Abstract
Background
Population based investigations suggest that red blood cells (RBCs) are therapeutically effective when collected, processed and stored for up to 42 days under validated conditions prior to transfusion. However, some retrospective clinical studies have shown worse patient outcomes when transfused RBCs have been stored for the longest times. Furthermore, studies of RBC persistence in the circulation after transfusion have suggested that considerable donor-to-donor variability exists, and may affect transfusion efficacy. To understand the limitations of current blood storage technologies and to develop approaches to improve RBC storage and transfusion efficacy, we investigated the global metabolic alterations that occur when RBCs are stored in AS-1 (AS1-RBC).
Methods
Leukoreduced AS1-RBC units prepared from 9 volunteer research donors (12 total donated units) were serially sampled for metabolomics analysis over 42 days of refrigerated storage. Samples were tested by GC/MS and LC/MS/MS, and specific biochemical compounds were identified by comparison to a library of purified standards.
Results
Over three experiments, 185–264 defined metabolites were quantified in stored RBC samples. Kinetic changes in these biochemicals confirmed known alterations in glycolysis and other pathways previously identified in RBCs stored in SAGM (SAGM-RBC). Furthermore, we identified additional alterations not previously seen in SAGM-RBCs (e.g., stable pentose phosphate pathway flux, progressive decreases in oxidized glutathione), and we delineated changes occurring in other metabolic pathways not previously studied (e.g., S-adenosyl methionine cycle). These data are presented in the context of a detailed comparison with previous studies of SAGM-RBCs from human donors and murine AS1-RBCs.
Conclusion
Global metabolic profiling of AS1-RBCs revealed a number of biochemical alterations in stored blood that may affect RBC viability during storage as well as therapeutic effectiveness of stored RBCs in transfusion recipients.
Significance
These results provide future opportunities to more clearly pinpoint the metabolic defects during RBC storage, to identify biomarkers for donor screening and prerelease RBC testing, and to develop improved RBC storage solutions and methodologies.
Keywords: Metabolomics, blood storage, AS1-RBCs, transfusion
INTRODUCTION
Red blood cell (RBC) transfusion is the most common therapeutic procedure performed in hospitals. Based on current regulations, RBC units collected for transfusion may be stored up to 42 days at refrigerated temperature prior to infusion. There is extensive evidence that RBCs undergo changes in proteins, lipids and other cellular constituents during storage [1–5]. Additionally, recent clinical studies indicate that patients infused with RBC units stored for longer pre-transfusion periods have worse clinical outcomes than do patients transfused relatively fresher units [6–8]. Furthermore, there is donor-specific variability in RBC survival during storage [9, 10] which may produce donor-dependent differences in transfusion outcomes in recipients. The biologic mechanisms that underlie biochemical changes in RBCs during storage are still poorly understood.
Although RBCs do not express DNA, transcribe RNA, or synthesize proteins, they are highly metabolically active. Thus metabolomics, the global profiling of biochemicals produced and consumed in cellular enzymatic processes, may be a powerful approach to understand RBC physiology by providing data for a comprehensive curation of changes that occur in the numerous interrelated metabolic pathways affected by RBC storage [11, 12]. Furthermore, dissection and analysis of RBC metabolomics should be a relatively tractable problem, since the RBC metabolome is much simpler than that of other eukaryotic cells, which contain a variety of organelles not found in RBCs (e.g., nuclei, mitochondria and endoplasmic reticulum).
We are seeking to develop a comprehensive picture of the metabolic alterations that occur in banked AS1-RBCs in order to develop a system-level model of cellular changes during RBC storage [13–15]. In the present study, applying global metabolomic profiling to AS1-RBCs collected from volunteer donors and stored up to 42 days in refrigerated conditions, we identified a number of biochemicals whose concentrations changed significantly during RBC storage and which may affect RBC viability during storage or cause physiologic effects of stored RBCs on transfusion recipients. Additionally, we reviewed previous metabolomic investigations of stored human RBCs, which were primarily performed in SAGM storage solution, as well as murine RBCs stored in AS-1. Many of the kinetic changes we detected were previously seen in SAGM-RBCs. However, there were unique metabolic patterns seen in AS1-RBCs but not in SAGM-RBCs, and vice versa. This finding suggests that results from studies using stored SAGM-RBCs, such as the ARIPI trial [16], may not necessarily be applicable to the clinical use of AS1-RBCs. Additionally, we detected potentially important similarities and differences between AS-1 stored human and murine RBCs that have implications for using mouse models of RBC transfusion to accurately model human RBC storage.
MATERIALS AND METHODS
Collection and Processing of Human RBC Units
All protocols were approved by the Institutional Review Board at Emory University. Research donors were consented to donate whole blood, and were screened by health history questionnaire and vital signs. Nine donors were studied: six volunteers each donated 1 unit; three additional volunteers (Donors 850, 867, and 1145) donated two units with several months between donations. At the time of each donation, 500 mL (+/− 10%) whole blood was drawn by peripheral venipuncture into CPD-containing blood collection sets (Fenwal, Inc., Lake Zurich, IL), stored at 2–6°C for 1–3 hours, and centrifuged at 4500 RPM for 10 minutes at 4°C. Platelet-rich plasma was removed and the residual RBC pellet was mixed with AS-1 additive solution, leukoreduced using integral filters, and then stored at 2–6°C for up to 42 days. At selected time points, RBC bags were gently but thoroughly mixed, and 1 mL samples were aseptically removed, added to labeled cryovials, snap frozen on liquid nitrogen, and stored at −80°C. Samples from each study were stored until all time points were collected, and then all samples from that study were analyzed together.
Mass Spectrometry Analysis of RBC Samples
Samples were solvent extracted and split into equal parts for analysis with gas chromatography/mass spectrometry (GC/MS) and liquid chromatography/tandem mass spectrometry (LC/MS/MS). For the former analysis, samples are dried, derivatized using bistrimethyl-silyl-triflouroacetamide, and run on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. LC/MS/MS uses a Waters ACQUITY UPLC and a Thermo-Finnigan LTQ mass spectrometer, consisting of an electrospray ionization (ESI) source and linear ion-trap (LIT) mass analyzer. The sample extracts were dried and reconstituted in acidic or basic LC-compatible solvents, each of which contained 11 or more injection standards at fixed concentrations. One aliquot was analyzed using acidic positive ion optimized conditions and the other using basic negative ion optimized conditions in two independent injections using separate dedicated columns. Extracts reconstituted in acidic conditions were gradient eluted using water and methanol both containing 0.1% formic acid, while the basic extracts, which also used water/methanol, contained 6.5mM ammonium bicarbonate. The MS analysis alternated between MS and data-dependent MS2 scans using dynamic exclusion.
Specific compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. Visualization and interpretation software was used to confirm the consistency of peak identification between samples. The peak areas for each named biochemical were log transformed, scaled to the median value for each compound observed in the experiment, and normalized to Bradford protein content. The platforms used for this analysis did not allow absolute quantitation of metabolite levels; therefore, the data are presented as arbitrary expression units derived by normalizing each data point to the average value for that metabolite (labeled as “Normalized Units” on the figures). For the three studies described herein, instrument variability ranged from 5–6% and total process variability ranged from 13–18%.
Data Graphs and Statistical Analyses
The Y-axes for each figure represent normalized units for each biochemical on a linear scale, since absolute quantitation was not possible; the X-axes represent days of storage. In figures displaying population results, the majority of data points represent mean results +/− standard deviation from 12 RBC units donated by nine volunteers, while other points represent data from three or six units. Paired t-tests were used to test significance at p < 0.05.
RESULTS
AS-1 RBC units were prepared from six age-, race- and sex-matched volunteers, and the units were serially sampled over 42 days of storage to survey the global metabolic changes that occurred during RBC storage. To investigate the stability of each donor’s metabotype, the same analysis was performed on RBC units from three different matched donors and, subsequently, on additional units collected from these same three donors several months later. The library of identified biochemicals corresponding to m/z histogram peaks expanded with time: 185 biochemicals were specifically detected and quantified for the first set of six donors; 221 defined metabolites were identified when units from the next 3 donors were tested the first time, which increased to 264 metabolites when subsequent units from these volunteers were tested the second time. Results were combined for the analyses below, unless otherwise stated.
The process of storing AS1-RBCs under standard blood bank conditions elicited changes in a large percentage of identifiable metabolites. By 3 days after collection, 25 biochemicals had increased significantly (p ≤ 0.05) in concentration compared to day 0, while 6 had decreased significantly. After 42 days of storage, 56 and 47 metabolites had increased or decreased significantly, respectively. Most of the observed metabolic alterations described below are consistent with previous metabolomic analysis of SAGM-RBCs [17]; instances in which metabolic kinetics differed between AS1-RBCs and SAGM-RBCs are described in the Discussion.
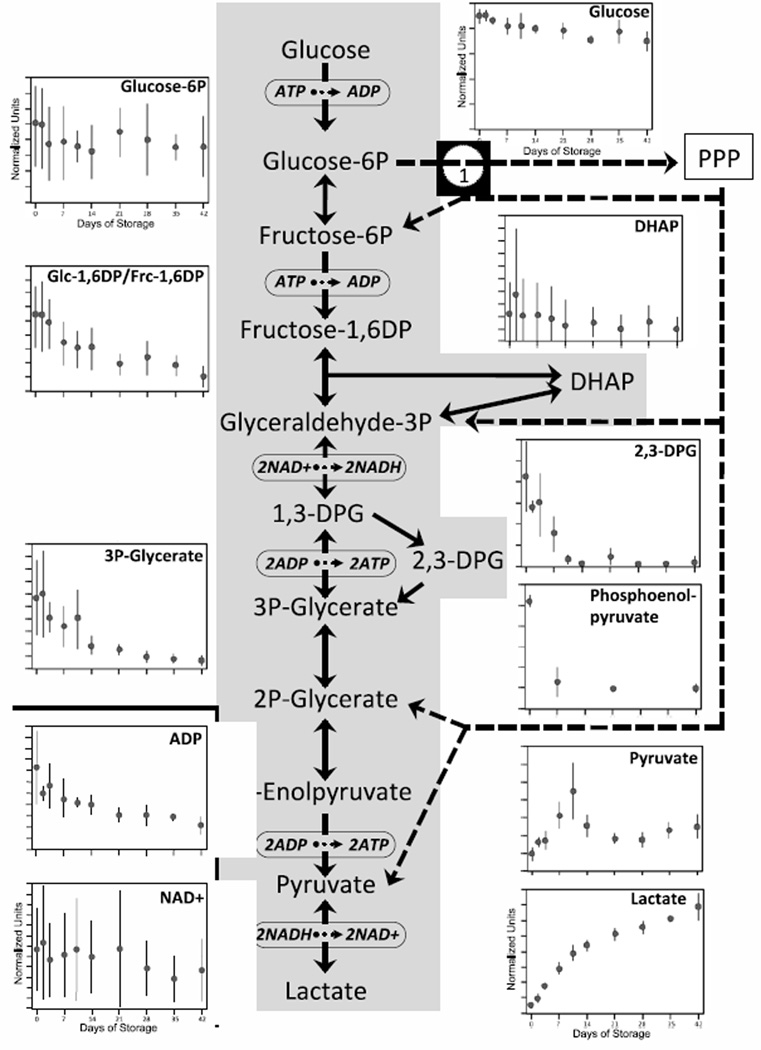
Glycolytic Pathway
All intermediary metabolites of the glycolysis pathway, including the Rapoport-Luebering shunt, were reduced throughout 42 days of storage, except for the terminal biochemicals pyruvate and lactate which were elevated (Figure 1). Glucose, which in blood units is primarily found extracellularly in the anticoagulant/preservative solution, is rapidly converted to glucose-6-phosphate (G6P) by hexokinase during internalization into RBCs, a step that requires ATP. We observed a small but consistent 23% decrease in glucose from day 0 to day 42 (P < 0.0001). Average G6P also decreased over the storage period (33% reduction from day 0 to 42; p=0.044). However, as compared to the changes in glucose, which were similar among the 5 units, there were up to 5-fold differences in G6P levels between different units at each time point leading to large observed standard deviations. As G6P is also a substrate in the pentose phosphate pathway (PPP; see below), donor differences in G6P levels may also reflect variable levels of metabolite shunting to this pathway.
Figure 1. Changes in glycolysis during 42-day storage of AS1-RBCs.
The glycolysis pathway, including points of interaction with the pentose phosphate pathway (PPP), is illustrated in the middle. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
In glycolysis, G6P is converted to fructose-6-phosphate (F6P), which is immediately upstream of phosphofructokinase (PFK), the key regulatory point and irreversible rate-limiting step of glycolysis (Fig 1). PFK utilizes ATP to convert F6P to fructose-1,6-diphosphate (F-1,6DP; detected as an isobar with glucose-1,6-diphosphate in this study). F-1,6DP decreased significantly (66%; P<0.00001) and consistently over the storage period. The precipitous fall in F-1,6DP in the face of only slightly reduced G6P levels may indicate that PFK activity is suppressed at later times of storage. PFK is negatively regulated by falling pH, ATP and 2,3- DPG levels, changes that have been well-documented during RBC storage [18–20]. Alternatively, G6P may be shunted to the PPP, depriving PFK of substrate.
F-1,6DP is split into two 3-carbon sugars, glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP), which can be interconverted by triosephosphate isomerase (TPI). While G3P was not specifically identified in this analysis, DHAP was quantified and showed a 57% reduction during storage (p=0.037) with marked donor variability. The conversion of G3P to 1,3-diphosphoglycerate (1,3-DPG, not quantified in this analysis) and then to 3-phosphoglycerate (3PG) initiates the pay-off phase of glycolysis, producing twice the amount of ATP consumed by hexokinase and PFK (Fig 1). Alternatively, 1,3-DPG can be converted to 2,3-DPG, which yields an important allosteric regulator of hemoglobin oxygen affinity but with the tradeoff of reduced ATP production. Overall reductions in 2,3-DPG and 3PG over the storage period were 94% and 87%, respectively, both of which were highly significant (p<0.0001 and p<0.01). 3PG is converted to 2-phosphoglycerate and then to phosphoenolpyruvate (PEP), which forms the substrate for the irreversible production of pyruvate and ATP by pyruvate kinase. We found a very significant 83% reduction in PEP (p < 0.001) which was apparent by 7 days of storage, and was very consistent between donors. The kinetics we observed for PEP are very similar to those of 3PG, consistent with the fact that 3PG, 2PG and PEP are interconvertible and thus in relative equilibrium.
In the terminal stages of glycolysis, producing the final yield of ATP, pyruvate and lactate showed highly significant 1.5- and 12-fold increases during storage, respectively (p< 0.0001 and p<0.000001). The reproducible two-phase kinetics observed for pyruvate metabolism suggest that pyruvate kinase activity, and thus ATP production, decline after 10 days of storage. However, this possibility will require more detailed investigations to map metabolite flux through glycolysis.
The GC/MS and LC/MS/MS methods used in this study detected adenosine diphosphate (ADP), but not ATP; similarly, these methods detected the oxidized form of nicotinamide adenine dinucleotide (NAD+) but not the reduced form (NADH). Mean ADP levels declined 64% during RBC storage (p< 0.01), and were consistent between donors. NAD+ decreased over 42 days of storage (p<0.05), but donor-donor variability was seen.
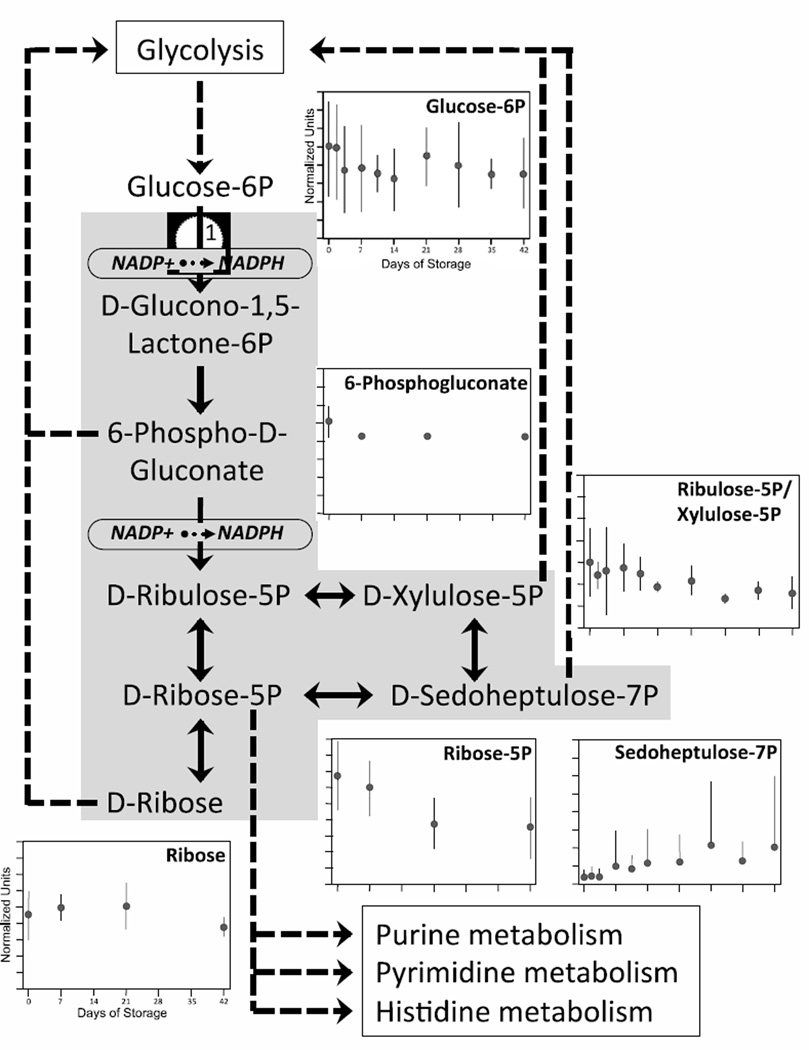
Pentose Phosphate Pathway (PPP)
PPP and glycolysis are closely interrelated metabolic pathways, with products from one pathway serving as substrates in the other (dotted lines in Figs 1 and 2). Under normal circumstances, up to 92% of glucose is utilized in glycolysis to produce ATP, while under oxidative conditions 90% of glucose can be shunted through PPP to produce NADPH in order to maintain levels of reduced glutathione (GSH) [21–24]. PPP is responsible for the majority of NADPH produced in the cell, yielding 2 molecules of NADPH for each G6P molecule that enters the pathway.
Figure 2. Changes in PPP during 42-day storage of AS1-RBCs.
PPP, including points of interaction with glycolysis, purine metabolism, pyrimidine metabolism, and histidine metabolism, is illustrated in the middle. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
In the initial oxidative phase of PPP, G6P from glycolysis is converted to D-glucono-1,5-lactone- 6P (G1,5L6P) by glucose-6-phosphate dehydrogenase (G6PD), a reaction that yields NADPH (Fig 2). G6PD (reaction # 1, Figs 1 and 2) is the rate-limiting enzyme in PPP. Deficiency of G6PD represents the most common human enzyme defect, occurring in 400 million individuals worldwide, likely because of the protection it confers against malaria [25]. While G6PD deficiency has been found in about 0.3% of randomly selected blood donors [26], the limited donor variability in PPP metabolites downstream of G6PD suggests that none of our study donors were G6PD deficient.
At the conclusion of the oxidative phase, G1,5L6P is converted to 6-phospho-D-gluconate (6PG) and then to D-ribulose-5P (R5P), with accompanying NADPH production. Over the storage period, an average 17% reduction in 6PG (p < 0.05) and 53% reduction in R5P (which was detected as an isobar with D-xylulose-5P [X5P]; p < 0.0001) were observed (Fig 2). In the second (non-oxidative) phase of PPP 5-carbon sugars are synthesized and interconverted. In addition to R5P and X5P, these sugars include D-ribose-5P, D-sedoheptulose-7P (SH7P), and D-ribose which were reduced 46% (p< 0.00001), increased 580% (p< 0.05), and decreased 4% (p > 0.05), respectively, during RBC storage. As shown in Fig 2, these 5 carbon sugars can be shunted into the glycolysis pathway.
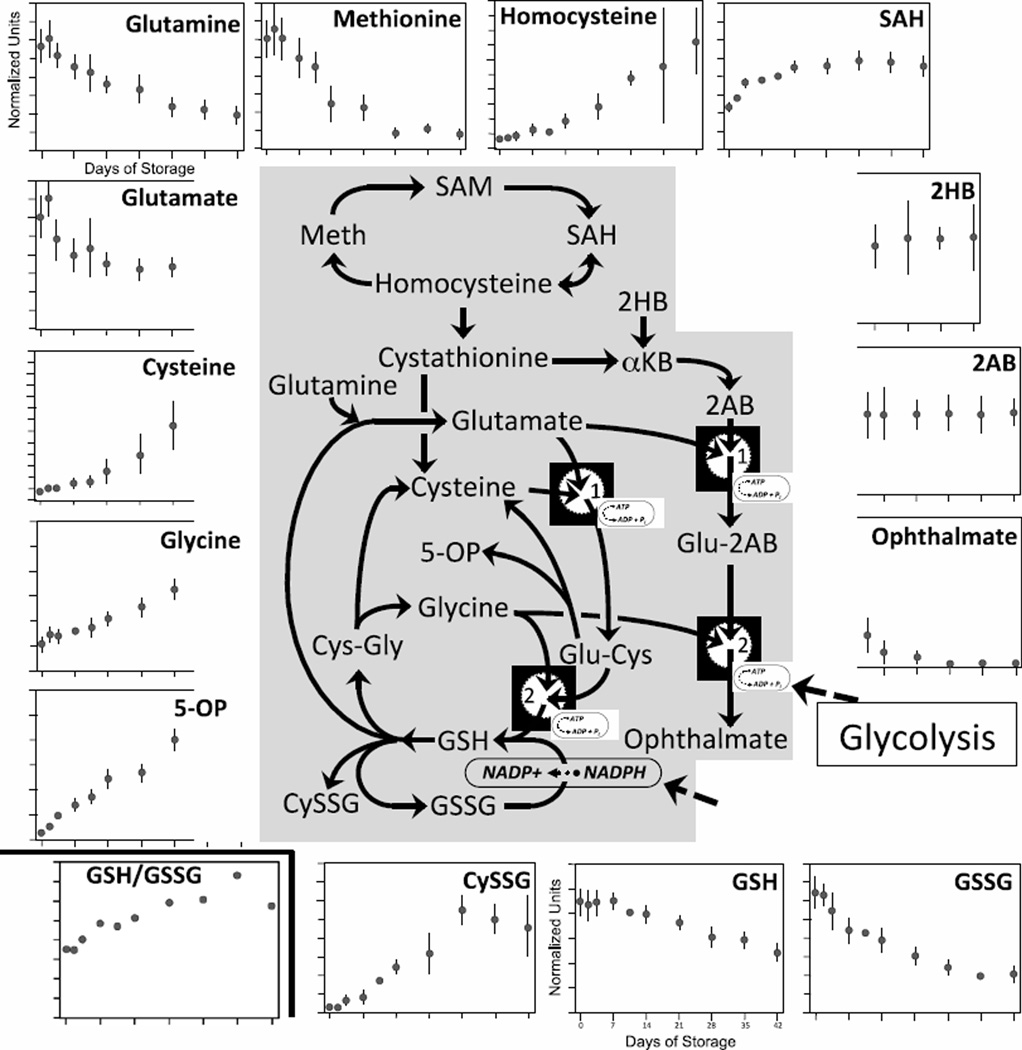
Glutathione Antioxidant Pathways
Banked RBCs are particularly susceptible to oxidative damage since they contain high intracellular concentrations of oxygen, continuously produce reactive oxygen species, and the efficacy of their antioxidant mechanisms declines as cellular energy stores decrease during storage [27, 28]. One of the primary intracellular antioxidants, GSH, is oxidized to glutathione disulfide (GSSG) during inactivation of oxygen radicals. While the pathways that regulate intracellular GSH levels are complex (Fig. 3), it is important to understand the dynamics of these pathways in order to develop improved approaches to augment GSH levels in stored RBCs [29, 30].
Figure 3. Changes in GSH synthesis pathways during 42-day storage of AS1-RBCs.
The GSH pathway, including points of interaction with glycolysis and PPP, is illustrated in the middle. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
At the core of these pathways, gamma-glutamylcysteine synthetase (GCS; reaction # 1 in Fig 3) catalyzes the ATP-dependent conversion of glutamate and cysteine into gamma-glutamylcysteine (Glu-Cys). GCS is the rate-limiting enzyme in GSH synthesis, and is subject to product inhibition by GSH [31]. Subsequently, Glu-Cys can then either combine with glycine to form GSH, in an ATP-dependent reaction catalyzed by glutathione synthetase (GS; reaction # 2 in Fig 3), or be broken down into cysteine and 5-oxoproline (5-OP). In most eukaryotic cells, 5-OP is then converted back to glutamate by ATP-dependent 5-oxoprolinase, producing a “futile cycle” that repetitively interconverts glutamate, cysteine and Glu-Cys, consuming ATP in the process. For reasons unclear, this futile cycle appears to be the dominant flux through the GSH synthesis pathway in most cells [32]. However, in RBCs 5-oxoprolinase is absent, breaking the cycle and rendering 5-OP a metabolic “dead-end”.
Over 42 days of RBC storage, GSH and GSSG levels declined 43% and 65%, respectively (p < 0.0001 for both). Under physiological circumstances, the decreasing levels of GSH should relieve feedback inhibition of GCS, leading to normalization of GSH levels [33]. However, the observed ongoing reductions in GSH levels indicate that prolonged storage of RBCs disrupts these complex regulatory pathways.
One potential cause for declining GSH could be reduced amino acid substrates. In fact, in previous studies GSH levels were better preserved in RBC units supplemented with GSH precursors [34]. However, our data lead us to question this explanation. In circulating RBCs, glutamate and glycine are both present at about 350–450 micromolar baseline concentrations [35], while cysteine is rate-limiting at about 5 micromolar [33, 36]. As compared to baseline conditions, cysteine and glycine levels both increased during storage (11-fold and 3-fold, respectively; both p < 0.0001). While a third GSH precursor, glutamate, decreased 35% during storage (p < 0.001), the totality of glutamate reduction was complete during the first week of storage, a time during which GSH levels were stable. The decreased glutamate level may be due to reductions in glutamine (67%; p < 0.00001), a glutamate precursor [37], or as mentioned above to the absence of 5-oxoprolinase which prevents the conversion of rising levels of 5-OP to glutamate. Given the likely sufficiency of endogenous amino acid precursors for GSH synthesis, it’s unclear why supplementation increased GSH synthesis in previous studies [34]. However, those investigators did not quantify cysteine, glycine or glutamate during RBC storage and it is possible that these precursors were depleted under their storage conditions.
It is also possible that reduced GSH synthesis may be due to alternative metabolic pathways competing for GSH precursors, such as the pathway that uses alpha-ketobutyrate (KB; derived from breakdown of cystathionine to form cysteine) and 2-hydroxybutyrate (2HB) to form 2-aminobutyrate (2AB) and then ophthalmate [38, 39]. Both the GSH and ophthalmate synthesis pathways require the GCS and GS enzymes (denoted as reactions # 1 and # 2, respectively, in Fig 3). However, the relatively constant levels of 2HB (reduced 4%, p = ns) and 2- aminobutyrate (2AB; increased 27%, p < 0.001) seen during storage (KB was not quantified in this analysis), along with the changes in GSH precursors described above, argue against this suggestion.
As an alternative possibility, the marked reductions in ophthalmate (reduced 96%, p < 0.0001) in parallel with the 43% reductions in GSH point to impaired GCS and/or GS activity during later RBC storage times. Possible reasons for reduced enzyme activity during RBC storage include ATP depletion, as both enzymes are ATP-dependent [34]. Since the “dead-end” metabolite 5- OP steadily rose 15-fold during storage (p < 0.00001), it seems likely that GCS continues to catalyze the conversion of glutamate and cysteine to form Glu-Cys, but that impaired GS activity shunts Glu-Cys to be broken down into cysteine and 5-OP. Because GSH provides feedback inhibition of GCS enzyme, falling GSH levels may further accentuate 5-OP production [31].
Ongoing inactivation of reactive oxygen species (ROS) during RBC storage also likely contribute to the decline in GSH levels, since GSH is consumed during these activities. Superoxide (O2−), a prominent cellular oxidant, is converted by superoxide dismutase to hydrogen peroxide (H2O2). H2O2 can then be inactivated either by catalase, forming H2O and O2, or by the enzyme glutathione peroxidase which combines H2O2 and GSH to form H2O and GSSG. GSSG is not an antioxidant, but can be reconverted to GSH by glutathione reductase, in a reaction that utilizes NADPH reducing-equivalents derived from the PPP.
Although we did not quantify cystine or cystathionine (cysteine and GSH precursor) in this analysis, homocysteine increased 10-fold during storage (p < 0.0005), while levels of Sadenosyl homocysteine (SAH), with which it can be interconverted, rose 84% (p < 0.00001). During the same timeframe there was a 90% decrease in methionine (p < 0.00001), indicating a block in the conversion from homocysteine, which is discussed in more detail below.
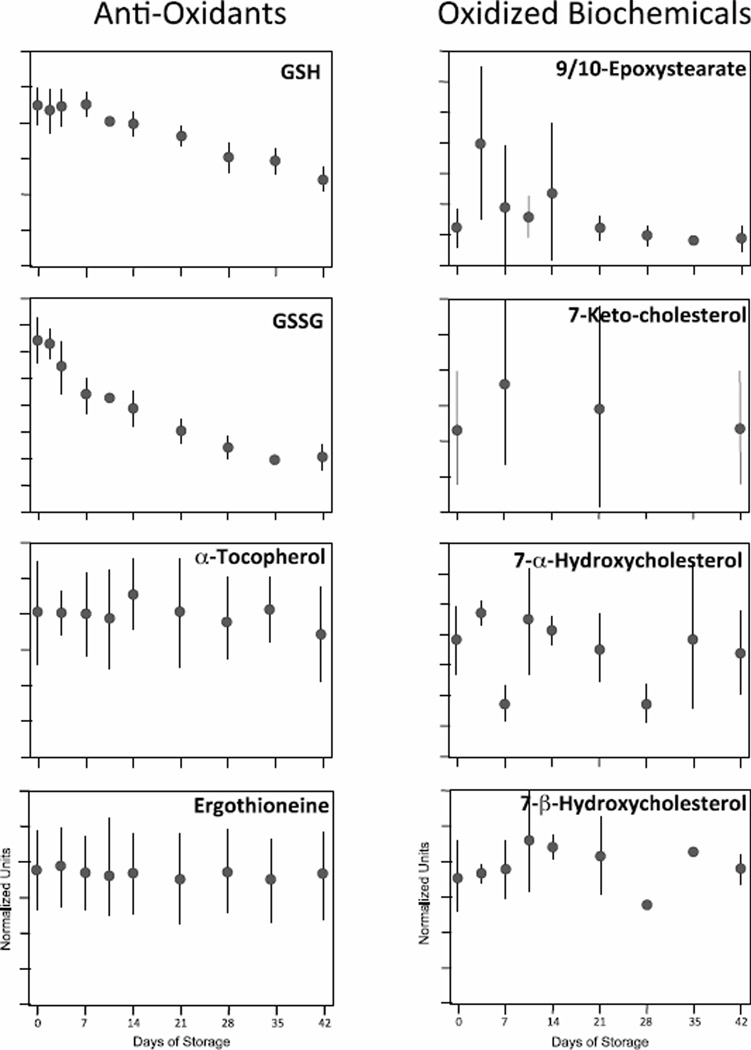
Antioxidants and oxidized biochemicals
A surprisingly large percentage of non-hemoglobin RBC proteins appear to play a role in the cellular response to oxidative stress [40]. However, despite the emphasis on redox control, ROS increase progressively in stored SAGM-RBCs reaching a plateau at day 21 that is maintained through day 42 [41]. In addition to GSH, two other antioxidants detected in our study are alpha-tocopherol (Vitamin E) and ergothioneine (Fig 4). Consistent with the fact that both of these biochemicals are obtained primarily, if not exclusively, from the diet, and not synthesized or catabolized in RBCs, average levels varied significantly at each time point due to donor-specific differences, but remained stable throughout storage.
Figure 4. Changes in anti-oxidant levels and oxidized metabolites during 42-day storage of AS1- RBCs.
Anti-oxidants (including GSH and it oxidized form, GSSG) are illustrated on the left, while a selected number of oxidized biochemicals are on the right. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
9,10-epoxystearate is an oxidized lipid while 7-ketocholesterol, 7-alphahydroxycholesterol and 7-betahydroxycholesterol are oxidized forms of cholesterol. These species can be used to gauge oxidative stress (the three cholesterol derivatives arise from auto-oxidation, although 7-alphahydroxycholesterol can also be enzymatically oxidized in the liver) [42]. Substantial variability was seen in these oxidized species, both in terms of changes during the storage period, and in donor-to-donor differences. Generally, there were increases in the species early during storage with a decline in their levels at later storage times. There were no obvious correlations between levels of anti-oxidants and levels of oxidized biochemicals in stored RBCs.
Synthesis of plasma membrane lipids
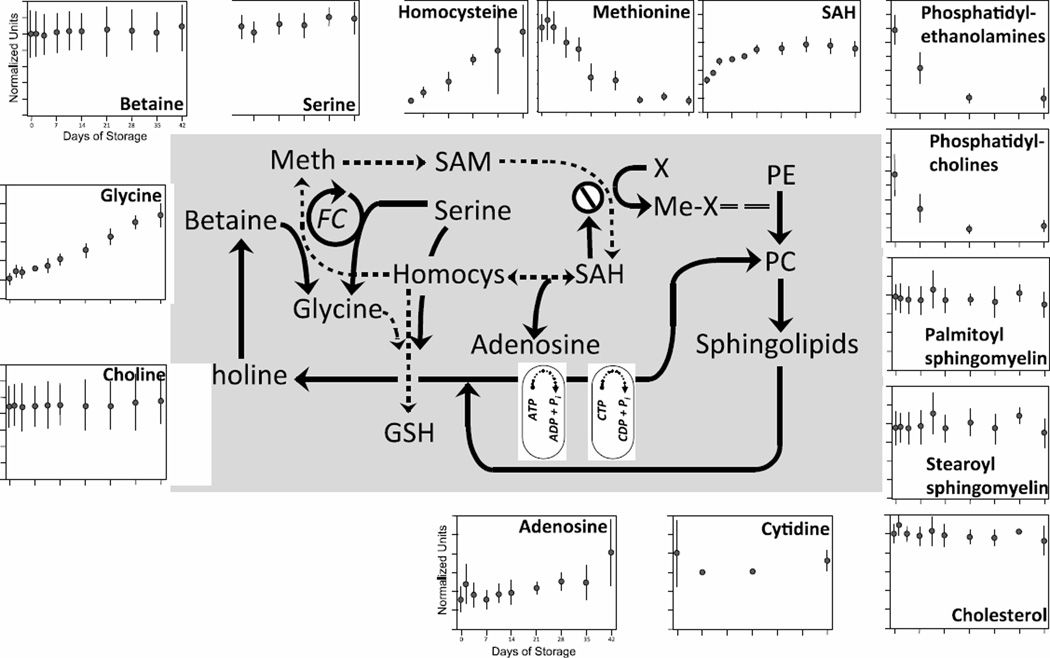
Lipids, primarily phospholipids and cholesterol, are the main constituents of the RBC membrane. The phospholipids critical to plasma membrane structure and function can be divided into phosphoglycerides (phosphatidylethanolamines [PE], phosphatidylcholines [PC], phosphatidylserines, and phosphatidylinositols [PI]) and sphingomyelins. In erythroid precursors, phospholipid synthesis involves contributions from both mitochondria and endoplasmic reticulum, as in other cell types. However, mature RBCs have neither mitochondria nor endoplasmic reticulum, indicating that this synthetic pathway is absent from mature RBCs in stored blood. There is evidence that mature RBCs have alternate pathways for synthesis, exchange and/or repair of PE, PC, and other membrane lipids [43]. However, these pathways may require greater quantities of plasma lipoproteins than are present in stored RBCs, possibly accounting for the significant decline in both PE and PC levels observed during storage (Fig 5; 76% and 72%, respectively; p < 0.005 and p < 0.05). Previous studies have likewise described progressive loss of RBC membrane lipids during storage, due in part to lipid peroxidation [3, 41].
Figure 5. Changes in SAM cycle and lipid expression during 42-day storage of AS1-RBCs.
The SAM cycle and its points of interaction with lipid synthesis pathways is illustrated in the middle; the portion of the SAM cycle previously illustrated in Figure 3 is shown here with dotted lines for clarity. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
In contrast to PE and PC, cholesterol levels are essentially unchanged during storage. As cholesterol is present in comparable amounts to phospholipids, the persistence of cholesterol may help to stabilize and maintain the fluidity of the RBC plasma membrane in the face of declining phospholipid levels.
The S-adenosyl methionine (SAM) cycle, which provides cysteine to support GSH synthesis as shown in Fig 3, also interacts with metabolic pathways of phospholipid synthesis (Fig 5; pathways previously described in Fig 3 are illustrated in Fig 5 with dotted lines for clarity) [44]. The conversion of SAM to SAH provides methyl groups for the majority of cellular methylation reactions [44], including the three methylation reactions involved in the conversion of PE to PC via the phosphatidylethanolamine methyltransferase (PEMT) pathway. While SAH is converted to homocysteine and adenosine during RBC storage, based on the observed 10-fold and 1.5- fold increases in these two products (p < 0.0005 and p < 0.001), SAH levels also increase.
Declining methionine levels during storage, along with elevations of SAH and homocysteine, suggest suppression of one or both of the pathways that remethylate homocysteine to form methionine. One pathway utilizes betaine as a substrate for homocysteine methylation. Levels of betaine did not change during storage, which indicates that its production and consumption are balanced, but does not address the degree of remethylation activity. None of the elements of the other pathway, the folate cycle which uses N5-methyltetrahydrofolate as a substrate for methionine synthase, were identified. Both remethylating pathways produce glycine which increased 3-fold during storage (p < 0.0001). While this observation supports the possibility of some homocysteine methylation during RBC storage, glycine is also processed in other metabolic pathways (eg, as a product of the metabolism of glutathione-conjugated compounds) so its elevation cannot definitively be ascribed to homocysteine remethylation.
PC can give rise to sphingolipids. Two sphingomyelins (palmitoyl and stearoyl sphingomyelin) were detected in this analysis, and neither showed significant changes during storage. PC, sphingolipids and choline are also interconvertible. Like sphingomyelin, levels of choline were essentially unchanged during storage, as were levels of cytidine which in the form of CTP is required for PC synthesis from PE in the Kennedy pathway, an alternative to PEMT synthesis.
Signaling and bioactive lipids
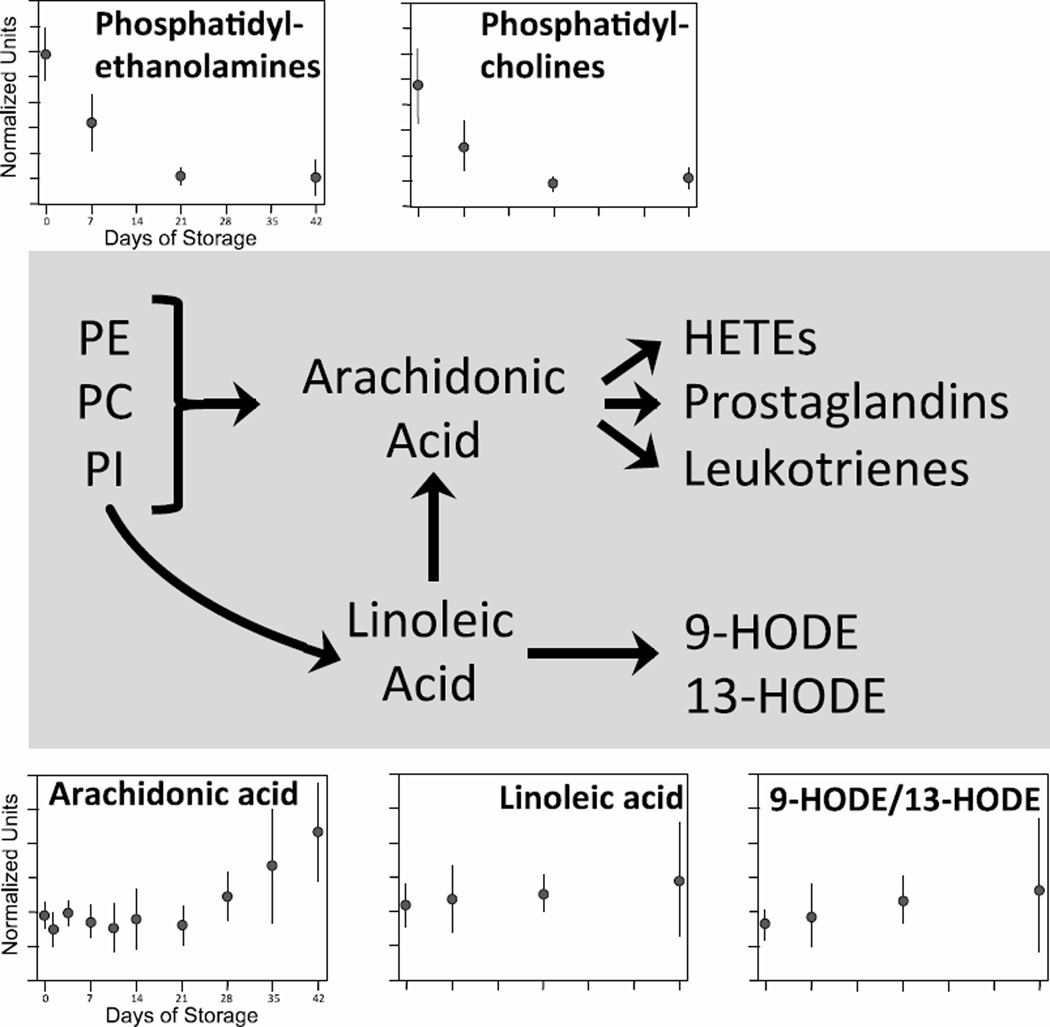
PE, PC and PI can be enzymatically converted to arachidonic acid (AA), a polyunsaturated fatty acid which can be further metabolized to bioactive eicosanoids including hydroxyeicosatetraenoic acids (HETEs), prostaglandins, and leukotrienes. PI can also be converted to linoleic acid and then to either AA or HODEs (Fig. 6). In stored RBCs, the declining levels of PE and PC may lead to AA production, the levels of which increased 126% on average over 42 days (p < 0.001). Changes in linoleic acid, 9-HODE and 13-HODE did not change significantly during RBC storage. While membrane phosphoinositides can also be metabolized to produce second messengers such as inositol triphosphate and diacylglycerol (DAG), none of these biochemicals were identified in our analysis.
Figure 6. Expression of inflammatory mediators during 42-day storage of AS1-RBCs.
The pathways for production of selected lipid-derived inflammatory mediators are illustrated in the middle. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
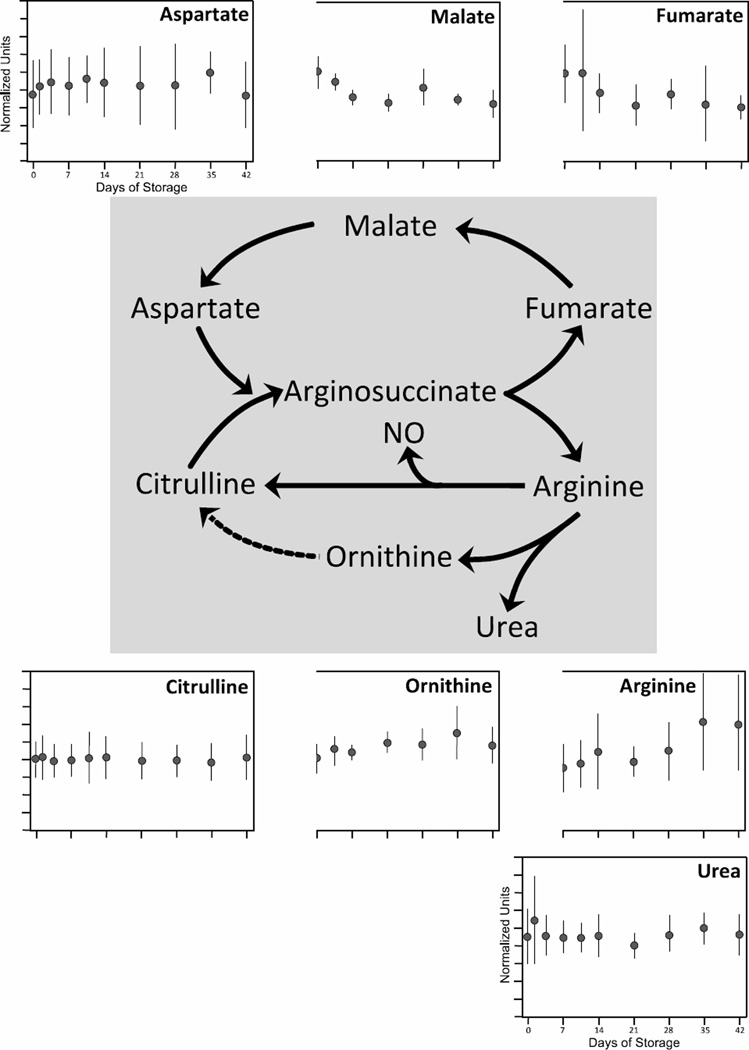
Urea cycle
The urea cycle (Figure 7) converts ammonium to urea, a less toxic biochemical. Since RBCs lack mitochondria, and thus the mitochondrial enzymes carbamoyl phosphate synthetase I and ornithine transcarbamoylase which convert ammonium, bicarbonate and ornithine to citrulline (dashed arrow), they have an incomplete urea cycle [45]. Consistent with this biology, the citrulline precursors arginine and ornithine increased 128% and 57% respectively (p < 0.01 and p < 0.05, respectively) during RBC storage. While increases in these biochemicals may suggest increased urea production during storage, urea levels remained relatively stable (p > 0.05). The other metabolites in this pathway were likewise relatively stable over 42 days of RBC storage, except for a 39% reduction in fumarate levels (p < 0.01).
Figure 7. Changes in the urea cycled during 42-day storage of AS1-RBCs.
The urea cycle is illustrated in the middle; the dotted line illustrates the portion of the cycle that takes place in mitochondria and is therefore absent in mature RBCs. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
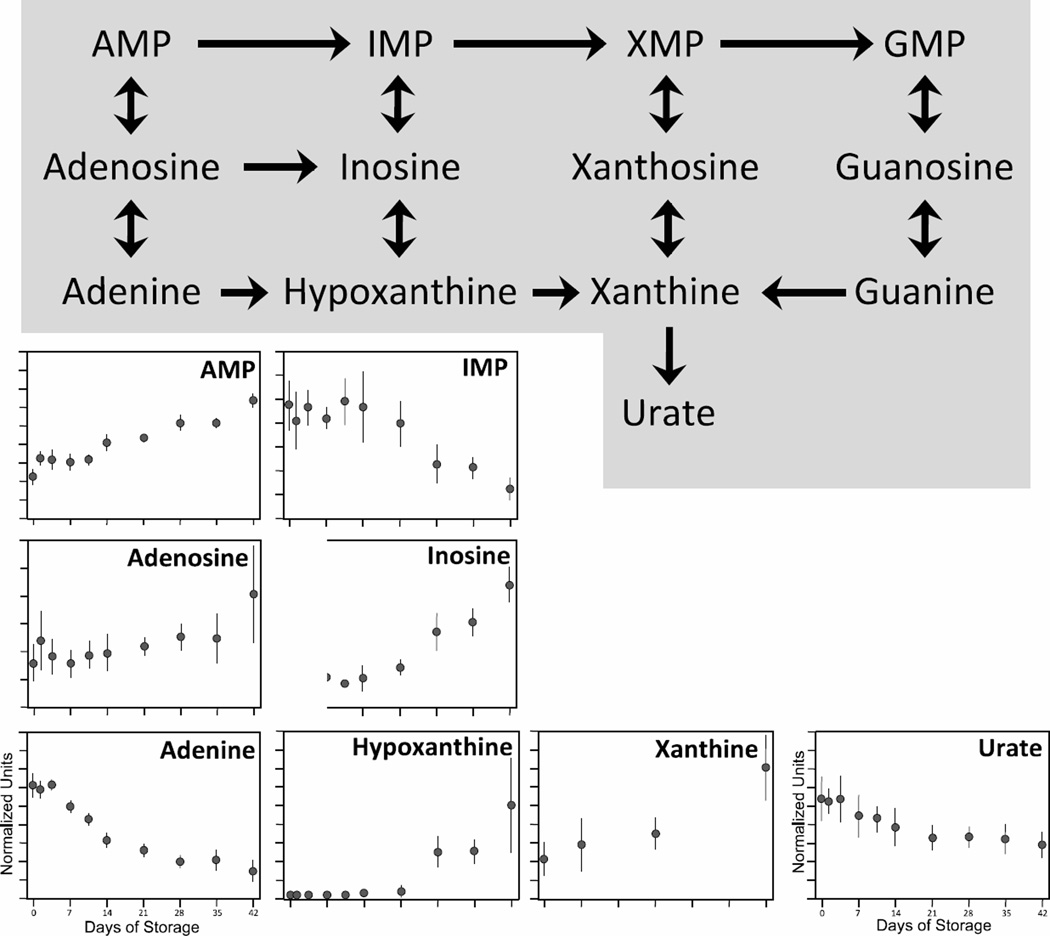
Nucleotide metabolism
Nucleotide salvage pathways are a critical feature of erythrocyte metabolism, since mature RBCs cannot synthesize ATP de novo but must recycle nucleosides and bases to provide substrates for ATP production [46]. Adenine, like glucose, is a key component of the red cell storage solution and is added to the RBCs at high levels during processing. Similar to changes in glucose levels seen in the glycolytic pathway (Figure 1), adenine levels also declined progressively during storage from their high day 0 levels (77% reduction, p < 0.0001; Figure 8). Nonetheless, high initial concentrations of adenine appear to drive significant flux through the biochemical pathways for purine salvage. Progressive increases in adenosine (150%; p < 0.005), adenosine monophosphate (AMP; 162%; p < 0.0001), inosine (203%; p < 0.0001), hypoxanthine (63-fold; p < 0.0005) and xanthine (247%; p < 0.005) are observed over 42 days of storage. The only exception was inosine monophosphate (IMP), which decreased 74% by day 42 (p < 0.0001). Other nucleotide intermediates (xanthine monophosphate [XMP], xanthosine, guanine monophosphate [GMP], guanosine, and guanine) are shown in Fig 8 for completeness, although they were not detected in our analysis.
Figure 8. Changes in purine metabolism during 42-day storage of AS1-RBCs.
The purine salvage pathway is illustrated at the top. Each inset figure displays levels of a different biochemical identified by metabolomic analysis (metabolite name above graphs). The Y-axes are in normalized expression units, while the X-axes represent days of storage. Each data point represents 3–12 units +/− SD.
The levels of adenosine, AMP, IMP, inosine and hypoxanthine were all relatively stable for the first 14 days of storage, at which time each of these biochemicals showed an inflection point followed by significant increases or reductions in levels. The biochemical changes underlying these kinetics are unclear, but seem unlikely to be related to adenine levels which had already declined about 50% by day 14 of storage.
Validation of results with an alternative platform
A subset of samples analyzed in this study were subsequently retested using an “undirected” metabolomics platform that detected nearly 20,000 discrete compounds, the majority of which are currently unidentified [47, 48]. The latter results confirmed the findings of our initial work (in cases where the same metabolite could be accurately identified with both systems) and also revealed hundreds of additional unidentified biochemicals that correlated with other metabolic changes during RBC storage and that represent interesting targets for future investigations of the physiologic changes that occur during RBC storage (data not shown; manuscript in preparation).
DISCUSSION
Biochemical measurements of selected metabolites during RBC storage have been performed for decades, and have been pivotal to improvements in storage solutions and methodologies [19]. However, much of this work only provided a limited glimpse of storage-related changes in RBC metabolism, because only a small number of metabolites could be accurately measured through enzymatic assays.
The “omics” revolution has produced a leap forward in experimental possibilities for probing cellular physiology at multiple levels: genomic, transcriptomic, proteomic, and metabolomic. Of these approaches, metabolomics is particularly well-suited to study RBC physiology since mature erythrocytes do not contain DNA or mRNA, and do not synthesize new proteins, but are highly active metabolically. Furthermore, changing fluxes through metabolic pathways during RBC storage likely precede other cellular changes that determine RBC survival and recipient effects such as the inhibition of vasodilation by stored AS1-RBC which we noted previously [18].
Metabolomics analysis uses the combination of chromatography and mass spectrometry to simultaneously quantify numerous small molecule metabolites present in experimental samples, including amino acids, sugars, lipids, and other endogenous biochemicals as well as xenobiotic agents. In the present study, we used a commercially available service to quantify nearly 300 metabolites (whose biochemical identities had been previously validated) in AS1-RBC units stored for 42 days under refrigerated conditions.
Primary findings of our studies
Previous metabolomics studies on stored RBCs were performed with SAGM additive solution, which as compared to AS-1 has lower concentrations of sodium chloride (8.77 vs. 9.00 g/L), dextrose (9.00 vs. 22.00 g/L), adenine (0.169 vs. 0.270 g/L), and mannitol (5.25 vs. 7.50 g/L). There are impairments in metabolic flux through glycolysis during AS1-RBC storage (Figure 1), as has been reported previously for SAGM-RBCs [17]. In particular, the marked reductions in metabolites downstream of PFK (the key regulatory point in glycolysis) in the face only modest reductions in glucose, indicate impaired PFK activity during storage. This may be due to a combination of reduced pH and ATP. Additionally, the interactions between PFK and the Band 3 membrane protein, which normally regulate enzyme activity, could be disrupted during RBC storage [49]. Suppression of glycolysis reduces production of ATP and 2,3-DPG, metabolites critical for RBC viability and function. Of note, rejuvenation raises pH and bypasses the putative block at PFK by feeding PEP and PPP intermediates directly into the latter part of glycolysis to increase ATP production [50, 51]. While highly effective at increasing ATP levels, rejuvenation is labor-intensive and difficult to implement for routine use. Alternatively, our data suggest that ATP production might be improved, without requiring rejuvenation, if approaches to increase PFK activity could be developed.
PPP (Figure 2) represents an interface between glycolysis and the pathways that regulate of GSH antioxidant levels (Figure 3). The relatively stable levels of PPP biochemical intermediates argues against significant dysregulation of this pathway, including NADPH synthesis, during AS1-RBC storage. However, GSH synthesis does appear to be disrupted: declining GSH levels should relieve feedback inhibition on the pathway, leading to increased GSH synthesis, but this effect was not observed. There are recent data indicating that supplementation of RBC units with amino acid precursors can stimulate GSH synthesis, however this approach will require more investigation to determine whether it has a beneficial effect on RBC function [34].
Since the RBC cytoplasm represents a strongly oxidizing environment, changes in antioxidant activity during storage may have a marked affect on cell viability. Many investigators consider the GSH/GSSG ratio to best reflect the reducing power in the cell [52]. This ratio increased by 70% (p < 0.001; see Fig 3) suggesting that GSH anti-oxidant activity may be relatively well maintained over 42 days of AS1-RBC storage. In contrast, others have suggested total free GSH (which equals GSH + 2×GSSG) as an alternative estimate of the cellular redox capacity of a cell [53]. By this measure, there is a decline of about 60% with storage (p < 0.00001), possibly resulting in impaired antioxidant activity. However, this latter calculation fails to take into account cysteine-glutathione disulfide (CySSG), a mixed disulfide with potent protective effects against acetaminophen liver toxicity [54], that increased by 11-fold over 42 days of storage (p < 0.005). Additionally, dietary antioxidants including alpha-tocopherol and ergothioneine can play a protective role (Figure 4), and their levels were maintained during storage.
As an alternative approach to assess antioxidant function during AS1-RBC storage, we examined our data for changes in markers of oxidative damage (Figure 4). Since the levels of 9,10-epoxystearate, 7-ketocholesterol, 7-alphahydroxycholesterol and 7-betahydroxycholesterol were quite variable over 42 days, no firm conclusions could be drawn from these metabolites. However, the substantial decreases observed in PE and PC, and the increase in AA, could indicate ongoing lipid oxidation (Figures 5 and 6) [3, 41]. Collectively, these data in conjunction with recent evidence that supplementation with the antioxidant ascorbic acid improves murine RBC storage [55] indicate that methods to supplement GSH and other antioxidant molecules in stored RBCs could have beneficial effects on transfusion therapy.
Since a functioning SAM cycle is required for both GSH synthesis and cellular methylation reactions (Figures 3 and 5) [44], it was surprising to observe a block in this cycle during storage that disrupted homocysteine remethylation to methionine, thus preventing regeneration of SAM. This metabolic defect was previously unrecognized in stored RBCs. While this disruption may not affect the GSH pathway, since cysteine levels continue to rise during storage, the combination of reduced SAM levels and elevated SAH, which inhibits SAM-dependent methylation, indicates that methylation-dependent pathways are likely impaired in stored RBCs [44, 56, 57]. Additionally, high levels of homocysteine may also reduce NO bioavailability [58]. The mechanisms underlying the SAM cycle block, as well as any effects of this disruption on RBC function, will require additional study.
As with the SAM cycle, global changes in the urea cycle (Figure 7) have not previously been investigated in stored RBCs. This cycle may be important for hypoxic vasodilation [59], since RBCs contain an endothelial type nitric oxide synthetase (eNOS) that converts arginine to citrulline with the elaboration of NO [60, 61]. Earlier work suggested that supplementing RBCs with arginine as an eNOS substrate can improve RBC membrane deformability presumably through elevated erythrocyte NO production [62, 63]. However, more recent studies have not shown an effect of arginine supplementation on stored RBCs [64] possibly because arginine, as shown in our data, does not diminish during RBC storage but instead progressively increases over 42 days.
The purine pool in RBCs is limited since mature erythrocytes cannot synthesize these nucleotides de novo, but must interconvert them. The high level of adenine added to RBCs during processing drives significant flux though the nucleotide salvage pathways (Figure 8), producing adenosine and AMP which support ATP production during the early storage period. However, these nucleotides are also converted irreversibly to IMP, inosine, hypoxanthine, and biochemicals shown further to the right in Figure 8 [65]. These latter purines thus represent a pool of nucleotides no longer available for ATP synthesis [46], which may represent a contributing factor for the declining ATP levels observed later during storage.
Comparison with other metabolomics investigations of stored human RBCs
While previous investigations have reported global metabolic profiling of stored SAGM-RBCs [17, 40, 41, 66], the present study is the first to describe metabolomics of human RBCs stored in AS1. In addition, our work differs from earlier studies in three other respects. First, we observed changes in a subset metabolites, averaged over 9 donors (12 donations), that were kinetically different from previous work on SAGM-RBCs. Second, we identified donor variability that had not been described before. And, third, we investigated some biochemical pathways that were not metabolically profiled in earlier work.
In the first category, kinetics of metabolic flux through glycolysis and PPP differed from previous investigations. For example, in SAGM-RBCs PEP levels were largely stable or modestly reduced [17], while we saw a very significant 83% reduction in PEP (p< 0.001) that was apparent by 7 days of storage, and was similar between donors. The kinetics we observed for PEP are comparable to those of 3PG, consistent with the fact that 3PG, 2PG and PEP are interconvertible and thus in equilibrium. In prior studies, pyruvate levels increased steadily [17], while in contrast we observed reproducible two-phase kinetics in pyruvate levels, with a peak at day 10 followed by a 50% decline and stabilization throughout the remainder of storage.
Under normal circumstances, RBCs can rapidly redirect metabolic flux through either glycolysis (yielding ATP) or PPP (producing NADPH) [21–23]. In earlier studies, stored SAGM-RBCs demonstrated a significant and stable increased flux through PPP [17]. Interestingly, while this shift should have lead to increased production of NADPH, these investigators also identified large increases in GSSG indicating that NAPDH levels were insufficient to maintain reduced GSH [17]. In contrast, we saw neither markedly increased flux through PPP nor an increase in GSSG. These results suggest that under AS-1 storage conditions, NADPH is produced in sufficient amounts to recycle GSSG to GSH without significant shunting of glycolytic metabolites through PPP.
Our investigations also identified a number of donor-specific differences (metabotypes) that were not described in earlier work. In the present analysis, these differences were manifest as large standard deviations in a small number of metabolites, not seen for the remainder of biochemicals. For example, in the glycolytic pathway we observed 5- to 10-fold differences in G6P, DHAP and NAD+ levels between different units at comparable time points. Substantial variability was also seen in other metabolites, including S7P, alpha-tocopherol, ergothioneine, 7-keto-cholesterol, and AA. In subsequent analysis, the wide variations seen for selected metabolites were due to individual donors whose metabolic kinetics differed significantly from other donors (data not shown; manuscript in preparation). The possibility that these differing metabotypes correlate with physiologic behavior of stored RBCs is under investigation.
The current study also presents a more comprehensive analysis of metabolic pathways than in earlier publications [17]. For example, herein we describe kinetic changes for many more of the metabolic intermediates in the complex GSH synthesis pathway, including the SAM cycle, as well as in the purine salvage pathway. In addition, changes in lipid synthesis, oxidation and the urea cycle during RBC storage have not previously been reported. Potentially important findings from these unstudied pathways include the observation that one or both of the mechanisms for remethylating homocysteine to form methionine are ineffective during RBC storage, possibly impairing methylation reactions in stored AS1-RBCs.
This work also generated observations suggesting future correlative investigations. For example, D’Alessandro [41] observed a combination of morphological, metabolic and proteomic changes in SAGM-RBCs that commenced about day 14 of storage. While the current work was confined to metabolomic investigations, we identified a number of metabolites that showed marked inflection points around day 14, including alpha-hydroxyisocaproate, arachidonate, cysteine, docosahexaenoate, docosapentaenoate, hypoxanthine and inosine. Whether any of these are markers or drivers of morphological or proteomic changes during storage will require future studies.
Comparison with studies of stored murine RBCs
The mouse transfusion model, using inbred and genetically engineered mouse strains, is a particularly powerful system for dissecting molecular mechanisms underlying differences in RBC storage, function, survival, and efficacy following transfusion [55, 67–69]. We previously utilized this model to identify metabolic patterns that correlated with good storage (RBCs from C57BL/6 mice) or poor storage (RBCs from FVB mice) [70]. Since the present data utilized the same analysis platform, we can compare the murine results with metabolic changes of human RBCs stored in AS-1.
In glycolysis, changes in glucose, 3PG, 2,3-DPG, and lactate were similar between both mouse strains and human donors. Furthermore, RBCs from good-storing C57BL/6 mice and human donors showed comparable increases in pyruvate during storage. However, FVB mice showed a decrease in pyruvate during storage which could suggest reduced pyruvate kinase activity and lower ATP synthesis in RBCs from this poor-storing strain.
Although biochemicals of PPP were not studied in mouse RBCs, metabolites in the GSH pathway were quantified and showed many similarities between both mouse strains and human RBCs. There were also differences, including increases in GSH in C57BL/6 mice which contrast with decreases seen in human and poor-storing FVB RBCs. While both mouse strains, like humans, showed reductions in GSSG during storage, the GSH/GSSG ratio increased in C57BL/6 mice suggesting an increase in reducing power during storage. While the underlying reasons for this finding are unclear, C57BL/6 mice also had significantly higher levels of ophthalmate than did FVB mice; this observation suggests that good-storing C57BL/6 mice have preserved levels of GCS and GS activity, which is not seen in either FVB or human RBCs. The preservation of GSH antioxidant activity is likely one of the underlying reasons that C57BL/6 mice had much lower levels of oxidized lipids than did FVB mice. Although human RBCs did not show a similar increase in the GSH/GSSG ratio, they showed stable levels of 9,10-epoxystearate and oxidized lipids, more consistent with the pattern seen in C57BL/6 than FVB RBCs. RBCs from both strains as well as human donors showed elevations in AA during storage, which may occur following membrane lipid oxidation.
Metabolites of the purine salvage pathway showed similar kinetics in RBCs from C57BL/6 and FVB mice, as well as in human donors. In all cases, the elevated levels of adenine in the storage solution appeared to drive flux through this pathway, resulting in elevated production of biochemicals in the pathway up to xanthine. However, beyond that point murine RBCs showed elevations of urate indicating that they maintain xanthine oxidase activity during storage, in contrast to human RBCs.
This comparison indicates that murine RBCs represent a powerful and tractable system for modeling the changes that occur during RBC storage, and for linking those changes to physiologic effects of transfusion [71].
Future opportunities
In conclusion, the results of global metabolic profiling of AS-1 RBCs have revealed a number of biochemical alterations in banked RBCs that may affect RBC viability during storage as well as physiologic effects of stored RBCs on transfusion recipients. Some of the alterations were also identified in stored SAGM-RBCs, although others have not previously been described. Our analysis is somewhat limited since the present metabolomics platform only determines relative changes in biochemical levels. We are addressing this issue with ongoing studies, and anticipate that when absolute molar changes for each metabolite are known specific alterations in enzyme activity can be calculated based on known Km and Vmax parameters. Based on the specifically detected perturbations of biochemical pathways, future opportunities have been identified to more clearly pinpoint the metabolic defects during RBC storage, to develop biomarkers for donor screening and prerelease RBC testing, and to develop improved RBC storage solutions and methodologies. Additionally, the data demonstrate potentially important uses for murine RBC storage studies to further optimize transfusion therapies.
Acknowledgements
This work was supported by R01 HL095479-01 and by an administrative supplement for metabolomics studies from the NIH Common Fund (both to JDR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- 1.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39(3):277–281. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91(1):13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 3.Bosman GJ, Lasonder E, Luten M, Roerdinkholder-Stoelwinder B, Novotny VM, Bos H, De Grip WJ. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48(5):827–835. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 4.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinalducci S, D'Amici GM, Blasi B, Zolla L. Oxidative stress-dependent oligomeric status of erythrocyte peroxiredoxin II (PrxII) during storage under standard blood banking conditions. Biochimie. 2011;93(5):845–853. doi: 10.1016/j.biochi.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 7.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 8.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51(4):859–866. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 10.Klein HG, Anstee DJ. Mollison's Blood Transfusion in Clinical Medicine. 11th ed. Malden Massachusetts: Blackwell Publishing Inc; 2005. [Google Scholar]
- 11.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for "omics" analyses. Blood Transfus. 2012;10(Suppl 2):s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cluitmans JC, Hardeman MR, Dinkla S, Brock R, Bosman GJ. Red blood cell deformability during storage: towards functional proteomics and metabolomics in the Blood Bank. Blood Transfus. 2012;10(Suppl 2):s12–s18. doi: 10.2450/2012.004S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamshidi N, Palsson BO. Systems biology of the human red blood cell. Blood Cells Mol Dis. 2006;36(2):239–247. doi: 10.1016/j.bcmd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Nishino T, Yachie-Kinoshita A, Hirayama A, Soga T, Suematsu M, Tomita M. In silico modeling and metabolome analysis of long-stored erythrocytes to improve blood storage methods. J Biotechnol. 2009;144(3):212–223. doi: 10.1016/j.jbiotec.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Bordbar A, Jamshidi N, Palsson BO. iAB-RBC-283: A proteomically derived knowledgebase of erythrocyte metabolism that can be used to simulate its physiological and pathophysiological states. BMC Syst Biol. 2011;5:110. doi: 10.1186/1752-0509-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308(14):1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 17.Gevi F, D'Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–180. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Alexander JT, El-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, Sutliff RL, Roback JD. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53(11):2619–2628. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess JR, Greenwalt TG. Storage of red blood cells: new approaches. Transfus Med Rev. 2002;16(4):283–295. doi: 10.1053/tmrv.2002.35212. [DOI] [PubMed] [Google Scholar]
- 20.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96(2):93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 21.Messana I, Misiti F, el-Sherbini S, Giardina B, Castagnola M. Quantitative determination of the main glucose metabolic fluxes in human erythrocytes by 13C- and 1H-MR spectroscopy. J Biochem Biophys Methods. 1999;39(1–2):63–84. doi: 10.1016/s0165-022x(99)00005-6. [DOI] [PubMed] [Google Scholar]
- 22.Messana I, Ferroni L, Misiti F, Girelli G, Pupella S, Castagnola M, Zappacosta B, Giardina B. Blood bank conditions and RBCs: the progressive loss of metabolic modulation. Transfusion. 2000;40(3):353–360. doi: 10.1046/j.1537-2995.2000.40030353.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuchel PW, Philp DJ. Isotopomer subspaces as indicators of metabolic-pathway structure. J Theor Biol. 2008;252(3):391–401. doi: 10.1016/j.jtbi.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, Spitalnik SL. Glucose-6- phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105(4):271–282. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 26.Francis RO, Jhang J, Hendrickson JE, Zimring JC, Hod EA, Spitalnik SL. Frequency of glucose-6-phosphate dehydrogenase-deficient red blood cell units in a metropolitan transfusion service. Transfusion. 2012;53(3):606–611. doi: 10.1111/j.1537-2995.2012.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baysal E, Rice-Evans C. Modulation of iron-mediated oxidant damage in erythrocytes by cellular energy levels. Free Radic Res Commun. 1987;3(1–5):227–232. doi: 10.3109/10715768709069787. [DOI] [PubMed] [Google Scholar]
- 28.Rice-Evans C, Baysal E. Iron-mediated oxidative stress in erythrocytes. Biochem J. 1987;244(1):191–196. doi: 10.1042/bj2440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geenen S, Taylor PN, Snoep JL, Wilson ID, Kenna JG, Westerhoff HV. Systems biology tools for toxicology. Arch Toxicol. 2012;86(8):1251–1271. doi: 10.1007/s00204-012-0857-8. [DOI] [PubMed] [Google Scholar]
- 30.Reed MC, Thomas RL, Pavisic J, James SJ, Ulrich CM, Nijhout HF. A mathematical model of glutathione metabolism. Theor Biol Med Model. 2008;5:8. doi: 10.1186/1742-4682-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975;250(4):1422–1426. [PubMed] [Google Scholar]
- 32.Raftos JE, Whillier S, Kuchel PW. Glutathione synthesis and turnover in the human erythrocyte: alignment of a model based on detailed enzyme kinetics with experimental data. J Biol Chem. 2010;285(31):23557–23567. doi: 10.1074/jbc.M109.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills BJ, Lang CA. Differential distribution of free and bound glutathione and cyst(e)ine in human blood. Biochem Pharmacol. 1996;52(3):401–406. doi: 10.1016/0006-2952(96)00241-9. [DOI] [PubMed] [Google Scholar]
- 34.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51(7):1450–1459. doi: 10.1111/j.1537-2995.2010.03026.x. [DOI] [PubMed] [Google Scholar]
- 35.Kiessling K, Roberts N, Gibson JS, Ellory JC. A comparison in normal individuals and sickle cell patients of reduced glutathione precursors and their transport between plasma and red cells. Hematol J. 2000;1(4):243–249. doi: 10.1038/sj.thj.6200033. [DOI] [PubMed] [Google Scholar]
- 36.Richie JP, Jr, Abraham P, Leutzinger Y. Long-term stability of blood glutathione and cysteine in humans. Clin Chem. 1996;42(7):1100–1105. [PubMed] [Google Scholar]
- 37.Whillier S, Garcia B, Chapman BE, Kuchel PW, Raftos JE. Glutamine and alphaketoglutarate as glutamate sources for glutathione synthesis in human erythrocytes. FEBS J. 2011;278(17):3152–3163. doi: 10.1111/j.1742-4658.2011.08241.x. [DOI] [PubMed] [Google Scholar]
- 38.Geenen S, Michopoulos F, Kenna JG, Kolaja KL, Westerhoff HV, Wilson I. HPLC-MS/MS methods for the quantitative analysis of ophthalmic acid in rodent plasma and hepatic cell line culture medium. J Pharm Biomed Anal. 2011;54(5):1128–1135. doi: 10.1016/j.jpba.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 39.Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T, Kakazu Y, Ishikawa T, Robert M, Nishioka T, Tomita M. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281(24):16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 40.D'Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and interactome: an update. J Proteome Res. 2010;9(1):144–163. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 41.D'Alessandro A, D'Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–115. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linseisen J, Wolfram G, Miller AB. Plasma 7beta-hydroxycholesterol as a possible predictor of lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1630–1637. [PubMed] [Google Scholar]
- 43.Strunecka A, Folk P. Phospholipid biosynthesis in mature human erythrocytes. Gen Physiol Biophys. 1988;7(2):205–216. [PubMed] [Google Scholar]
- 44.Tehlivets O, Malanovic N, Visram M, Pavkov-Keller T, Keller W. S-adenosyl-Lhomocysteine hydrolase and methylation disorders: yeast as a model system. Biochim Biophys Acta. 2013;1832(1):204–215. doi: 10.1016/j.bbadis.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishibe H. Urea cycle enzymes in human erythrocytes. Clinica Chimica Acta. 1974;50:305–310. [Google Scholar]
- 46.Schuster S, Kenanov D. Adenine and adenosine salvage pathways in erythrocytes and the role of S-adenosylhomocysteine hydrolase. A theoretical study using elementary flux modes. FEBS J. 2005;272(20):5278–5290. doi: 10.1111/j.1742-4658.2005.04924.x. [DOI] [PubMed] [Google Scholar]
- 47.Roede JR, Uppal K, Park Y, Lee K, Tran V, Walker D, Strobel FH, Rhodes SL, Ritz B, Jones DP. Serum metabolomics of slow vs. rapid motor progression Parkinson's disease: a pilot study. PLoS One. 2013;8(10):e77629. doi: 10.1371/journal.pone.0077629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. doi: 10.1186/1471-2105-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers SC, Ross JG, d'Avignon A, Gibbons LB, Gazit V, Hassan MN, McLaughlin D, Griffin S, Neumayr T, Debaun M, DeBaun MR, Doctor A. Sickle hemoglobin disturbs normal coupling among erythrocyte O2 content, glycolysis, and antioxidant capacity. Blood. 2013;121(9):1651–1662. doi: 10.1182/blood-2012-02-414037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011;51(7):1574–1579. doi: 10.1111/j.1537-2995.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 51.Dumont LJ, Herschel L, Roback JD, Zimring JC, Gray AD. Changes in the metabolomic profiles of stored RBC following treatment with Rejuvesol® Solution. Transfusion. 2012;52(Suppl.):78A. [Google Scholar]
- 52.Lang CA, Mills BJ, Mastropaolo W, Liu MC. Blood glutathione decreases in chronic diseases. J Lab Clin Med. 2000;135(5):402–405. doi: 10.1067/mlc.2000.105977. [DOI] [PubMed] [Google Scholar]
- 53.Raftos JE, Whillier S, Chapman BE, Kuchel PW. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int J Biochem Cell Biol. 2007;39(9):1698–1706. doi: 10.1016/j.biocel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Berkeley LI, Cohen JF, Crankshaw DL, Shirota FN, Nagasawa HT. Hepatoprotection by L-cysteine-glutathione mixed disulfide, a sulfhydryl-modified prodrug of glutathione. J Biochem Mol Toxicol. 2003;17(2):95–97. doi: 10.1002/jbt.10069. [DOI] [PubMed] [Google Scholar]
- 55.Stowell SR, Smith NH, Zimring JC, Fu X, Palmer AF, Fontes J, Banerjee U, Yazer MH. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;53(10):2248–2257. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- 56.Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009;583(8):1215–1225. doi: 10.1016/j.febslet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 57.Perna AF, Ingrosso D, Zappia V, Galletti P, Capasso G, De Santo NG. Enzymatic methyl esterification of erythrocyte membrane proteins is impaired in chronic renal failure. Evidence for high levels of the natural inhibitor S-adenosylhomocysteine. J Clin Invest. 1993;91(6):2497–2503. doi: 10.1172/JCI116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upchurch GR, Jr, Welch GN, Loscalzo J. Homocysteine, EDRF, and endothelial function. J Nutr. 1996;126(4 Suppl):1290S–1294S. doi: 10.1093/jn/126.suppl_4.1290S. [DOI] [PubMed] [Google Scholar]
- 59.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107(2):566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiss C, Kroncke KD, Hogg N, Feelisch M, Kelm M. Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood. 2012;120(20):4229–4237. doi: 10.1182/blood-2012-07-442277. [DOI] [PubMed] [Google Scholar]
- 61.Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FM, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase. Circ Res. 2008;103(9):957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107(7):2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 63.Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol. 2003;284(5):H1577–H1584. doi: 10.1152/ajpheart.00665.2002. [DOI] [PubMed] [Google Scholar]
- 64.Kanias T, Wang L, Lippert A, Kim-Shapiro DB, Gladwin MT. Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis. Transfusion. 2013;53(5):981–999. doi: 10.1111/j.1537-2995.2012.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simmonds HA, Fairbanks LD, Duley JA, Morris GS. ATP formation from deoxyadenosine in human erythrocytes: evidence for a hitherto unidentified route involving adenine and Sadenosylhomocysteine hydrolase. Biosci Rep. 1989;9(1):75–85. doi: 10.1007/BF01117513. [DOI] [PubMed] [Google Scholar]
- 66.Rinalducci S, D'Amici GM, Blasi B, Vaglio S, Grazzini G, Zolla L. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51(7):1439–1449. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 67.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110(7):2736–2743. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 68.Hod EA, Cadwell CM, Liepkalns JS, Zimring JC, Sokol SA, Schirmer DA, Jhang J, Spitalnik SL. Cytokine storm in a mouse model of IgG-mediated hemolytic transfusion reactions. Blood. 2008;112(3):891–894. doi: 10.1182/blood-2008-01-132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimring JC, Hair GA, Chadwick TE, Deshpande SS, Anderson KM, Hillyer CD, Roback JD. Nonhemolytic antibody-induced loss of erythrocyte surface antigen. Blood. 2005;106(3):1105–1112. doi: 10.1182/blood-2005-03-1040. [DOI] [PubMed] [Google Scholar]
- 70.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, Hod EA, Hendrickson JE, Roback JD, Spitalnik SL. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2013;54(1):137–148. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimring JC, Spitalnik SL. On the appropriate use and interpretation of animal models in transfusion medicine research. Transfusion. 2013;53(10):2334–2339. doi: 10.1111/trf.12131. [DOI] [PubMed] [Google Scholar]