Abstract
Corneal nerves are responsible for the sensations of touch, pain, and temperature and play an important role in the blink reflex, wound healing, and tear production and secretion. Corneal nerve dysfunction is a frequent feature of diseases that cause opacities and result in corneal blindness. Corneal opacities rank as the second most frequent cause of blindness. Technological advances in in vivo corneal nerve imaging, such as optical coherence tomography and confocal scanning, have generated new knowledge regarding the phenomenological events that occur during reinnervation of the cornea following disease, injury, or surgery. The recent availability of transgenic neurofluorescent murine models has stimulated the search for molecular modulators of corneal nerve regeneration. New evidence suggests that neuro-regenerative and inflammatory pathways in the cornea are intertwined. Evidence-based treatment of neurotrophic corneal diseases includes using neuro-regenerative (blood component-based and neurotrophic factors), neuroprotective, and ensconcing (bandage contact lens and amniotic membrane) strategies and avoiding anti-inflammatory therapies, such as cyclosporine and corticosteroids.
Keywords: corneal nerves, nerve regeneration, neurotrophic keratitis, inflammation, neuropathic pain
A. INTRODUCTION
The cornea is the most densely innervated structure in the human body. In 1831, Schlemm discovered nerves in the cornea. Prior to this discovery, the cornea was thought to be entirely without nerves. Several years after Schlemm’s original observation, Bochdalek dissected the ciliary nerves within the cornea and found that they divided into corneal and iris branches and that the corneal nerves entered at the anterior thickness. 29,198 Corneal nerves are responsible for sensations of touch, pain, and temperature and play an important role in blink reflex, wound healing, 28 and tear production and secretion. 88,171 Numerous studies have established that corneal nerve dysfunction is a frequent pathobiological feature of corneal diseases that cause opacities and result in blindness. Approximately 285 million people worldwide suffer from impaired vision; of these, 2.85 million have corneal opacities. 178 Likewise, of the 39.3 million worldwide who are blind, in 1.57 million this is the result of corneal opacities. 178 In conditions that cause corneal blindness, sensation is frequently diminished or absent because of nerve dysfunction or degeneration. Several infectious and non-infectious inflammatory corneal diseases can lead to lost or compromised innervation and result in neurotrophic keratopathy and blindness. 52,216 Although the subbasal nerve density is variably reduced in these inflammatory corneal diseases, 24,52,216,219 the number of antigen presenting dendritic cells is increased, 52,216 suggesting that the immune and nervous system pathways in the cornea are intertwined.
Despite the high prevalence of corneal blindness and nerve dysfunction and the clinical need to promote corneal nerve regeneration in neurotrophic corneas, there are relatively few specific therapeutic interventions. Therefore, it is not surprising that the National Eye Institute (NEI) Cornea Disease Panel in its July, 2012, Vision Research: Needs, Gaps and Opportunities document highlights the need to develop novel agents capable of stimulating appropriate corneal nerve regeneration. Additionally, this panel has emphasized the importance of correlating the molecular and structural composition of corneal nerves with their function. These recommendations build upon those of the NEI Workshop on Ocular Pain and Sensitivity. This panel concluded that the role of neurons in the health, healing, scarring, and immunology of the cornea, as well as the responsible molecular and cellular mechanisms, has yet to be determined. Therefore, further understanding the molecular and cellular changes that occur in primary sensory neurons as a result of disease or trauma, including their regenerative mechanisms, constitute a high priority.
B. CORNEAL NERVE BIOLOGY
B1. Corneal nerve organization
B1a. Corneal nerve anatomy
In the cornea, 50–450 sensory trigeminal neurons transmit nerve fibers via the ophthalmic division of the trigeminal nerve (cranial nerve V) and terminate in free nerve endings in the corneal epithelium.156 From trigeminal ganglion cells, nerve fibers travel suprachoroidally and branch to form nerve bundles that come to rest uniformly around the corneoscleral limbus to form the limbal plexus. 6 Autonomic innervation may travel along with these nerve fibers, though it is believed to be scarce (sympathetic) or unknown (parasympathetic). 156 Stromal nerve trunks arise from the limbal plexus and enter the peripheral corneal stroma radially at a depth of 293 ± 106 μm before progressing anteriorly.138 There are discrepancies among studies regarding the number of stromal nerve bundles located at the corneo-scleral junction, perhaps due to differences in the points where the nerve bundles are imaged and counted. 87 The stromal nerves enter the cornea like a tree dividing into several branches in the peripheral area close to the limbus. Because the number of nerve divisions increases in relation to the distance from the corneo-scleral junction, more nerves will appear to be present than what are really in the cornea.19 The reported average number of nerve bundles entering the human cornea ranges from 33 to 71. 6,87,138
Stromal nerve trunks are comprised of 900–1200 myelinated and unmyelinated axons with diameters of 0.5–5 μm. 160 Although myelinated NF-200 positive nerve fibers in the periphery of the murine cornea are present, 165 the peripheral and central nerve fibers of the human cornea are nociceptive Aδ and C fibers. 158 Prototypical Aδ fibers are myelinated straight nerves with a relatively large diameter, whereas C fibers are smaller, beaded fibers with no myelination. 160 In the stroma, beaded fibers may arise from straight fibers, 157 suggesting an overlap in morphology or the presence of multiple fiber types in straight nerve bundles. One millimeter after entering the corneal limbus, corneal nerve fibers lose their perineurium and myelin sheaths, and continue on surrounded solely by Schwann cells. 156 As in other peripheral innervation, non-myelinating Schwann cells “wrap” several C-type nerve fibers together to form a “Remak bundle.” 48,156 These Schwann cells and Remak bundles are essential for maintenance and function of unmyelinated axons and nociceptors and may assist in electrical conduction. 48 In the stroma, nerves organize in parallel to collagen lamellae, branch into smaller fascicles as they proceed toward the superficial stroma, and form interconnections within the stroma to create the anterior stromal plexus. 87,156,157 In other words, nerve density increases while nerve diameter thins as one moves anteriorly through the stroma. A small number of stromal nerves terminate as free nerve endings, 137 whereas others directly innervate keratocytes. 157 Such direct keratocyte innervation alludes to the complex dependency of these tissue types; in fact, a trophic interdependence exists between ocular tissues and their sensory innervation. 23 Additional stromal nerves defasciculate as they progress anteriorly towards the anterior stroma, subbasal, and epithelial layers of the cornea.
The corneal epithelium derives innervation from the subbasal nerve plexus. Although the existence of the epithelial nerve plexus has been known for more than a century, 93,174 until recently its detailed architecture and exact location was unclear.87 He et al. have shown that the plexus forms a delicate three-dimensional network in the corneal epithelium.87 The plexus originates from the branches of the peripheral stromal nerves. The tips of the stromal nerves penetrate the Bowman layer into the epithelium, predominantly in the peripheral cornea, and give rise to long bundles that run from the periphery to the center close to the subbasal epithelia like wavy lines. Along their course, these long nerve bundles divide into numerous smaller branches that connect to each other, constituting a delicate nerve network within the epithelium. These nerves form a whorl-like pattern approximately 1 – 2.5 mm inferonasal to the corneal apex. 138,181 The subbasal plexus nerves are thinner than the stromal nerves, and seem to be comprised predominantly of C fibers. 158 At the level of the subbasal plexus, straight Aδ fibers within the basal epithelial cell layer course parallel to the corneal surface, and only beaded unmyelinated C fibers travel for a short distance along the subbasal plexus before turning upward and terminating perpendicularly just beneath the epithelial surface as free nerve endings 157 (Figure 1). Density of epithelial nerves is greater centrally than peripherally. 87 Free nerve endings display a density of approximately 605.8 terminals/mm2 in central epithelial suprabasal cells, 138 resulting in a large receptive field overlap. 160 Both the subbasal plexus and epithelial nerves display unique centripetal migration during development (in tandem with epithelial cells). 13 These nociceptive fibers collectively respond to mechanical, thermal, and chemical stimuli. Examples of chemical stimuli include acetylcholine (ACh), prostaglandins, and bradykinin. 23 As discussed by Belmonte et al., 21 some fibers are stimulus-specific, although the majority are polymodal. Approximately 20% of corneal nociceptors are Aδ mechanoreceptors that generate acute pain. Another approximately 70% are polymodal. The remaining 10% are C fiber cold receptors. A separate, “silent” class of nociceptors that are activated only by local inflammation may also exist. Collectively, these fiber types have been implicated in animal models of neuropathic pain, possibly via expression of transient receptor potential (TRP) family members such as the TRP vanilloid receptor (TRPV1), 93,214 which may be absent from Aδ mechanoreceptors. 159 In the cornea, inflammatory cells may trigger neuropathic pain, or extracellular chemicals may act on TRPV1 to produce pathologically sensitized signaling. 159 In addition to anatomical location and nerve fiber type, corneal nerve fibers can be classified based on their neurochemical profiles and have been studied in response to neurotrophic factors.
Figure 1.
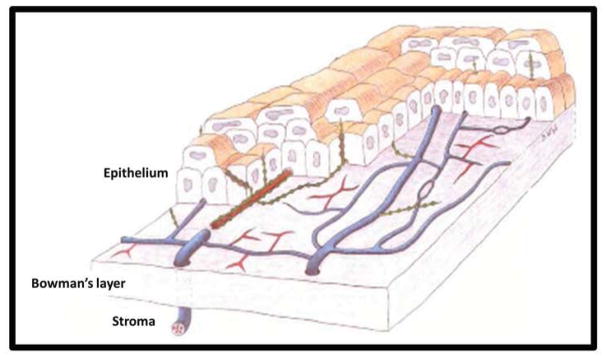
Three-dimensional schematic of human corneal nerves. Stromal nerve bundles traverse Bowman’s membrane to form the subbasal nerve plexus and innervate the epithelium. Unmyelinated nerve fibers (blue) bifurcate, and are composed of several straight (red) and beaded (green) nerve fibers. Beaded fibers branch orthogonally to terminate in free nerve endings in the anterior epithelium. Adapted from Müller et al. 158 Copyright Association for Research in Vision and Ophthalmology.
The various nerve populations in the cornea are distinguishable by their neurochemical markers (Table 1). Some neurochemical markers also serve as neurotrophic factors. Animal studies show neurotrophic factors assist in maintenance of the healthy cornea and modulate wound repair. 162 For example, substance P (SP), released directly from C fibers following inflammation 21 in synergism with epidermal growth factor (EGF), stimulates epithelial proliferation and wound healing. Neuropeptide Y (NPY) has a similar role elsewhere in the body. 106 SP and calcitonin gene-related peptide (CGRP) are involved in neurogenic inflammation. 21 Other corneal neurotrophic factors include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin (NT)-3, Semaphorin (Sema) 3A, 3F, and 7A, Slits 1–3, Netrin 2, and Ephrin (Eph) B2. 113,164 A patterned expression of factors and receptors is observed between corneal nerves and epithelium. 188 Loss of neurotrophic signaling negatively impacts corneal nerve function. For example, inactivation of tyrosine receptor kinase (Trk) A, a high-affinity NGF tyrosine kinase receptor, results in decreases in nociceptive neurons, stromal nerves, and corneal epithelium, as well as a reduced response to noxious stimuli. 56 Changes in neurotrophic signaling drive corneal nerve development and may underlie changes in corneal nerves during the aging process.
TABLE 1.
. Select neurochemical markers expressed in the cornea*
| Nerve fiber type | Associated markers |
|---|---|
|
| |
| Somatosensory (mechanical) | NF-200165 |
|
| |
| Somatosensory (nociceptive) | |
|
| |
| Peptidergic | CGRP |
| SP | |
| PACAP | |
| Galanin | |
| 156 | |
| Non-peptidergic | FRAP |
| 156 | |
|
| |
| Autonomic | |
|
| |
| Sympathetic | NE |
| 5-HT | |
| NPY | |
| 156 | |
| Parasympathetic | ACh |
| VIP | |
| NPY | |
| Galanin | |
| 156 | |
NF-200 = neurofilament-200 kDa; CGRP = calcitonin gene-related peptide; SP = substance P; PACAP = pituitary adenylate cyclase-activating peptide; FRAP = fluoride-resistant acid phosphatase; NE = norepinephrine; 5-HT = serotonin; NPY = neuropeptide Y; ACh = acetylcholine; VIP = vasoactive intestinal peptide.
In addition to these markers, the cornea contains neuropeptides of unspecified origin. These include cholecystokinin (CCK), neurotensin, brain natriuretic peptide (BNP), vasopressin, and β-endorphin. 156
Based on information obtained from human and animal models.
B1b. Corneal nerves in development and aging
Corneal nerve development has been predominantly studied in chicks, with fewer studies in mice. Corneal nerves derive from neural crest cells residing in the ophthalmic lobe of the trigeminal ganglion. 133 The proto-corneal neural crest derivatives migrate with placode-derived cells to the periocular mesenchyme, possibly led by EphA3 receptor kinase axon guidance. 104,133 In chicks, corneal nerve migration is differentially regulated by Sema3A and Slit2 interactions with their respective receptors, Neuropilin (Npn)-1 and transmembrane roundabout (ROBO). 113 Negative regulation coincides with pericorneal nerve ring development, allowing nerve fascicles to accumulate and extend dorsally and ventrally around the cornea to cover uniformly greater than three-quarters of its circumference. 134 (Figure 2) This is followed by positive regulation allowing corneal nerves to invade the stroma. 113,134 Extracellular levels of highly sulfated keratan sulfate proteoglycans (KSPGs) also regulate the initial process of invasion. 46 Following stromal invasion, further nerve bifurcation leads to peripheral corneal innervation in chicks by embryonic day 10 (E10), and guarantees the entire circumference is innervated by E15. 134 Complete penetration of corneal nerves to the center of the cornea with branching into the corneal epithelium is achieved by E16. 18 In mice nerve bundles directly innervate the cornea under the regulation of Sema3A/Npn1 and Sema3F/Npn2 without first forming a pericorneal nerve ring. 146,147 Nerve development relies on nerve bundles from all four quadrants of the eye and progresses from a radial pattern to a more central orientation, branching to form a subbasal “swirl” network akin to the subbasal plexus in human cornea. 146 In humans, both the subbasal plexus and epithelial nerves display centripetal migration during development (in tandem with epithelial cells). 13 The radial progression of innervation may be equivalent to the chick pericorneal nerve ring.
Figure 2.
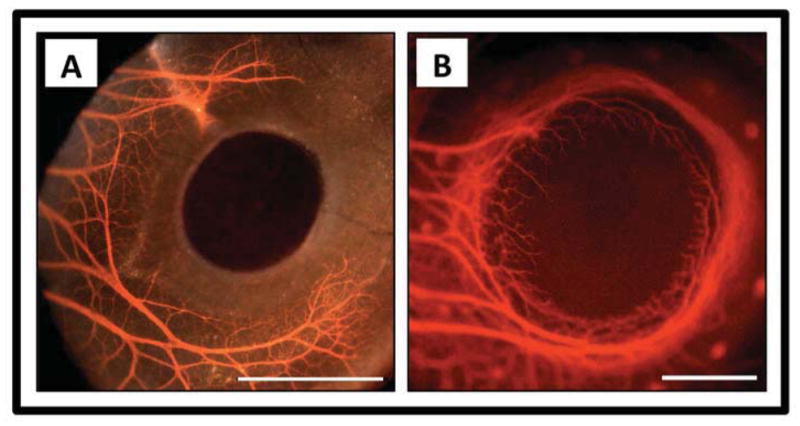
Corneal nerves during development. A) Formation of the chick pericorneal nerve ring at embryonic day 6 (E6). Fluorescence image with brightfield overlay. B) Completion of the chick pericorneal nerve ring and subsequent penetration of corneal nerves into the stroma at E8. Fluorescence image only. Scale bar for A and B: 1 mm. Courtesy of Dr. James K. Kubilus, Department of Anatomy and Cell Biology, Tufts University School of Medicine, Boston, MA. 113
On the opposite spectrum of corneal nerve development are corneal nerves during the aging process. It is unknown whether healthy corneal nerves exhibit uncompromised function throughout life or if they are subject to the typical degenerative processes of aging. Current research yields conflicting results. Data from tandem scanning and slit scanning confocal microscopy suggest that corneal nerves (particularly subbasal nerve density) are maintained in an age-independent manner, 64,91,183 whereas laser scanning confocal and fluorescence microscopy demonstrate pronounced loss of corneal epithelial nerve terminals and subbasal nerve fiber density with age. 87,169 Increased subbasal nerve tortuosity has also been observed with age. 183 Such mixed results are most likely due to differences in imaging techniques (discussed in greater detail below). An alternative measure of age-related corneal nerve changes is testing the functionality of corneal nerves. Using a Cochet-Bonnet aesthesiometer to test Aδ fiber mechanical sensitivity, corneal sensitivity seems to decrease gradually with age, beginning in the periphery and progressing centrally. 160,192 Using the Belmonte non-contact aesthesiometer, which measures mechanical stimulation to Aδ fibers and C fibers as well as thermo- and chemoreceptor sensitivity in C fibers, corneal sensitivity begins to decline in the patient’s second decade, with major changes (presumably those registered by the Cochet-Bonnet aesthesiometer) becoming apparent by age 50. 160 It remains unknown whether C fibers are preferentially targeted earlier in aging, but these findings suggest a dual decline in Aδ and C fiber sensitivity with age.
B2. Imaging corneal nerves
B2a. In vivo Confocal Microscopy (IVCM) and Optical Coherence Tomography (OCT)
As reviewed by others, 51,180 IVCM has been used to assess corneal nerves in healthy subjects, contact lens wearers, keratoconus patients, dry eye disease, diabetics, and other pathologic states. IVCM findings clinically correlate to slit-lamp microscopy and post-operative findings and highlight the utility of consistent imaging methodology. 51,180 Several confocal microscopes exist that image corneal nerves. Some instruments utilize a confocal slit principle, such as the ConfoScan 4 (Nidek, Japan), whereas in tandem scanning microscopes, images are captured using a rotating Nipkow disc. Other instruments, such as the Rostock Cornea Module-HRT (Heidelberg, Germany), utilize a laser. Figure 3 shows sample imaging from these modalities. Automated and manual tracing of nerves in confocal images have well-correlated length estimates, but discrepancies exist in image quantification, including minimum nerve lengths and maximum number of nerves compared to “most representative” images. 180 Differences also arise due to confocal design. 180 A tandem scanning microscope has a narrow depth of field (11 μm), which reduces brightness and contrast, as well as limited magnification such that structures <5 μm are difficult to resolve. 65 The tandem scanning method yields a lower subbasal nerve plexus density than other confocal microscopes. 180 A slit scanning microscope with a wider aperture of 300 μm (ConfoScan4 Nidek Technologies) compared to the tandem scanning aperture of 30 μm produces images of higher contrast, brightness, sharpness, and detail. 65 A combination of high light throughput and magnification, lower resolution, and variable optical section thickness allows visualization of subbasal nerves and possibly sub-Bowman stromal nerves, which are unlikely to be seen in tandem scanning microscopy. 61 A third type of confocal microscope, a laser scanning microscope, offers high-contrast, high quality images with the greatest resolution. 65 Laser-scanning shows whorl-like subbasal nerves (architecture not seen with slit-scanning) 51 and increased nerve densities. 65,181 A comparison of confocal microscopy modalities can be found in Table 2. Recently, optical coherence tomography (OCT) has been investigated as an alternative to confocal microscopy for corneal imaging. OCT is typically used to assess tissue thickness, such as that of the cornea and retina. Full-field (FF) OCT produces high-resolution images of corneal nerve fibers. 4
Figure 3.
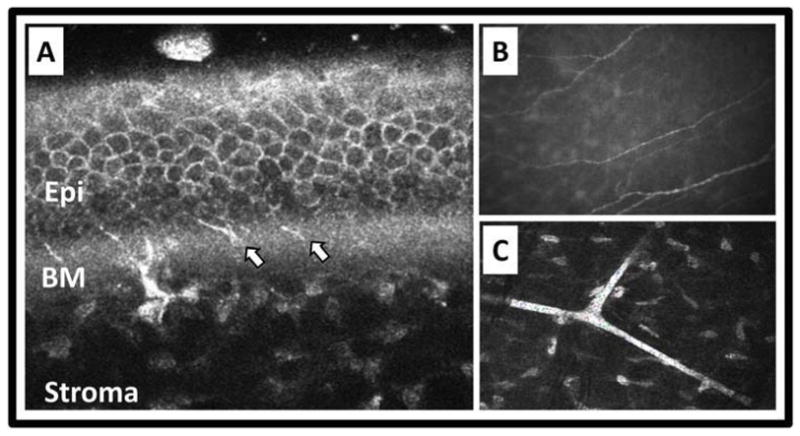
In vivo imaging of corneal nerves. A) Heidelberg Retina Tomograph II with Rostock Corneal Module optical coherence tomography showing corneal nerves traversing Bowman’s membrane (BM) to innervate the epithelium (Epi). Arrows point to nerves in the BM area. B and C) Nidek ConfoScan 4 slit-scanning confocal microscopy showing parallel nerves of the subbasal nerve plexus (B) and branching thick nerves of stroma (C). Courtesy of Heidelberg Engineering, Inc. and Nidek, Inc.
TABLE 2.
In vivo confocal microscopy of corneal nerves: a comparison of imaging modalities
| Type of Imaging | Principal of Image Capture | Aperture (μm) | Depth of field (μm) | Anterior stroma and subbasal nerve resolution* | Measurement of subbasal nerve density (mm/mm2) | Epithelial nerve imaging |
|---|---|---|---|---|---|---|
| In vivo confocal microscopy | Tandem scanning | 30.065 | 7.0 –11.065,210 | Poor resolution (nerves) High quality (stroma) 102,174 |
5.5 – 8.4 ± 2.064,65 | No 65,156 |
| Slit-scan (e.g., Confo-Scan) | Variable: e.g., 300.065 | 10.0 – 26.065,102 | High quality (nerves) 13 Poor resolution (stroma) 101,102 |
11.1 – 14.765,183 | Possible, but unreliable 156 | |
| Laser (e.g., HRT-RCM) | Variable | 4.0 – 7.065,210 | High quality (nerves) 180 | 21.7181 | No 65,201 |
HRT-RCM = Heidelberg Retina Tomograph with Rostock Cornea Module.
Statements regarding resolution have been extrapolated from several articles. Oliveira-Soto et al. 174 observed 57.1% of subbasal plexus nerve images obtained with a tandem scanning microscope were blurred with heterogenous reflectivity. Simultaneously, 50% of nerves in the subepithelial plexus of the anterior stroma exhibited beads, suggesting higher quality resolution in the stroma. Tandem scanning also offers good repeatability when measuring thickness of corneal layers. 102 Slit-scanning confocal microscopy demonstrates subbasal plexus resolution at values greater than or equal to histologic analysis. 102 However, poor repeatability of corneal layer thickness measurements suggest inadequate stromal resolution. 101,102 Laser scanning confocal microscopy exhibits high quality resolution with increased visibility of finer subbasal nerve branches. 180 Differences in nerve resolution may also be supported by the variable measurements of subbasal nerve density.
Corneal epithelial innervation, as estimated by confocal microscopes, is 15–20 times greater than that found by immunohistochemical staining techniques. 87 This discrepancy may be partly because IVCM images of human corneas are recorded at or near the corneal apex in areas most densely innervated. 87
B2b. Transgenic neurofluorescent murine models
Neurofluorescent mouse models allow in vivo visualization of sensory nerves. The thy1-YFP transgenic mouse is a neurofluorescent murine model in which yellow fluorescent protein (YFP) is tagged to the thy1 gene promoter. 235 (Figure 4) In the thy1-YFP mouse model a proportion of sensory nerves are fluorescent, so axonal regeneration and degeneration can be visualized and quantified in vivo. Yu and Rosenblatt were the first to use these mice for studying corneal nerves. 235 They reported that only half of the corneal subbasal nerves are YFP-labeled. In addition, changes in the density and pattern of the YFP-labeled subbasal nerve plexus may occur with sequential stereofluorescence microscope imaging without any intervention. 165 Therefore, investigation of the subbasal nerve plexus should account for any limitations inherent in the thy1-YFP mouse model as well in vivo visualization methodology limitations. In contrast to the subbasal nerve plexus, nearly all stromal nerves can be visualized in the thy1-YFP mouse model. 235 The stromal nerves have YFP-labeled and unlabeled fibers; however, there are enough YFP-labeled fibers in each stromal nerve to allow their visualization. The pattern and density of stromal nerves remains constant during sequential visualization. 165,197 YFP fluorescence disappears in transected stromal nerves distal to the transection site and reappears in regenerating neurites. 235 By visualizing the density and pattern of regenerating YFP-labeled neurites, it is possible to investigate the neurotrophic potential of molecular or pharmacological interventions on stromal nerve regeneration. 163
Figure 4.
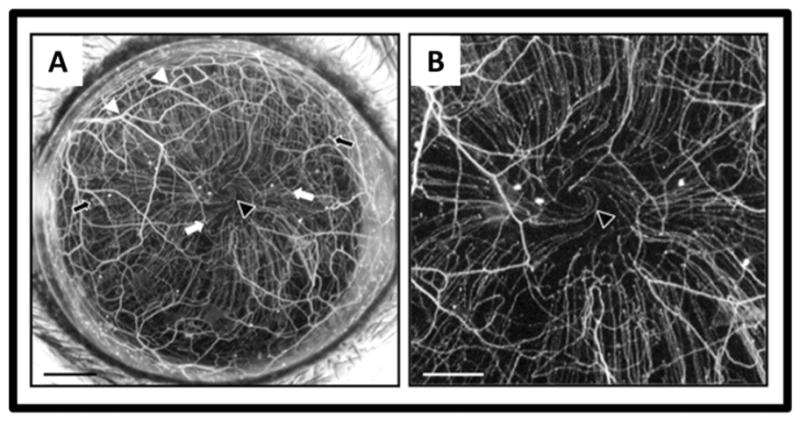
In vivo maximum intensity projection image of fluorescent nerves in the normal thy1-YFP mouse cornea. A stromal network is formed by thick nerve trunks that traverse the cornea, branching frequently (white arrowheads) and anastomosing with adjacent nerve trunks. A second network is formed by thinner subbasal hairpin-like nerves that project centripetally and run roughly parallel to one another. The subbasal nerves arise from the stromal nerve trunks at variable distances from the periphery (black arrows indicate more peripheral origins and white arrows more central origins) and terminate as free nerve endings. In some corneas, the subbasal nerves show swirling at the corneal apex (black arrowhead). Intraepithelial nerves are not resolved with the stereo fluorescence microscope at the magnification used. Scale bar A, 500 μm; B, 250 μm.
B3. Signs and symptoms of corneal nerve dysfunction
Several methods are available for testing corneal nerve function. Direct measures employ aesthesiometry, whereas indirect tests rely on lacrimation and fluorescent dyes. The Cochet-Bonnet contact aesthesiometer (CBA) primarily detects mechano-nociceptor response to an invasive mechanical stimulus, thereby quantifying Aδ fiber function while C fiber activity is undetermined. 160 The tip of the aesthesiometer filament stimulates nerve terminal endings over a test area of 0.011 mm2 in the cornea, corresponding to approximately 6–77 terminal endings per square millimeter (based on current estimates of terminal nerve fiber density). 138,156,160 Corneal sensitivity is highest in the central cornea, 192 thus requiring appropriate placement of the aesthesiometer tip for accurate results. The direct contact of the CBA can damage the corneal epithelium, 160 which may be a source of decreased sensitivity from loss of nerve endings or increased sensitivity due to pathologic nerve damage. The Belmonte non-contact corneal aesthesiometer (NCCA) non-invasively stimulates corneal nerves over a test area of 0.196 mm2 with variable force, composition, and temperature using a pulse (or “puff”) of air to allow multimodal testing (mechanical, chemical, and thermal stimuli). 21,160 NCCA testing provides more information regarding polymodal and thermal receptor functionality, as well as C fibers, than does the CBA. 160 The NCCA is more accurate and sensitive than the CBA. 160
Indirect tests of corneal nerve function reveal general information about the state of corneal nerves. Lacrimation tests (Schirmer and phenol red thread tests) assess tear production, whereas vital dyes reveal damaged corneal epithelium. Dyes include fluorescein, rose bengal, and lissamine green. Fluorescein is often preferred for corneal epithelial staining and may identify contact lens-associated pathologies without requiring further diagnostic intervention, while lissamine green stains correlate highly with dry eye syndromes. 60 In addition to direct and indirect assessments of corneal nerve function, patients’ symptoms reflect ocular surface distress.
Corneal nerve damage may be associated with symptoms such as ocular irritation, photophobia, or pain; 23 however, symptoms may not correlate with the degree of ocular surface disease. Because of hypoesthesia, patients with neurotrophic keratitis may have significant ocular surface disease, but may be quite comfortable. 226 Alternatively, some patients report significant pain in the absence of ocular surface disease. This neuropathic pain typically follows nerve damage, for example, from surgical transection such as LASIK or cataract surgery. 21
B4. Modulators of corneal nerve growth and function
B4a. Neurotrophins
Nerve growth factor (NGF)
As discovered through in vitro and in vivo animal studies, nerve growth factor (NGF) is critical for corneal nerve survival and maintenance, as well as axonal branching and elongation, neuronal sprouting, and regeneration following nerve damage. 135 Multiple studies have shown that NGF augments corneal wound healing, as evidenced by recovery of corneal sensitivity and photophobia in association with increased NGF and corneal epithelium healing. 9,156 NGF is expressed in the cornea during reinnervation after nerve surgical transection. 42 Nerve regeneration may be partially attributable to neurogenic inflammation, such as interleukin (IL)-1-induced NGF. 120,129 NGF reportedly promotes the human cornea’s healing process via pro-neural molecules like SP and insulin-like growth factor (IGF)-1, as well as by stimulation of epithelial proliferation. 36,156 Interestingly, NGF expression is elevated in inflammatory conditions like dry eye, and this elevation in NGF may contribute to the pathogenesis of dry eye. 121 NGF also has potent antiviral properties and restricts viral infections like HSV-1 to the cornea. 118
Brain-derived neurotrophic factor (BDNF)
Similar to NGF, brain-derived neurotrophic factor (BDNF) is found in corneal epithelium and stromal keratocytes and is believed to also originate from corneal sensory neurons. 156,234 In vitro studies of human cell lines and in vivo animal models demonstrate that BDNF is capable of inducing central and peripheral nerve growth and regeneration, 45,132 and stimulates other neurotrophins (including NT-4/5) and neurotrophic factors (such as Gap-43) to achieve neurite re-growth. 112 BDNF is neuroprotective in retinal ganglion cells, possibly via delayed apoptosis of neurons. 98 Although BDNF expression has been observed after experimental flap surgery in putative corneal stromal and/or inflammatory cells in a positive association with neurite extension, its exact role related to corneal nerves is unclear. 42
Glial cell-derived neurotrophic factor (GDNF)
Glial cell-derived neurotrophic factor (GDNF) is a pro-neural member of the TGF-β superfamily. 156,234 Elsewhere in the nervous system, GDNF applied to in vitro chick dorsal root ganglia explants promotes axonal elongation. 135 GDNF is expressed in human corneal stromal keratocytes and may operate similarly to or synergistically with NGF by triggering gene transcription governing epithelial cell migration and wound healing. 135,233,234 It has been shown that topical GDNF produces complete epithelial healing in a patient with a progressive neurotrophic ulcer. 233
Neurotrophins 3, 4/5 (NT-3, -4)
In the cornea, neurotrophin-3 (NT-3) is transcribed in epithelial cells and stromal keratocytes. 234 In vitro mouse studies show that elsewhere in the nervous system, NT-3 regulates neuronal cell differentiation and survival and induces neurite outgrowth in mouse neural stem cells. 128 NT-3 is also a chemoattractant for dorsal root ganglion cells. 185 A recent animal model, however, demonstrated only minimal changes in NT-3 gene expression following surgical transection of corneal nerves. 42 These data suggest that NT-3 may be preferentially involved in corneal epithelial maintenance. NT-4 is also present in corneal epithelium and is a neurotrophic factor that may be involved in the regulation of stromal keratocytes by epithelial cells. 234 In development, NT-4 collapses neural growth cones and causes transient neural growth inhibition in NGF- and NT-3-dependent rat dorsal root ganglion cells. 185 NT-4 is neuroprotective, however, in rat explants of retinal ganglion cells and enhances neurite outgrowth in the retina. 45 Little is known about the specific roles of NT-3 and -4 with regards to corneal nerves.
B4b. Neurotrophic factors, nerve guidance factors, and regeneration-associated genes
Vascular endothelial growth factor (VEGF)
As shown in murine models, vascular endothelial growth factor (VEGF) and its receptors are minimally present in healthy and damaged corneas, and VEGF is upregulated in the injured cornea. 127,236 VEGF is pro-neural via neurotrophic and neuroprotective actions. Trigeminal neurons respond to and secrete VEGF, and VEGF is required for efficient corneal nerve regeneration. 127,236 VEGF supplementation promotes trigeminal nerve repair, and abrogation of VEGF signaling reduces corneal nerve growth. Because topical VEGF neutralization using bevacizumab eye drops has been proposed as a treatment for corneal neovascularization, the finding that VEGF signaling modulates corneal nerve repair warrants consideration of the anti-neuronal effects of antiangiogenesis treatment in the cornea. In the normal murine cornea, topical application of bevacizumab eye drops does not reduce nerve fiber density. 30 Topical application of bevacizumab eye drops to wounded murine corneas, however, results in VEGF depletion, NGF downregulation, and development of a neurotrophic keratitis-like ulcer. 109,236 In humans, topical bevacizumab is associated with increased corneal epitheliopathy. 111
Semaphorins
Semaphorins were identified originally as axon guidance factors. Through animal models, it now is known that several semaphorins, the “immune semaphorins,” are involved in various phases of the immune response by regulating immune cell contacts or cell migration. 173,207 These “immune semaphorins” include Sema3A, 4A, 4D, 6D, and 7A. 173 Unlike many semaphorins that act as repulsive guidance cues, the GPI-anchored membrane-associated Sema7A is neither an axonal repellent nor attractant. 179 Instead, it promotes axon outgrowth in a direction that is likely dictated by other cues. Sema7A is expressed constitutively in the cornea, predominantly concentrated in the epithelium with less expression in the stroma. 164 Corneal Sema7A expression increases after nerve transecting lamellar surgery and is localized near the regenerating nerve fronds. 164 Sema7A induces trigeminal ganglion cell neurite growth in vitro as potently as NGF. 164 Additionally, it induces significant nerve regeneration in vivo, accompanied by significant inflammatory cell influx to the cornea. 164 This suggests that Sema7A acts as a neurotrophic factor in the cornea that can also influence inflammatory processes. In contrast to Sema7A, Sema3A is a negative regulator of innervation. During development, Sema3A repulses innervation and collapses neural growth cones. 113 Blocking Sema3A results in overwhelmingly disorganized corneal innervation during development. 113 Sema3A also blocks the positive neuromodulator VEGF and repulses growing nerves in vitro. 82 A recent study suggests that blocking Sema3A aids corneal nerve regeneration into donor tissue and recovery of corneal nerve sensation in a mouse model of corneal transplantation. 175 Although the role of Sema3A in the human cornea is unclear, it is possible that Sema3A negatively counterbalances positive VEGF neural regulation to ensure controlled neural regeneration after wounding.
Growth-associated protein 43 (GAP-43)
Growth-associated protein 43 (GAP-43) is a rapidly transported axonal protein induced after nerve injury and localized to the axonal growth cone. 200 Studies in animals show GAP-43 is constitutively expressed in corneal sensory nerves, and GAP-43 levels are elevated in response to infection with HSV-1 and for approximately 2 weeks following surgical transection. 42,139,140 GAP-43 plays a role in neural regeneration stimulated by BDNF and NT-4/5. 112 In contrast to the effect of BDNF on GAP-43 expression, high doses of NGF suppress early GAP-43 production following peripheral axotomy and are associated with decreased nerve regeneration in animal models. 92,135 This may be due to exogenous NGF delaying neuronal recognition of an injury state and the subsequent conversion to a neuronal re-growth state. 92,135 Overall, relatively little work has been done to elucidate the roles of constitutive or elevated GAP-43 expression in response to corneal injury.
Small proline-rich protein 1a (Sprr1A)
A member of the small proline-rich protein (Sprr) family, Sprr1A expression has been observed in animal models of injured peripheral nerves, as well as in the cornea following surgical transection. 42,202 Sprr1A expression is accompanied by elevated BDNF and GAP-43 levels in the injured cornea, suggesting a regenerative neurotrophic property. 42 Sprr1a is not expressed during development or in naive adult nervous tissue. 31 It is not normally present in sensory neurons, but its delayed de novo expression after peripheral nerve injury, as well as the fact that overexpression increases neurite outgrowth, suggest Sprr1a is a regeneration-specific protein. 31,202 Sprr1A is robustly and significantly expressed during corneal nerve regeneration in vivo. 42 Sprr1a has been localized in human corneal epithelium in the context of envelope proteins, but unlike other members of the Sprr family, its expression does not change in response to epithelial stress injury. 215 Sprr1b, but not Sprr1a, is a proposed biomarker for corneal epithelium squamous metaplasia and keratinization. 126 Sprr1a is induced during epithelial differentiation in rat keratinocytes, and may contribute to the permeability barrier function of corneal epithelium. 190 Given that corneal epithelial cells undergo continuous turnover, 84 and the sensory endings in the corneal epithelium also undergo continual rearrangement, 85 it is possible that different family members of the small proline rich proteins may differentially regulate epithelial and nerve remodeling. The common properties of Sprr1a with other cornified epithelium genes (e.g., upregulation of Sprr family genes with ultraviolet light stress) raise the possibility that peripheral axonal regeneration uses a shared gene program with epithelial differentiation. 31 Therefore, epithelial innervation, differentiation, and permeability barrier function are interlinked processes.
β-tubulin III (Tubb3)
Tubulins are structural proteins involved in nerve growth, and neurotrophins like NGF promote tubulin expression. 70,152 β-tubulin III (Tubb3) expression is significantly increased in the mouse cornea approximately 2 weeks after surgical transection and positively correlates with BDNF and Sprr1A expression. 42 Tubb3 upregulation is associated with nerve regeneration in the injured peripheral nervous system, and loss of Tubb3 upregulation after peripheral nerve injury is associated with attenuated nerve regeneration in a diabetic animal model. 229
B4c. Inflammatory mediators
T lymphocytes
In the murine cornea, a subset of helper T cells (IL-17+ γδ T cells) is pivotal in corneal nerve repair and regeneration. 127 Loss of these T cells reduces the inflammatory response with >50% decrease in nerve regeneration, likely due to loss of T cell-induced cytokine signaling and related reduction in VEGF. This suggests a pro-neural role for both these T cells and inflammation in general.
Leukemia inhibitory factor (LIF)
Leukemia inhibitory factor (LIF) is a neuropoietic cytokine expressed in a variety of cell types, including inflammatory cells, epithelium, and corneal tissues. 189 LIF enhances in vitro stem cell proliferation in human epithelial limbal cells, and is involved in pro-inflammatory signaling cascades. In the healthy murine cornea, LIF is expressed in vivo in epithelial cells, 189 suggesting a role for corneal health maintenance and function as well as a potential reservoir for immediate chemoattractant signaling upon injury. In a LASIK rabbit model, following injury LIF accelerates corneal nerve regeneration. 177
Interleukins 6, 17 (IL-6, 17)
Interleukin-6 (IL-6), a neuropoietic cytokine involved in immunomodulation as well as neuronal survival and function, 238 is synthesized in peripheral sensory neurons and corneal epithelium and promotes stromal inflammation, neuronal differentiation via the NGF Trk receptor, and neuronal survival with concurrent upregulation of NGF. 204,205,238 IL-6 synthesis increases in pathological states; 238 however, IL-6 has been implicated in pathologic chronic inflammation of keratoconus. 124 Another cytokine involved in neurogenic inflammation is IL-17. In the murine cornea, injury (including surgical transection) is followed by accumulation of IL-17+ γδ helper T cells and inflammatory cells that promote complete epithelial and near-complete nerve recovery. 127 Loss of these helper T cells and/or IL-17 leads to a >50% reduction in corneal nerve regeneration. 127
B4d. Hormonal regulators
Thyroxine
Thyroxine increases NGF and augments nerve regeneration in the central and peripheral nervous systems, respectively. 172,223 Little is known about thyroxine in the cornea. In the developing chick, exogenous thyroxine increases nerve elongation, possibly via increased actin cytoskeleton polymerization that is required for neuronal outgrowth. 46 In the human peripheral nervous system, thyroxine may signal through VEGF or EGFR to modulate neuronal migration and wound healing. 40,55 Thyroid hormone receptor is present in all layers of the cornea, 47 although the effects of thyroxine on corneal nerve regeneration are unknown.
Corticosteroids
Corticosteroids regulate inflammation and cell proliferation and can be synthesized by peripheral nerves. 148 Neural roles for endogenous corticosteroids include changes in Schwann cell morphology and proliferation in the peripheral nervous system, serving as coactivators required for myelination. 148 Little is known about the role of endogenous steroids in the cornea; however, the cornea has numerous steroid hormone receptors, and ocular tissues express mRNA for 5α-reductase, an enzyme required for metabolizing steroid hormones to biologically active molecules. 81 For information regarding the role of exogenous corticosteroids, see the “Treatment methods” section of this review.
B4e. Miscellaneous neuromodulators
Pituitary adenylate cyclase-activating polypeptide (PACAP)
Pituitary adenylate cyclase-activating polypeptide (PACAP) acts as a neurotransmitter (a C fiber neuropeptide 224) and has neuromodulating effects. PACAP induces corneal neurite outgrowth and accelerates the return of corneal sensitivity after surgical transection. 76 PACAP responds to nitric oxide (NO) levels, and NO may activate ocular C fibers following injury. 224 PACAP increases concomitantly with NO in response to inflammation. 224 PACAP may prove to be a viable pro-neural factor once its role following injury in corneal nerves is elucidated.
Collagen XVIII
Components of the extracellular matrix play pivotal roles in peripheral nervous system growth and regeneration. 194 Collagen XVIII localizes to the basement membrane of corneal nerves, and is required for proper corneal nerve organization and appropriate regeneration after surgical transection. 194 Re-growth of corneal nerves appears to occur with the laying down of new basement membrane, 13 suggesting the importance of a healthy membrane and the presence of collagen XVIII in the regeneration process.
C. CORNEAL NERVE DYSFUNCTION
C1. Corneal diseases causing nerve dysfunction
Neurotrophic keratopathy can be caused by a variety of infectious agents, as well as congenital, ocular, and systemic diseases, pharmaceutical agents, trauma, and corneal dystrophies. 226 Sensory nerves in the cornea deploy afferent stimulation signals to the brain, which then returns an efferent signal; for example, to the lacrimal gland via autonomic nerves to drive tear production and secretion. When the cornea is damaged, these neural circuits are disrupted. Corneal nerve damage leads to neurotrophic keratopathy either directly (via loss of supportive neuropeptides) or indirectly (via disruption of neural circuits resulting in, for example, dry eye). Thus, corneal nerve impairment is responsible for epithelial defects, ulcerations, corneal perforations, and reduced function of corneolimbal stem cells 33,217 (Figure 5). Late-stage neurotrophic keratitis patients are often asymptomatic because of significantly decreased corneal sensation. 226 We will now elucidate the interdependence between corneal nerves and epithelium and highlight the complicated role of inflammation in the pathophysiology of neurotrophic keratopathies.
Figure 5.
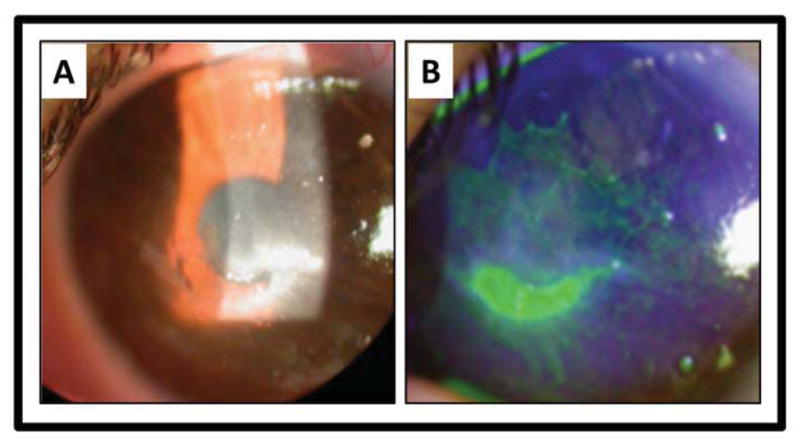
Clinical photograph of a patient with neurotrophic keratitis. A) A central epithelial defect is surrounded by corneal haze. B) Fluorescein stains the central epithelial defect and reveals extensive superficial punctate keratitis.
Infection negatively affects corneal tissues and corneal nerves. Neurotrophic keratitis can be caused by herpes viruses [herpes-simplex virus type 1 (HSV 1) and varicella-zoster virus (VZV)], Mycobacterium leprae, Acanthamoeba, and fungal infections. Acanthamoeba and fungal keratitis more profoundly impact corneal nerves. 116 In these infections, with the exception of leprosy, the subbasal plexus exhibits at least a 2- to 3-fold reduction in the number of nerve fiber bundles, total nerve number, and branching, along with increased tortuosity. 83,116 Similar changes are seen in contralateral, uninfected eyes. 83 Interestingly, in both the infected and uninfected eyes of HSV1 patients, subbasal plexus alterations directly correlate with corneal hypoesthesia related to disease duration and number of recurrences, 83 while Acanthamoeba keratitis patients often present with significant pain, possibly neuropathic in nature. 116 The location of corneal nerves within the layers of the cornea and the type of herpetic keratitis determine the extent of corneal nerve changes in response to herpetic infection. The subepithelial plexus exhibits destructive changes in epithelial and stromal HSV keratitis, but appears preserved in clinical endothelial keratitis. 161
Leprosy is associated with changes in stromal nerve density, irregularities in epithelial nerves, and corneal nerve thickening, tortuosity, and beading, accompanied by hypoesthesia. 237 Inflammatory mediators have conflicting roles in infectious neurotrophic keratopathy. Activation of latent VZV causes inflammatory destruction of corneal nerves, 196 whereas inflammation-related neuropeptides can be beneficial in reducing herpetic keratopathies. Trigeminal and corneal sympathetic nervous system activity and increased interferon-γ levels ward off infection in mice, 212 thus preserving corneal nerves. Combination treatment with SP and IGF-1 completely resolves epithelial defects in neurotrophic keratopathy patients, though SP may worsen microbial keratitis by promoting excess inflammation. 72,212,230 Non-infectious disease processes, both corneal and systemic, also negatively affect corneal nerves.
Keratoconus, bullous keratopathy (e.g., in the setting of Fuchs’ dystrophy), and atopic keratoconjunctivitis (AKC) are associated with inflammation of the ocular surface and are accompanied by pathological alterations of corneal tissues and loss of corneal sensitivity. 3,5,95,124,136,170 Nerves of the subbasal plexus exhibit significant loss of density, branching, and diameter, with increased tortuosity in conjunction with the stromal nerve changes of localized thickening, twisting, or looping. 3,5,95,136,170 In keratoconus, subbasal nerves may also be thickened. 170 Thickened subbasal and stromal nerves appear approximately 1.5- to 2-fold wider than those of healthy controls, which may be due to keratocyte “wrapping” of the nerves rather than genuine hypertrophy. 136,170 Subbasal nerves in the keratoconic corneal apex exhibit altered architecture, consisting of horizontal and oblique orientations of closed loop nerve fibers and abrupt terminations instead of the whorl-like arrangement of the healthy cornea. 182 Epithelial nerves display density loss in the subepithelial plexus. 136 Corneal nerves have been implicated in keratoconus pathophysiology through nerve damage (such as a cranial nerve V palsy), proteolytic enzyme activity (cathepsin B and G) in keratocytes and nerve terminals associated with thickened nerves, or loss of neurotrophic signaling (NGF and its receptors). 35,119,193 It is unclear if these changes are underlying etiology or merely a result of the disease. Non-infectious systemic diseases, including autoimmune diseases and systemic polyneuropathies, have similar detrimental effects on corneal nerves.
Autoimmune diseases negatively affect corneal nerve parameters including density, tortuosity, beading, and function. Graves’ disease, or any significant thyroid dysfunction, may be accompanied by dysthyroid ophthalmopathy with signs and symptoms of corneal nerve dysfunction. 14 Decreased serum NGF is associated with the occurrence of Graves’ ophthalmopathy, and there are fewer subbasal nerve fibers and increased tortuosity and beading. 155,220 Nerve function is reduced, which may be due to neurotrophic factor deprivation from loss of corneal epithelium integrity. 89,220 Epithelial defects have been reported in cases of severe Graves’ ophthalmopathy. 89 These defects may worsen with corticosteroid therapy, likely due in part to suppression of proneural cytokines. 89,220 The autoimmune Sjögren’s syndrome (SS) exemplifies the relationship among corneal nerves, tear secretion, and epithelial integrity. Corneal nerve damage, for example from refractive surgery, may lead to tear hyposecretion. The resultant dry eye and dry eye epitheliopathy further damages corneal nerves, perpetuating the dry eye disease state and predisposing to neurotrophic keratopathy. In fact, SS is associated with compromised nerve function, thickened nerves, increased subbasal nerve tortuosity, and beading. 24,216 Disagreement exists (perhaps due to different testing modalities) over changes in subbasal nerve density and beading and whether there is increased or decreased corneal sensitivity. 24,216 Inflammation in dry eye disease like SS may be a response to efferent nerve stimulation in the cornea that results in the release of neuropeptides such as CGRP and SP. 203 Inflammation may be beneficial for nerve regeneration. 127 Activated keratocytes, induced by pro-inflammatory cytokines, are capable of secreting NGF, which may improve corneal sensitivity and restore corneal integrity, possibly via ACh and SP production with concurrent sympathetic nervous system modulation. 24,33 Nerve growth cones and sprouts have been observed in conjunction with keratocyte activation. 24,216 In animal studies, epithelial damage is exacerbated by sympathetic innervation and ameliorated by CGRP, SP, ACh, and IGF-1. 33 It is unclear if corneal nerve damage occurs as a primary component of these autoimmune diseases, or whether it is secondary to other orbital pathophysiology. Other, systemic polyneuropathies exhibit similar negative impacts on corneal nerves.
Systemic polyneuropathies that can lead to neurotrophic corneal ulceration include peripheral diabetic neuropathy (PDN), amyloidosis, sarcoidosis, vitamin B12 deficiency, and alcoholism. The majority of corneal symptoms experienced by polyneuropathy patients like diabetics are the result of damage of the cornea’s small Aδ and C nerve fibers that can lead to neurotrophic keratopathy. 86,94 Central corneal epithelial nerve density is markedly decreased. 86 The subbasal plexus exhibits both reduced density and branching with increased tortuosity and nerve thickening. 153 These findings positively correlate to decreased corneal sensitivity and systemic disease severity. 94 Stromal nerves appear thickened and may have increased looping or curving of nerve fibers, and diabetic corneas exhibit aberrant nerve regeneration. 86 Aδ mechanoreceptors may be affected prior to and differently from polymodal C fiber nociceptors. 160 NGF is elevated in diabetic patients, though levels of NGF and its receptors decrease in proportion to disease severity. 110 PDN can lead to corneal epithelial disease even before the underlying diabetes has been diagnosed, 137 and neurotrophic keratopathy should be considered when a known diabetic develops corneal epithelial disease.
Hereditary sensory and autonomic neuropathies (HSAN, types IV and V) exhibit compromised nerve function with corneal alacrima and analgesia, and may even lead to neurotrophic ulceration and corneal opacities. 151 In the cornea, there is central loss of Aδ fibers and impairment of the remaining Aδ and C fibers with a reduction in subbasal nerve bundles and decrease in corneal sensation. 151 HSAN IV and V display mutations in NGF and its Trk receptor, resulting in impaired nociceptor development and function. 151 Congenital aniridia is often accompanied by corneal changes, and 80% of patients display keratopathy (aniridia-related keratopathy [ARK]) that correlates with a loss in corneal sensitivity. 59 ARK also correlates with, and may be brought on by, intraocular surgery. 59 ARK may be a type of neurotrophic keratitis due to a Pax6 abnormality of corneolimbal stem cells and aberrant nerve projection, though a Pax6 animal model suggests the keratopathy is not neurotrophic in nature. 59,123 Another rare disease that involves corneal nerve dysfunction is ocular-auriculo-vertebral dysplasia (OAV or Goldenhar syndrome). Caused by a trigeminal nerve malformation, patients may present with corneal hypoesthesia and keratopathy. 221 Multiple endocrine neoplasia (MEN) 2A and B are associated with corneal nerve thickening due to a putative genetic alteration and axon and Schwann cell abundance, respectively. 103,206 In MEN2B, keratoconjunctivitis sicca and meibomian gland dysfunction may accompany nerve thickening, though epithelial changes may be a result of lid and meibomian gland malfunction and not corneal nerve dysfunction. 103 In other polyneuropathies, such as small fiber neuropathies of autoimmune, pharmacologic, or idiopathic origin, patients also demonstrate reduced sensitivity, nerve fiber density, branching, and length, with a concomitant increase in tortuosity. 211
Pharmacological agents may also lead to keratitis and compromise corneal nerves. Topical analgesics, notably the non-steroidal anti-inflammatory drug (NSAID) diclofenac, can delay corneal wound healing and result in epithelial breakdown and corneal melting. 77,227 Direct damage from anesthetics, such as proparacaine, retards healing and causes epithelial defects. 187,227 The epithelial damage negatively affects corneal nerves, which elicits pain. The pain prompts further anesthetic use, which exacerbates the initial epithelial defect. Topical morphine does not appear to be toxic in experimental corneal abrasions in rabbits. 187 Analgesic use is warranted for corneal nerve injuries from many causes, including chemical and physical trauma.
Corneal nerves can be injured by chemical and physical trauma. Exposure to chemical agents, such as carbon disulfide and hydrogen sulfide, reduces corneal sensitivity and can irritate the cornea at high concentrations. 141 Exposure to mustard gas [bis- (2-chloroethyl) sulfide] results in corneal epithelial ulceration and destruction. 100 Patients with chronic mustard gas keratopathy exhibit thickened midstromal nerves and loss of the subbasal nerve plexus; however, this latter finding could be explained by concordant dry eye. 100 In addition to chemical factors, physical trauma to corneal nerves ranges from accidental abrasions to planned surgical transection of corneal nerves. As with corneal nerve damage by previously discussed modalities, traumatic injury to corneal nerves can lead to altered function and neurotrophic keratitis.
C2. Corneal surgeries causing nerve dysfunction
C2a. Corneal nerve transection in refractive vision correction
In 2007, the American Academy of Ophthalmology estimated 42.5 million patients in the United States over the age of 40 years had refractive disorders. 10 Laser in situ keratomileusis (LASIK) is the most common surgical procedure for vision correction, with 1.5 million procedures performed in the United States in 2005. 10 Laser sub-epithelial keratomileusis (LASEK) and photorefractive keratectomy (PRK) are alternatives. Comparing the effects of these refractive surgeries on corneal nerves is difficult because of the variability among studies regarding the type of surgery, depth of excision, and extent of nerve damage already present or inflicted. 21 In PRK and LASEK, superficial and subbasal nerves are ablated, whereas the majority of stromal nerves remain intact. In PRK, subbasal nerves regenerate from stromal nerves to near pre-operative density by 3 years 63,180 and restore corneal sensitivity after an average of approximately 2 months. 34,62,114,142,168,181 LASEK demonstrates a recovery of corneal sensitivity to the pre-operative state within approximately 3 months, 54 though a recent review suggests anatomical nerve recovery may take 6 months or more. 180 In contrast, during LASIK procedures, stromal nerves through the stromal bed are transected. 21,63,181 Post-LASIK, the subbasal plexus density decreases more than 80%. 57,154 Nerve regrowth is observed in the central cornea by 6 months post-procedure, though it may be 5 years before nerve densities achieve pre-operative levels. 54,63,154,180 (refer to Table 3 for a comparison of subbasal nerve changes after refractive surgeries) Nerve regrowth may not mimic the native state. 180 In a murine model undergoing lamellar corneal dissection, regenerating nerves in the central stromal were comprised of non-myelinated nociceptive fibers as well as myelinated fibers. 165 In the pre-operative native state, myelinated fibers are limited to the peripheral corneal stroma. Discrepancies exist regarding subepithelial plexus regrowth as well after refractive surgery, 107 although it is generally accepted that central regrowth is present by 6 months post-operatively and corneal sensitivity returns approximately 3 to 9 months post-LASIK and arguably does not depend on long-term nerve abnormalities. 54,63,154,180,191 Depth of ablation is debatably a significant factor in time to recovery of corneal sensitivity. 25,167 Figure 6 illustrates post-LASIK changes in the subbasal plexus. LASEK appears to have a more favorable recovery profile, whereas LASIK may be associated with LASIK-induced neurotrophic epitheliopathy (LINE), dry eye, and neuropathic pain. 21,122,191
Table 3.
Subbasal nerves after refractive surgery
| Type of refractive surgery | Percent recovery of subbasal density from baseline | Recovery time of corneal sensitivity (mean from available studies) | ||
|---|---|---|---|---|
| 6 months | 1 year | >3 years | ||
| LASIK | 33.6%38,54,122 | 43.8%38,63 | 58.6%38,63 | 5.5 months25,34,44,54,114,150,166,167,168,181 |
| LASEK | 57.6%54,122 | Data not available | Data not available | 3.6 months 54,90,122,228 |
| PRK | 25.3% 62 | 40.5% 62,63 | 100%* 62,63 | 2.3 months 34,62,114,142,168,181 |
LASIK = laser in situ keratomileusis; LASEK = laser sub-epithelial keratomileusis; PRK = photorefractive keratectomy.
Typically observed at “no significant difference” with pre-PRK subbasal nerve density.
Figure 6.
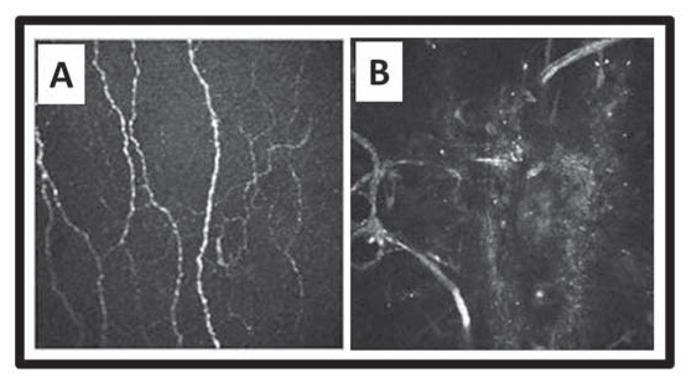
Subbasal nerves before and after LASIK. Heidelberg Retina Tomograph II with Rostock Corneal Module optical coherence tomography showing subbasal corneal nerves A) before LASIK, and B) regenerated nerve loops in the flap area 4 years postoperatively. Courtesy of Heidelberg Engineering, Inc. and Dr. E.M. Messmer, Munich, Germany.
Transection of corneal nerves in LASIK disrupts the regulatory loops of neurotrophins, growth factors, and cytokines across the cornea, nervous system, and lacrimal gland. 191 Figure 7 demonstrates superficial punctate keratitis associated with LINE. LINE improves with nerve healing 6 months post-procedure, but dry eye and neuropathic pain may become chronic. 21,57 One study reports that nearly 50% of LASIK patients experience dry eye 6 months post-operatively. 191 Damaged nerves exhibit altered ion channel conductivity resulting in aberrant hypersensitivity firing and dysesthesias. 21 The extent of side effects reportedly depend upon flap hinge width, depth, and location. 157 Increased incidence of LASIK-induced dry eye has been shown in flaps created with a microkeratome compared to a femtosecond laser, 195 though reinnervation rates remain similar. 184 While recent studies find no effect of hinge position on nerve recovery, 96 earlier reports suggested that corneal flaps with superior hinges have a greater risk of dry eye and reduced corneal sensation compared to nasal hinged flaps. 57 The performance of a horizontal flap is supported by the model of corneal innervation proposing that the long ciliary nerves enter the cornea at the 3- and 9-o’clock positions, suggesting that there is greater susceptibility to corneal nerve damage with vertically than horizontally hinged flaps; 57 however, He et al. 87 have recently reported that corneal nerves enter the stroma in a radial pattern, and the distributions of stromal nerves and epithelial nerve are similar in each corneal quadrant; thus providing direct anatomical evidence that the position of the corneal flap is irrelevant.
Figure 7.
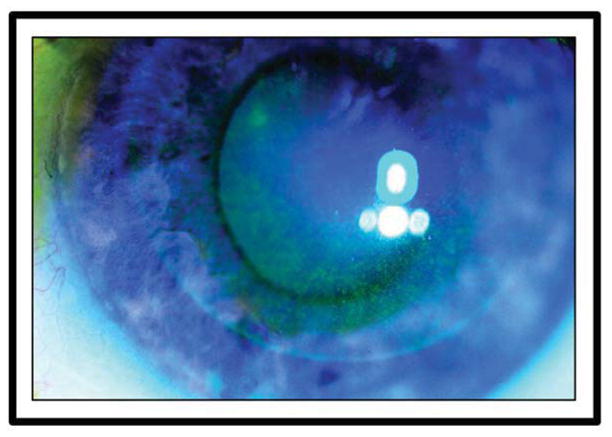
Clinical photograph of a patient with LASIK-induced neurotrophic epitheliopathy (LINE). Superficial punctate keratitis stained with fluorescein is limited to the flap area of the cornea.
C2b. Reinnervation following corneal transplant surgery
Corneal allograft procedures include penetrating keratoplasty (PK), lamellar keratoplasty (LK), deep anterior lamellar keratoplasty (DALK), Descemet stripping automated endothelial keratoplasty (DSAEK), and epikeratophakia. PK involves full thickness trephination of the host cornea to prepare the bed for transplantation. Trephination transects the stromal nerves circumferentially. 7 Following PK, subbasal and epithelial re-innervation of the donor graft occurs to some degree, however, stromal innervation is quite limited. In a recent review, 7 Al-Aqaba et al. note that subbasal nerve density does not recover by 30 years after PK, and significant alterations to the subbasal nerve plexus are apparent even 40 years later. In full thickness corneal grafts, the majority of regenerated nerves in the donor tissue are a continuation of host subbasal nerves, which cross the graft-host junction to innervate the peripheral part of the grafts. Stromal nerve trunks from the host tissue may stop at the edge of the graft and in some instances are completely absent in the donor tissue. When stromal nerves do invade the donor tissue, they frequently innervate only the peripheral areas. Regenerated stromal nerves do not contribute to the epithelial innervation. In normal cornea, the subbasal nerves arise from the stromal nerves and there are numerous connections between them. Following PK, these normal links between the subbasal and stromal nerves are absent in donor tissue. 7 Recovery of corneal sensation is of some debate, returning by 1 year or reduced for decades after PK, and does not seem to correspond to the degree of anatomic re-innervation. 7,53 Epithelial and subbasal innervation in the donor graft is generally necessary for some degree of corneal sensation restoration. 7 In DALK, a full thickness donor cornea (with Descemet membrane and endothelium removed) is transplanted over host Descemet membrane and endothelium. The recovery of corneal sensitivity in the graft following PK or DALK is similar, with one comparative study obtaining good corneal sensitivity by 2 years after surgery. 41 This is because in both procedures, all stromal nerve trunks are transected in the process of preparing the host bed. DSAEK is a technique of corneal transplantation that allows for selective replacement of diseased endothelium and Descemet membrane. In contrast to PK and DALK, DSAEK does not cause corneal hypesthesia. 115 This is not surprising because corneal nerves do not innervate Descemet membrane or the corneal endothelium. In epikeratophakia, epithelial nerves are lost in conjunction with long-term reduction in subbasal nerve density. 19,68
C2c. Corneal nerves following collagen crosslinking (CXL)
Riboflavin UVA-induced corneal collagen crosslinking (CXL) is a relatively new treatment for progressive keratoconus and post-LASIK corneal ectasia that may reduce the need for keratoplasty. 144 CXL combines riboflavin treatment with UVA irradiation to promote connections, or crosslinking, among collagen fibers to strengthen the disease-weakened cornea. CXL can be performed either transepithelial (without epithelial removal) or after de-epithelialization (with epithelial removal). CXL after epithelial removal results in loss of subepithelial plexus and anterior-midstromal nerves. 144 By 6 months post-CXL, sensitivity is restored, and nerve fiber regeneration is nearly complete. 144 In contrast, following transepithelial CXL, subepithelial and stromal nerve plexi remain intact. 39 CXL postoperative pain is intense and worse than pain after PRK, particularly during the first 3 days after surgery, and necessitates an aggressive postoperative pain control regimen. 78 The pain is neuropathic in nature and mitigated by age. 78 Re-epithelialization is accompanied by a significant decrease in both pain and the need for analgesia on each consecutive day. 78 One of the sources of intense pain might be the destructive effect that CXL has on the subbasal nerve plexus while keeping the majority of stromal nerves intact within a postoperative milieu. 78
C2d. Recombinant human collagen (RHC) corneal substitutes
Recombinant human collagen (RHC) corneal substitutes are being tested as therapeutic corneal replacements. RHC implants have minimal inflammation and/or neovascularization and exhibit partial regeneration of subbasal nerves as well as a return of corneal sensitivity. 67
D. THE LINK BETWEEN INFLAMMATION AND NERVE REGENERATION PATHWAYS
Inflammation plays a key role in peripheral nerve regeneration. 26 Corneal nerve regeneration and inflammation pathways appear to be intertwined. (Table 4) Corneal nerve regeneration is enhanced by a γδ T cell-dependent inflammatory cascade that involves IL-17, neutrophils, platelets, and VEGF-A. 127 A strong and significant correlation has been reported between increased numbers of dendritic-shaped cells of the central cornea and decreased subbasal corneal nerves, suggesting a potential interaction of the immune and nervous systems during corneal infections. 52 Loss of the subbasal nerve plexus occurs in non-infectious inflammatory eye diseases as well. 24,219 In addition, we reported previously that immunomodulation with cyclosporine eye drops in an animal model reduces cytokine expression in the cornea and retards regenerative sprouting from transected corneal stromal nerve trunks. 163 Our data also revealed that topical benzalkonium application to the eye increases corneal inflammation and induces neurotoxocity. 197 These findings suggest the presence of molecular regulators in the cornea that straddle the immune and nervous systems.
TABLE 4.
Modulators that affect neuronal as well as inflammatory pathways
| Factor | Effect on corneal nerves | Effect on inflammation |
|---|---|---|
| Biological factors | ||
| NGF | Nerve regeneration 66 | Pro 36,120,129 |
| VEGF | Efficient regeneration 127 | Pro 99 |
| DHA | Enhances regeneration 66 | Anti 49 |
| PACAP | Neurite outgrowth Accelerates return of corneal sensitivity after injury 76 |
Pro 224 |
| Slit2 | Axonal repulsion (early development) 113 Epithelial nerve branching (late development) 113 |
Anti 213 |
| Sema7A | Axonal elongation Neurite outgrowth Nerve regeneration 164 |
Pro 164 |
| T lymphocytes | Enhances regeneration 127 | Pro 127 |
| LIF | Accelerates regeneration 177 | Pro or anti 189 |
| IL-17 | Enhances regeneration 127 | Pro 127 |
| Pharmacologic factors | ||
| Cyclosporine A | Mixed: Improved indirect measures of corneal nerve function58* Retards regeneration 163 |
Anti 58,163 |
| Corticosteroids | Mixed: May reverse neuropathic morphologies 117 Improvement or no change in indirect corneal nerve function tests121* |
Anti 121,125 |
| Opioids | Corneal reflex blockade 218 Analgesia 187 |
Anti 222† |
| Benzalkonium chloride | Neurotoxicity 197 | Pro 197 |
NGF= nerve growth factor; VEGF = vascular endothelial growth factor; DHA = docosahexanoic acid; PACAP = pituitary adenylate cyclase-activating peptide; Sema7A = semaphorin 7A; LIF = leukemia inhibitory factor; IL-17 = interleukin 17. “ – ” = no known effect.
In these publications, corneal nerve function was measured indirectly by tests that assess elements of the nerve-epithelium-tear film axis, (such as Schirmer’s, tear film breakup time, and/or fluorescent dyes for epithelial integrity), as well as subjective symptoms (such as dry eye and foreign body sensation).
Evidence supports the anti-inflammatory properties of kappa opioids, while the effect of other opioid classes on inflammation remains unclear.
Taken together, these studies suggest that some degree of inflammation (inflammatory cells, molecules, or chemokines) promotes corneal nerve regeneration, whereas excessive inflammation may lead to loss of corneal innervation and subsequent neurotrophic keratopathy. The nervous and immune systems communicate biochemically. Neurons, including nociceptors, express functional receptors for cytokines, whereas cells of the immune system may recognize, and are modulated by, neuropeptides (reviewed by Li et al. 125). In fact, neural signaling with SP and/or VIP promotes resistance to infection by stimulating immune system effectors (reviewed by Cruzat et al. 52). We reported that one molecule, Sema7A, is shared by the nervous and immune systems and links nerve regeneration and inflammation in the murine cornea. 164 In the peripheral nervous system, Sema7A enhances axonal outgrowth. In the immune system, Sema7A is expressed in activated T cells and plays a role in T cell-mediated inflammation. In the cornea, Sema7A is constitutively expressed in the epithelium and stroma. Following corneal wounding, Sema7A stimulates nerve regeneration in association with inflammatory cell influx. From the narrow perspective of nerve regeneration, the most direct practical implication of these studies is that avoiding long-duration immunomodulation or anti-inflammatory treatment may benefit corneal reinnervation if postoperative healing is uncomplicated.
E. EVIDENCE-BASED TREATMENT OF NEUROTROPHIC CORNEAL DISEASE
Corneal nerves have complex interactions with resident cells (limbal stem cells, epithelium, and antigen-presenting cells) as well as bone marrow-derived inflammatory cells that migrate into the cornea. Corneal nerve dysfunction from injury or disease perturbs these interactions and leads to neurotrophic keratitis, characterized by reduced corneal sensation, epithelial defects, and corneal scarring. The pathways controlling corneal nerve regeneration molecularly link with pathways that control corneal epithelium differentiation as well as its permeability barrier function. Furthermore, corneal nerves are essential for maintaining limbal stem cell homeostasis and function. 217 Corneal nerve regeneration is enhanced by the presence of some degree of concomitant inflammation.
The therapeutic strategy to treat neurotrophic keratitis must consider the interactions of nerve regeneration and inflammatory pathways. In general, patients should discontinue use of all preserved eye drops. At least one common preservative, benzalkonium chloride, increases corneal inflammation and additionally has direct neurotoxicity. 197 Topical corticosteroid and cyclosporine eye drops should also be avoided, as they can excessively retard inflammation and some inflammation appears facilitative of nerve regeneration. 121,125,127,163 Corneal nerve regeneration can be stimulated by topical application of platelet-rich plasma (PRP) because platelets contain neurotrophic factors like VEGF and Sema7A. 73,74 Silicone hydrogel soft contact lenses can be used as a bandage, and non-preserved artificial tears may be used for lubrication to promote healing. 79,143,197 Oral minocycline may be used for neuroprotection. 225 Treatment for refractory neurotrophic keratitis may include the ProKera amniotic membrane contact “lens,” amniotic membrane transplantation, and/or tarsorrhaphy. Additional treatment includes topical NGF to promote corneal nerve regeneration. 135 Taken together, the data support a multipronged approach to treat neurotrophic keratitis. The available therapeutic interventions are detailed individually below.
E1. Neuro-regenerative
E1a. Blood component-based therapy
E1a1. Serum eye drops
Autologous serum eye drops contain a variety of factors, including epidermal growth factor (EGF), TGF-β, vitamin A, SP, IGF-1, and NGF, that are pro-epithelial and pro-neural. 232 Autologous serum eye drops effectively treat some cases of neurotrophic keratitis, as well as promote corneal epithelial healing in other ocular disorders such as dry eye. 232 Tissue healing is accompanied by a return of corneal sensitivity. 143 To prepare serum eye drops, blood samples are collected, clotted, and centrifuged before being diluted with balanced salt solution (BSS) or sterile saline. Therapeutic concentrations range from 20% to 100% autologous serum. In practice, preparations can be dispensed in 5-ml bottles to be stored at −20°C, although storage at −70°C is also recommended. When in use, the unused portion is stored at 4°C, and opened bottles should be discarded after 16 hours. 131 Appropriate preparation and serum concentration will optimize treatment of neurotrophic keratopathy with autologous serum eye drops. Liu et al. 131 demonstrated that serum dilution in BSS promotes epithelial cell proliferation better than serum dilution in saline, as does centrifugation at higher speeds, (e.g., 3,000 × g). Prolonged clotting times (~120 minutes) enhance cell migration and differentiation. These improvements are thought to result from increased release of epithelial and neurotrophic factors from serum components. 131 One study reported that dry eye disease and some neurotrophic keratopathies with epithelial defects were successfully treated with 20% autologous serum eye drops in sterile saline. 143 Use of 50% serum eye drops diluted in sodium hyaluronate or balanced salt solution treats a similarly high percentage of epithelial defects, and patients enjoy the increased viscosity, suggesting that a 50% dilution of autologous serum eye drops should be the first-line treatment for neurotrophic keratopathy. 43,105 Autologous serum eye drops are similar to umbilical cord serum eye drops (see below) because both improve recurrent epithelial erosions and promote complete epithelial healing in dry eye and non-dry eye keratopathies. 231
E1a2. Umbilical cord serum eye drops
Like autologous serum, umbilical cord serum eye drops contain growth factors like EGF, vitamin A, TGF-β, SP, IGF-1, and NGF. They are used in the successful treatment of recurrent corneal erosion, neurotrophic keratitis, and dry eye syndrome. 232 Umbilical cord serum generally appears more efficient in epitheliopathy symptom relief and healing time compared to autologous serum, 231 likely because of the higher levels of EGF, TGF-β, SP, and NGF in umbilical cord serum. 231 In addition, this is likely why a 20% dilution of umbilical cord serum eye drops successfully treats persistent neurotrophic keratitis refractory to conventional treatment. 232
E1a3. Platelet-rich plasma (PRP)
Platelets contain many bioactive compounds that play a role in inflammation and wound healing. 74 These include platelet-derived growth factor (PDGF), TGF-β, VEGF, Sema7A, cytokines, and active metabolites. 73,74 Platelet rich plasma (PRP) assists in cranial nerve regeneration in experimental models, 69 and platelets are associated with murine corneal nerve regeneration. 127 PRP successfully treats symptomatic dry eye, with a decrease in inflammation and fluorescein staining. 8 Because it contains VEGF and Sema7A, two potent neurotrophic factors, PRP is theoretically a more powerful therapy than serum to treat neurotrophic keratitis. There are commercially available methods to produce PRP, though clinicians may also generate PRP in the clinic. Blood is collected and anticoagulated with citrate, followed by centrifugation to separate the PRP. Aspirin and NSAIDs cause permanent platelet dysfunction, and at least 1 week is required for megakaryocyte precursors to replenish the platelet population. Therefore, clinicians must ensure patients have discontinued aspirin or NSAID for at least 1 week prior to drawing blood for PRP.
E1b. Neurotrophic factors
NGF is responsible for axonal elongation and branching stimulation in chick explants. 135 Exogenous NGF reverses damage to peripheral nerves and completely heals corneal epithelial defects such as those observed in neurotrophic keratitis. 9 NGF eye drops improve corneal sensitivity and promote corneal epithelial healing in patients with moderate and severe neurotrophic keratitis. 32 Topical application of NGF to the cornea has been reported to be safe and efficacious for treating epithelial defects in patients with congenital corneal anesthesia. 209 NGF, in combination with neuromediators, may potentiate regenerative effects. NGF and decosahexanoic acid (DHA), an omega-3 fatty acid postulated to have neuroprotective activity, exhibit enhanced nerve and epithelial regeneration in animal experiments. 66 DHA may aid recovery of corneal sensitivity recovers to near-normal levels, perhaps mediated via the DHA-derived lipid mediator neuroprotectin D1 (NPD1), a docosanoid with neuroprotective actions. 49
VEGF is another growth factor with positive effects on corneal nerve healing. VEGF appears necessary for corneal nerve regeneration following wounding. Blocking VEGF-A results in reduced murine subbasal neuron regeneration by nearly 80% of total corneal nerves, and exogenous VEGF doubles regenerating nerve density. 127,236 The VEGF antibody bevacizumab binds VEGF and inhibits inflammation-induced angiogenesis and lymphangiogenesis in corneal experimental models. 236 The effects of bevacizumab treatment on human corneal nerves remain unknown.
E2. Ensconcing
E2a. Contact lenses
Contact lenses can be used in a protective manner for neurotrophic keratopathy. Choi et al. successfully treated persistent epithelial defects of neurotrophic keratopathy with a combination of silicone hydrogel lenses with 50% autologous serum eye drops. 43 Silicone hydrogel lenses maintain hydration and have increased oxygen transmissibility, which lessens hypoxic conditions. 43 Silicon hydrogel contact lens materials include senofilcon A (Acuvue Oasys ®) and narafilcon B (Acuvue TruEye ®). A special contact lens, ProKera ® (Bio-Tissue, Inc., Miami, FL) is designed specifically to promote corneal healing. The ProKera lens, comprised of amniotic membrane stretched within a polycarbonate ring conformer, speeds healing and improves visual outcome in patients with corneal wounding such as neurotrophic keratitis, likely via its delivery of amniotic membrane growth factors. 176 Prosthetic replacement of the ocular surface ecosystem (PROSE, Boston Foundation for Sight), is a scleral contact lens-based strategy that is another effective alternative for recalcitrant neurotrophic corneal disease. 130
E2b. Amniotic membrane transplant (AMT)
Preserved human amniotic membrane can be transplanted as a basement membrane substitute. Indications for AMT include corneal ulceration, persistent epithelial defects, band keratopathy, limbal stem cell deficiencies, acute herpetic keratitis, and Stevens-Johnson syndrome. AMT promotes ocular epithelialization, most likely because of intrinsic EGF, keratocyte growth factor, and neurotrophins. 108,149
E2c. Tarsorrhaphy
Tarsorrhaphy facilitates healing of recalcitrant epithelial defects in neurotrophic keratitis. By decreasing the width of the palpebral fissure, tarsorrhaphy decreases the evaporation rate of tears. Tarsorrhaphy also minimizes the traumatic effect of lid movement over a healing epithelial defect. 50 Tarsorrhaphy is successful in healing more than 90% of persistent epithelial defects from neurotrophic ulcers that are refractory to conventional therapies. 50,75,97
E3. Anti-inflammatory
E3a. Cyclosporine A
Cyclosporine A (CSA), an anti-inflammatory immunosuppressant drug that is potentially neuroprotective, 58 resolves inflammatory T-cell infiltrates 58,163 and is believed to increase tear production by reducing ocular inflammation of dry eye disease. 58 CSA also promotes lymphocyte apoptosis and inhibits epithelial apoptosis. 58 CSA use in dry eye patients results in improved patient comfort and tear production and decreased pathologic staining in corneal epithelium. 58 Because the goal of treatment is similar in patients with neurotrophic keratitis and dry eye disease, (that is, improved comfort, reduced photophobia, and decreased corneal staining), it seems logical to use CSA to treat neurotrophic keratitis; however, in the neurotrophic keratitis setting, the direct effect of CSA on corneal nerves may be detrimental, retarding nerve fiber regeneration due to loss of pro-neural IL-6 signaling and IL-17+ γδ helper T cells, as well as decreased transcription factors [NFATC1 and MAPK14 (p38)] and neurotrophins (BDNF and NT 4/5). 58,163 A concomitant increase in c-fos and pro-neural GAP43 may explain why some neural regeneration still occurs with CSA treatment. 163
Ocular surface surgeries may sever corneal nerves and interrupt a neural feedback loop between the ocular surface and lacrimal glands. Therefore, to reduce the occurrence of dry eye symptoms after LASIK and cataract surgery, a preoperative and postoperative topical CSA regimen is recommended, particularly in patients who have pre-existing dry eyes or who are at risk of developing dry eye. 58 Although topical CSA use in the context of dry eye disease is beneficial, its benefits in the context of established neurotrophic keratitis are unknown. Until clinical data become available, the current data from experimental studies support limiting CSA use in neurotrophic keratitis.
E3b. Corticosteroids
The effects of corticosteroids on corneal nerves are mixed. Steroids are associated with ocular surface epithelial toxicity and delayed epithelial healing; 145 however, post-wounding, steroids may speed epithelial recovery and reduce pain by inhibiting prostaglandins and sympathetic nerve sprouting (identified in animal models), 33,125 improve corneal nerve health in diseases such as autoimmune neuropathy, and help reverse the neuropathic findings of abnormal nerve thickening and tortuosity. 117 Corticosteroids, however, suppress pro-neural NGF and inflammatory cytokines such as IL-6, reduce T cell-mediated inflammation, and inhibit sympathetic nerve sprouting in response to injury in both animal models and human cornea. 121,125 In patients with dry eye disease, corticosteroids result in epithelial healing and subjective improvement of dry eye symptoms, although measures of corneal nerve function like tear film breakup time and Schirmer tear tests do not significantly improve. 121 Corticosteroids exert their effects in a dose- or time-dependent manner, 145 which may explain the seemingly conflicting results in nerves following wounding.
Topical application of corticosteroid eye drops reduces corneal inflammation but can produce epithelial toxicity. Because corneal epithelial cells, as well as sensory nerve endings, undergo continuous turnover 84,85 and share molecular mechanisms of regeneration, delayed epithelial healing from steroid use may concomitantly cause delayed nerve regeneration. In animal studies, corticosteroids retard corneal epithelial regeneration, inhibit stromal healing, and even cause superficial epithelial necrosis. 145 Therefore, topical corticosteroid eye drop use should be minimized in neurotrophic keratitis.
E3c. Corneal analgesics
Several pharmacological mediators are used for corneal analgesia, including NSAIDs, resiniferatoxin (RTX), opioids, and dilute anesthetics. NSAIDs reduce pain associated with corneal injury, such as occurs after refractive surgery or inflammatory processes. 2 NSAIDs, particularly diclofenac, inhibit inflammatory mediators in a nociceptor-specific manner, resulting in decreased responsiveness of corneal Aδ and C polymodal nociceptors to pain, chemical, mechanical, and thermal stimuli. 2 NSAIDs reduce post-surgical hypersensitivity, but some debate exists regarding compromise of the neurotrophic signaling required for nerve growth. 27,77 Significant adverse effects have been reported, including corneal perforation or “melting.” 80 These harmful effects may be the result of inhibition of beneficial cyclooxygenase products or of analgesia that prevents a protective response to external traumatic forces. Therefore, the use of topical NSAIDs should be avoided in neurotrophic keratitis. Whereas NSAIDs rely on blocking inflammatory mediators, other analgesics target TRPV1, a signaling calcium-permeable channel highly expressed in nociceptive neurons. 17,159 RTX is a TRPV1 agonist. RTX binding leads to calcium influx and subsequent dose-dependent cytotoxicity, resulting in ablation of nociceptive neurons and nerve terminals to produce analgesia. 17 Morphine may also act by inhibiting TRPV1 and/or inhibiting high-voltage activated calcium channels. 239 Corneal nerves contain opioid receptors, 239 and opioid analgesics may induce a corneal reflex blockade. 218 Narcotics also exhibit a range of effects on corneal epithelium. In animal models, an endogenous opioid, called opioid growth factor (OGF), inhibits re-epithelialization following injury, while the OGF antagonist naltrexone enhances re-epithelialization. 1 While it may not have a negative effect on corneal wound healing, fentanyl’s analgesic capacity remains unproven. 239 Anesthetics have a proven analgesic capacity, yet are toxic at typical doses. Application of dilute anesthetics circumvents their toxicity and delivers adequate corneal analgesia. 15
F. EVIDENCE-BASED TREATMENT OF CORNEAL NEUROPATHIC PAIN
Neuropathic pain arises from damaged or dysfunctional sensory nerves. Patients with corneal neuropathic pain report dysesthesias perceived as ocular dryness or phantom pain. 20 Receptors on free nerve endings in the corneal epithelium are implicated in neuropathic pain. Neuropathic pain may be triggered by damage, inflammatory cells, or extracellular chemicals such as glutamate that, through signaling cascades, increase receptor excitability and/or receptor expression. 199 Corneal nerve injury or transection (such as LASIK) may impair nerve sensitivity and increase the pain threshold while formation of microneuromas and altered nerve excitability likely generate aberrant nerve impulses and neuropathic sensations in the eye. 22 Patients with corneal neuropathic pain complain of photophobia and eye discomfort without reduced tear production or surface staining that typically explain such symptoms. Although there is some understanding of the pathogenesis and management of neuropathic pain in the setting of peripheral nerve injury elsewhere, little is known in the context of corneal nerve injury. Therefore, current management of corneal neuropathic pain borrows from treatments available for other conditions such as trigeminal neuralgia.
If corneal neuropathic pain is refractory to topical treatments including contact lenses, then pharmacotherapy that is typically used for pain management may be considered. Treatments may include tricyclic antidepressants (TCAs) such as amitriptyline and the serotonin noradrenaline reuptake inhibitor (SNRI) duloxetine, or anticonvulsant GABA analogs such as gabapentin (Neurontin, Pfizer) and pregabalin (Lyrica, Pfizer). 12,71 The anticonvulsant carbamazepine may be another option, but its efficacy is offset by poor tolerability. 12,71 Opioids like tramadol can effectively treat neuropathic pain; however, opioids are not regarded as first-line therapy due to side effects of tolerance, cognitive impairment, and dependence. 12,71
Several other agents may have a beneficial effect on treating neuropathic pain; however there is insufficient clinical data supporting their use in routine clinical practice. These investigational agents can act by nociceptive block or ablation or by attenuating putative neuropathic signaling molecules and include the alpha2 adrenergic agonist brimonidine (Alphagan; Allergan), grape seed extract, minocycline, botulinum toxin, ceftriaxone, and RTX. Brimonidine is topically administered to reduce intraocular pressure. A model of trigeminal-derived neuropathic pain suggests that activation of alpha-2 adrenergic receptors via binding of an alpha-2 adrenergic agonist may inhibit nociceptive transmission. 208 Grape seed extract and minocycline have anti-hypernociceptive and pro-neural functions. Grape seed extract may suppress neuropathic sensitization of trigeminal-derived nerves by suppressing CGRP expression and upregulating the glutamate transporter GLAST. 37 The neuroprotective minocycline also has known antihypernociceptive functions, possibly via anti-inflammatory action. 16 Experimental models reviewed by Bastos et al. 16 show the effects of minocycline on neuropathic pain are inconsistently proven; however, a narrow therapeutic window exists for minocycline application to effectively treat hypernociceptive pathologic states. 16 Ceftriaxone upregulates the glutamate clearance transporter GLT1 in the nervous system, and the combination of minocycline with ceftriaxone potentiates the antihypernociceptive effects of each drug alone. 11 RTX binds TRPV1, a nociceptive receptor implicated in neuropathic pain. 22 In experimental animals, RTX provides dose-dependent analgesia and preserves the corneal epithelium, blink reflex, and sensation of non-TRPV1 neurons. 17
G. FUTURE DIRECTIONS
The role of neurons in the health, healing, scarring, and immunology of the cornea, as well as the responsible molecular and cellular mechanisms, have yet to be fully determined. Therefore, the molecular and cellular changes that occur in primary sensory neurons as a result of disease or trauma, including their regenerative mechanisms, constitute high priority needs, gaps, and opportunities in the field and require further study. Recent laboratory investigations have revealed additional corneal nerve modulators; for example, pigment epithelial-derived factor (PEDF) with DHA enhances regeneration of corneal nerves and return of corneal sensitivity following corneal nerve damage. 49 Technological advances are revealing the positive role of inflammation in corneal nerve regeneration. 163 The potential clinical impact is great, as visual impairment as the result of corneal nerve dysfunction adversely affects the qualify of life of millions worldwide. Continued laboratory and clinical investigations are needed to advance our understanding of corneal nerves in health and disease.
H. METHOD OF LITERATURE SEARCH
In preparing this review, we conducted a Medline and PubMed search of medical literature for the period between 1960 and 2013 using the following key words in various combinations: cornea, corneal nerves, nerve regeneration, neurotrophic keratitis, neurotrophic factors, platelet-rich plasma, autologous serum, umbilical cord serum, alphagan, contact lens, corticosteroids, and minocycline. Individual molecules, disease processes, and surgical procedures as discussed in the review were also used as search terms. In addition, reference lists from the selected articles were used to obtain further references not included in the electronic databases. Articles were critically appraised, and pertinent information was included in this review and cited accordingly.
Acknowledgments
We thank James Kubilus, Ph.D. (Tufts University) for contributing to the section on corneal nerve development and Nitin K. Garg (University of Illinois at Chicago) for contributing to the literature search in the section on corneal surgeries causing nerve dysfunction.
Supported by National Eye Institute (NEI) Grants K08EY018874 and R01EY023656 (SJ), NEI core grant EY001792, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abelson MB, Dewey-Mattia D, Shapiro A. Finding new uses for ancient drugs. [Accessed online Jan 2012.];Review of Ophthalmology. Online June 11, 2009. http://www.revophth.com/content/d/therapeutic_topics/i/1215/c/22882/
- 2.Acosta MC, Berenguer-Ruiz L, García-Gálvez A, Perea-Tortosa D, Gallar J, Belmonte C. Changes in mechanical, chemical, and thermal sensitivity of the cornea after topical application of nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci. 2005 Jan;46(1):282–6. doi: 10.1167/iovs.04-0884. [DOI] [PubMed] [Google Scholar]
- 3.Ahuja Y, Baratz KH, McLaren JW, Bourne WM, Patel SV. Decreased corneal sensitivity and abnormal corneal nerves in Fuchs Endothelial Dystrophy. Cornea. 2012;31(11):1257–63. doi: 10.1097/ICO.0b013e31823f7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiba M, Maeda N, Yumikake K, Soma T, Nishida K, Tano Y, Chan KP. Ultrahigh-resolution imaging of human donor cornea using full-field optical coherence tomography. J Biomed Opt. 2007 Jul-Aug;12(4):041202. doi: 10.1117/1.2764461. [DOI] [PubMed] [Google Scholar]
- 5.Al-Aqaba M, Alomar T, Lowe J, Dua HS. Corneal nerve aberrations in bullous keratopathy. Am J Ophthalmol. 2011;151(5):840–49.e1. doi: 10.1016/j.ajo.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–9. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 7.Al-Aqaba MA, Otri AM, Fares U, Miri A, Dua HS. Organization of the regenerated nerves in human corneal grafts. Am J Ophthalmol. 2012;153:29–37. doi: 10.1016/j.ajo.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Alio JL, Colecha JR, Pastor S, Rodriguez A, Artola A. Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res. 2007;39:124–9. doi: 10.1159/000100933. [DOI] [PubMed] [Google Scholar]
- 9.Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008 Apr;57(4):253–8. doi: 10.1016/j.phrs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Ophthalmology. [Accessed online Feb 2012.];Eye Health Statistics at a Glance. http://www.aao.org/newsroom/press_kit/upload/Eye_Stats_3-5-07.
- 11.Amin B, Hajhashemi V, Hosseinzadeh H, Abnous Kh. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neurosci. 2012;224:15–25. doi: 10.1016/j.neuroscience.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 13.Auran JD, Koester CJ, Kleiman NJ, Rapaport R, Bomann JS, Wirotsko BM, Florakis GJ, Koniarek JP. Scanning slit confocal microscopic observation of cell morphology and movement within the normal human anterior cornea. Ophthalmology. 1995;102:33–41. doi: 10.1016/s0161-6420(95)31057-3. [DOI] [PubMed] [Google Scholar]
- 14.Bahn RS. Graves’ Ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball IM, Seabrook J, Desai N, Allen L, Anderson S. Dilute proparacaine for the management of acute corneal injures in the emergency department. CJEM. 2010;12(5):389–96. doi: 10.1017/s1481803500012537. [DOI] [PubMed] [Google Scholar]
- 16.Bastos LF, de Oliveira AC, Watkins LR, Moraes MF, Coelho MM. Tetracyclines and pain. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(3):225–41. doi: 10.1007/s00210-012-0727-1. [DOI] [PubMed] [Google Scholar]
- 17.Bates BD, Mitchell K, Keller JM, Chan CC, Swaim WD, Yaskovich R, Mannes AJ, Iadarola MJ. Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain. 2010;149:522–8. doi: 10.1016/j.pain.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bee J. The development and pattern of innervation of the avian cornea. Dev Biol. 1982;92(1):5–15. doi: 10.1016/0012-1606(82)90145-2. [DOI] [PubMed] [Google Scholar]
- 19.Belmonte C. Corneal Nerves: Function. In: Dartt DA, Bex P, D’Amore P, et al., editors. Ocular Periphery and Disorders. Academic Press; San Diego, CA: 2011. p. 162. [Google Scholar]
- 20.Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007 Jun;23(6):598–602. doi: 10.3928/1081-597X-20070601-11. [DOI] [PubMed] [Google Scholar]
- 21.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004 Mar;78(3):513–25. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf. 2004 Oct;2(4):248–53. doi: 10.1016/s1542-0124(12)70112-x. [DOI] [PubMed] [Google Scholar]
- 23.Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Prog Ret Eye Res. 1997;16(1):117–56. [Google Scholar]
- 24.Benítez-del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, García-Sánchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007 Jan;48(1):173–81. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 25.Benítez-del-Castillo JM, del Rio T, Iradier T, Hernández JL, Castillo A, Garcia-Sanchez J. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20:30–32. doi: 10.1097/00003226-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24:577–83. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 27.Beuerman RW, McDonald MB, Zhang D, Varnell RJ, Thompson HW. Diclofenac sodium attenuates neural activity after photorefractive keratectomy in rabbits. J Refract Surg. 1996;12(7):783–91. doi: 10.3928/1081-597X-19961101-10. [DOI] [PubMed] [Google Scholar]
- 28.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- 29.Bochdalek V, et al. Über die Nerven der durchsichtigen Hornhaut and über einiges anderweitiges Verhalten der letzteren. Med Jb Oest Staates. 1839;29:185–94. 398–412. [Google Scholar]
- 30.Bock F, Onderka J, Rummelt C, Dietrich T, Bachmann B, Kruse FE, Schlötzer-Schrehardt U, Cursiefen C. Safety profile of topical VEGF neutralization at the cornea. Invest Ophthalmol Vis Sci. 2009;50(5):2095–102. doi: 10.1167/iovs.07-1129. [DOI] [PubMed] [Google Scholar]
- 31.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–51. doi: 10.1016/s0161-6420(00)00163-9. discussion 1351–2. [DOI] [PubMed] [Google Scholar]
- 33.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond) 2003 Nov;17(8):989–95. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 34.Bragheeth MA, Dua HS. Corneal sensation after myopic and hyperopic LASIK: clinical and confocal microscopic study. Br J Ophthalmol. 2005 May;89(5):580–5. doi: 10.1136/bjo.2004.046888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brookes NH, Loh IP, Clover GM, Poole CA, Sherwin T. Involvement of corneal nerves in the progression of keratoconus. Exp Eye Res. 2003;77:515–524. doi: 10.1016/s0014-4835(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 36.Brown SM, Lamberts DW, Reid TW, Nishida T, Murphy CJ. Neurotrophic and anhidrotic keratopathy treated with substance P and insulinlike growth factor 1. Arch Ophthalmol. 1997;115:926–7. doi: 10.1001/archopht.1997.01100160096021. [DOI] [PubMed] [Google Scholar]
- 37.Cady RJ, Hirst JJ, Durham PL. Dietary grape seed polyphenols repress neuron and glia activation in trigeminal ganglion and trigeminal nucleus caudalis. Mol Pain. 2010;6:91. doi: 10.1186/1744-8069-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calvillo MP, McLaren JW, Hodge DO, Bourne WM. Corneal reinnervation after LASIK: prospective 3-year longitudinal study. Invest Ophthalmol Vis Sci. 2004 Nov;45(11):3991–6. doi: 10.1167/iovs.04-0561. [DOI] [PubMed] [Google Scholar]
- 39.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T, Paradiso AL. Transepithelial corneal collagen crosslinking for keratoconus: qualitative investigation by in vivo HRT II confocal analysis. Eur J Ophthalmol. 2012;22(Suppl 7):S81–8. doi: 10.5301/ejo.5000125. [DOI] [PubMed] [Google Scholar]
- 40.Carrasco E, Blum M, Weickert CS, Casper D. Epidermal growth factor receptor expression is related to post-mitotic events in cerebellar development: regulation by thyroid hormone. Brain Res Dev Brain Res. 2003;140(1):1–13. doi: 10.1016/s0165-3806(02)00539-4. [DOI] [PubMed] [Google Scholar]
- 41.Ceccuzzi R, Zanardi A, Fiorentino A, Tinelli C, Bianchi PE. Corneal sensitivity in keratoconus after penetrating and deep anterior lamellar keratoplasty. Ophthalmologica. 2010;224(4):247–50. doi: 10.1159/000277615. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhary S, Namavari A, Yco L, Chang JH, Sonawane S, Khanolkar V, Sarkar J, Jain S. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea. 2012;31(12):1460–7. doi: 10.1097/ICO.0b013e318247b60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi JA, Chung SH. Combined application of autologous serum eye drops and silicone hydrogel lenses for the treatment of persistent epithelial defects. Eye & Contact Lens. 2011;37:370–3. doi: 10.1097/ICL.0b013e318233c9bb. [DOI] [PubMed] [Google Scholar]
- 44.Chuck RS, Quiros PA, Perez AC, McDonnell PJ. Corneal sensation after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:337–9. doi: 10.1016/s0886-3350(99)00416-2. [DOI] [PubMed] [Google Scholar]
- 45.Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25(8):953–9. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- 46.Conrad AH, Strafuss JM, Wittman MD, Conway S, Conrad GW. Thyroxine increases the rate but does not alter the pattern of innervation during embryonic chick corneal development. Invest Ophthalmol Vis Sci. 2008;(1):139–53. doi: 10.1167/iovs.07-0800. [DOI] [PubMed] [Google Scholar]
- 47.Conrad AH, Zhang Y, Walker AR, Olberding LA, Hanzlick A, Zimmer AJ, Morffi R, Conrad GW. Thyroxine affects expression of KSPG-related genes, the carbonic anhydrase II gene, and KS sulfation in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 2006;47(1):120–32. doi: 10.1167/iovs.05-0806. [DOI] [PubMed] [Google Scholar]
- 48.Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. Journ Neurosci. 2004;24(42):9250–60. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortina MS, He J, Li N, Bazan NG, Bazan HEP. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves, and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. 2012;130(1):76–83. doi: 10.1001/archophthalmol.2011.287. [DOI] [PubMed] [Google Scholar]
- 50.Cosar CB, Cohen EJ, Rapuano CJ, Maus M, Penne R, Flanagan JC, Laibson PR. Tarsorrhaphy: clinical experience from a cornea practice. Cornea. 2001;20(8):787–91. doi: 10.1097/00003226-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25(5–6):171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, Chodosh J, Pavan-Langston D, Dana R, Hamrah P. Inflammation and the nervous system: The connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52(8):5136–43. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darwish T, Brahma A, Efron N, O’Donnell C. Subbasal nerve regeneration after penetrating keratoplasty. Cornea. 2007 Sep;26(8):935–40. doi: 10.1097/ICO.0b013e3180de493f. [DOI] [PubMed] [Google Scholar]
- 54.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: prospective study. J Cataract Refract Surg. 2007 Sep;33(9):1515–21. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Davis PJ, Davis FB, Mousa SA. Thyroid hormone-induced angiogenesis. Curr Cardiol Rev. 2009;5(1):12–16. doi: 10.2174/157340309787048158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Castro F, Silos-Santiago I, De Armentia MI, Barbacid M, Belmonte C. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. European Journal of Neuroscience. 1998;10(1):146–52. doi: 10.1046/j.1460-9568.1998.00037.x. [DOI] [PubMed] [Google Scholar]
- 57.Donnenfeld ED, Ehrenhaus M, Solomon R, Mazurek J, Rozell JC, Perry HD. Effect of hinge width on corneal sensation and dry eye after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30(4):790–7. doi: 10.1016/j.jcrs.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 58.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–38. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Edén U, Riise R, Tornqvist K. Corneal involvement in congenital aniridia. Cornea. 2010;29(10):1096–1102. doi: 10.1097/ICO.0b013e3181d20493. [DOI] [PubMed] [Google Scholar]
- 60.Efron N. Putting vital stains in context. Clin Exp Optom. 2012 Oct 10; doi: 10.1111/j.1444-0938.2012.00802.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.Erie EA, McLaren JW, Kittleson KM, Patel SV, Erie JC, Bourne WM. Corneal subbasal nerve density: a comparison of two confocal microscopes. Eye Contact Lens. 2008 Nov;34(6):322–5. doi: 10.1097/ICL.0b013e31818b74f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erie JC. Corneal wound healing after photorefractive keratectomy: a 3-year confocal microscopy study. Transactions of the American Ophthalmological Society. 2003;101:293–333. [PMC free article] [PubMed] [Google Scholar]
- 63.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005 Dec;140(6):1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 64.Erie JC, McLaren JW, Hodge DO, Bourne WM. The effect of age on the corneal subbasal nerve plexus. Cornea. 2005 Aug;24(6):705–9. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]
- 65.Erie JC, McLaren JW, Patel SV. Confocal microscopy in ophthalmology. Am J Ophthalmol. 2009;148(5):639–46. doi: 10.1016/j.ajo.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 66.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–7. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 67.Fagerholm P, Lagali NS, Carlsson DJ, Merrett K, Griffith M. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clin Transl Sci. 2009;2:162–4. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan JC, Patel DV, McGhee CN. Long-term microstructural changes following epikeratophakia: in vivo confocal microscopy study. J Cataract Refract Surg. 2008 Oct;34(10):1793–8. doi: 10.1016/j.jcrs.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 69.Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157–65. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 70.Fernyhough P, Mill JF, Roberts JL, Ishii DN. Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Brain Res Mol Brain Res. 1989;6(2–3):109–20. doi: 10.1016/0169-328x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 71.Finnerup NB, Otto M, Jensen TS, Sindrup SH. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36. [PMC free article] [PubMed] [Google Scholar]
- 72.Foldenauer ME, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Substance P affects growth factors in Pseudomonas aeruginosa-infected mouse corneas. Cornea. 2012;31(10):1176–88. doi: 10.1097/ICO.0b013e31824d6ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, Brass LF. Deciphering the human platelet sheddome. Blood. 2010;117(1):e15–26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma. From basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 75.Fu Y, Liu J, Tseng SC. Ocular surface deficits contributing to persistent epithelial defect after penetrating keratoplasty. Cornea. 2012;31(7):723–9. doi: 10.1097/ICO.0b013e31821142ee. [DOI] [PubMed] [Google Scholar]
- 76.Fukiage C, Nakajima T, Takayama Y, Minagawa Y, Shearer TR, Azuma M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol. 2007 Feb;143(2):255–262. doi: 10.1016/j.ajo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 77.Gaynes BI, Onyekwuluje A. Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. Clin Ophthalmol. 2008;2(2):355–68. doi: 10.2147/opth.s1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghanem VC, Ghanem RC, de Oliveira R. Postoperative pain after corneal collagen cross-linking. Cornea. 2013;32(1):20–4. doi: 10.1097/ICO.0b013e31824d6fe3. [DOI] [PubMed] [Google Scholar]
- 79.Gil-Cazorla R, Teus MA, Arranz-Márquez E. Comparison of silicone and non-silicone hydrogen soft contact lenses used as a bandage after LASEK. J Refract Surg. 2008;24:199–203. doi: 10.3928/1081597X-20080201-12. [DOI] [PubMed] [Google Scholar]
- 80.Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108(5):936–44. doi: 10.1016/s0161-6420(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 81.Gulati A, Dana R. Ch. 28 Keratoconjuctivitis sicca: Clinical Aspects. In: Smolin G, Foster CS, Azar DT, Dohlman CH, editors. Smolin and Thoft’s The cornea: scientific foundations and clinical practice. 4. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. p. 607. [Google Scholar]
- 82.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282(36):26294–305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 83.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with Herpes Simplex Keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–6. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanna C, Bicknell DS, O’Brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695, 8. doi: 10.1001/archopht.1961.01840020697016. [DOI] [PubMed] [Google Scholar]
- 85.Harris LW, Purves D. Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci. 1989;9:2210–4. doi: 10.1523/JNEUROSCI.09-06-02210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He J, Bazan HE. Mapping the nerve architecture of diabetic human corneas. Ophthalmology. 2012;119(5):956–64. doi: 10.1016/j.ophtha.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91(4):513–23. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heigle TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15(2):135–8. doi: 10.1097/00003226-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Heinz C, Eckstein A, Steuhl KP, Meller D. Amniotic membrane transplantation for reconstruction of corneal ulcer in Graves ophthalmopathy. Cornea. 2004;23(5):524–6. doi: 10.1097/01.ico.0000114128.63670.e2. [DOI] [PubMed] [Google Scholar]
- 90.Herrmann WA, Shah C, Gabler B, Winkler von Mohrenfels C, Hufendiek K, Lohmann CP. Corneal sensation after laser epithelial keratomileusis for the correction of myopia. Graefes Arch Clin Exp Ophthalmol. 2005 Jan;243(1):33–7. doi: 10.1007/s00417-004-0974-z. [DOI] [PubMed] [Google Scholar]
- 91.Hillenaar T, van Cleynenbreugel H, Remeijer L. How Normal is the Transparent Cornea? Effects of Aging on Corneal Morphology. Ophthalmology. 2012;119(2):241–8. doi: 10.1016/j.ophtha.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 92.Hirata A, Masaki T, Motoyoshi K, Kamakura K. Intrathecal administration of nerve growth factor delays GAP 43 expression and early phase regeneration of adult rat peripheral nerve. Brain Res. 2002;19;944(1–2):146–56. doi: 10.1016/s0006-8993(02)02739-7. [DOI] [PubMed] [Google Scholar]
- 93.Hiura A, Nakagawa H. Innervation of TRPV1-, PGP-, and CGRP-immunoreactive fibers in the subepithelial layer of a whole mount preparation of the rat cornea. Okajimas Folia Anat Jpn. 2012;89(2):47–50. doi: 10.2535/ofaj.89.47. [DOI] [PubMed] [Google Scholar]
- 94.Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005 Oct 15–21;366(9494):1340–3. doi: 10.1016/S0140-6736(05)67546-0. [DOI] [PubMed] [Google Scholar]
- 95.Hu Y, Matsumoto Y, Adan ES, Dogru M, Fukagawa K, Tsubota K, Fujishima H. Corneal in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Ophthalmology. 2008;115(11):2004–12. doi: 10.1016/j.ophtha.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Huang JC, Sun CC, Chang CK, Ma DH, Lin YF. Effect of hinge position on corneal sensation and dry eye parameters after femtosecond laser-assisted LASIK. J Refract Surg. 2012;28(9):625–31. doi: 10.3928/1081597X-20120815-07. [DOI] [PubMed] [Google Scholar]
- 97.Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33(9):1644–6. doi: 10.1016/j.jcrs.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 98.Isenmann S, Klöcker N, Gravel C, Bähr M. Protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci. 1998;10(8):2751–56. doi: 10.1046/j.1460-9568.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 99.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44(5):2155–62. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 100.Jafarinasab MR, Zarei-Ghanavati S, Kanavi MR, Karimian F, Soroush MR, Javadi MA. Confocal microscopy in chronic and delayed mustard gas keratopathy. Cornea. 2010;29(8):889–94. doi: 10.1097/ICO.0b013e3181ca324c. [DOI] [PubMed] [Google Scholar]
- 101.Jalbert I, Papas E, Sweeney DF, Stapleton F. Instrument, diurnal, and day to day repeatability of in vivo measurements of the central human cornea using slit scanning confocal microscopy and modified optical pachometry. Invest Ophthalmol Vis Sci. 2002;43 E–Abstract 1713. [Google Scholar]
- 102.Jalbert I, Stapleton F, Papas E, Sweeney DF, Coroneo M. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225–36. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Javadi MA, Rezaei Kanavi M, Faramarzi A, Feizi S, Azizi F, Javadi F. Confocal scan imaging and impression cytology of the cornea in a case of multiple endocrine neoplasia type-2b. J Ophthalmic Vis Res. 2012;7(2):176–9. [PMC free article] [PubMed] [Google Scholar]
- 104.Jayasena CS, Flood WD, Koblar SA. High EphA3 expressing ophthalmic trigeminal sensory axons are sensitive to ephrin-A5-Fc: implications for lobe specific axon guidance. Neuroscience. 2005;135(1):97–109. doi: 10.1016/j.neuroscience.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 105.Jeng BH, Dupps WJ., Jr Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28(10):1104–8. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 106.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66(4):421–35. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 107.Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. Ger J Ophthalmol. 1996;5(6):508–12. [PubMed] [Google Scholar]
- 108.Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005 Aug;24(6):654–60. doi: 10.1097/01.ico.0000153102.19776.80. [DOI] [PubMed] [Google Scholar]
- 109.Kim EC, Lee WS, Kim MS. The inhibitory effects of bevacizumab eye drops on NGF expression and corneal wound healing in rats. Invest Ophthalmol Vis Sci. 2010;51(9):4569–73. doi: 10.1167/iovs.09-4937. [DOI] [PubMed] [Google Scholar]
- 110.Kim HC, Cho YJ, Ahn CW, Park KS, Kim JC, Nam JS, Im YS, Lee JE, Lee SC, Lee HK. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet Med. 2009;26(12):1228–34. doi: 10.1111/j.1464-5491.2009.02856.x. [DOI] [PubMed] [Google Scholar]
- 111.Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115(6):e33–8. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Tα1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17(24):9583–95. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kubilus JK, Linsenmayer TF. Developmental guidance of embryonic corneal innervation: roles of Semaphorin 3A and Slit2. Dev Biol. 2010;344(1):172–84. doi: 10.1016/j.ydbio.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumano Y, Matsui H, Zushi I, Mawatari A, Matsui T, Nishida T, Miyazaki M. Recovery of corneal sensation after myopic correction by laser in situ keratomileusis with a nasal or superior hinge. J Cataract Refract Surg. 2003;29:757–61. doi: 10.1016/s0886-3350(02)01840-0. [DOI] [PubMed] [Google Scholar]
- 115.Kumar RL, Koenig SB, Covert DJ. Corneal sensation after descemet stripping and automated endothelial keratoplasty. Cornea. 2010;29(1):13–8. doi: 10.1097/ICO.0b013e3181ac052b. [DOI] [PubMed] [Google Scholar]
- 116.Kurbanyan K, Hoesl LM, Schrems WA, Hamrah P. Corneal nerve alterations in acute Acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye. 2012;26:126–32. doi: 10.1038/eye.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lalive PH, Truffert A, Magistris MR, Landis T, Dosso A. Peripheral autoimmune neuropathy assessed using corneal in vivo confocal microscopy. Arch Neurol. 2009;66(3):403–5. doi: 10.1001/archneurol.2008.587. [DOI] [PubMed] [Google Scholar]
- 118.Lambiase A, Coassin M, Costa N, Lauretti P, Micera A, Ghinelli E, Aloe L, Rama P, Bonini S. Topical treatment with nerve growth factor in an animal model of herpetic keratitis. Graefes Arch Clin Exp Ophthalmol. 2008;246(1):121–7. doi: 10.1007/s00417-007-0593-6. [DOI] [PubMed] [Google Scholar]
- 119.Lambiase A, Merlo D, Mollinari C, Bonini P, Rinaldi AM, D’Amato M, Micera A, Coassin M, Rama P, Bonini S, Garaci E. Molecular basis for keratoconus: Lack of TrkA expression and its transcriptional repression by Sp3. PNAS. 2005;102(46):16795–800. doi: 10.1073/pnas.0508516102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–80. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 121.Lee HK, Ryu IH, Seo KY, Hong S, Kim HC, Kim EK. Topical 0. 1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113(2):198–205. doi: 10.1016/j.ophtha.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 122.Lee SJ, Kim JK, Seo KY, Kim EK, Lee HK. Comparison of corneal nerve regeneration and sensitivity between LASIK and laser epithelial keratomileusis (LASEK) Am J Ophthalmol. 2006 Jun;141(6):1009–1015. doi: 10.1016/j.ajo.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 123.Leiper LJ, Ou J, Walczysko P, Kucerova R, Lavery DN, West JD, Collinson JM. Control of patterns of corneal innervation by Pax6. Invest Ophthalmol Vis Sci. 2009;50(3):1122–8. doi: 10.1167/iovs.08-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lema I, Duran JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112(4):654–9. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 125.Li H, Xie W, Strong JA, Zhang JM. Systemic anti-inflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107(3):469–77. doi: 10.1097/01.anes.0000278907.37774.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li S, Nikulina K, DeVoss J, et al. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178(3):1106–16. doi: 10.1016/j.ajpath.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim MS, Nam SH, Kim SJ, Kang SY, Lee YS, Kang KS. Signaling pathways of the early differentiation of neural stem cells by neurotrophin-3. Biochem Biophys Res Com. 2007;357(4):903–9. doi: 10.1016/j.bbrc.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 129.Lindholm D, Heumann R, Hengerer B, Thoenen H. Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem. 1988;263:16348–51. [PubMed] [Google Scholar]
- 130.Ling JD, Gire A, Pflugfelder SC. PROSE therapy used to minimize corneal trauma in patients with corneal epithelial defects. Am J Ophthalmol. 2013;155(4):615–9. doi: 10.1016/j.ajo.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 131.Liu L, Hartwig D, Harloff S, Herminghaus P, Wedel T, Geerling G. An optimized protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):706–14. doi: 10.1007/s00417-004-1106-5. [DOI] [PubMed] [Google Scholar]
- 132.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;14;6(3):e17899. doi: 10.1371/journal.pone.0017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lwigale PY. Embryonic origin of avian corneal sensory nerves. Dev Biol. 2001;239(2):323–37. doi: 10.1006/dbio.2001.0450. [DOI] [PubMed] [Google Scholar]
- 134.Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007 Jun 15;306(2):750–9. doi: 10.1016/j.ydbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 135.Madduri S, Papaloïzos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65(1):88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 136.Mannion LS, Tromans C, O’Donnell C. Corneal nerve structure and function in keratoconus: a case report. Eye Contact Lens. 2007 Mar;33(2):106–8. doi: 10.1097/01.icl.0000235270.45379.9c. [DOI] [PubMed] [Google Scholar]
- 137.Marfurt CF. Corneal Nerves: Anatomy. In: Dartt DA, Bex P, D’Amore P, et al., editors. Ocular Periphery and Disorders. Academic Press; San Diego, CA: 2011. p. 150. [Google Scholar]
- 138.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–92. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 139.Martin RE, Bazan NG. Growth-associated protein GAP-43 and nerve cell adhesion molecule in sensory nerves of cornea. Exp Eye Res. 1992;55(2):307–14. doi: 10.1016/0014-4835(92)90195-x. [DOI] [PubMed] [Google Scholar]
- 140.Martin RE, Henken DB, Hill JM. Altered expression and changing distribution of the nerve growth associated protein GAP-43 during ocular HSV-1 infection in the rabbit. J Neurovirol. 1996;2(2):127–35. doi: 10.3109/13550289609146546. [DOI] [PubMed] [Google Scholar]
- 141.Martin XY, Safran AB. Corneal hypoesthesia. Surv Ophthalmol. 1988;33(1):28–40. doi: 10.1016/0039-6257(88)90070-7. [DOI] [PubMed] [Google Scholar]
- 142.Matsui H, Kumano Y, Zushi I, Yamada T, Matsui T, Nishida T. Corneal sensation after correction of myopia by photorefractive keratectomy and laser in situ keratomileusis. Journ Cat Refract Surg. 2001;27:370–3. doi: 10.1016/s0886-3350(00)00756-2. [DOI] [PubMed] [Google Scholar]
- 143.Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, Tsubota K. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004 Jun;111(6):1115–20. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 144.Mazzotta C, Traversi C, Baiocchi S, Caporossi O, Bovone C, Sparano MC, Balestrazzi A, Caporossi A. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008 Oct;146(4):527–533. doi: 10.1016/j.ajo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 145.McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 146.McKenna CC, Lwigale PY. Innvervation of the mouse cornea during development. Invest Ophthalmol Vis Sci. 2011;52(1):30–35. doi: 10.1167/iovs.10-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McKenna CC, Munjaal RP, Lwigale PY. Distinct roles for neuropilin1 and neuropilin2 during mouse corneal innervation. PLoS One. 2012;7(5):e37175. doi: 10.1371/journal.pone.0037175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melcangi RC, Giatti S, Pesaresi M, Calabrese D, Mitro N, Caruso D, Garcia-Segura LM. Role of neuroactive steroids in the peripheral nervous system. Front Endocrinol (Lausanne) 2011;2:104. doi: 10.3389/fendo.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meller D, Pauklin M, Thomasen H, Westekemper H, Steuhl KP. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108(14):243–8. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Michaeli A, Slomovic AR, Sakhichand K, Rootman DS. Effect of laser in situ keratomileusis on tear secretion and corneal sensitivity. J Refract Surg. 2004;20:379–83. doi: 10.3928/1081-597X-20040701-12. [DOI] [PubMed] [Google Scholar]
- 151.Mimura T, Amano S, Fukuoka S, Honda N, Arita R, Ochiai M, Yanagisawa M, Usui T, Ono K, Araki F, Yamagami S, Araie M, Awaya Y. In vivo confocal microscopy of hereditary sensory and autonomic neuropathy. Curr Eye Res. 2008 Nov;33(11):940–5. doi: 10.1080/02713680802450992. [DOI] [PubMed] [Google Scholar]
- 152.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1(9):761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 153.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006 Aug;25(7):769–73. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 154.Moilanen JA, Holopainen JM, Vesaluoma MH, Tervo TM. Corneal recovery after lasik for high myopia: a 2-year prospective confocal microscopic study. Br J Ophthalmol. 2008 Oct;92(10):1397–402. doi: 10.1136/bjo.2007.126821. [DOI] [PubMed] [Google Scholar]
- 155.Molnár I, Bokk A. Decreased nerve growth factor levels in hyperthyroid Graves’ ophthalmopathy highlighting the role of neuroprotective factor in autoimmune thyroid diseases. Cytokine. 2006;35(3–4):109–11. doi: 10.1016/j.cyto.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 156.Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp eye Research. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 157.Müller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Opthalmol Visc Sci. 1996;37(4):476–88. [PubMed] [Google Scholar]
- 158.Müller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–94. [PubMed] [Google Scholar]
- 159.Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085(1):87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 160.Murphy PJ, Patel S, Kong N, Ryder RE, Marshall J. Noninvasive assessment of corneal sensitivity in young and elderly diabetic and nondiabetic subjects. Invest Ophthalmol Vis Sci. 2004 Jun;45(6):1737–42. doi: 10.1167/iovs.03-0689. [DOI] [PubMed] [Google Scholar]
- 161.Nagasato D, Araki-Sasaki K, Kojima T, Ideta R, Dogru M. Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Japanese Journal of Ophthalmology. 2011;55(5):444–50. doi: 10.1007/s10384-011-0068-5. [DOI] [PubMed] [Google Scholar]
- 162.Nakamura M, Nishida T, Ofuji K, Reid TW, Mannis MJ, Murphy CJ. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp Eye Res. 1997;65(3):321–9. doi: 10.1006/exer.1997.0345. [DOI] [PubMed] [Google Scholar]
- 163.Namavari A, Chaudhary S, Chang JH, Yco L, Sonawane S, Khanolkar V, Yue BY, Sarkar J, Jain S. Cyclosporine immunomodulation retards regeneration of surgically transected corneal nerves. Invest Ophthalmol Vis Sci. 2012;53(2):732–40. doi: 10.1167/iovs.11-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Namavari A, Chaudhary S, Ozturk O, Chang JH, Yco L, Sonawane S, Katam N, Khanolkar V, Hallak J, Sarkar J, Jain S. Semaphorin 7a links nerve regeneration and inflammation in the cornea. Cornea. 2012;53(8):4575–85. doi: 10.1167/iovs.12-9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Namavari A, Chaudhary S, Sarkar J, Yco L, Patel K, Han KY, Yue BY, Chang JH, Jain S. In vivo serial imaging of regenerating corneal nerves after surgical transection in transgenic thy1-YFP mice. Invest Ophthalmol Vis Sci. 2011;52(11):8025–32. doi: 10.1167/iovs.11-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nassaralla BA, McLeod SD, Boteon JE, Nassaralla JJ., Jr The effect of hinge position and depth plate on the rate of recovery of corneal sensation following LASIK. Am J Ophthalmol. 2005;139:118–24. doi: 10.1016/j.ajo.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 167.Nassaralla BA, McLeod SD, Nassaralla J. Effect of myopic LASIK on human corneal sensitivity. Opthalmology. 2003;110:497–502. doi: 10.1016/s0161-6420(02)01897-3. [DOI] [PubMed] [Google Scholar]
- 168.Nejima R, Miyata K, Tanabe T, Okamoto F, Hiraoka T, Kiuchi T, Oshika T. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am Journ Ophthalmol. 2005;139:64–71. doi: 10.1016/j.ajo.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 169.Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007 Sep;91(9):1165–9. doi: 10.1136/bjo.2006.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Niederer RL, Perumal D, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest Ophthalmol Vis Sci. 2008 Jul;49(7):2964–70. doi: 10.1167/iovs.07-0968. [DOI] [PubMed] [Google Scholar]
- 171.Nishida T, Chikama T, Sawa M, Miyata K, Matsui T, Shigeta K. Differential contributions of impaired corneal sensitivity and reduced tear secretion to corneal epithelial disorders. Jpn J Ophthalmol. 2012;56(1):20–5. doi: 10.1007/s10384-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 172.Oble DA, Burton L, Maxwell K, Hassard T, Nathaniel EJH. A comparison of thyroxine- and polyamine-mediated enhancement of rat facial nerve regeneration. Exp Neurol. 2004;189(1):105–11. doi: 10.1016/j.expneurol.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 173.Okuno T, Nakatsuji Y, Kumanogoh A. The role of immune semaphorins in multiple sclerosis. FEBS Lett. 2011;585:3829–35. doi: 10.1016/j.febslet.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 174.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–84. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 175.Omoto M, Yoshida S, Miyashita H, Kawakita T, Yoshida K, Kishino A, Kimura T, Shibata S, Tsubota K, Okano H, Shimmura S. The semaphorin 3A inhibitor SM-345431 accelerates peripheral nerve regeneration and sensitivity in a murine corneal transplantation model. PLoS One. 2012;7(11):e47716. doi: 10.1371/journal.pone.0047716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pachigolla G, Prasher P, Di Pascuale MA, McCulley JP, McHenry JG, Mootha VV. Evaluation of the role of ProKera in the management of ocular surface and orbital disorders. Eye & Contact Lens. 2009;35(4):172–5. doi: 10.1097/ICL.0b013e3181a66a12. [DOI] [PubMed] [Google Scholar]
- 177.Pan S, Li L, Xu Z, Zhao J. Effect of leukemia inhibitory factor on corneal nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Letters. 2011;499:99–103. doi: 10.1016/j.neulet.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 178.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 179.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 180.Patel DV, McGhee CNJ. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–60. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 181.Patel DV, McGhee CN. Mapping of the normal human corneal subbasal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4485–8. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 182.Patel DV, McGhee CN. Mapping the corneal subbasal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2006;47(4):1348–51. doi: 10.1167/iovs.05-1217. [DOI] [PubMed] [Google Scholar]
- 183.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CJ. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28:735–40. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]
- 184.Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after laser in situ keratomileusis: femtosecond laser vs mechanical microkeratome. Arch Opthalmol. 2010;128:1413–19. doi: 10.1001/archophthalmol.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290(2):285–97. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- 186.Perez-Santonja JJ, Sakla HF, Cardona C, Chipont E, Alio JL. Corneal sensitivity after photorefractive keratectomy and laser in situ keratomieusis for low myopia. Am J Ophthalmol. 1999;127:497–504. doi: 10.1016/s0002-9394(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 187.Peyman GA, Rahimy MH, Fernandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. Br Journ Opthalmol. 1994;78:138–41. doi: 10.1136/bjo.78.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qi H, Chuang EY, Yoon KC, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, Li DQ. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007 Oct 16;13:1934–41. [PMC free article] [PubMed] [Google Scholar]
- 189.Ramaesh K, Ramaesh T, West JD, Dhillon B. Immunolocalisation of leukaemia inhibitory factor in the cornea. Eye. 2004;18:1006–9. doi: 10.1038/sj.eye.6701394. [DOI] [PubMed] [Google Scholar]
- 190.Rosen CF, Poon R, Drucker DJ. UVB radiation-activated genes induced by transcriptional and posttranscriptional mechanisms in rat keratinocytes. Am J Physiol. 1995;268:C846–55. doi: 10.1152/ajpcell.1995.268.4.C846. [DOI] [PubMed] [Google Scholar]
- 191.Rosenfeld SI. Evaluation and management of post-LASIK dry eye syndrome. Int Ophthalmol Clin. 2010;50(3):191–9. doi: 10.1097/IIO.0b013e3181e2469b. [DOI] [PubMed] [Google Scholar]
- 192.Roszkowska AM, Colosi P, Ferreri FM, Galasso S. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004 Sep-Oct;218(5):350–5. doi: 10.1159/000079478. [DOI] [PubMed] [Google Scholar]
- 193.Ruddle JB, Mackey DA, Downie NA. Clinical progression of keratoconus following a Vth nerve palsy. Clin Exp Ophthalmol. 2003;31:363–5. doi: 10.1046/j.1442-9071.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 194.Sakimoto T, Kim TI, Ellenberg D, Fukai N, Jain S, Azar DT, Chang JH. Collagen XVIII and corneal reinnervation following keratectomy. FEBS Lett. 2008 Oct 29;582(25–26):3674–80. doi: 10.1016/j.febslet.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Salomão MQ, Ambrósio R, Jr, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cat Refract Surg. 2009;35(10):1756–60. doi: 10.1016/j.jcrs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanjay S, Huang P, Lavanya R. Herpes zoster ophthalmicus. Curr Treat Options Neurol. 2011;13(1):79–91. doi: 10.1007/s11940-010-0098-1. [DOI] [PubMed] [Google Scholar]
- 197.Sarkar J, Chaudhary S, Namavari A, Ozturk O, Chang JH, Yco L, Sonawane S, Khanolkar V, Hallak J, Jain S. Corneal neurotoxicity due to topical benzalkonium chloride. Invest Ophthalmol Vis Sci. 2012;53(4):1792–1802. doi: 10.1167/iovs.11-8775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schlemm TFW. Nerven der Cornea. Ammon’ Z Ophthalmol. 1831;1:113–4. [Google Scholar]
- 199.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 200.Skene JH, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stachs O, Zhivov A, Kraak R, Stave J, Guthoff R. In vivo three-dimensional confocal laser scanning microscopy of the epithelial nerve structure in the human cornea. Graefes Arch Clin Exp Ophthalmol. 2007 Apr;245(4):569–75. doi: 10.1007/s00417-006-0387-2. [DOI] [PubMed] [Google Scholar]
- 202.Starkey ML, Davies M, Yip PK, Carter LM, Wong DJN, McMahon SB, Bradbury EJ. Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury. J Comp Neurol. 2009;513(1):51–68. doi: 10.1002/cne.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stern ME. Pathophysiology and allergy of the lacrimal functional unit: what goes wrong with our tear-secreting apparatus? Asian Journal of Ophthalmology. 2005;7(2):5–8. [Google Scholar]
- 204.Sterneck E, Kaplan DR, Johnson PF. Interleukin-6 induces expression of Peripherin and cooperates with Trk receptor signaling to promote neuronal differentiation in PC12 cells. J Neurochem. 1996;67(4):1365–74. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- 205.Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55(3):277–82. doi: 10.1007/s10384-011-0002-x. [DOI] [PubMed] [Google Scholar]
- 206.Takai S, Kinoshita S, Tanaka F, Ikeda M, Tanaka N, Kobayashi T. Prominent corneal nerves in patients with multiple endocrine neoplasia type 2A: diagnostic implications. World J Surg. 1992;16(4):620–3. doi: 10.1007/BF02067337. [DOI] [PubMed] [Google Scholar]
- 207.Takamatsu H, Kumanogh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33:127–35. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 208.Takeda M, Ikeda M, Tanimoto T, Lipski J, Matsumoto S. Changes of the excitability of rat trigeminal root ganglion neurons evoked by alpha(2)-adrenreceptors. Neuroscience. 2002;115(3):731–41. doi: 10.1016/s0306-4522(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 209.Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25(3):352–5. doi: 10.1097/01.ico.0000176609.42838.df. [DOI] [PubMed] [Google Scholar]
- 210.Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2(2):435–45. doi: 10.2147/opth.s1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tavakoli M, Marhsall A, Pitceathly R, Gow D, Roberts ME, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223(1):245–250. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Templeton A, Nguyen G, Ash JD, Straub RH, Carr DJ. Chemical sympathectomy increases susceptibility to ocular herpes simplex virus type 1 infection. J Neuroimmunol. 2008 Jun 15;197(1):37–46. doi: 10.1016/j.jneuroim.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tole S, Mukovozov IM, Huang YW, Magalhaes MA, Yan M, Crow MR, Liu GY, Sun CX, Durocher Y, Glogauer M, Robinson LA. The axonal repellent, Slit2, inhibits directional migration of circulating neutrophils. J Leukoc Biol. 2009;86(6):1403–15. doi: 10.1189/jlb.0609391. [DOI] [PubMed] [Google Scholar]
- 214.Tominaga M, Julius D. Capsaicin receptor in the pain pathway. Jpn J Pharmacol. 2000;83:20–4. doi: 10.1254/jjp.83.20. [DOI] [PubMed] [Google Scholar]
- 215.Tong L, Corrales RM, Chen Z, et al. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:1938–46. doi: 10.1167/iovs.05-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008 Jun;86(6):879–85. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 217.Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Invest Ophthalmol Vis Sci. 2012;53(2):867–72. doi: 10.1167/iovs.11-8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vanbever R, Langers G, Montmayeur S, Préat V. Transdermal delivery of fentanyl: rapid onset of analgesia using skin electroporation. Journal of Controlled Release. 1998;50:225–35. doi: 10.1016/s0168-3659(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 219.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007 May;48(5):2017–22. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 220.Villani E, Viola F, Sala R, Salvi M, Mapelli C, Currò N, Vannucchi I, Beck-Peccoz P, Ratiglia R. Corneal involvement in Graves’ orbitopathy: an in vivo confocal study. Invest Ophthalmol. 2010;51(9):4574–8. doi: 10.1167/iovs.10-5380. [DOI] [PubMed] [Google Scholar]
- 221.Villanueva O, Atkinson DS, Lambert SR. Trigeminal nerve hypoplasia and aplasia in children with goldenhar syndrome and corneal hypoesthesia. J AAPOS. 2005;9(2):202–4. doi: 10.1016/j.jaapos.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 222.Walker JS. Anti-inflammatory effects of opioids. Adv Exp Med Biol. 2003;521:148–60. [PubMed] [Google Scholar]
- 223.Walker P, Weischel ME, Jr, Fisher DA, Guo SM, Fisher DA. Thyroxine increases nerve growth factor concentration in adult mouse brain. Science. 1979;204(4391):427–9. doi: 10.1126/science.441732. [DOI] [PubMed] [Google Scholar]
- 224.Wang ZY, Alm P, Håkanson R. The contribution of nitric oxide to endotoxin-induced ocular inflammation: interaction with sensory nerve fibres. Br J Pharmacol. 1996;118(6):1537–43. doi: 10.1111/j.1476-5381.1996.tb15571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wells J, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126(7):1628–37. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 226.Wells JR, Michelson MA. EyeNet Magazine. the American Academy of Ophthalmology; Jul-Aug. 2008. [Accessed online Dec. 2011.]. Diagnosing and treating neurotrophic keratopathy. http://www.aao.org/publications/eyenet/200807/pearls.cfm. [Google Scholar]
- 227.Wilson SA, Last A. Management of corneal abrasions. Am Fam Physician. 2004;70(1):123–8. [PubMed] [Google Scholar]
- 228.Wu Y, Chu RY, Zhou XT, Dai JH, Qu XM, Rao S, Lam D. Recovery of corneal sensitivity after laser-assisted subepithelial keratectomy. Journ Cat Refract Surg. 2006;32:785–8. doi: 10.1016/j.jcrs.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 229.Xu G, Pierson CR, Murakawa Y, Sima AA. Altered tubulin and neurofilament expression and impaired axonal growth in diabetic nerve regeneration. J Neuropathol Exp Neurol. 2002;61(2):164–75. doi: 10.1093/jnen/61.2.164. [DOI] [PubMed] [Google Scholar]
- 230.Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, Ishimitsu T, Kamiya A. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92(7):896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]
- 231.Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144(1):86–92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 232.Yoon KC, You IC, Im SK, Jeong TS, Park YG, Choi J. Application of umbilical cord serum eye drops for the treatment of neurotrophic keratitis. Ophthalmology. 2007 Sep;114(9):1637–42. doi: 10.1016/j.ophtha.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 233.You L, Ebner S, Kruse FE. Glial cell-derived neurotrophic factor (GDNF)-induced migration and signal transduction in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2001;4:2496–2504. [PubMed] [Google Scholar]
- 234.You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 235.Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48(4):1535–42. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]
- 236.Yu CQ, Zhang M, Matis KI, Kim C, Rosenblatt MI. Vascular endothelial growth factor mediates corneal nerve repair. Invest Ophthalmol Vis Sci. 2008;49(9):3870–8. doi: 10.1167/iovs.07-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhao C, Lu S, Tajouri N, Dosso A, Safran AB. In vivo confocal laser scanning microscopy of corneal nerves in leprosy. Arch Ophthalmol. 2008 Feb;126(2):282–4. doi: 10.1001/archophthalmol.2007.67. [DOI] [PubMed] [Google Scholar]
- 238.Zhong J, Dietzel ID, Wahle P, Kopf M, Heumann R. Sensory impairments and delayed regeneration of sensory axons in interleukin-6-deficient mice. J Neurosci. 1999;19:4305–13. doi: 10.1523/JNEUROSCI.19-11-04305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zöllner C, Mousa S, Klinger A, Förster M, Schäfer M. Topical fentanyl in a randomized, double-blind study in patients with corneal damage. Clin J Pain. 2008;24(8):690–6. doi: 10.1097/AJP.0b013e318175929e. [DOI] [PubMed] [Google Scholar]


