Abstract
Wild-type transcriptional activation by Gcn4p is dependent on multiple coactivators, including SAGA, SWI/SNF, Srb mediator, CCR4-NOT, and RSC, which are all recruited by Gcn4p to its target promoters in vivo. It was not known whether these coactivators are required for assembly of the preinitiation complex (PIC) or for subsequent steps in the initiation or elongation phase of transcription. We find that mutations in subunits of these coactivators reduce the recruitment of TATA binding protein (TBP) and RNA polymerase II (Pol II) by Gcn4p at ARG1, ARG4, and SNZ1, implicating all five coactivators in PIC assembly at Gcn4p target genes. Recruitment of Pol II at SNZ1 and ARG1 was eliminated by mutations in TBP or by deletion of the TATA box, indicating that TBP binding is a prerequisite for Pol II recruitment by Gcn4p. However, several mutations in SAGA subunits and deletion of SRB10 had a greater impact on promoter occupancy of Pol II versus TBP, suggesting that SAGA and Srb mediator can promote Pol II binding independently of their stimulatory effects on TBP recruitment. Our results reveal an unexpected complexity in the cofactor requirements for the enhancement of PIC assembly by a single activator protein.
Transcription by RNA polymerase II (Pol II) is dependent on a set of general transcription factors (GTFs), including the TATA binding protein (TBP) and TFIIB, that recognize the core promoter and catalyze transcription initiation from the correct start site. The stimulation of transcription by activator proteins is dependent on coactivator complexes, several of which function as adapters to facilitate the recruitment of Pol II or GTFs by activators bound to their target sites in the promoter. Other coactivators stimulate preinitiation complex (PIC) assembly by remodeling the nucleosome structure of the promoter DNA (38). Coactivators may also be required to stimulate postrecruitment steps of the initiation process, such as promoter clearance, or for optimal rates of transcription elongation once Pol II leaves the promoter (25, 55, 62).
The recruitment of TBP is tightly associated with transcriptional activation of genes in yeast (34, 41). The TFIID complex is an important coactivator for recruitment of TBP to a subset of yeast genes that lack canonical TATA sequences (21, 33, 42, 47). The SAGA complex, containing the histone acetyltransferase (HAT) Gcn5p and several TBP-associated factors (TAFs) shared with TFIID, is recruited by activators in vivo and stimulates TBP recruitment at numerous TATA-containing yeast genes (5, 35, 36). TBP recruitment at such genes is also facilitated by the Srb mediator (34, 41, 42, 47).
Srb mediator is found associated with Pol II in a holoenzyme that also contains TFIIB and TFIIF (23, 29, 39, 44). In vitro, this coactivator enhances basal transcription and promotes phosphorylation of the C-terminal domain of the largest Pol II subunit by TFIIH, a reaction involved in promoter clearance (reviewed in reference 50). The mediator also supports the stimulation of Pol II function by activators in vitro, at least partly by enhancing recruitment of Pol II to the promoter (57). In vivo, it was shown that Srb mediator is recruited by activators (6, 53, 55, 63), in some cases independently of Pol II (6, 54), but there is little evidence that mediator directly promotes Pol II recruitment in living yeast cells. On the other hand, it appears to do so indirectly by stimulating TBP recruitment (34, 41, 42, 47), in accordance with in vitro data indicating that TBP binding is a prerequisite for Pol II recruitment (24, 57).
We have been analyzing the coactivator requirements for Gcn4p, a transcriptional activator of amino acid biosynthetic genes in yeast (52) that is induced at the translational level by starvation for any amino acid (26). Gcn4p activity is dependent on functionally redundant clusters of bulky hydrophobic residues in its activation domain (15, 27) that are required for specific interactions of Gcn4p with various coactivators in vitro. These include Srb mediator, SAGA, the ATP-dependent chromatin remodeling complexes SWI/SNF and RSC, and the CCR4-NOT complex (16, 51, 63, 67). By chromatin immunoprecipitation (ChIP) analysis, we and others have shown that all five of these coactivators, plus the Paf1 complex, can be recruited by Gcn4p to one or more of its target genes in living cells (31, 32, 63, 64). Furthermore, mutations have been identified in various subunits of these coactivators that confer sensitivity to inhibitors of amino acid biosynthetic enzymes (Gcn− phenotype) and reduce transcriptional activation of one or more Gcn4p-dependent target genes or reporters (see reference 63 and references therein).
While it was clear from the previous work that Gcn4p requires multiple coactivators for efficient induction of its target genes, little was known about which steps in the transcription process are stimulated by each coactivator. It was possible, for example, that only SAGA and Srb mediator would be required for recruitment of TBP and Pol II at the stage of promoter recognition and PIC assembly, whereas the other coactivators would be required for the postassembly steps of promoter melting, promoter clearance, or transcription elongation. Using ChIP analysis, we have investigated which coactivators are required for optimal recruitment of TBP and Pol II by Gcn4p. Surprisingly, we found that SAGA, Srb mediator, SWI/SNF, RSC, and CCR4-NOT are all necessary for optimal recruitment of TBP and Pol II by promoter-bound Gcn4p at three different target genes. By analyzing mutations in TBP or the TATA element, we further demonstrate that TBP binding is a prerequisite for recruitment of Pol II to the promoter by Gcn4p. However, it appears that SAGA and Srb mediator can also promote Pol II recruitment independently of their stimulatory effects on TBP recruitment. Our results establish that a multiplicity of cofactors and the TATA element are required in vivo for optimal stimulation of PIC assembly by Gcn4p.
MATERIALS AND METHODS
Yeast strains and plasmids.
All strains and plasmids used in this study are listed in Tables 1 and 2. The wild-type (WT) parent strain (BY4741 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and deletion derivatives thereof, generated by the Saccharomyces Genome Deletion Project, were purchased from Research Genetics. The presence of all reported deletion alleles was confirmed by PCR amplification or phenotype complementation by plasmid-bearing WT genes as described previously (63). Strains carrying gcn4Δ::hisG were created by transformation with plasmid pHQ1240 (68). TBP1 and RPB3 were tagged at the 3′ ends with the myc13-HIS3 cassette in pFA6a-13Myc-HIS3 (45), and the insertions were confirmed as described previously (63). Strains HQY647 (rsc1Δ gcn4Δ) and HQY648 (rsc2Δ gcn4Δ) were created by transforming strain 249 (gcn4Δ::kanMX4) with plasmids pHQ1333 and pHQ1334 bearing rsc1Δ::hisG::URA3::hisG and rsc2Δ::hisG::URA3::hisG, respectively, and the deletions in the resulting strains were confirmed by PCR amplification. Strains HQY692 (TBP1-myc ΔTATAARG1) and HQY693 (RPB3-myc ΔTATAARG1) were constructed as follows. Strains HQY366 (TBP1-myc) and HQY403 (RPB3-myc) were transformed to Ura+ with the integrative plasmid pHQ1344 bearing the ΔTATAARG1 mutation. The resulting transformants were grown on medium containing 5-fluoroorotic acid (5-FOA) to evict the wild-type copy of ARG1 together with the URA3 plasmid. The presence of the ΔTATAARG1 mutation was confirmed by PCR analysis of total genomic DNA and was shown to confer an Arg− phenotype. Strains BYΔ2, BYΔ2 ts-1 (a strain carrying a temperature-sensitive mutation [Ts−]) and BYΔ2 ts-2 were kindly provided by Kevin Struhl.
TABLE 1.
Yeast strains used in this study
| Strain | Parent | Relevant genotypec | Source or reference |
|---|---|---|---|
| TBP1-myc | |||
| HQY366 | BY4741a | TBP1-myc13::HIS3* | This work |
| HQY382 | HQY366b | TBP1-myc13::HIS3* gcn4Δ::hisG | This work |
| HQY473 | 1799a | TBP1-myc13::HIS3* ahc1Δ::kanMX4 | This work |
| HQY583 | 4282a | TBP1-myc13::HIS3* ada2Δ::kanMX4 | This work |
| HQY584 | 3534a | TBP1-myc13::HIS3* ada3Δ::kanMX4 | This work |
| HQY406 | 7285a | TBP1-myc13::HIS3* gcn5Δ::kanMX4 | This work |
| HQY426 | 1038a | TBP1-myc13::HIS3* ada1Δ::kanMX4 | This work |
| HQY407 | 7309a | TBP1-myc13::HIS3* ada5Δ::kanMX4 | This work |
| HQY533 | 3218a | TBP1-myc13::HIS3* spt7Δ::kanMX4 | This work |
| HQY427 | 4228a | TBP1-myc13::HIS3* spt3Δ::kanMX4 | This work |
| HQY530 | 2666a | TBP1-myc13::HIS3* spt8Δ::kanMX4 | This work |
| HQY424 | 1742a | TBP1-myc13::HIS3* gall1Δ::kanMX4 | This work |
| HQY405 | 4393a | TBP1-myc13::HIS3* pgd1Δ::kanMX4 | This work |
| HQY425 | LSO2b | TBP1-myc13::HIS3* med2Δ::kanMX4 | This work |
| HQY594 | 6611a | TBP1-myc13::HIS3* srb2Δ::kanMX4 | This work |
| HQY431 | 4734a | TBP1-myc13::HIS3* srb5Δ::kanMX4 | This work |
| HQY534 | 3119a | TBP1-myc13::HIS3* rox3Δ::kanMX4 | This work |
| HQY423 | 2786a | TBP1-myc13::HIS3* srb10Δ::kanMX4 | This work |
| HQY408 | 1586a | TBP1-myc13::HIS3* swi2Δ::kanMX4 | This work |
| HQY410 | 7123a | TBP1-myc13::HIS3* caf1Δ::kanMX4 | This work |
| HQY369 | 3858a | TBP1-myc13::HIS3* dhh1Δ::kanMX4 | This work |
| HQY373 | 387a | TBP1-myc13::HIS3* ccr4Δ::kanMX4 | This work |
| HQY429 | 4686a | TBP1-myc13::HIS3* rsc1Δ::kanMX4 | This work |
| HQY430 | 5266a | TBP1-myc13::HIS3* rsc2Δ::kanMX4 | This work |
| HQY692 | HQY366b | TBP1-myc13::HIS3* ΔTATAARGI | This work |
| RPB3-myc | |||
| HQY403 | BY4741a | RPB3-myc13::HIS3* | This work |
| HQY422 | HQY403b | RPB3-myc13::HIS3* gcn4Δ::hisG | This work |
| HQY560 | 1799a | RPB3-myc13::HIS3* ahc1Δ::kanMX4 | This work |
| HQY589 | 4282a | RPB3-myc13::HIS3* ada2Δ::kanMX4 | This work |
| HQY590 | 3534a | RPB3-myc13::HIS3* ada3Δ::kanMX4 | This work |
| HQY445 | 7285a | RPB3-myc13::HIS3* gcn5Δ::kanMX4 | This work |
| HQY432 | 1038a | RPB3-myc13::HIS3* ada1Δ::kanMX4 | This work |
| HQY414 | 7309a | RPB3-myc13::HIS3* ada5Δ::kanMX4 | This work |
| HQY535 | 3218a | RPB3-myc13::HIS3* spt7Δ::kanMX4 | This work |
| HQY433 | 4228a | RPB3-myc13::HIS3* spt3Δ::kanMX4 | This work |
| HQY531 | 2666a | RPB3-myc13::HIS3* spt8Δ::kanMX4 | This work |
| HQY412 | 1742a | RPB3-myc13::HIS3* gal11Δ::kanMX4 | This work |
| HQY413 | 4393a | RPB3-myc13::HIS3* pgd1Δ::kanMX4 | This work |
| HQY421 | LSO2b | RPB3-myc13::HIS3* med2Δ::kanMX4 | This work |
| HQY596 | 6611a | RPB3-myc13::HIS3* srb2Δ::kanMX4 | This work |
| HQY559 | 4734a | RPB3-myc13::HIS3* srb5Δ::kanMX4 | This work |
| HQY574 | 3119a | RPB3-myc13::HIS3* rox3Δ::kanMX4 | This work |
| HQY411 | 2786a | RPB3-myc13::HIS3* srb10Δ::kanMX4 | This work |
| HQY415 | 1586a | RPB3-myc13::HIS3* swi2Δ::kanMX4 | This work |
| HQY437 | 7123a | RPB3-myc13::HIS3* caf1Δ::kanMX4 | This work |
| HQY575 | 3858a | RPB3-myc13::HIS3* dhh1Δ::kanMX4 | This work |
| HQY444 | 387a | RPB3-myc13::HIS3* ccr4Δ::kanMX4 | This work |
| HQY417 | 4686a | RPB3-myc13::HIS3* rsc1Δ::kanMX4 | This work |
| HQY558 | 5266a | RPB3-myc13::HIS3* rsc2Δ::kanMX4 | This work |
| HQY651 | BYΔ2 | RPB3-myc13::URA3 gcn4Δ::hisG TBP1 | This work |
| HQY641 | BYΔ2 | RPB3-myc13::URA3 TBP1 | This work |
| HQY642 | BYΔ2 ts-1 | RPB3-myc13::URA3 tbp1-ts1(T111I) | This work |
| HQY643 | BYΔ2 ts-2 | RPB3-myc13::URA3 tbp1-ts2(S136N) | This work |
| HQY693 | HQY403b | RPB3-myc13::HIS3* ΔTATAARG1 | This work |
| gcn4Δ | |||
| 249a | BY4741a | gcn4Δ::kanMX4 | Research Genetics |
| HQY577 | 1742a | gcn4Δ::hisG gal11Δ::kanMX4 | This work |
| HQY513 | 4393a | gcn4Δ::hisG pgd1Δ::kanMX4 | This work |
| HQY591 | LSO2b | gcn4Δ::hisG med2Δ::kanMX4 | This work |
| HQY522 | 4734a | gcn4Δ::hisG srb5Δ::kanMX4 | This work |
| HQY592 | 3119a | gcn4Δ::hisG rox3Δ::kanMX4 | This work |
| HQY523 | 1038a | gcn4Δ::hisG ada1Δ::kanMX4 | This work |
| HQY516 | 7309a | gcn4Δ::hisG ada5Δ::kanMX4 | This work |
| HQY578 | 3218a | gcn4Δ::hisG spt7Δ::kanMX4 | This work |
| HQY515 | 7285a | gcn4Δ::hisG gcn5Δ::kanMX4 | This work |
| HQY514 | 1586a | gcn4Δ::hisG swi2Δ::kanMX4 | This work |
| HQY532 | 387a | gcn4Δ::hisG ccr4Δ::kanMX4 | This work |
| HQY647 | 249a | gcn4Δ::kanMX4 rsc1Δ::hisG | This work |
| HQY648 | 249a | gcn4Δ::kanMX4 rsc2Δ::hisG | This work |
| HQY644 | BYΔ2 | gcn4Δ::hisG | This work |
| BYΔ2 | TBP1 | 12 | |
| BYΔ2 ts-1 | BYΔ2 | tbp1-ts1(T111I) | 13 |
| BYΔ2 ts-2 | BYΔ2 | tbp1-ts2(S136N) | 13 |
Strains are purchased from Research Genetics.
Strains are isogenic to the Research Genetics’ strain.
HIS3* designates the HIS3 allele from S. kluyveri (45).
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| YEplac195 | Vector | 20 |
| pSK1 | GCN4-myc in YCp50 | 63 |
| pHQ1240 | gcn4Δ::hisG::URA3::hisG in pUC18 | 68 |
| pHQ1303 | GCN4 in YEplac195 | 68 |
| pHQ1304 | gcn4-14Ala in YEplac195 | 68 |
| pHQ1333 | rsc1Δ::hisG::URA3::hisG in pUC18 | This work |
| pHQ1334 | rsc2Δ::hisG::URA3::hisG in pUC18 | This work |
| pHQ1344 | arg1-ΔTATA in YIplac211 | This work |
Plasmids pHQ1333 and pHQ1334 were created as follows: the 5′ and 3′ noncoding regions (∼300 to 400 bp) of RSC1 and RSC2 were synthesized by PCR using genomic DNA of strain BY4741 as template, adding EcoRI, BamHI, or XbaI sites at the ends of PCR products, and inserted into pUC18 to leave a unique BamHI site between the 5′ and 3′ fragments of each gene, producing pHQ1329 and pHQ1330, respectively. The 3.8-kb BamHI fragment containing hisG::URA3::hisG from pNKY51 (2) was then inserted at the BamHI site to create plasmids pHQ1333 and pHQ1334, respectively. Plasmids pHQ1303 and pHQ1304 were described previously (68), as was pSK1 (63). To construct pHQ1344, the EcoRI-SphI fragment of wild-type ARG1 from M13mp7-ARG1 (a gift from Marjolaine Crabeel) was cloned into YIplac211 (20) to produce plasmid pHQ1342. The PstI-ScaI fragment (−438 to −152) and the SmaI-Asp718 fragment (−144 to +170) from WT ARG1 were amplified by PCR and inserted into pUC18, joining the ScaI and SmaI ends of the fragments to delete the TATA sequence (TATATTAA) in plasmid pHQ1343. The PstI-Asp718 fragment of pHQ1342 was replaced with that of pHQ1343 to produce pHQ1344.
Biochemical methods.
The ChIP experiments were conducted as described previously (63). A primer pair (5′ TAATCTGAGCAGTTGCGAGA 3′ at −197 to −178 and 5′ ATGTTCCTTATCGCTGCACA 3′ at −51 to −70, relative to the ATG codon) was used to amplify sequences −197 to −51 of the ARG1 promoter (ARG1TATA). The primers used to amplify nucleotides −235 to −62 at the ARG4 promoter were 5′ GTTCTTGTGGTGGTTACTCA 3′ (−235 to −216) and 5′ CCCTAGCTAAAGAAAGGTAG 3′ (−62 to −81). The primers used to amplify the POL1ORF and the SNZ1 promoter were described previously (63, 68).
Northern analysis was carried out as described previously (56) with the following modifications. ULTRAhyb ultrasensitive hybridization buffer (Ambion) was used for prehybridization and hybridization as described by the vendor except for the washing conditions. After hybridization, the membranes were washed once with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate (SDS) at room temperature, once with the same buffer at 55°C, and once with 1× SSC-0.1% SDS buffer at 55°C for 15 min each and then washed twice with 0.1× SSC-0.1% SDS at 55°C for 20 min each. The washed membranes were subjected to phosphorimaging analysis for quantification of signals and also autoradiography. For Western analysis, whole-cell extracts (WCEs) were prepared as described previously (58) and analyzed by using polyclonal anti-TBP antibodies (gift from Joe Reese) and anti-Gcd6p antibodies (10).
RESULTS
Gcn4p recruits TBP and Pol II to target promoters in vivo through its activation domain.
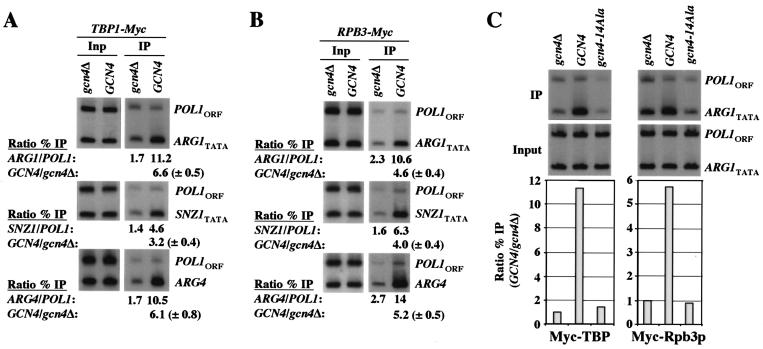
To determine whether binding of Gcn4p to the promoters of its target genes leads to increased binding of TBP and Pol II, we conducted ChIP analysis on isogenic GCN4 and gcn4Δ strains containing TBP or Rpb3p (a subunit of Pol II) tagged at their C termini with myc epitopes. We found that myc tagging of TBP or Rpb3p had little or no effect on cell growth in medium containing sulfometuron-methyl (SM), an inhibitor of isoleucine/valine biosynthesis used to induce Gcn4p synthesis. After inducing with SM for 2 h, cells were treated with formaldehyde and processed for ChIP analysis to measure the binding of myc-TBP and myc-Rpb3p to the promoters of the Gcn4p target genes ARG1, SNZ1, and ARG4 (52). The results in Fig. 1A show that specific binding of myc-TBP to these promoters was between 3.2-fold (SNZ1) and 6.6-fold (ARG1) higher in the WT than in the gcn4Δ strain (see GCN4/gcn4Δ ratios in Fig. 1A). Similar results were obtained for the strains containing myc-Rpb3p (Fig. 1B), where the levels of binding in the gcn4Δ strain were 20 to 25% of those seen in the GCN4 strain at all three genes. Thus, recruitment by Gcn4p accounts for the majority of myc-TBP and myc-Rpb3p associated with these genes under SM-inducing conditions.
FIG. 1.
Gcn4p recruits TBP and Rpb3p to ARG1, SNZ1, and ARG4, dependent on hydrophobic residues in the activation domain. Strains HQY382 (gcn4Δ) and HQY366 (GCN4) in panel A, HQY422 (gcn4Δ) and HQY403 (GCN4) in panel B, and HQY382 and HQY422 carrying empty vector (gcn4Δ), pHQ1303 (GCN4), or pHQ1304 (gcn4-14Ala) in panel C were grown to optical densities at 600 nm (OD600) of 0.8 to 1.0 in SC-Ilv medium at 30°C and treated with SM at 0.6 μg/ml for 2 h. Cells were harvested, treated with formaldehyde, and lysed with glass beads, and the extracts were sonicated to produce chromatin fragments of ∼500 bp. Aliquots (5% of total) were immunoprecipitated with myc antibodies, and DNA was extracted from the immunoprecipitate (IP) after reversing the cross-links. DNA was extracted directly from another aliquot of chromatin (5% of the total) to serve as the input (Inp) control. A 1,000-fold dilution of the Inp and the undiluted IP samples were PCR amplified using primers specific for the POL1ORF, the ARG1TATA, the SNZ1TATA, or ARG4 promoter (UAS and TATA sequence), in the presence of [33P]dATP. The PCR products were resolved by polyacrylamide gel electrophoresis (PAGE), quantified by phosphorimaging, and visualized by autoradiography. The ratios of the ARG1TATA, SNZ1TATA, or ARG4 promoter signals to the POL1 signals in the IP were calculated and normalized for the corresponding ratios for the input samples from >9 independent measurements to produce the average ratio percent IP (ARG1/POL1) values listed immediately below the IP autoradiograms in panels A and B. The average ratios of the percent IP (ARG1/POL1) values in the GCN4 versus gcn4Δ strains were calculated and are listed on the second line below the IP autoradiograms in panels A and B, with standard errors given in parentheses. The corresponding ratio percent IP (GCN4/gcn4Δ) values are plotted in the histogram in panel C.
To examine whether recruitment of TBP and Pol II is dependent on the critical residues in the Gcn4p activation domain, we conducted ChIP experiments using TBP1-myc gcn4Δ and RPB3-myc gcn4Δ strains harboring multicopy plasmids bearing GCN4 or gcn4-14Ala. The latter contains 14 alanine substitutions (14Ala), which inactivate all 7 hydrophobic clusters in the activation domain (27). Both myc-TBP and myc-Rpb3p showed high-level binding to the ARG1 promoter in the GCN4 strain but no significant promoter binding in the gcn4-14Ala strain (Fig. 1C). We showed recently that these 14 Ala substitutions do not impair binding of Gcn4p itself to ARG1 (68). Thus, the hydrophobic clusters in the activation domain are required for recruitment of TBP and Pol II by promoter-bound Gcn4p.
Gcn4p requires an array of coactivators for optimal recruitment of TBP and RNA Pol II in vivo.
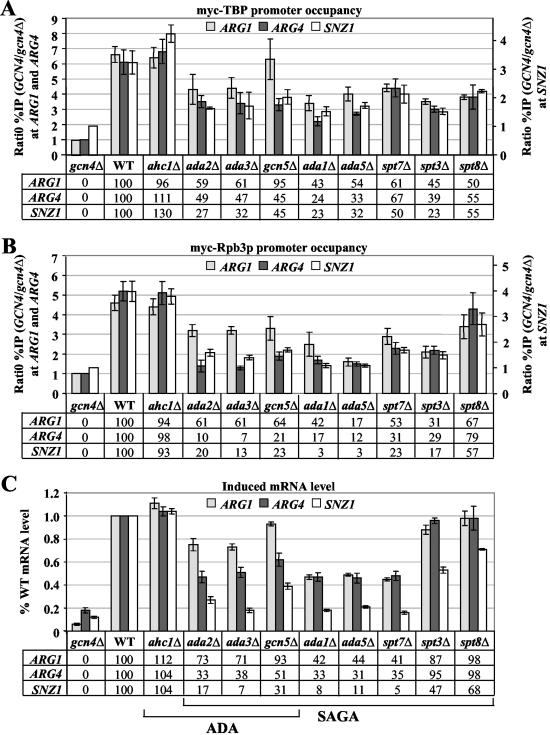
Gcn4p is dependent on the coactivators Srb mediator, SWI/SNF, SAGA, RSC, and CCR4-NOT for WT transcription of one or more target genes in vivo (see reference 63 and references therein). To determine which of these coactivators is necessary for recruitment of TBP and Pol II by Gcn4p, we conducted ChIP analysis on GCN4 strains containing myc-TBP or myc-Rpb3p and lacking different nonessential subunits of these coactivators following induction of Gcn4p with SM. The majority of the subunit deletions examined below were shown previously to impair transcriptional activation by Gcn4p at one or more target genes in vivo (63). Based on the results of multiple experiments with independent cultures, we calculated the amounts of myc-TBP and myc-Rpb3p binding to the ARG1, ARG4, and SNZ1 promoters for each mutant and plotted the results in the histograms shown in panels A and B of Fig. 2 to 4. The values below the histograms give the Gcn4p-dependent component of the myc-TBP or myc-Rpb3p binding in the mutants expressed as a percentage of that seen in WT cells (see Fig. 2 legend for details).
FIG. 2.
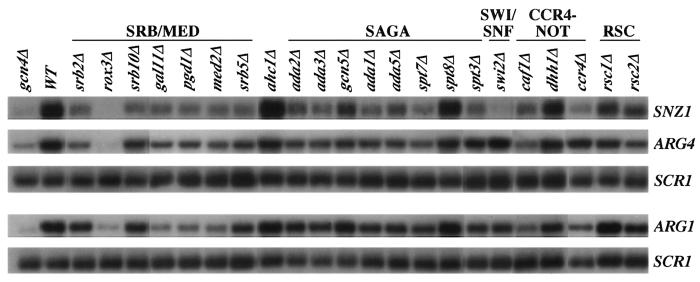
Deletions of multiple SAGA subunits decrease the recruitment of myc-TBP and myc-Rpb3p by Gcn4p and reduce mRNA levels at ARG1, ARG4, and SNZ1. (A and B) ChIP analysis of TBP1-myc strains (A) HQY382 (gcn4Δ), HQY366 (WT), HQY473 (ahc1Δ), HQY583 (ada2Δ), HQY584 (ada3Δ), HQY406 (gcn5Δ), HQY426 (ada1Δ), HQY407 (ada5Δ), HQY533 (spt7Δ), HQY427 (spt3Δ) and HQY530 (spt8Δ), and of RPB3-myc strains (B) HQY422 (gcn4Δ), HQY403 (WT), HQY560 (ahc1Δ), HQ589 (ada2Δ), HQY590 (ada3Δ), HQY445 (gcn5Δ), HQY432 (ada1Δ), HQY414 (ada5Δ), HQY535 (spt7Δ), HQY433 (spt3Δ), and HQY531 (spt8Δ) conducted as described in the legend to Fig. 1. The ratio percent IP (GCN4/gcn4Δ) values were calculated (as defined in the legend to Fig. 1) for the ARG1TATA, SNZ1TATA, and ARG4 probes. The average results obtained from ≥2 independent cultures and ≥2 PCR amplifications for each culture were plotted in the histograms with standard errors shown as error bars. Note the different scale on the y axis for the ratio percent IP (GCN4/gcn4Δ) for SNZ1. The numbers under the histograms, corresponding to the percentages of the WT Gcn4p-dependent binding of myc-TBP or myc-Rpb3p, were calculated by subtracting unity from all of the ratio percent IP (GCN4/gcn4Δ) values in the histogram and calculating the percentage of the resulting WT value obtained for each mutant. (C) Duplicate cultures of the TBP1-myc strains listed in panel A were grown under the same conditions employed for ChIP analysis, and total RNA samples were extracted and subjected to Northern analysis of ARG1, ARG4, and SNZ1 mRNAs with the SCR1 transcript as a loading control (see Fig. 5). The Northern signals from two independent blots for each duplicate culture were measured by phosphorimaging analysis and normalized for SCR1 RNA levels, and the mean value with standard error was plotted in the histogram as a fraction of the WT value. The numbers under the histograms were calculated by subtracting the values shown in the histogram for the gcn4Δ strain from the corresponding values for the other strains and expressing the resulting differences as percentages of the value obtained for the WT. None of the SAGA mutants showed any significant differences in mRNA levels among the TBP1-myc, RPB3-myc, and untagged strains (data not shown).
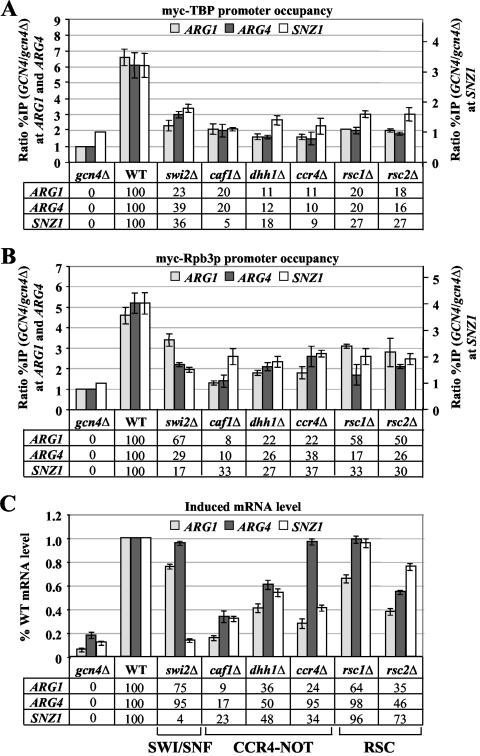
FIG. 4.
Deletions affecting SWI/SNF, CCR4-NOT, and RSC decrease the recruitment of myc-TBP and myc-Rpb3p by Gcn4p and reduce mRNA levels at ARG1, ARG4, and SNZ1. (A and B) ChIP analysis of TBP1-myc strains HQY382 (gcn4Δ), HQY366 (WT), HQY408 (swi2Δ), HQY410 (caf1Δ), HQY369 (dhh1Δ), HQY373 (ccr4Δ), HQY429 (rsc1Δ), and HQY430 (rsc2Δ) in panel A and of RPB3-myc strains HQY422 (gcn4Δ), HQY403 (WT), HQY415 (swi2Δ), HQY437 (caf1Δ), HQY575 (dhh1Δ), HQY444 (ccr4Δ), HQY417 (rsc1Δ), and HQY558 (rsc2Δ) in panel B was conducted as described in the legends to Fig. 2A and B. (C) Northern analysis of the TBP1-myc strains listed in panel A, as described in the legend to Fig. 2C. Northern analysis of the RPB3-myc and untagged strains (data not shown) revealed no significant differences between the mRNA levels observed in the TBP1-myc strains versus the RPB3-myc and untagged strains.
Figure 2A shows the effects of deleting eight nonessential SAGA subunits or Ahc1p on binding of myc-TBP to ARG1, ARG4, and SNZ1. Ada2p, Ada3p, and Gcn5p are shared between the SAGA and ADA complexes, whereas Ada1p, Ada5p, Spt7p, Spt3p, and Spt8p reside only in SAGA. Ahc1p is unique to the ADA complex (19). With one exception (gcn5Δ), all of the SAGA subunit mutations led to moderate reductions in Gcn4p-dependent myc-TBP binding at all three genes, decreasing the binding to 23 to 67% of the WT level. Interestingly, gcn5Δ had little effect on myc-TBP binding at ARG1, even though it substantially impaired TBP binding at the other two genes. The ahc1Δ mutation had no effect on myc-TBP binding at any of the genes, consistent with the fact that this mutation does not impair transcriptional activation by Gcn4p in vivo (63). Considering that deletion of AHC1 destabilizes the ADA complex (19), it appears that SAGA, but not ADA, is required for optimal recruitment of TBP by Gcn4p.
Figure 2B shows the effects of the same SAGA subunit deletions on myc-Rpb3p promoter occupancy. Considering first ARG4 and SNZ1, all of the SAGA mutations except spt8Δ had a greater effect on recruitment of myc-Rpb3p versus myc-TBP, reducing the amount of Gcn4p-dependent myc-Rpb3p binding to 3 to 30% of the WT level. In fact, ada3Δ, ada1Δ, and ada5Δ reduced myc-Rpb3p binding at ARG4 and SNZ1 to nearly the same low level seen in gcn4Δ cells, which lack the activator (Fig. 2B). At the ARG1 gene, the ada5Δ and gcn5Δ mutations again had a greater effect on binding of myc-Rpb3p versus myc-TBP, although the other SAGA mutations impaired myc-Rpb3p and myc-TBP binding by similar amounts at this gene (cf. Fig. 2A and B). Overall, it appears that the SAGA subunits are more important for recruitment of Pol II than TBP by Gcn4p.
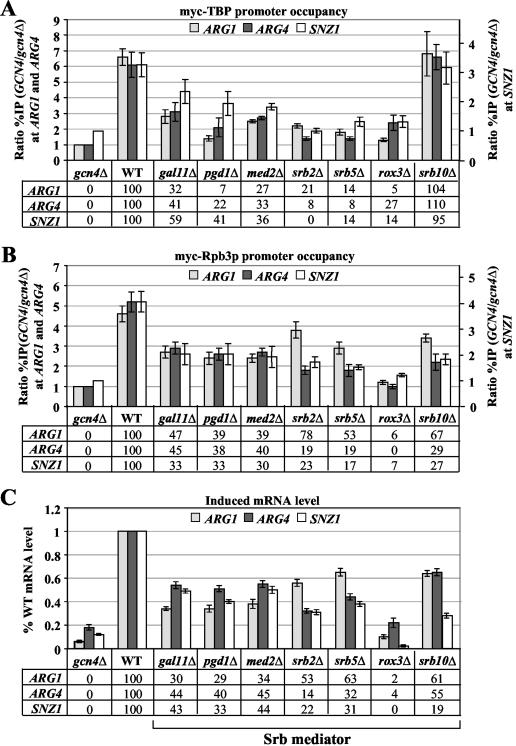
Considering next the Srb mediator, deletions of all subunits examined, except srb10Δ, reduced myc-TBP occupancy at all three target genes (Fig. 3A). These reductions were substantial in the case of rox3Δ, srb2Δ, and srb5Δ, which diminished Gcn4p-dependent myc-TBP binding in several instances to <10% of the WT level. All of the mediator mutations also reduced myc-Rpb3p recruitment, including srb10Δ; however, most of the mutations had a smaller effect on recruitment of myc-Rpb3 versus myc-TBP. This was especially clear for srb2Δ at all three genes and also for srb5Δ and pgd1Δ at ARG1 and ARG4 (Fig. 3B). rox3Δ was the only mediator mutation that strongly decreased recruitment of both Pol II and myc-TBP (Fig. 3B). The srb10Δ mutation was unique in reducing myc-Rpb3p binding without having any effect on myc-TBP recruitment. These findings suggest that Srb mediator is required for optimal recruitment of both TBP and Pol II by Gcn4p but that the subunits we studied differ in their relative importance for recruitment of one factor versus the other. Whereas Srb2p and Srb5p are relatively more important for TBP binding, Srb10p is required exclusively for efficient Pol II recruitment.
FIG. 3.
Deletions of multiple Srb mediator subunits decrease the recruitment of myc-TBP and myc-Rpb3p by Gcn4p and reduce mRNA levels at ARG1, ARG4, and SNZ1. (A and B) ChIP analysis of TBP1-myc strains (A) HQY382 (gcn4Δ), HQY366 (WT), HQY424 (gal11Δ), HQY405 (pgd1Δ), HQY425 (med2Δ), HQY594 (srb2Δ), HQY431 (srb5Δ), HQY534 (rox3Δ), and HQY423 (srb10Δ) and of RPB3-myc strains (B) HQY422 (gcn4Δ), HQY403 (WT), HQY412 (gal11Δ), HQY413 (pgd1Δ), HQY421 (med2Δ), HQY596 (srb2Δ), HQY559 (srb5Δ), HQY574 (rox3Δ), and HQY411 (srb10Δ) was conducted as described in the legends to Fig. 2A and B. (C) Northern analysis of the TBP1-myc strains listed in panel A, as described in the legend to Fig. 2C. Northern analysis of the RPB3-myc and untagged strains (data not shown) revealed that the reductions in mRNA levels observed in the TBP1-myc strains harboring gal11Δ, med2Δ, or pgd1Δ were more pronounced than those seen in the corresponding RPB3-myc and untagged strain versions of these mutants.
The swi2Δ mutation, which impairs SWI/SNF ATPase activity, also produced significant reductions in myc-TBP recruitment at all three genes (Fig. 4A), comparable to the defects observed in the ada1Δ and med2Δ mutations in SAGA and Srb mediator, respectively (Fig. 2A and 3A). The same was true for deletions of the nonessential RSC subunits Rsc1p and Rsc2p and for deletion of the CCR4-NOT subunit Caf1p. Even stronger reductions in myc-TBP binding were conferred by deletions of Ccr4p itself and of the CCR4-NOT-associated factor Dhh1p (Fig. 4A). The foregoing mutations affecting SWI/SNF, CCR4-NOT, or RSC subunits also reduced the level of myc-Rpb3p binding at all three promoters, but these reductions were generally smaller than those observed in the same mutants for myc-TPB binding (cf. Fig. 4A and B). There were only two gene-specific exceptions to this last generalization—swi2Δ at SNZ1 and caf1Δ at ARG1, where the promoter occupancy of myc-Rpb3p was impaired to a greater extent than that of myc-TBP.
In summary, the results in Fig. 2 to 4 indicate that deleting subunits of SAGA, Srb mediator, SWI/SNF, CCR4-NOT, or RSC all produced significant reductions in Gcn4p-dependent promoter occupancy by myc-TBP and myc-Rpb3p. In most cases, the mutations had a relatively greater effect on TBP recruitment, but the srb10Δ mutation affecting Srb mediator and multiple mutations in SAGA subunits, particularly ada5Δ and gcn5Δ, had a decidedly stronger impact on Pol II recruitment.
Effects of coactivator mutations on mRNA accumulation from the target genes.
We conducted Northern analysis of ARG1, ARG4, and SNZ1 mRNA expression in the coactivator mutants cultured under the same conditions used for ChIP analysis (see Fig. 5 for typical results). RNA samples from duplicate cultures of the TBP1-myc strains were analyzed on two independent blots, and the signals were quantified and normalized for the amount of a Pol III transcript, SCR1. The mean values for each mutant were plotted as a fraction of the WT value in the histograms shown in panels C of Fig. 2 to 4. We also conducted Northern analysis on the RPB3-myc and untagged mutant strains (data not shown) and, with only a few exceptions, obtained results very similar to those shown in Fig. 2 to 4 for the TBP1-myc strains (see Fig. 3 legend 3 for exceptions). The results summarized below show that the majority of coactivator mutations led to significant reductions in mRNA accumulation from all three genes. However, there were certain mutants in which reduced promoter occupancy by myc-TBP or myc-Rpb3p was not associated with a commensurate decrease in mRNA induction for one or more of the three target genes.
FIG. 5.
Effects of coactivator mutations on the induction of ARG1, ARG4, and SNZ1 mRNAs by Gcn4p. Representative results from the Northern analysis of TBP1-myc strains summarized in panels C of Fig. 2 to 4. The indicated mutant and WT strains were grown under the same inducing conditions used for ChIP assays. The SNZ1 and ARG4 mRNAs were probed along with SCR1 RNA on one set of blots, whereas ARG1 mRNA and SCR1 RNA were probed on a replicate blot.
Considering the SAGA mutations first, the ada1Δ, ada5Δ, and spt7Δ mutations substantially reduced mRNA levels at all three genes but had the greatest effects at SNZ1, where the mRNA levels approached the low basal level seen in gcn4Δ cells. The ada2Δ, ada3Δ, and gcn5Δ mutations also substantially reduced ARG4 and SNZ1 mRNA levels (again with the greater effects for SNZ1 mRNA) but had relatively small effects on ARG1 mRNA induction. Surprisingly, spt3Δ and spt8Δ had little effect on ARG1 and ARG4 mRNA accumulation, despite significant decreases in myc-TBP and myc-Rpb3p recruitment at these two genes (cf. Fig. 2A to C).
Turning to the Srb mediator, the rox3Δ strain showed drastically reduced mRNA levels for all three genes, commensurate with the strong reductions in TBP and Pol II binding observed in this mutant (cf. Fig. 3A to C). The gal11Δ, pgd1Δ, and med2Δ mutations produced moderate reductions in mRNA levels that were comparable in magnitude to the corresponding decreases in myc-Rpb3p and myc-TBP binding generated by these mutations. In the srb2Δ and srb5Δ mutants, the reductions in mRNA levels seem to correlate more closely with the defects in myc-Rpb3p recruitment versus the more drastic reductions in myc-TBP binding observed in these strains.
Finally, considering the effects of mutations in CCR4-NOT, SWI/SNF, or RSC subunits, we found that deletions of CCR4-NOT subunit Caf1p and the associated protein Dhh1p reduced mRNA levels in parallel with the corresponding reductions in myc-TBP and myc-Rpb3p recruitment (cf. Fig. 4A to C). However, we observed little or no decrease in mRNA accumulation despite strong reductions in myc-TBP or myc-Rpb3p recruitment for the swi2Δ mutant at ARG1 and ARG4, ccr4Δ at ARG4, and rsc1Δ at ARG4 and SNZ1. Below, we consider two alternative mechanisms to explain how mRNA levels can be nearly unaffected in these three mutants and in spt3Δ cells, despite marked reductions in TBP or Pol II recruitment to the promoter.
Gcn4p binding to the promoter is largely independent of coactivator function.
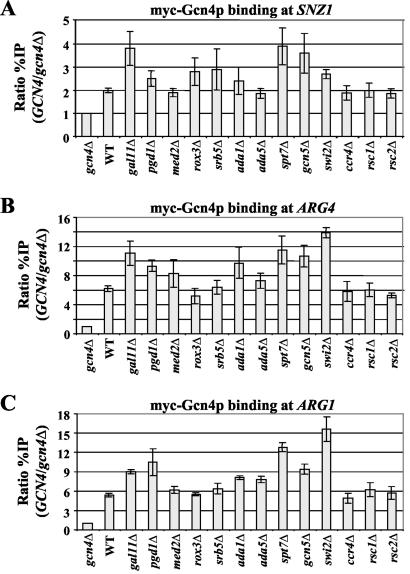
A reduction in the promoter occupancy of myc-TBP or myc-Rpb3p in a given mutant could arise from a decrease in the expression of these myc-tagged proteins. We eliminated this possibility by conducting Western analysis on all of the WT and mutant TBP1-myc and RPB3-myc strains by using anti-myc antibodies (data not shown). It was also possible that the coactivator mutations lowered the amount of Gcn4p bound to the promoter, which in turn would decrease the recruitment of TBP and Pol II. To address the second possibility, we introduced a GCN4-myc allele into the gcn4Δ versions of the coactivator mutant strains and conducted ChIP analysis of myc-Gcn4p binding to the upstream activation sequence (UAS) of each gene. None of the mutations affecting SAGA, Srb mediator, SWI/SNF, or RSC significantly reduced myc-Gcn4p binding at the three target genes examined. The same conclusion holds for the Ccr4p subunit of the CCR4-NOT complex (Fig. 6). We conclude that SAGA, Srb mediator, SWI/SNF, RSC, and CCR4-NOT all function to promote TBP and Pol II recruitment by promoter-bound Gcn4p.
FIG. 6.
ChIP analysis of myc-Gcn4p binding to the SNZ1, ARG4, and ARG1 promoters in coactivator mutants. (A to C) A transformant of strain 249 carrying empty vector (gcn4Δ) and transformants of the strains containing single-copy GCN4-myc plasmid pSK1: HQY577 (gal11Δ), HQY513 (pgd1Δ), HQY591 (med2Δ), HQY592 (rox3Δ), HQY522 (srb5Δ), HQY523 (ada1Δ), HQY516 (ada5Δ), HQY578 (spt7Δ), HQY515 (gcn5Δ), HQY514 (swi2Δ), HQY532 (ccr4Δ), HQY647 (rsc1Δ), and HQY648 (rsc2Δ) were subjected to ChIP analysis as described in the legend to Fig. 1 to measure myc-Gcn4p binding at SNZ1 (A), ARG4 (B), and ARG1 (C).
TBP binding is a prerequisite for Pol II recruitment by Gcn4p.
It is generally considered that PIC formation is initiated by the binding of TBP to the TATA element, with TFIIB bridging the interaction between TBP and Pol II (18). Consistent with this, it was shown that a Ts− mutation in TBP (tbp1-ts2) that reduced promoter occupancy by TBP impaired Pol II recruitment at the GAL1 promoter in vivo (42). On the other hand, there is strong evidence that recruitment of Pol II by the enhanceosome complex precedes TBP binding to the promoter of the beta interferon (IFN-β) gene in human cells (1). Having found that the majority of the coactivator mutants analyzed above impaired recruitment of both TBP and Pol II, we wondered whether the reductions in TBP recruitment could account for the decreased promoter occupancy by Pol II observed in these strains.
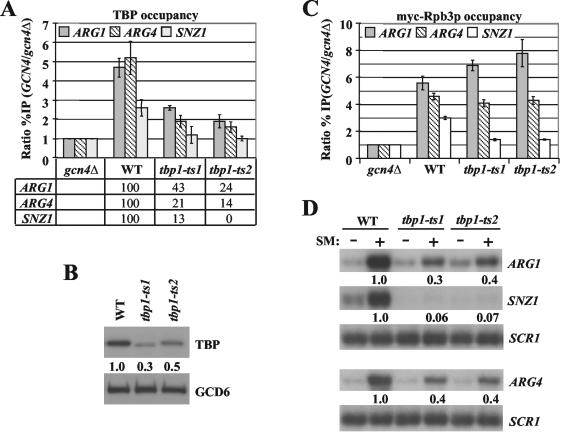
To address this question, we first asked whether Ts− mutations in TBP (tbp1-ts1 and tbp1-ts2) would impair myc-Rpb3 recruitment by Gcn4p. The mutant strains were grown under nonstarvation conditions at the nonpermissive temperature (37°C), and Gcn4p was induced with SM for 2 h at 37°C prior to fixation of the cells for ChIP analysis. Using antibodies against TBP, we found that Gcn4p-dependent binding of the mutant TBP proteins was greatly reduced at ARG1 and ARG4 and essentially abolished at SNZ1 when Gcn4p was induced at the restrictive temperature (Fig. 7A). Consistent with these results, we observed decreased induction of ARG1 and ARG4 mRNAs and completely impaired induction of SNZ1 mRNA by Gcn4p in both mutants at the restrictive temperature 37°C (Fig. 7D). Interestingly, Western analysis revealed a marked reduction in the steady-state levels of TBP in both tbp1 mutants at 37°C (Fig. 7B), which may partly explain the reductions in TBP recruitment we observed under these conditions. ChIP analysis of myc-Rpb3p binding revealed a strong reduction in Pol II promoter occupancy at SNZ1 in both tbp1 mutants; however, myc-Rpb3p binding occurred at WT or higher levels at ARG1 and ARG4 (Fig. 7C). The last results raised the possibility of TBP-independent recruitment of Pol II by Gcn4p at a subset of its target genes.
FIG. 7.
Promoter occupancy of Pol II at ARG1 and ARG4 is not diminished by reductions in TBP recruitment. (A) ChIP analysis of TBP recruitment in tbp1 mutants. Strains HQY644 (gcn4Δ), BYΔ2 (WT), BYΔ2 ts-1 (tbp1-ts1) and BYΔ2 ts-2 (tbp1-ts2) were grown at 25°C to an optical density at 600 nm of 0.6 to 0.8, shifted to 37°C for 20 min, and cultured for 2 h at 37°C in the presence of SM to induce Gcn4p. ChIP analysis was conducted using TBP antibodies to measure TBP recruitment at ARG1, ARG4, and SNZ1 as described in the legend to Fig. 1. The results from duplicate cultures and replicate PCR amplifications for each culture were averaged and plotted as described in the legend to Fig. 2A. (B) Western analysis of TBP expression in tbp1 mutants. WCEs were prepared from the same cultures described in panel A were subjected to Western blot analysis using antibodies against TBP and Gcd6p, the latter serving as loading control. The ratios of TBP to Gcd6p were calculated by video densitometry using NIH image software and are listed beneath the TBP blot. (C) ChIP analysis of myc-Rpb3p recruitment in RPB3-myc strains HQY651 (gcn4Δ), HQY641 (WT), HQY642 (tbp1-ts1), and HQY643 (tbp1-ts2), conducted as described for panel A. (D) Northern analysis of tbp1 mutants. Strains BYΔ2 (WT), BYΔ2 ts-1 (tbp1-ts1) and BYΔ2 ts-2 (tbp1-ts2) were grown at 25°C to an optical density at 600 nm of 0.6 to 0.8, split into halves, and incubated at 37°C for 20 min. After adding SM to one half (+SM), both halves were incubated at 37°C for 2 h longer. Total RNA was subjected to Northern analysis, and the mean ARG1/SCR1, ARG4/SCR1, and SNZ1/SCR1 ratios, measured by phosphorimaging analysis of two independent blots, are listed as relative to the WT value beneath the autoradiograms.
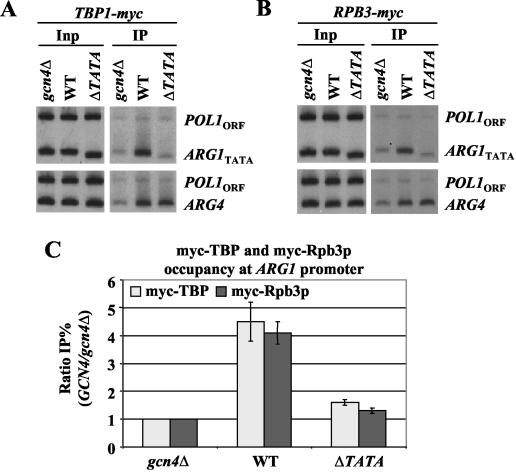
To pursue this possibility further, we deleted the TATA element at ARG1 (ΔTATA) in the WT strain and conducted ChIP analysis of myc-TBP and myc-Rpb3p recruitment. The results in Fig. 8 show clearly that deletion of the TATA element nearly eliminated recruitment of both myc-TBP and myc-Rpb3p by Gcn4p. As expected, the ΔTATA mutation at ARG1 had no effect on recruitment of TBP and Pol II to ARG4. These findings strongly suggest that TBP binding is a prerequisite for Pol II recruitment by Gcn4p at ARG1. To account for the WT level of Pol II recruitment that occurred at ARG1 in the tbp1-ts1 and -ts2 mutants (Fig. 7), we note that TBP binding at this gene was reduced but not abolished in both mutants. Thus, Pol II recruitment should still proceed, but at reduced rates. We propose that the tbp1 mutations interfere with promoter melting or promoter clearance by assembled PICs, decreasing the rate at which Pol II is released from the promoter. This defect would compensate for the decreased rate of Pol II recruitment that results from diminished TBP binding to yield no net change in promoter occupancy by Pol II. At SNZ1, by contrast, the tbp1 mutations abolish TBP binding and thus mimic the effect of deleting the TATA element in preventing Pol II recruitment.
FIG. 8.
TBP binding to TATA box is a prerequisite for recruitment of Pol II by Gcn4p. ChIP analysis of TBP1-myc strains (A) HQY382 (gcn4Δ), HQY366 (WT), and HQY692 (ΔTATA) and of RPB3-myc strains (B) HQY422 (gcn4Δ), HQY403 (WT), and HQY693 (ΔTATA) was conducted as described in the legend to Fig. 1. The ratio percent IP (GCN4/gcn4Δ) values were calculated (as defined in Fig. 1) for the ARG1TATA probe, and the average results obtained from two independent cultures and two PCR amplifications for each culture were plotted in the histogram (C) with standard errors shown as error bars.
DISCUSSION
Using the ChIP assay, we found that induction of Gcn4p by amino acid starvation leads to the recruitment of TBP and the Rpb3p subunit of Pol II to the ARG1, ARG4, and SNZ1 promoters in vivo. The recruitment of TBP and Pol II, but not promoter binding by Gcn4p, requires the hydrophobic residues in the Gcn4p activation domain necessary for transcriptional activation. We and others have shown previously that transcriptional activation by Gcn4p is dependent on multiple coactivators (see reference 63 and references therein.). It was not known, however, whether these cofactors enhance the ability of Gcn4p to stimulate PIC assembly, postassembly steps of initiation (promoter DNA melting and promoter clearance), or the elongation phase of transcription. Our results show that the functions of SAGA, SWI/SNF, Srb mediator, RSC, and the CCR4/NOT complex are required for optimal recruitment of TBP and Pol II by Gcn4p at all three target genes we analyzed. These requirements cannot be explained by the involvement of the coactivators in binding of Gcn4p itself to the UAS elements at its target genes or for expression of wild-type levels of TBP or Rpb3p. Thus, it appears that all five coactivators are required to support the recruitment of TBP and Pol II by promoter-bound Gcn4p. Of course, it is likely that some of these coactivators also regulate downstream steps in the transcription process following PIC assembly.
The ts1 and ts2 mutations in TBP produced strong reductions in the recruitment of both TBP and myc-Rpb3p at SNZ1 by Gcn4p. Similarly, deletion of the TATA element at ARG1 nearly abolished recruitment of both myc-TBP and myc-Rpb3p at this gene. Thus, we conclude that TBP binding to the TATA element is a prerequisite for recruitment of Pol II by Gcn4p in vivo. This situation is similar to that described previously for the activator Gal4p in yeast (42) but contrasts with the TFIID-independent recruitment of Pol II by the enhanceosome at the human IFN-β gene (1). The dependence of Pol II recruitment on TBP binding may be explained by the ability of TFIIB to interact with both TBP and Pol II (24), although Srb mediator could also serve as an adapter between TBP and Pol II (28) to stabilize Pol II association with the promoter.
It is surprising that the promoter occupancy of Pol II at ARG1 and ARG4 was not diminished by the tbp1 mutations, even though binding of the mutant TBPs at these genes was substantially reduced. How can we reconcile this result with our finding that Pol II occupancy at the ARG1 promoter requires the TATA element and TBP binding? We propose that the ts1 and ts2 mutations impair not only TBP binding to the TATA box but also promoter melting or promoter clearance following PIC assembly. Because TBP recruitment was not completely eliminated at ARG1 and ARG4, Pol II recruitment should continue at a reduced rate in the mutants. The proposed defect in promoter opening or clearance caused by the tbp1 mutations would lead to Pol II accumulation in the promoter, offsetting the defect in Pol II recruitment. Our findings underscore the importance of analyzing mutations that completely eliminate TBP binding to the TATA box to assess the importance of TBP in Pol II recruitment.
The fact that SAGA is required for optimal TBP recruitment by Gcn4p is in agreement with previous results showing that TBP recruitment by Gal4p was greatly reduced in ada1Δ, spt20Δ/ada5Δ, spt7Δ, and spt3Δ mutants (4, 5, 17). There is strong evidence supporting a direct role for Spt3p in TBP recruitment by Gal4p, with a smaller contribution provided by the HAT activity of Gcn5p (35). Relative to Gal4p, it appears that Gcn4p is less dependent on SAGA for TBP recruitment and that Gcn5p plays a more prominent role in the contribution of SAGA to TBP recruitment by Gcn4p. Histone acetylation by Gcn5p could increase accessibility of the TATA element by enhancing nucleosome displacement by SWI/SNF (22, 59, 64). In addition, we found recently that ada1Δ and ada5Δ reduced SWI/SNF recruitment by Gcn4p (68). Thus, SAGA may stimulate both the recruitment and remodeling function of SWI/SNF as an indirect means of stimulating TBP recruitment by Gcn4p.
Our finding that recruitment of TBP by Gcn4p was strongly impaired in Srb mediator mutants also fits well with previous observations that TBP binding in vivo to various promoters was eliminated by a Ts− mutation (srb4-138) in an essential mediator subunit (33, 42), although it was unclear whether activator binding occurred in the srb4-138 cells. It was surprising, however, to find that deletions of Srb mediator subunits Srb2p and Srb5p have greater effects on recruitment of TBP than Pol II. Srb mediator might stimulate TBP binding indirectly by enhancing the recruitment SWI/SNF, as we found that efficient SWI/SNF recruitment by Gcn4p also requires Srb mediator subunits (68). An adapter mechanism for TBP recruitment by Srb mediator is also possible considering the physical interaction detected between TBP and Srb mediator (65), possibly involving the mediator head domain (28).
It was shown recently that TBP recruitment is dependent on Pol II binding to the RNR3 promoter using the Ts− rpb1-1 mutation to impair Pol II binding (61). We could not address whether recruitment of TBP by Gcn4p is dependent on Pol II because no binding of Gcn4p to its target promoters occurred in rpb1-1 cells at the restrictive temperature (data not shown). The latter most likely reflects the rapid turnover of Gcn4p (30) and the complete impairment of GCN4 mRNA synthesis in this mutant at 37°C.
Given that Pol II recruitment by Gcn4p is absolutely dependent on TBP binding to the TATA box, it is possible that the defects in Pol II recruitment we observed in the various coactivator mutants were secondary to the defects in TBP recruitment occurring in these strains. The srb10Δ mutation is a likely exception to this interpretation because it reduced Pol II recruitment without decreasing TBP binding to the promoters of all three genes. A TBP-independent mechanism of Pol II recruitment by mediator was indicated previously by in vitro findings on activator recruitment of holoenzyme to immobilized promoters (57). Given the tight physical association between mediator and Pol II, an adapter mechanism is an obvious possibility for the role of mediator in stimulating Pol II recruitment by Gcn4p.
We found that certain deletions of SAGA subunits, notably ada5Δ and gcn5Δ, impaired recruitment of Pol II to a larger extent than they reduced TBP recruitment at ARG4 and SNZ1. This suggests that SAGA also can enhance Pol II binding independently of its stimulatory effects on TBP recruitment. It could be argued instead that SAGA mutations reduce Pol II promoter occupancy by increasing the rate of promoter clearance in addition to the decrease in Pol II binding, which results indirectly from impaired TBP recruitment. In this event, the SAGA mutations would be expected to have a smaller effect on transcription rates than on TBP recruitment, as the decreased Pol II recruitment resulting from impaired TBP binding would be offset by increased rates of promoter clearance. However, the reductions in mRNA levels were either comparable (ARG1 and ARG4) or much more extensive (SNZ1) than the reductions in TBP recruitment in most SAGA mutants (Fig. 2). In addition, ChIP analysis of Pol II binding to the coding sequences showed that ada5Δ produced a greater reduction in transcription rate than in TBP binding to the promoter at all three target genes (data not shown). Thus, it seems likely that SAGA can enhance Pol II recruitment independently of its role in TBP recruitment. Although most of the available evidence points to an adapter function for SAGA in recruitment of TBP (5, 35, 36), our conclusion does not conflict with previous results on the role of SAGA in PIC assembly. Bhaumik and Green showed that spt20Δ and spt3Δ produced strong reductions in recruitment of both TBP and Pol II by the activator Gal4p, and Pol II binding seemed to be reduced more than that of TBP in spt3Δ cells (5). It is conceivable that SAGA promotes Pol II recruitment indirectly by stimulating recruitment of mediator. Although this possibility was ruled out for Gal4p (7), we have preliminary evidence that SAGA is required for optimal mediator recruitment by Gcn4p. It is also possible that histone acetylation by Gcn5p increases accessibility of the core promoter to Pol II binding (22, 59, 64).
Other yeast activators have been shown to require SAGA, Srb mediator, or SWI/SNF for optimal recruitment of TBP and Pol II (see the references cited above and reference 61); however, this is the first report implicating RSC and CCR4-NOT in stimulating PIC assembly. As Gcn4p binds specifically to CCR4-NOT and RSC in vitro and can recruit CCR4-NOT and RSC to the ARG1 promoter in vivo (63), it seems likely that both cofactors function at the promoter to stimulate PIC formation after being recruited by Gcn4p. The RSC complex could enhance TBP and Pol II recruitment by displacing nucleosomes from the promoter, as proposed for the SWI/SNF complex. A possible mechanism for the function of CCR4-NOT in PIC assembly is less obvious. Previous work has indirectly implicated CCR4-NOT in regulating TBP binding, including its role in repressing promoters that lack a canonical TATA sequence (37), its interactions with TFIID subunits TAF13 (40) and TAF1, and its influence on TAF1 promoter occupancy (14). Subunits of CCR4-NOT also interact with Srb mediator components Srb9p and Srb10p (43). On the other hand, CCR4-NOT functions in the cytoplasm as an mRNA deadenylase (8, 66), and we suggested previously that recruitment by Gcn4p might facilitate its association with nascent transcripts to control their deadenylation, translation, and degradation in the cytoplasm (63). In this event, recruitment of CCR4-NOT to the promoter would not necessarily signify a role in PIC assembly. Hence, it is prudent to consider that a defect in CCR4-NOT deadenylase function might impair PIC assembly indirectly by altering the expression of one or more proteins with a more direct role in this process.
We reported previously that the Paf1 complex is also required for WT transcriptional activation of certain Gcn4p targets. However, we found recently that deletion of the Cdc73p subunit of this complex has no effect on myc-TBP or myc-Rpb3p recruitment by Gcn4p (data not shown); hence, Paf1 complex probably stimulates only the elongation phase of transcription at Gcn4p target genes, in accordance with recent findings (25, 55, 62).
All of the coactivator mutations that reduced recruitment of TBP and Pol II by Gcn4p led to a significant reduction in the mRNA produced from at least one of the three target genes. This is consistent with previous results indicating that these mutations increased sensitivity to SM and impaired induction of Gcn4p-dependent reporter genes (63). However, in several instances (e.g. srb2Δ at SNZ1), very low levels of myc-TBP recruitment were associated with more appreciable levels of mRNA accumulation. It is possible that a portion of the TBP binding is below the detection limit of the ChIP assay so that the apparent reduction in TBP recruitment is exaggerated for such mutants with very strong defects in TBP binding. Nevertheless, we saw almost no decrease in mRNA levels for the spt3Δ and swi2Δ mutations at ARG1 and ARG4, ccr4Δ at ARG4, and rsc1Δ at ARG4 and SNZ1, despite marked reductions in recruitment of TBP or Pol II. One way to account for the last findings is to propose that these coactivator mutations indirectly impair an mRNA degradation pathway and thus stabilize the mRNAs produced from one or more Gcn4p target genes. This hypothesis is particularly relevant to the ccr4Δ mutation in view of the mRNA deadenylase function of CCR4-NOT complex. A more intriguing possibility is based on the possibility that promoter melting and promoter clearance are rate-limiting steps in vivo (see reference 9 and references therein). The coactivator mutations under consideration could allow these steps to occur more rapidly by eliminating a function of the WT coactivator that negatively regulates these reactions. In this way, the reduction in PIC assembly (as manifested by reduced TBP binding to the promoter) would be offset by an increase in the rate of promoter clearance or elongation. For example, it was shown recently that mutations affecting the ISW1 nucleosome-remodeling complex eliminate a barrier to transcription elongation at the 5′ end of the MET16 open reading frame (ORF) (49). This hypothesis is also consistent with the fact that subunits of SAGA (3, 60), SWI/SNF (46), CCR4-NOT (11), and RSC (48) have been implicated previously in repression of transcription and thus may have opposing positive and negative effects on transcription of a given gene.
If the coactivator mutations reduce the transcription rate but produce a compensatory defect in mRNA degradation, then the number of Pol II molecules associated with the transcribed portion of the gene should be reduced compared to that of the WT. If instead the compensatory defects in the mutants lead to elevated promoter clearance or elongation rates, then the number of Pol II molecules in the coding region should be close to the WT level. To distinguish between these possibilities, we have recently conducted ChIP analysis on Rpb3p binding to the 3′ ends of the Gcn4p target genes in the spt3Δ, swi2Δ, rsc1Δ, and ccr4Δ mutants. Interestingly, we found a WT level of Pol II association with the coding sequences in the rsc1Δ mutant (data not shown), consistent with an elevated rate of promoter clearance or elongation in this strain. Hence, the Rsc1p-containing complex likely promotes both PIC assembly and promoter clearance. In the spt3Δ, swi2Δ, and ccr4Δ mutants, the amount of Pol II associated with the coding sequences was lower than in the WT, suggesting a decrease in the transcription rate, but it was not reduced to the large extent seen at the promoter in these mutants (data not shown). Thus, these three mutations probably increase the rate of promoter clearance or elongation and thereby partly compensate for reduced PIC formation, but they likely also extend the half-life of the transcript to yield a net level of mRNA accumulation similar to that seen in WT cells.
Acknowledgments
We thank Joe Reese, Kevin Struhl, and Marjolaine Crabeel for generous gifts of strains, antibodies, and plasmids and Joe Reese and Chhabi Govind for critical reading of the manuscript and many helpful suggestions.
REFERENCES
- 1.Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-beta promoter. Cell 103:667-678. [DOI] [PubMed] [Google Scholar]
- 2.Alani, E., L. Cao, and N. Kleckner. 1987. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics 116:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman, and S. L. Berger. 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20:634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaumik, S. R., and M. R. Green. 2002. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22:7365-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaumik, S. R., and M. R. Green. 2001. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhoite, L. T., Y. Yu, and D. J. Stillman. 2001. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15:2457-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant, G. O., and M. Ptashne. 2003. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11:1301-1309. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., Y. C. Chiang, and C. L. Denis. 2002. CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 21:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, C., and P. A. Sharp. 2003. RNA polymerase II accumulation in the promoter-proximal region of the dihydrofolate reductase and γ-actin genes. Mol. Cell. Biol. 23:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cigan, A. M., M. Foiani, E. M. Hannig, and A. G. Hinnebusch. 1991. Complex formation by positive and negative translational regulators of GCN4. Mol. Cell. Biol. 11:3217-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collart, M. A., and K. Struhl. 1994. NOT1(CDC39), NOT2(CDC36), NOT3, and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 8:525-537. [DOI] [PubMed] [Google Scholar]
- 12.Cormack, B. P., M. Strubin, A. S. Ponticelli, and K. Struhl. 1991. Functional differences between yeast and human TFIID are localized to the highly conserved region. Cell 65:341-348. [DOI] [PubMed] [Google Scholar]
- 13.Cormack, B. P., and K. Struhl. 1992. The TATA-binding protein is required for transcription by all three nuclear RNA polymerases in yeast cells. Cell 69:685-696. [DOI] [PubMed] [Google Scholar]
- 14.Deluen, C., N. James, L. Maillet, M. Molinete, G. Theiler, M. Lemaire, N. Paquet, and M. A. Collart. 2002. The Ccr4-Not complex and yTAF1 (yTafII130p/yTafII145p) show physical and functional interactions. Mol. Cell. Biol. 22:6735-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drysdale, C. M., E. Dueñas, B. M. Jackson, U. Reusser, G. H. Braus, and A. G. Hinnebusch. 1995. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 15:1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drysdale, C. M., B. M. Jackson, R. McVeigh, E. R. Klebanow, Y. Bai, T. Kokubo, M. Swanson, Y. Nakatani, P. A. Weil, and A. G. Hinnebusch. 1998. The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex. Mol. Cell. Biol. 18:1711-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley, A. M., C. Rougeulle, and F. Winston. 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvir, A., J. W. Conaway, and R. C. Conaway. 2001. Mechanism of transcription initiation and promoter escape by RNA polymerase II. Curr. Opin. Genet. Dev. 11:209-214. [DOI] [PubMed] [Google Scholar]
- 19.Eberharter, A., D. E. Sterner, D. Schieltz, A. Hassan, J. R. Yates, S. L. Berger, and J. L. Workman. 1999. The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:6621-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 21.Green, M. R. 2000. TBP-associated factors (TAFIIs): multiple, selective transcriptional mediators in common complexes. Trends Biochem. Sci. 25:59-63. [DOI] [PubMed] [Google Scholar]
- 22.Gregory, P. D., A. Schmid, M. Zavari, M. Munsterkotter, and W. Horz. 1999. Chromatin remodelling at the PHO8 promoter requires SWI-SNF and SAGA at a step subsequent to activator binding. EMBO J. 18:6407-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson, C. M., L. C. Myers, J. Beve, H. Spahr, M. Lui, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 1998. Identification of new mediator subunits in the RNA polymerase II holoenzyme from Saccharomyces cerevisiae. J. Biol. Chem. 273:30851-30854. [DOI] [PubMed] [Google Scholar]
- 24.Hampsey, M. 1998. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol. Mol. Biol. Rev. 62:465-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampsey, M., and D. Reinberg. 2003. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell 113:429-432. [DOI] [PubMed] [Google Scholar]
- 26.Hinnebusch, A. G. 1996. Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2, p. 199-244. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Jackson, B. M., C. M. Drysdale, K. Natarajan, and A. G. Hinnebusch. 1996. Identification of seven hydrophobic clusters in GCN4 making redundant contributions to transcriptional activation. Mol. Cell. Biol. 16:5557-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang, J. S., K. S. H., M. S. Hwang, S. J. Han, Y. C. Lee, and Y. J. Kim. 2001. The structural and functional organization of the yeast mediator complex. J. Biol. Chem. 276:42003-42010. [DOI] [PubMed] [Google Scholar]
- 29.Kim, Y. J., S. Bjorklund, Y. Li, M. H. Sayre, and R. D. Kornberg. 1994. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77:599-608. [DOI] [PubMed] [Google Scholar]
- 30.Kornitzer, D., B. Raboy, R. G. Kulka, and G. R. Fink. 1994. Regulated degradation of the transcription factor Gcn4. EMBO J. 13:6021-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo, M. H., E. vom Bauer, K. Struhl, and C. D. Allis. 2000. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6:1309-1320. [DOI] [PubMed] [Google Scholar]
- 32.Kuo, M. H., J. Zhou, P. Jambeck, M. E. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuras, L., P. Kosa, M. Mencia, and K. Struhl. 2000. TAF-containing and TAF-independent forms of transcriptionally active TBP in vivo. Science 288:1244-1248. [DOI] [PubMed] [Google Scholar]
- 34.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609-613. [DOI] [PubMed] [Google Scholar]
- 35.Larschan, E., and F. Winston. 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15:1946-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, T. I., H. C. Causton, F. C. Holstege, W. C. Shen, N. Hannett, E. G. Jennings, F. Winston, M. R. Green, and R. A. Young. 2000. Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405:701-704. [DOI] [PubMed] [Google Scholar]
- 37.Lee, T. I., J. J. Wyrick, S. S. Koh, E. G. Jennings, E. L. Gadbois, and R. A. Young. 1998. Interplay of positive and negative regulators in transcription initiation by RNA polymerase II holoenzyme. Mol. Cell. Biol. 18:4455-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, T. I., and R. A. Young. 2000. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 34:77-134. [DOI] [PubMed] [Google Scholar]
- 39.Lee, Y. C., J. M. Park, S. Min, S. J. Han, and Y. J. Kim. 1999. An activator binding module of yeast RNA polymerase II holoenzyme. Mol. Cell. Biol. 19:2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaire, M., and M. A. Collart. 2000. The TATA-binding protein-associated factor yTAFII19p functionally interacts with components of the global transcriptional regulator Ccr4-Not complex and physically interacts with the Not5 subunit. J. Biol. Chem. 275:26925-26934. [DOI] [PubMed] [Google Scholar]
- 41.Li, X., A. Virbasius, X. Zhu, and M. R. Green. 1999. Enhancement of TBP binding by activators and general transcription factors. Nature 399:605-609. [DOI] [PubMed] [Google Scholar]
- 42.Li, X. Y., S. R. Bhaumik, and M. R. Green. 2000. Distinct classes of yeast promoters revealed by differential TAF recruitment. Science 288:1242-1244. [DOI] [PubMed] [Google Scholar]
- 43.Liu, H. Y., Y. C. Chiang, J. Pan, J. Chen, C. Salvadore, D. C. Audino, V. Badarinarayana, V. Palaniswamy, B. Anderson, and C. L. Denis. 2001. Characterization of CAF4 and CAF16 reveals a functional connection between the CCR4-NOT complex and a subset of SRB proteins of the RNA polymerase II holoenzyme. J. Biol. Chem. 276:7541-7548. [DOI] [PubMed] [Google Scholar]
- 44.Liu, Y., J. A. Ranish, R. Aebersold, and S. Hahn. 2001. Yeast nuclear extract contains two major forms of RNA polymerase II mediator complexes. J. Biol. Chem. 276:7169-7175. [PubMed] [Google Scholar]
- 45.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 46.Martens, J. A., and F. Winston. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 16:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mencia, M., Z. Moqtaderi, J. V. Geisberg, L. Kuras, and K. Struhl. 2002. Activator-specific recruitment of TFIID and regulation of ribosomal protein genes in yeast. Mol. Cell 9:823-833. [DOI] [PubMed] [Google Scholar]
- 48.Moreira, J. M., and S. Holmberg. 1999. Transcriptional repression of the yeast CHA1 gene requires the chromatin-remodeling complex RSC. EMBO J. 18:2836-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morillon, A., N. Karabetsou, J. O'Sullivan, N. Kent, N. Proudfoot, and J. Mellor. 2003. Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell 115:425-435. [DOI] [PubMed] [Google Scholar]
- 50.Myers, L. C., and R. D. Kornberg. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69:729-749. [DOI] [PubMed] [Google Scholar]
- 51.Natarajan, K., B. M. Jackson, E. Rhee, and A. G. Hinnebusch. 1998. yTAFII61 has a general role in RNA polymerase II transcription and is required by Gcn4p to recruit the SAGA coactivator complex. Mol. Cell 2:683-692. [DOI] [PubMed] [Google Scholar]
- 52.Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts, A. G. Hinnebusch, and M. J. Marton. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21:4347-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park, J. M., H.-S. Kim, S. J. Han, M.-S. Hwang, Y. C. Lee, and Y.-J. Kim. 2000. In vivo requirement of activator-specific binding targets of mediator. Mol. Cell. Biol. 20:8709-8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park, J. M., J. Werner, J. M. Kim, J. T. Lis, and Y. J. Kim. 2001. Mediator, Not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol. Cell 8:9-19. [DOI] [PubMed] [Google Scholar]
- 55.Pokholok, D. K., N. M. Hannett, and R. A. Young. 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9:799-809. [DOI] [PubMed] [Google Scholar]
- 56.Qiu, H., E. Park, L. Prakash, and S. Prakash. 1993. The Saccharomyces cerevisiae DNA repair gene RAD25 is required for transcription by RNA polymerase II. Genes Dev. 7:2161-2171. [DOI] [PubMed] [Google Scholar]
- 57.Ranish, J. A., N. Yudkovsky, and S. Hahn. 1998. Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev. 13:49-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reid, G. A., and G. Schatz. 1982. Import of proteins into mitochondria. Extramitochondrial pools and post-translational import of mitochondrial precursors in vivo. J. Biol. Chem. 257:13062-13067. [PubMed] [Google Scholar]
- 59.Reinke, H., P. D. Gregory, and W. Horz. 2001. A transient histone hyperacetylation signal marks nucleosomes for remodeling at the PHO8 promoter in vivo. Mol. Cell 7:529-538. [DOI] [PubMed] [Google Scholar]
- 60.Ricci, A. R., J. Genereaux, and C. J. Brandl. 2002. Components of the SAGA histone acetyltransferase complex are required for repressed transcription of ARG1 in rich medium. Mol. Cell. Biol. 22:4033-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma, V. M., B. Li, and J. C. Reese. 2003. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAFIIs and the general transcription machinery. Genes Dev. 17:502-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Squazzo, S. L., P. J. Costa, D. L. Lindstrom, K. E. Kumer, R. Simic, J. L. Jennings, A. J. Link, K. M. Arndt, and G. A. Hartzog. 2002. The Paf1 complex physically and functionally associates with transcription elongation factors in vivo. EMBO J. 21:1764-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swanson, M. J., H. Qiu, L. Sumibcay, A. Krueger, S.-J. Kim, K. Natarajan, S. Yoon, and A. G. Hinnebusch. 2003. A multiplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol. Cell. Biol. 23:2800-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syntichaki, P., I. Topalidou, and G. Thireos. 2000. The Gcn5 bromodomain co-ordinates nucleosome remodeling. Nature 404:414-417. [DOI] [PubMed] [Google Scholar]
- 65.Thompson, C. M., A. J. Koleske, D. M. Chao, and R. A. Young. 1993. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell 73:1361-1375. [DOI] [PubMed] [Google Scholar]
- 66.Tucker, M., R. R. Staples, M. A. Valencia-Sanchez, D. Muhlrad, and R. Parker. 2002. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21:1427-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394:498-502. [DOI] [PubMed] [Google Scholar]
- 68.Yoon, S., H. Qiu, M. J. Swanson, and A. G. Hinnebusch. 2003. Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol. Cell. Biol. 23:8829-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]