reduced insulin sensitivity in adolescent and adult subjects with type 1 diabetes stands in contrast with previous cross-sectional studies that showed a close relation between SUA and reduced insulin sensitivity in adolescents and adults without type 1 diabetes (35–37).
Insulin sensitivity is recognized to play an important role in the development of vascular complications in type 1 diabetes (2, 38, 39), and SUA has been shown to predict both the development of albuminuria (40–42) and progression of coronary artery calcification (19) in subjects with type 1 diabetes. In non-diabetic subjects and subjects with type 2 diabetes, there is a strong association between SUA and reduced insulin sensitivity. For that reason one could hypothesize that insulin sensitivity and SUA are two components of the same pathway leading to vascular complications in type 1 diabetes. However, the evidence of a significantly weaker association between SUA and reduced insulin sensitivity in type 1 diabetes, suggests SUA and insulin sensitivity may represent separate pathways in type 1 diabetes, and offer promise for their role as independent therapeutic targets in the prevention of vascular complications. There are clinical trials under way to lower SUA and to improve insulin sensitivity in type 1 diabetes, including The Preventing Early Renal Function Loss (PERL) Allopurinol Study (17) and Reducing with Metformin Vascular Adverse Lesions in Type 1 Diabetes (REMOVAL) trial (43), respectively.
An important strength of our study is the large number of subjects with data available on important confounders across a wide age distribution. Unlike some previous studies, we did not limit our data to those with abnormally high levels of SUA and instead looked at SUA as a continuous variable and quartiles to explore the association with insulin sensitivity across the whole distribution of SUA. Our cohorts also offer important longitudinal data, which imply a causal relationship between SUA and insulin sensitivity in adults without type 1 diabetes.
The limitations of our study also deserve comment. The sample size of adolescent controls (n=50) with follow-up data was too small for any meaningful longitudinal multivariable analysis. The adolescent subjects with type 1 diabetes had higher BMI z-score than controls, but we adjusted for BMI in our multivariable models to account for this. Although we adjusted for a variety of important confounding variables, we cannot rule out the presence of unknown risk factors that may have biased or confounded the present analyses. Another important limitation is the lack of direct measures of insulin sensitivity due our large cohorts making hyperinsulinemic-euglycemic clamp studies too cumbersome; however both eIS and ISI have been validated in clamp studies and shown to explain 63% and 74% of the variability of insulin sensitivity in their respective cohorts. We only had FeUA data in our adolescent cohort, but adjusting for this variable neither changed the associations observed between SUA and insulin sensitivity, nor the difference observed between adolescent subjects with and without type 1 diabetes. It is uncertain whether adjusting for FeUA would have explained our observations in the adult cohort, but cannot be ruled out due to the significantly longer diabetes duration and renal involvement in our adult subjects. This is also to our knowledge the first clinical study adjusting for this FeUA in an attempt to account for the uricosuric effect.
In conclusion, insulin sensitivity and levels of SUA were significantly lower in adolescents and adults with type 1 diabetes. In contrast to non-diabetic subjects, SUA only accounted for a small amount of the variability of insulin sensitivity in adolescents and adults with type 1 diabetes. There is compelling observational evidence linking both SUA and reduced insulin sensitivity respectively with vascular complications in type 1 diabetes. In this study we present evidence of a very weak association between SUA and reduced insulin sensitivity in type 1 diabetes, which is unlikely to be of clinical significance, and therefore stands in contrast to the strong association observed in non-diabetic subjects. The absence of a clinically significant association between SUA and reduced insulin sensitivity in type 1 diabetes is promising, and may suggest the independence of SUA and reduced insulin sensitivity as mediators of vascular pathogenesis in type 1 diabetes.
Acknowledgements
Support for this study was provided by NHLBI grants R01 HL61753, HL79611, and HL113029, JDRF grant 17-2013-313, and DERC Clinical Investigation Core P30 DK57516. The study was performed at the Adult CTRC at UCD support by NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes and at Colorado Heart Imaging Center in Denver, CO. Dr. Maahs was supported by a grant from NIDDK (DK075360), Dr. Snell-Bergeon by an American Diabetes Association Junior Faculty Award (1-10-JF-50) and Dr. Wadwa by an early career award from the Juvenile Diabetes Research Foundation (11-2007-694).
Drs. David M Maahs, R. Paul Wadwa, Petter Bjornstad and Janet K. Snell-Bergeon are guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
PB researched, wrote, contributed to discussion, and reviewed/edited the manuscript; JKSB researched, contributed to the discussion, reviewed/edited the manuscript; MR designed the CACTI Study, researched, contributed to the discussion and reviewed/edited the manuscript; KM formulated the analytic plan, performed the statistical analyses and reviewed/edited the manuscript; RPW designed the Determinants of Macrovascular Disease in in Adolescents with Type 1 Diabetes Study, researched, contributed to the discussion and reviewed/edited the manuscript; DJ contributed to the discussion and reviewed/edited the manuscript; CJR was responsible for the laboratory analyses, contributed to the discussion and reviewed/edited the manuscript; MC contributed to the discussion and reviewed/edited the manuscript; RJJ contributed to the discussion and reviewed/edited the manuscript; DMM researched, contributed to discussion, and reviewed/edited the manuscript.
Duality of interest
RJJ has patent applications related to the lowering of uric acid and or blocking fructose metabolism as a means for slowing diabetic nephropathy or improving insulin resistance, and has shares with XORT Therapeutics related to these patents. Drs. Bjornstad, Snell-Bergeon, McFann, Wadwa, Rewers, Rivard, Jalal, Chonchol and Maahs have no conflict of interest to disclose.
References
- 1.Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is 'double diabetes' and what are the risks? Diabetologia. 2013;56(7):1462–1470. doi: 10.1007/s00125-013-2904-2. Epub 2013/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, et al. Early Diabetic Nephropathy: A complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013 doi: 10.2337/dc13-0631. Epub 2013/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Eckel RH, et al. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2012;97(5):1663–1672. doi: 10.1210/jc.2011-3172. Epub 2012/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman BC, Howard D, Schauer IE, Maahs DM, Snell-Bergeon JK, Clement TW, et al. The importance of palmitoleic acid to adipocyte insulin resistance and whole-body insulin sensitivity in type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2013;98(1):E40–E50. doi: 10.1210/jc.2012-2892. Epub 2012/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, et al. Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study) Diabetes Care. 2005;28(8):2019–2024. doi: 10.2337/diacare.28.8.2019. Epub 2005/07/27. [DOI] [PubMed] [Google Scholar]
- 6.Erbey JR, Kuller LH, Becker DJ, Orchard TJ. The association between a family history of type 2 diabetes and coronary artery disease in a type 1 diabetes population. Diabetes Care. 1998;21(4):610–614. doi: 10.2337/diacare.21.4.610. Epub 1998/05/08. [DOI] [PubMed] [Google Scholar]
- 7.Roglic G, Colhoun HM, Stevens LK, Lemkes HH, Manes C, Fuller JH. Parental history of hypertension and parental history of diabetes and microvascular complications in insulin-dependent diabetes mellitus: the EURODIAB IDDM Complications Study. Diabetic medicine : a journal of the British Diabetic Association. 1998;15(5):418–426. doi: 10.1002/(SICI)1096-9136(199805)15:5<418::AID-DIA604>3.0.CO;2-P. Epub 1998/06/03. [DOI] [PubMed] [Google Scholar]
- 8.Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180–187. doi: 10.1161/CIRCULATIONAHA.111.077487. Epub 2012/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62(10):3307–3315. doi: 10.2337/db12-1814. Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, et al. Effect of insulin on renal sodium and uric acid handling in essential hypertension. American journal of hypertension. 1996;9(8):746–752. doi: 10.1016/0895-7061(96)00098-2. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, et al. Perspectives: Sugar, Uric acid, and the Etiology of Diabetes and Obesity. Diabetes (In Review) 2013 doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. The American journal of medicine. 2010;123(10):957–961. doi: 10.1016/j.amjmed.2010.03.027. Epub 2010/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism: clinical and experimental. 2008;57(6):845–852. doi: 10.1016/j.metabol.2008.01.030. Epub 2008/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995;310(6979):560–564. doi: 10.1136/bmj.310.6979.560. Epub 1995/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdberg A, Boner G, van Dyk DJ, Carel R. Urine uric acid excretion in patients with insulin-dependent diabetes mellitus. Nephron. 1992;60(2):134–137. doi: 10.1159/000186728. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 16.Nan H, Dong Y, Gao W, Tuomilehto J, Qiao Q. Diabetes associated with a low serum uric acid level in a general Chinese population. Diabetes Res Clin Pract. 2007;76(1):68–74. doi: 10.1016/j.diabres.2006.07.022. Epub 2006/09/12. [DOI] [PubMed] [Google Scholar]
- 17.Maahs DM, Caramori L, Cherney DZ, Galecki AT, Gao C, Jalal D, et al. Uric Acid Lowering to Prevent Kidney Function Loss in Diabetes: The Preventing Early Renal Function Loss (PERL) Allopurinol Study. Curr Diab Rep. 2013 doi: 10.1007/s11892-013-0381-0. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanbay M, Yilmaz MI, Sonmez A, Solak Y, Saglam M, Cakir E, et al. Serum uric acid independently predicts cardiovascular events in advanced nephropathy. American journal of nephrology. 2012;36(4):324–331. doi: 10.1159/000342390. Epub 2012/09/26. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues TC, Maahs DM, Johnson RJ, Jalal DI, Kinney GL, Rivard C, et al. Serum uric acid predicts progression of subclinical coronary atherosclerosis in individuals without renal disease. Diabetes Care. 2010;33(11):2471–2473. doi: 10.2337/dc10-1007. Epub 2010/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabelea D, D'Agostino RB, Jr, Mason CC, West N, Hamman RF, Mayer-Davis EJ, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54(1):78–86. doi: 10.1007/s00125-010-1911-9. Epub 2010/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care. 2005;28(2):301–306. doi: 10.2337/diacare.28.2.301. Epub 2005/01/29. [DOI] [PubMed] [Google Scholar]
- 22.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol M, Johnson RJ, et al. Early Diabetic Nephropathy - a Complication of Insulin Resistance in Type 1 Diabetes. Diabetes Care. 2013 doi: 10.2337/dc13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60(1):306–314. doi: 10.2337/db10-0328. Epub 2010/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snell-Bergeon JK, Maahs D, Schauer IE, Bergman BC, Rewers M. Practical ways to estimate insulin sensitivity in adults with type 1 diabetes. In Review with Diabetologia. 2013 [Google Scholar]
- 25.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA : the journal of the American Medical Association. 2011;305(15):1545–1552. doi: 10.1001/jama.2011.468. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60(4):1258–1269. doi: 10.2337/db10-0916. Epub 2011/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA. Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. Journal of the American Society of Nephrology : JASN. 2006;17(6):1703–1709. doi: 10.1681/ASN.2005080872. [DOI] [PubMed] [Google Scholar]
- 28.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. The Journal of clinical investigation. 1986;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. American journal of physiology Renal physiology. 2006;290(3):F625–F631. doi: 10.1152/ajprenal.00140.2005. Epub 2005/10/20. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nature clinical practice Nephrology. 2005;1(2):80–86. doi: 10.1038/ncpneph0019. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez G, Soriano LC, Choi HK. Impact of diabetes against the future risk of developing gout. Annals of the rheumatic diseases. 2010;69(12):2090–2094. doi: 10.1136/ard.2010.130013. Epub 2010/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira RI, Snell-Bergeon JK, Erickson C, Schauer IE, Bergman BC, Rewers M, et al. Adiponectin dysregulation and insulin resistance in type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2012;97(4):E642–E647. doi: 10.1210/jc.2011-2542. Epub 2012/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. Epub 2009/07/09. [DOI] [PubMed] [Google Scholar]
- 34.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin Resistance in Adolescents with Type 1 Diabetes and Its Relationship to Cardiovascular Function. Journal of Clinical Endocrinology & Metabolism. 2010;95(2):513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Annals of epidemiology. 1998;8(4):250–261. doi: 10.1016/s1047-2797(97)00204-4. Epub 1998/05/20. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. Epub 2007/05/02. [DOI] [PubMed] [Google Scholar]
- 37.Pauly RP, Tonelli M. Predicting development of CKD in the general population--early days in a rapidly evolving field. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(6):805–807. doi: 10.1053/j.ajkd.2011.02.378. Epub 2011/05/24. [DOI] [PubMed] [Google Scholar]
- 38.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. The Journal of pediatrics. 2013;162(2):297–301. doi: 10.1016/j.jpeds.2012.07.036. Epub 2012/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snell-Bergeon JK, Hokanson JE, Jensen L, MacKenzie T, Kinney G, Dabelea D, et al. Progression of coronary artery calcification in type 1 diabetes: the importance of glycemic control. Diabetes Care. 2003;26(10):2923–2928. doi: 10.2337/diacare.26.10.2923. Epub 2003/09/30. [DOI] [PubMed] [Google Scholar]
- 40.Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(6):1865–1869. doi: 10.1093/ndt/gfp740. Epub 2010/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33(6):1337–1343. doi: 10.2337/dc10-0227. Epub 2010/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58(7):1668–1671. doi: 10.2337/db09-0014. Epub 2009/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrie JR. REducing With MetfOrmin Vascular Adverse Lesions in Type 1. Diabetes (REMOVAL) 2013 [Google Scholar]