Abstract
Pathogen induced migration of neutrophils across mucosal epithelial barriers requires epithelial production of the chemotactic lipid mediator, hepoxilin A3 (HXA3). HXA3 is an eicosanoid derived from arachidonic acid. Although eosinophils are also capable of penetrating mucosal surfaces, eosinophilic infiltration occurs mainly during allergic processes whereas neutrophils dominate mucosal infection. Both neutrophils and eosinophils can respond to chemotactic gradients of certain eicosanoids, however, it is not known whether eosinophils respond to pathogen induced lipid mediators such as HXA3. In this study, neutrophils and eosinophils were isolated from human blood and placed on the basolateral side of polarized epithelial monolayers grown on permeable Transwell filters and challenged by various chemotactic gradients of distinct lipid mediators. We observed that both cell populations migrated across epithelial monolayers in response to a leukotriene B4 (LTB4) gradient, whereas only eosinophils migrated towards a prostaglandin D2 (PGD2) gradient. Interestingly, while pathogen induced neutrophil trans-epithelial migration was substantial, pathogen induced eosinophil trans-epithelial migration was not observed. Further, gradients of chemotactic lipids derived from pathogen infected epithelial cells known to be enriched for HXA3 as well as purified HXA3 drove significant numbers of neutrophils across epithelial barriers, whereas eosinophils failed to respond to these gradients. These data suggest that although the eicosanoid HXA3 serves as an important neutrophil chemo-attractant at mucosal surfaces during pathogenic infection, HXA3 does not appear to exhibit chemotactic activity towards eosinophils.
Keywords: neutrophils, eosinophils, eicosanoids, hepoxilin A3, leukotriene B4, prostaglandin D2
Introduction
Infection of polarized mucosal epithelial barriers on the apical surfaces with pathogenic bacteria instigates neutrophils to migrate from the basolateral to the apical side 1, 2. This process has been shown to be facilitated by release of lipid mediators from epithelial cells, specifically the eicosanoid hepoxilin A3 (HXA3)1, 3, 4. HXA3 serves multiple roles during inflammatory processes including contributing towards neutrophil recruitment to infected sites4–11. Lung epithelial barriers infected with Pseudomonas aeruginosa, Streptococcus pneumoniae, or Klebsiella pneumoniae and intestinal epithelial monolayers infected with Salmonella Typhimurium, Shigella flexneri, or pathogenic Escherichia coli facilitate neutrophil trans-epithelial migration in a HXA3-dependant manner1, 4, 5, 10, 12–15. Mucosal breach resulting from excessive neutrophil trans-epithelial migration can lead to tissue damage and mucosal barrier dysfunction3, 16.
Eicosanoids are lipid mediators synthesized from the precursor arachidonic acid that exhibit a diversity of functions17–19. Many eicosanoids are thought to act during inflammatory processes as a consequence of infection or otherwise, and several eicosanoids have been described to function as chemo-attractants for inflammatory leukocytes3, 18, 19. For example, leukotriene B4 (LTB4) derived from the 5-lipoxygenase eicosanoid synthetic pathway is well-known to attract neutrophils, but also is capable of attracting eosinophils18, 20. Prostaglandin D2 (PGD2) derived from the cyclo-oxygenase pathway has been established as an eosinophil chemo-attractant not thought to be capable of attracting neutrophils18, 20. HXA3 has been established as a neutrophil chemo-attractant, but whether HXA3 is capable of attracting eosinophils has not been considered, nor has it been determined whether pathogens are capable of driving eosinophil trans-epithelial migration.
Eosinophilic esophagitis (EoE) has been recently characterized as a discrete disorder distinguishable from gastroesophageal reflux disease (GERD)21. EoE is characterized by eosinophilic infiltration of the mucosal esophageal epithelium in the absence of acid reflux21. A recent microarray analysis revealed that several genes are uniquely modulated in the esophagus of EoE patients22. One of the genes shown to be highly upregulated in esophageal biopsy tissue from EoE patients was 15-lipoxygenase (alox15)22. The alox15 gene encodes a key enzyme that exhibits dual 12-lipoxygenase and 15-lipoxygenase activity and is thought to be involved in HXA3 synthesis23. Given the capacity of HXA3 to drive neutrophils through mucosal surfaces during pathological conditions, we sought to determine whether eosinophil trans-epithelial migration would occur in a similar fashion. Such a finding could implicate HXA3 as a potential mediator in eosinophil-driven mucosal disease24. Comparative analysis of the ability of structurally and functionally distinct eicosanoids LTB4, PGD2 and HXA3 to facilitate either neutrophil or eosinophil migration across epithelial barriers has potential to contribute towards the understanding granulocyte infiltration during mucosal inflammation.3, 8, 17, 24.
Materials and Methods
Neutrophil & Eosinophil Isolation
Unpurified leukocyte buffy coats from de-identified normal donors were purchased from Research Blood Components, LLC (Boston, MA). Buffy coats were processed using a Ficoll-Paque™ PLUS (GE Healthcare, Uppsala, Sweden) density gradient followed by RBC lysis of the granulocyte and red blood cell layer using an ammonium chloride buffer. After washing in cold phosphate buffered saline (PBS) (Dulbecco's Phosphate Buffered Saline, Mediatech, Manassas, VA), granulocytes were resuspended in PBS buffer containing 0.5% BSA + 4 mM EDTA and separated into 2 groups. The first group of granulocytes was resuspended in Hank's balanced salt solutions without calcium and magnesium (HBSS-). No further processing of the first group of granulocytes was performed as unpurified granulocytes represent mostly neutrophils. We therefore refer to the unpurified granulocyte population as neutrophils in subsequent sections. The second group of granulocytes was further processed to isolate eosinophils, which represent a small fraction of the granulocyte population. Eosinophils were purified using immunomagnetic negative selection (EasySep® Human Eosinophil Enrichment Kit, Stem Cell Technologies, Vancouver, Canada). Eosinophil purity was 90–98%. Purity of unstained preparations was determined by flow cytometry (BD™ LSR II, Franklin Lakes, New Jersey) using forward scatter, side scatter, and autofluorescence, as detected on the FITC channel25. For histology, cytospin preparations fixed with 100% methanol were stained with Accustain modified Wright-Giemsa (Sigma-Aldrich, St. Louis, MO). Images were acquired using a Nikon Eclipse ME600 light microscope25.
Granulocyte Quantification by Peroxidase Activity
A standard curve was generated for both neutrophils and eosinophils by adding 2×106 cells to 2 ml HBSS- and preparing nine 2-fold serial dilutions. Granulocytes from samples and standards were lysed by 0.5 % TritonX-100 with moderate agitation at 4°C for 20 minutes. Lysed granulocytes (100 μl in duplicate) were transferred to a 96-well plate and 100 μl ABTS peroxidase substrate solution (Sigma-Aldrich, St. Louis, MO) was added to each well. After 10 minutes, optical density in each well was measured at 405 nm (OD@405)1, 10.
Lung Epithelial Cell Culture
The H292 lung epithelial cell line was purchased from American Type Tissue Culture (Manassas, VA) and maintained in RPMI-1640 supplemented with 10% heat inactivated Fetal Bovine Serum and antibiotics. H292 monolayers were grown on the underside of 0.33 cm2 collagen coated Transwell filters purchased from Corning Incorporated (Corning, NY) to study eosinophil and neutrophil migration in the physiological basolateral to apical direction10.
Bacterial Strains
P. aeruginosa strain PAO1 was grown aerobically in Luria–Bertani broth overnight at 37°C. For infection of epithelial cells, overnight cultures were washed once in HBSS and resuspended at a concentration of 6×107 bacteria / ml of HBSS10.
Extraction of Lipid-Associated chemotactic activity from Supernatants
H292 cells were seeded on 162 cm2 flasks and grown to confluence. Confluent monolayers were treated with or without 6×107 PAO1 / ml HBSS for 1 hr at 37°C, washed three times with HBSS, and incubated an additional 2 hrs at 37°C. Supernatants were collected and acidified to pH 4.0. Acidified supernatants were passed through a Supelco Discovery® DSC-18 SPE Column and eluted with methanol. The lipid fraction suspended in methanol was dried under a stream of nitrogen to 100 μl methanol and stored at −80°C until processed further. On the day of the neutrophil / eosinophil migration experiment, each extracted lipid sample for H292 lung epithelial cells with or without PAO1 infection (prepared in triplicate and stored at −80°C) was dried under a stream of nitrogen and re-suspended in 1.3 ml HBSS. Each volume of 1.3 ml re-suspended extracted lipid supernatants was split into 2 × 600 μl / well to the apical well of a Transwell with a H292 lung epithelial barrier prior to adding either neutrophils or eosinophils to the top basolateral well10, 26.
Neutrophil / Eosinophil Trans-epithelial Migration Assay
Transwell filters containing H292 cell monolayers seeded on the underside were washed two times in HBSS and placed in a 24-well plate with HBSS to pre-equilibrate. In a separate 24-well plate, 600 μl / well HBSS with or without chemo-attractant gradient was prepared. The following chemo-attractant gradients were applied; 1.25 μg/ml eotaxin-3 (R&D Systems), 100 ng/ml IL-8 (eBioscience), 5 ng/ml LTB4 (Enzo Life Science), 100 ng/ml PGD2 (Enzo Life Science), 16.7 μg/ml HXA3 (Enzo Life Science), or HBSS resuspended lipid extracts from uninfected and PAO1 infected H292 cell supernatants prepared as described above. Alternatively, the apical surface of H292 monolayers was exposed to 25 μl of 6×107 bacteria / ml or mock infected with 25 μl HBSS and incubated for 1 hr, followed by washing three times in HBSS. Uninfected Transwells were transferred to a 24-well plate with or without chemo-attractant in each well. PAO1 infected monolayers were transferred to a 24 well plate with HBSS alone. A volume of 100 μl HBSS was added to the top (basolateral) well of all transferred Transwells followed by 20 μl of 5×107 cells/ml, either neutrophils or eosinophils. The number of neutrophils or eosinophils that fully migrated across the lung epithelial barrier in response to various chemo-attractants was quantified following incubation for 2 hours at 37°C in a 5% carbon dioxide environment10, 26. Neutrophils and eosinophils were quantified by the peroxidase assay described above.
Data Analysis
Each figure represents data from an individual internally controlled experiment using granulocytes from a single donor with a mean (standard deviation) of at least two independent data points per condition. Each experiment was performed on at least two different occasions yielding similar results. Statistical analysis was performed by a two-tailed paired Student's t test combining data from at least two independent experiments representing different donors and considered significant when p values were less than 0.05. In aggregate, experiments represent data from over a dozen different normal donors.
Results
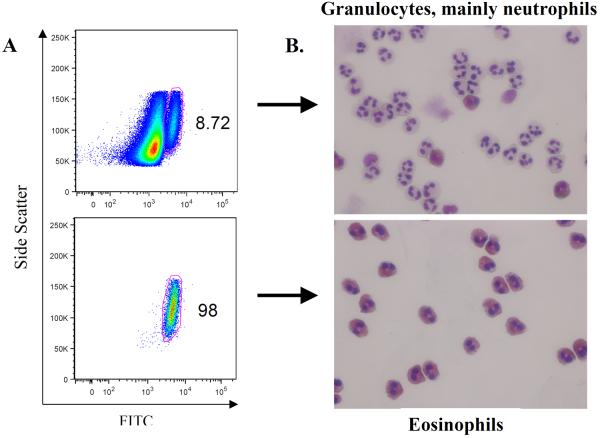
It is critical to ensure the integrity of the neutrophil and eosinophil populations for accurate interpretation of data generated by the granulocyte trans-epithelial migration assay. Therefore, granulocyte populations were characterized based on two distinct methods in addition to inclusion of functional controls for trans-epithelial migration experiments. Neutrophils and eosinophils are distinguishable from one another based on multiple characteristics. Firstly, the degree of autofluorescence allows one to discern neutrophils from eosinophils. Our granulocyte preparations consisted of mainly neutrophils, with less than 10% of the population exhibiting autofluorescence that is associated with eosinophils (figure 1A, top panel). Isolation of eosinophils from the total granulocyte population using the eosinophil enrichment kit efficiently removes the bulk neutrophils resulting in a highly pure (98%) population of eosinophils (figure 1A, bottom panel). In addition to flow cytometry, we exploited the distinctive appearance exhibited by neutrophils and eosinophils following modified Wright Giemsa staining to characterize the isolated granulocyte populations25. Neutrophils clearly dominated the total granulocyte population as would be expected (figure 1B, top panel). When total granulocytes were subjected to the eosinophil enrichment kit, the resultant cell population was primarily eosinophils (figure 1B, bottom panel).
Figure 1. Purity of isolated human eosinophils.
(A) Eosinophils were identified by their forward scatter, side scatter, and autofluorescent properties on flow cytometry of unstained human granulocyte and isolated eosinophil preparations. While neutrophils and isolated eosinophils have similar forward and side scatter characteristics, eosinophils have higher autofluorescence, as captured by the FITC channel. (B) Cytospin preparations of granulocytes and eosinophils underwent modified Wright-Giemsa staining. Neutrophils can be distinguished from the characteristic pink, granular cytoplasm of the eosinophils. The upper panel for both (A) & (B) represents cells derived from the initial granulocyte isolation which consists of primarily neutrophils. The lower panel for both (A) & (B) represents the granulocyte cell population after eosinophil enrichment.
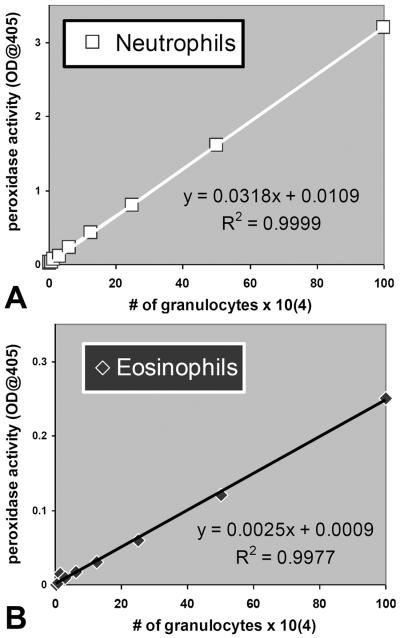
Both neutrophils and eosinophils are known to exhibit peroxidase activity. Neutrophils abundantly express myeloperoxidase (MPO), which is a major component of their antibacterial arsenal27. Eosinophils express an enzyme known as eosinophil-derived peroxidase (EPO) that is thought to assist in the defense against parasitic organisms28. Both neutrophils (figure 2A) and eosinophils (figure 2B) exhibited peroxidase activity whereby the magnitude of peroxidase activity was linearly proportional to the number of cells. The peroxidase activity per cell was substantially greater for neutrophils compared to eosinophils, 1 × 106 cells resulted in an optical density of 3.2 for neutrophils and only 0.25 for eosinophils when measured at 405 nm (figure 2). Peroxidase activity was used in subsequent experiments to quantify transmigrated neutrophils and eosinophils in order to explore whether each of these granulocytes responded to chemotactic gradients of selected eicosanoids2.
Figure 2. Characterization of granulocyte peroxidase activity.
The amount of peroxidase activity (measured as optical density @ 405 nm) per granulocyte represents a linear relationship within the 0.5×104 to 100×104 range and facilitates quantification of unknown numbers of granulocytes. Neutrophils depicted as white squares (A) exhibit considerably more activity / cell than eosinophils depicted as black diamonds (B).
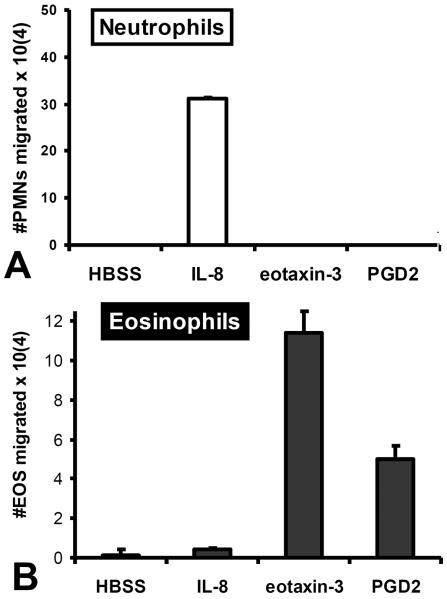
Granulocytes invade mucosal barriers and can cause pathology16, 21, 29. We sought to determine if chemotactic gradients of selected eicosanoids could drive either neutrophils or eosinophils across an epithelial barrier. In addition to the flow analysis described in the methods section, we included functional controls in each experiment to be sure that we were assaying exclusively either neutrophils or eosinophils. IL-8 (CXCL8) is a well described neutrophil CXC chemokine that is known to be capable of driving neutrophils across cellular barriers and eotaxin-3 (CCL26) is a well described CC chemokine known to promote eosinophil chemotaxis30, 31. Substantial trans-epithelial migration from the basolateral to the apical side was observed in response to a gradient of IL-8 for the neutrophils, but not the purified eosinophils (Figure 3). In response to a gradient of eotaxin-3 added to the apical side of an epithelial monolayer, purified eosinophils, but not neutrophils transmigrated from the basolateral side in large numbers (Figure 3). These data suggest that we are able to successfully resolve migratory responses of functionally distinct granulocyte populations following isolation. Very few eosinophils are functionally detected in the total granulocyte preparations “neutrophils” as indicated by a complete lack of measurable cell migration following exposure of neutrophils to an eotaxin-3 gradient (figure 3A). Despite presence of a minority population of eosinophils following isolation of total granulocytes (generally less than 10% eosinophils), the inability to detect migrating eosinophils is most likely due to not only their low abundance amongst granulocytes, but also a substantially lower peroxidase / cell activity compared to neutrophils (figure 2). For assaying eosinophils, the large majority of neutrophils are removed during eosinophils enrichment procedures (Figure 1) and, as a consequence, migrating neutrophils are not observed as demonstrated by a lack of cell migration following exposure of eosinophils to an IL-8 gradient (figure 3B).
Figure 3. PGD2 exhibits specificity towards eosinophils.
The ability of an established PGD2 gradient across H292 epithelial barriers to promote (A) neutrophil or (B) eosinophil trans-epithelial migration is evaluated. Established gradients of the neutrophil chemokine IL-8 and the eosinophil chemokine eotaxin-3 are included as positive controls for neutrophil specific and eosinophil specific trans-epithelial migration respectively to demonstrate purity of each granulocyte population. Absence of a chemotactic gradient (HBSS) serves as a negative control.
Having established appropriate functional controls, we examined the relative response of neutrophils and eosinophils to select eicosanoids. The eicosanoid PGD2 has been previously demonstrated to act as an eosinophil chemo-attractant32. We investigated whether PGD2 added as a gradient was capable of driving either neutrophils or eosinophils from the basolateral to the apical side of an epithelial barrier. In response to an established gradient of PGD2 across epithelial barriers, neutrophils did not transmigrate, as evidenced by the lack of detectable numbers of neutrophils in the apical well (figure 3A). In contrast, eosinophils were clearly capable of migrating across in response to a gradient of PGD2, as a number of eosinophils were detected in the apical well (figure 3B).
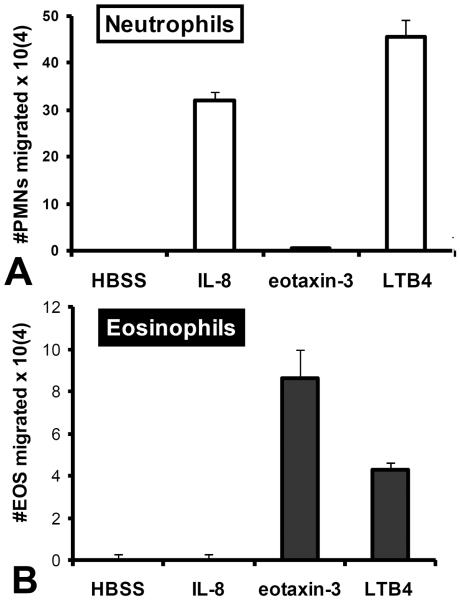
Next we explored the ability of the eicosanoid LTB4 to serve as a chemo-attractant capable of driving granulocytes across epithelial monolayers. Previous literature suggests that LTB4 is capable of serving as a chemo-attractant for both neutrophils and eosinophils33, 34. As depicted in figure 4, an apical LTB4 gradient was capable driving either neutrophils (figure 4A) or eosinophils (figure 4B) across the epithelial monolayer towards the LTB4 gradient. Neutrophil and eosinophil populations subjected to LTB4 gradients were functionally pure as evidenced by the differential and appropriate responses to IL-8 and eotaxin-3 respectively (figure 4).
Figure 4. LTB4 attracts both neutrophils and eosinophils.
The ability of an established LTB4 gradient across H292 epithelial barriers to promote (A) neutrophil or (B) eosinophil trans-epithelial migration is evaluated. Established gradients of the neutrophil chemokine IL-8 and the eosinophil chemokine eotaxin-3 are included as positive controls for neutrophil specific and eosinophil specific trans-epithelial migration respectively to demonstrate purity of each granulocyte population. Absence of a chemotactic gradient (HBSS) serves as a negative control.
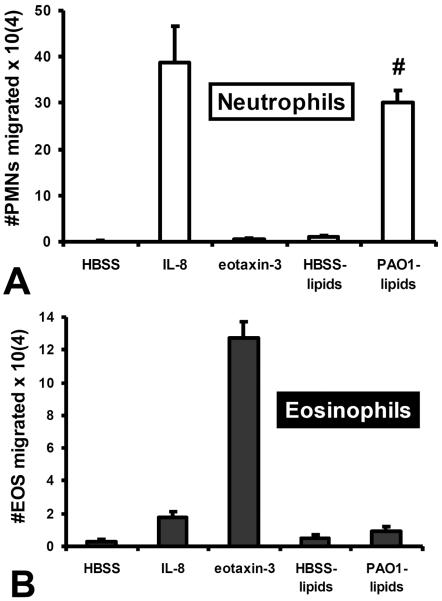
Infection of polarized epithelial cells with pathogen induces apical release of HXA3, which serves as a chemo-attractant for neutrophils1, 4, 5, 10, 26. Studies have demonstrated that lipid fractions of conditioned supernatant from P. aeruginosa (PAO1) infected lung epithelial cells display substantial neutrophil chemotactic activity owing to PAO1 induced release of HXA310, 26. Consistent with the hypothesis, PAO1 induced epithelial derived lipid based chemotactic activity was demonstrated to be sensitive to the HXA3 degrading enzyme, soluble epoxide hydrolase10, 35. We therefore explored whether eosinophils were capable of responding to PAO1 induced lipid associated chemotactic activity. As with previous reports, lipid mediator enriched fractions from PAO1 infected H292 cells display significant neutrophil chemotactic activity when applied as a gradient across epithelial monolayers in contrast to fractions from uninfected H292 cells (figure 5A). Interestingly, eosinophils responded poorly to these lipid extracts whether they were derived from either infected or uninfected cells (figure 5B). The functional purity of the neutrophil and eosinophil populations was internally controlled in the experiment as neutrophils responded to IL-8, but not eotaxin-3 and eosinophils were far more sensitive to eotaxin-3 compared with IL-8 (figure 5).
Figure 5. Lipid fractions from pathogen-infected epithelial supernatants exhibit chemotactic activity towards neutrophils, but not eosinophils.
Lipid enriched fractions from the supernatant of uninfected (HBSS lipids) and P. aeruginosa infected (PAO1 lipids) is added to the apical well of H292 epithelial barriers to establish a gradient and determine whether lipid fraction gradients are capable of driving either (A) neutrophils or (B) eosinophils across H292 barriers. Established gradients of the neutrophil chemokine IL-8 and the eosinophil chemokine eotaxin-3 are included as positive controls for neutrophil specific and eosinophil specific trans-epithelial migration respectively to demonstrate purity of each granulocyte population. Absence of a chemotactic gradient (HBSS) serves as a negative control. Data represent a single internally controlled experiment (n = 3). The symbol (#) represents a statistically significant increase in the number of transmigrated granulocytes in response to a gradient of lipids derived from PAO1 infected epithelial cells compared to lipids derived from uninfected epithelial cells for a compilation of two independent experiments (n = 5), P = 0.013).
Since PAO1 induced lipid associated chemotactic activity has been previously ascribed to the presence of HXA3, the observation that eosinophils, unlike neutrophils, fail to respond to gradients of this lipid fraction suggests that eosinophils do not exhibit a chemotactic response to HXA3. Multiple reports have demonstrated that establishment of a gradient of HXA3 on the apical side of an epithelial barrier is capable of driving basolateral to apical neutrophil trans-epithelial migration 1, 4, 10, 36. We therefore explored whether eosinophils were capable of migrating towards a gradient of HXA3 in the context of an internally controlled experiment. In the absence of the HXA3 gradient, neutrophil trans-epithelial migration was not detectable, whereas a significant number of migrated neutrophils in response to an imposed HXA3 gradient were quantified (2.3×104 neutrophils +/− 1.4 ×104). The number of eosinophils migrating across the epithelial barrier was below the threshold of detection either in the presence or absence of an imposed HXA3 gradient, however, a significant number of eosinophils transmigrated in response to a gradient of LTB4 assayed in parallel (7.6×104 eosinophils +/− 2.1 ×104).
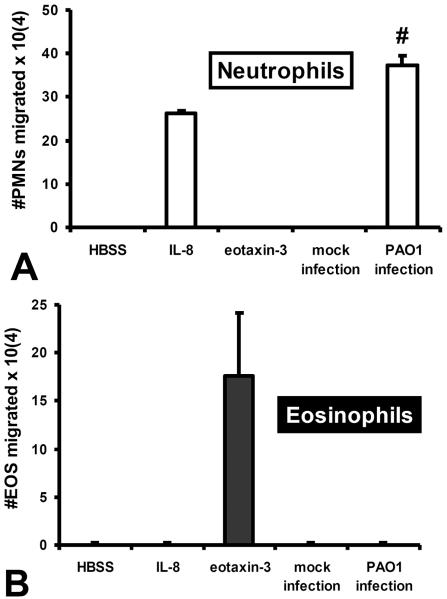
PAO1 infection on the apical surface of lung epithelial monolayers results in trans-epithelial migration of neutrophils. Investigation of the molecular mechanism underlying this event revealed that production of HXA3 in response to infection is critical for driving neutrophils across an infected monolayer as inhibitors of the 12-lipoygenase pathway and structural based HXA3 antagonists potently and specifically block this process1, 10, 36. Thus, we sought to determine whether infection of polarized lung epithelial cells with pathogen is capable of driving eosinophil trans-epithelial migration. As expected, PAO1 infection of H292 epithelial monolayers resulted in a significant number of neutrophils migrating across, whereas monolayers mock infected with HBSS did not facilitate neutrophil trans-epithelial migration (figure 6A). In contrast, eosinophils failed to migrate across either infected or mock infected monolayers, further suggesting a lack of responsiveness to HXA3 (figure 6B). Again IL-8 and eotaxin-3 were included as internal controls to demonstrate purity at a functional level for neutrophils and eosinophils (figure 6). In aggregate, these findings indicate that although HXA3 serves as a chemo-attractant capable of driving neutrophils across mucosal surfaces, HXA3 fails to engage eosinophils and promote eosinophil trans-epithelial migration.
Figure 6. Pathogen infection of epithelial cells drive neutrophil, but not eosinophil trans-epithelial migration.
H292 epithelial barriers not infected (mock infection) or infected apically with P. aeruginosa (PAO1 infection) are examined for their ability to promote basolateral to apical trans-epithelial migration of either (A) neutrophils or (B) eosinophils. Established gradients of the neutrophil chemokine IL-8 and the eosinophil chemokine eotaxin-3 are included as positive controls for neutrophil specific and eosinophil specific trans-epithelial migration respectively to demonstrate purity of each granulocyte population. Absence of a chemotactic gradient (HBSS) serves as a negative control. Data represent a single internally controlled experiment (n = 3). The symbol (#) represents a statistically significant increase in the number of migrated granulocytes across PAO1 infected epithelial monolayers compared to mock infected epithelial monolayers for a compilation of two independent experiments (n = 5), P = 0.002).
Discussion
Granulocytes respond to external threats at mucosal surfaces and are capable of migrating through mucosal epithelial barriers and accessing space within the lumen3, 16, 21, 37. Neutrophils respond vigorously to bacterial infection whereas eosinophils accumulate following exposure to allergens3, 21. Although granulocyte deployment is meant to assist in host defense, excessive breach of mucosal barriers without proper resolution adds to tissue destruction compounding pathology initiated by exposure to harmful organisms or agents3, 21. Signals that orchestrate specific cellular recruitment through mucosal barriers are not fully understood, but represent attractive therapeutic targets to control inflammatory mediated damage.
Several diseases have been described that involve granulocyte infiltration of mucosal surfaces16, 21, 29, 38. Infection of the airway by pathogenic bacteria characterizing diseases such as pneumonia and cystic fibrosis results in marked and pathological accumulation of neutrophils in the airway29, 39, 40. Enteric infections often result in inflammation of the gut marked by neutrophil mediated intestinal barrier breach3, 16. Colonization of parasitic organisms in the digestive tract can instigate eosinophil infiltration of the mucosa41. Further, granulocyte recruitment into and through mucosal epithelial barriers is not exclusively a consequence of infection as this process occurs in a number of idiopathic inflammatory conditions. For example, in the lung, acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD) involve pathological neutrophil accumulation within the airspace42. Asthma is marked by eosinophilic recruitment to the airway, with neutrophil involvement in severe cases38. Inflammatory bowel disease is characterized by massive neutrophilic mediated damage to the intestinal barrier whereas allergic colitis involves eosinophil recruitment16, 43. In the esophagus, a relatively newly defined disease known as eosinophilic esophagitis (EoE) is marked by eosinophil infiltration of the esophageal epithelial mucosa and is thought to be instigated by exposure to allergens21. Taken together, a better understanding of the molecular mechanisms that underlie granulocyte migration into and across mucosal surface epithelia may reveal targets to quell excessive recruitment and granulocyte mediated mucosal tissue destruction in a wide array of disease processes.
We have previously identified the lipid mediator hepoxilin A3 (HXA3) as a neutrophil chemo-attractant produced by epithelial cells at the mucosal surface1, 4. HXA3 is a member of the eicosanoid class of lipid mediators8. Eicosanoids are well known for their prominent and diverse roles in either promoting or resolving inflammation44. HXA3 represents a novel and attractive therapeutic target to potentially control neutrophilic breach of the mucosa. As described above, eosinophils are also capable of infiltrating mucosal barriers in certain disease contexts, with or without accompanying neutrophils. We therefore sought to determine whether HXA3 was capable of driving eosinophils across epithelial barriers.
Certain eicosanoids have demonstrated chemotactic activity for granulocytes. For example, the prostaglandin PGD2 is capable of attracting eosinophils18, 20. The receptors DP1 and DP2 (CRTH2) expressed on eosinophils bind PGD2 to promote chemotaxis18, 20. The leukotriene LTB4 serves as a chemo-attractant, capable of attracting both neutrophils and eosinophils18, 20. LTB4 associates with the BLT receptors (BLT1 & BLT2) expressed on granulocytes to facilitate this process18, 20. Given that these eicosanoids are known to serve as granulocyte chemo-attractants, we hypothesized that establishing a gradient of either PGD2 or LTB4 across an epithelial barrier would drive trans-epithelial migration of granulocytes in a mediator specific fashion. Using neutrophils and eosinophils isolated from normal donors, we demonstrate that an established gradient of PGD2 is capable of driving eosinophils, but not neutrophils, across epithelial barriers. These data are consistent with the previously described eosinophil chemotactic potential of this eicosanoid 18, 20. In response to an imposed gradient of LTB4, both neutrophils and eosinophils migrated across the epithelial barrier. Previous studies have reported the chemotactic potential of LTB4 for both granulocyte subtypes and our observation that neutrophils and eosinophils migrate across an epithelial barrier towards LTB4 is consistent with these findings 18, 20. Although concentration gradients of HXA3 are known to promote neutrophil trans-epithelial migration, the chemotactic potential of HXA3 towards eosinophils had not been specifically explored. Further, the receptor for HXA3 has not yet been identified making it difficult to speculate which cell types might respond to HXA3.
We have previously shown that infection of lung epithelial cells with P. aeruginosa results in the release of HXA3 1, 10. Establishment of a concentration gradient of extracted lipid mediators from P. aeruginosa infected epithelial cell supernatant is capable of driving neutrophils across uninfected epithelial monolayers and this lipid-associated activity is sensitive to the enzyme, soluble epoxide hydrolase 10. Soluble epoxide hydrolase specifically deactivates HXA3 by degrading a functionally critical epoxide group within HXA3 that is not present in other lipid chemo-attractants such as LTB4 45. Thus induced HXA3 release represents the key driver of neutrophil trans-epithelial migration amongst the extracted lipid mediators produced by P. aeruginosa infected epithelial cells. Data presented herein demonstrate that gradients of lipids derived from PAO1 infected epithelial cell supernatant instigate a substantial number of neutrophils to migrate across uninfected epithelial monolayers, while eosinophils fail to transmigrate towards gradients derived from identical lipid extracts assayed in parallel. This observation suggests that eosinophils may not be responsive to HXA3. Consistent with this hypothesis, HXA3 from a commercial source presented as a concentration gradient was able to cause neutrophils to migrate across epithelial monolayers, whereas eosinophils did not respond to HXA3 within an internally controlled experiment. Although a significant and quantifiable number of neutrophils transmigrated in response to the commercial source of HXA3, it was notable that the magnitude of the response was less than the response observed to gradients of lipid mediators derived from PAO1 infected epithelial cells as well as being reduced when compared to the response associated with commercial HXA3 tested at lower concentration gradients in previous studies 1, 10, 36. There are several possible explanations for this, including potential variability in bio-activity of HXA3 from different commercial sources or variation introduced by different methods of handling and processing this labile epoxide containing lipid mediator. Alternatively, there exists the possibility that the magnitude of neutrophil migration follows a bell curve pattern whereby the number of neutrophils migrating diminishes not only if the concentration gradient is too low, but also if it is too high. We have previously observed a bell curve pattern of response to a range of infection doses during P. aeruginosa induced neutrophil trans-epithelial migration, although this does not appear to be the case for other pathogens such as K. pneumoniae 1, 14, 46. Direct comparison of neutrophil and eosinophil trans-epithelial migration within an internally controlled experiment is limited to the examination of only a single concentration gradient of HXA3 per experiment as a consequence of the low yield in number of eosinophils that can be reasonably isolated from a single donor. For this reason a higher than previously reported concentration gradient of HXA3 was selected to explore neutrophil versus eosinophil transmigration in the present study. Regardless of the explanation underlying a decreased magnitude of neutrophil trans-epithelial migration towards an increased concentration gradient of commercial HXA3 that was observed in the current study, the critical observation described herein is that while the commercial source of HXA3 remained capable of directing a significant number of neutrophils across the epithelial monolayer, eosinophil trans-epithelial migration was not detected in response to an equivalent concentration gradient of HXA3 assayed in parallel.
Pathogen induced neutrophil trans-epithelial migration has been reported to occur for a wide variety of pathogens exposed to the epithelial barrier models of different mucosal surfaces 3, 47. Several of these studies have implicated the 12-lipoxygenase metabolite HXA3 as the major factor produced by epithelial cells during infection that serves to direct trans-epithelial migration of neutrophils 1, 5, 9, 10, 12, 14, 15, 26, 36. Multiple 12-lipoxygenase inhibitors have been shown to potently and specifically inhibit pathogen induced neutrophil trans-epithelial migration 1, 4, 5, 12, 15. Epithelial monolayers with targeted reduction in the expression of alox15 (a dual 12/15-lipoxygenase) experience a significantly reduced pathogen induced neutrophil trans-epithelial migratory response 5, 12, 15. Structural analogues of HXA3 that lack the epoxide group serve to specifically antagonize pathogen induced neutrophil trans-epithelial migration 10. Whether other 12-lipoxygenase metabolites (12-HETEs or HXB3) or hepoxilin breakdown products (trioxilins) exhibit modulating effects during pathogen induced neutrophil trans-epithelial migration is not entirely clear and requires further exploration.
In this study, pathogen induced trans-epithelial migration was examined for neutrophils and eosinophils in parallel. Consistent with previous reports, epithelial monolayers infected with PAO1 facilitated significant neutrophil trans-epithelial migration that was not observed in mock infected epithelial cells. No measurable eosinophil migration was observed to occur across either infected or mock infected epithelial cells. Given previous evidence implicating HXA3 as the key factor driving pathogen induced neutrophil trans-epithelial migration combined with the observation herein that pathogen induced eosinophil migration does not occur in parallel suggest that eosinophil lack responsiveness to HXA3 gradients. In aggregate, this study provides evidence to suggest that while HXA3 serves as a neutrophil chemo-attractant, it does not appear to be capable of attracting eosinophils.
In this study, granulocytes were obtained from normal donors. It is important to consider the possibility that granulocytes may respond differently if isolated from individuals experiencing certain disease processes or if isolated granulocytes are treated with inflammatory stimuli prior to assaying the response to various lipid mediators. Activation of eosinophils by cytokines or allergens could impact their ability to respond to HXA3. Since the receptor for HXA3 has not yet been identified, it cannot be currently determined whether HXA3 receptor expression can be modulated during a disease process or by external stimuli. Based on the current study using non-stimulated eosinophils from normal volunteers we were unable to find any evidence that eosinophils were capable of responding to HXA3. Future studies aim to address whether eosinophils isolated from EoE patients or eosinophils stimulated with cytokines or allergens are capable of responding to gradients of HXA3 by migrating across epithelial barriers. However, it remains possible that HXA3 functions exclusively as a neutrophil chemo-attractant and therapies targeting this molecule would be most effective against neutrophil dominant disease processes.
Conclusion
Our investigation has revealed that neutrophils, but not eosinophils, isolated from the bloodstream of normal donors are capable of responding to chemotactic gradients of HXA3. These results could have significant implications when considering a therapeutic approach based on the modulation of HXA3 during inflammatory disease processes. Further, it is interesting to note the specificity exhibited by distinct eicosanoids capable of attracting granulocytes. PGD2 appears to favor eosinophils, whereas HXA3 would appear from studies described herein to be specific for neutrophils. LTB4 appears to be capable of attracting either granulocyte. Increased knowledge regarding the functional specificity of each chemotactic eicosanoid, combined with information regarding location of synthesis and action within tissue compartments during an inflammatory process occurring at different organ systems, will better inform development and implementation of eicosanoid based therapeutic interventions for a diverse variety of inflammatory diseases.
Highlights
Infection of epithelial barriers promotes neutrophil, but not eosinophil transmigration.
Hepoxilin A3 fails to elicit a trans-epithelial chemotactic response from eosinophils.
Eicosanoids exhibit unique specificity for mediating trans-epithelial migration of distinct granulocyte populations.
Strategies to alleviate inflammation will benefit from elucidation of eicosanoid specificity for leukocyte types.
Acknowledgements
We thank Waheed Pirzai for expert technical assistance. We thank Mark E. Kusek MD and Michael A. Pazos PhD for critical review of the manuscript. This work acknowledges NIH grant R01 AI095338 for financial support. We also acknowledge support from Nutrition Obesity Research Center at Harvard (P30 DK040561) and we thank Dr. Ronald E. Kleinman MD, Physician in Chief of Massachusetts General Hospital for Children for support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173(9):5712–20. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 2.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131(6 Pt 1):1599–608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick BA. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J. 2007;274(14):3513–8. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- 4.Mrsny RJ, Gewirtz AT, Siccardi D, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci U S A. 2004;101(19):7421–6. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhowmick R, Tin Maung NH, Hurley BP, et al. Systemic Disease during Streptococcus pneumoniae Acute Lung Infection Requires 12-Lipoxygenase-Dependent Inflammation. J Immunol. 2013 doi: 10.4049/jimmunol.1300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284(32):21599–612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregus AM, Doolen S, Dumlao DS, et al. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci U S A. 2012;109(17):6721–6. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins. A review. Adv Exp Med Biol. 1999;447:123–32. [PubMed] [Google Scholar]
- 9.Pazos M, Siccardi D, Mumy KL, et al. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J Immunol. 2008;181(11):8044–52. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamang DL, Pirzai W, Priebe GP, et al. Hepoxilin a3 facilitates neutrophilic breach of lipoxygenase-expressing airway epithelial barriers. J Immunol. 2012;189(10):4960–9. doi: 10.4049/jimmunol.1201922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland M, Schewe T, Nigam S. Biological actions of the free acid of hepoxilin A3 on human neutrophils. Biochem Pharmacol. 2000;59(4):435–40. doi: 10.1016/s0006-2952(99)00345-7. [DOI] [PubMed] [Google Scholar]
- 12.Boll EJ, Struve C, Sander A, Demma Z, Krogfelt KA, McCormick BA. Cell Microbiol. 2012. pp. 120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurley BP, Thorpe CM, Acheson DW. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun. 2001;69(10):6148–55. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley BP, Williams NL, McCormick BA. Involvement of phospholipase A2 in Pseudomonas aeruginosa-mediated PMN transepithelial migration. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L703–L9. doi: 10.1152/ajplung.00390.2005. [DOI] [PubMed] [Google Scholar]
- 15.Mumy KL, Bien JD, Pazos MA, Gronert K, Hurley BP, McCormick BA. Distinct isoforms of phospholipase A2 mediate the ability of Salmonella enterica serotype typhimurium and Shigella flexneri to induce the transepithelial migration of neutrophils. Infect Immun. 2008;76(8):3614–27. doi: 10.1128/IAI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–43. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 17.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 18.Sadik CD, Luster AD. Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J Leukoc Biol. 2012;91(2):207–15. doi: 10.1189/jlb.0811402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshikai Y. Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr Opin Infect Dis. 2001;14(3):257–63. doi: 10.1097/00001432-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kim N, Luster AD. Regulation of immune cells by eicosanoid receptors. ScientificWorldJournal. 2007;7:1307–28. doi: 10.1100/tsw.2007.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder DJ, Justinich CJ. Understanding eosinophilic esophagitis: the cellular and molecular mechanisms of an emerging disease. Mucosal Immunol. 2011;4(2):139–47. doi: 10.1038/mi.2010.88. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:245–62. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 24.Luna-Gomes T, Bozza PT, Bandeira-Melo C. Eosinophil recruitment and activation: the role of lipid mediators. Front Pharmacol. 2013;4:27. doi: 10.3389/fphar.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carulli G, Sbrana S, Azzara A, et al. Detection of eosinophils in whole blood samples by flow cytometry. Cytometry. 1998;34(6):272–9. doi: 10.1002/(sici)1097-0320(19981215)34:6<272::aid-cyto5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Hurley BP, Pirzai W, Mumy KL, Gronert K, McCormick BA. Selective eicosanoid-generating capacity of cytoplasmic phospholipase A2 in Pseudomonas aeruginosa-infected epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(2):L286–94. doi: 10.1152/ajplung.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Arch Biochem Biophys. 2010;500(1):92–106. doi: 10.1016/j.abb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Malik A, Batra JK. Antimicrobial activity of human eosinophil granule proteins: involvement in host defence against pathogens. Crit Rev Microbiol. 2012;38(2):168–81. doi: 10.3109/1040841X.2011.645519. [DOI] [PubMed] [Google Scholar]
- 29.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15(2):194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–60. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan Q, Campanella GS, Colvin RA, et al. Membrane-bound eotaxin-3 mediates eosinophil transepithelial migration in IL-4-stimulated epithelial cells. Eur J Immunol. 2006;36(10):2700–14. doi: 10.1002/eji.200636112. [DOI] [PubMed] [Google Scholar]
- 32.Schuligoi R, Sturm E, Luschnig P, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85(6):372–82. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- 33.Cheraim AB, Xavier-Elsas P, de Oliveira SH, et al. Leukotriene B4 is essential for selective eosinophil recruitment following allergen challenge of CD4+ cells in a model of chronic eosinophilic inflammation. Life Sci. 2008;83(5–6):214–22. doi: 10.1016/j.lfs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int. 2008;57(4):291–8. doi: 10.2332/allergolint.08-RAI-0019. [DOI] [PubMed] [Google Scholar]
- 35.Cronin A, Decker M, Arand M. Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res. 2011;52(4):712–9. doi: 10.1194/jlr.M009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley BP, Sin A, McCormick BA. Adhesion molecules involved in hepoxilin A3-mediated neutrophil transepithelial migration. Clin Exp Immunol. 2008;151(2):297–305. doi: 10.1111/j.1365-2249.2007.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83(2):309–36. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 38.Macdowell AL, Peters SP. Neutrophils in asthma. Curr Allergy Asthma Rep. 2007;7(6):464–8. doi: 10.1007/s11882-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 39.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346(6):429–37. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 40.Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3(2):e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makepeace BL, Martin C, Turner JD, Specht S. Granulocytes in helminth infection -- who is calling the shots? Curr Med Chem. 2012;19(10):1567–86. doi: 10.2174/092986712799828337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgos RA, Hidalgo MA, Figueroa CD, Conejeros I, Hancke JL. New potential targets to modulate neutrophil function in inflammation. Mini Rev Med Chem. 2009;9(2):153–68. doi: 10.2174/138955709787316092. [DOI] [PubMed] [Google Scholar]
- 43.Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Adv Anat Pathol. 2011;18(5):335–48. doi: 10.1097/PAP.0b013e318229bfe2. [DOI] [PubMed] [Google Scholar]
- 44.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–33. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 46.Hurley BP, Goodman AL, Mumy KL, Murphy P, Lory S, McCormick BA. The two-component sensor response regulator RoxS/RoxR plays a role in Pseudomonas aeruginosa interactions with airway epithelial cells. Microbes Infect. 2010;12(3):190–8. doi: 10.1016/j.micinf.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mumy KL, McCormick BA. The role of neutrophils in the event of intestinal inflammation. Curr Opin Pharmacol. 2009;9(6):697–701. doi: 10.1016/j.coph.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]